Optimization of Solar Photocatalytic Degradation of Chloroxylenol Using TiO2, Er3+/TiO2, and Ni2+/TiO2 via the Taguchi Orthogonal Array Technique
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD) of TiO2, Er3+/TiO2 and Ni2+/TiO2 NPs
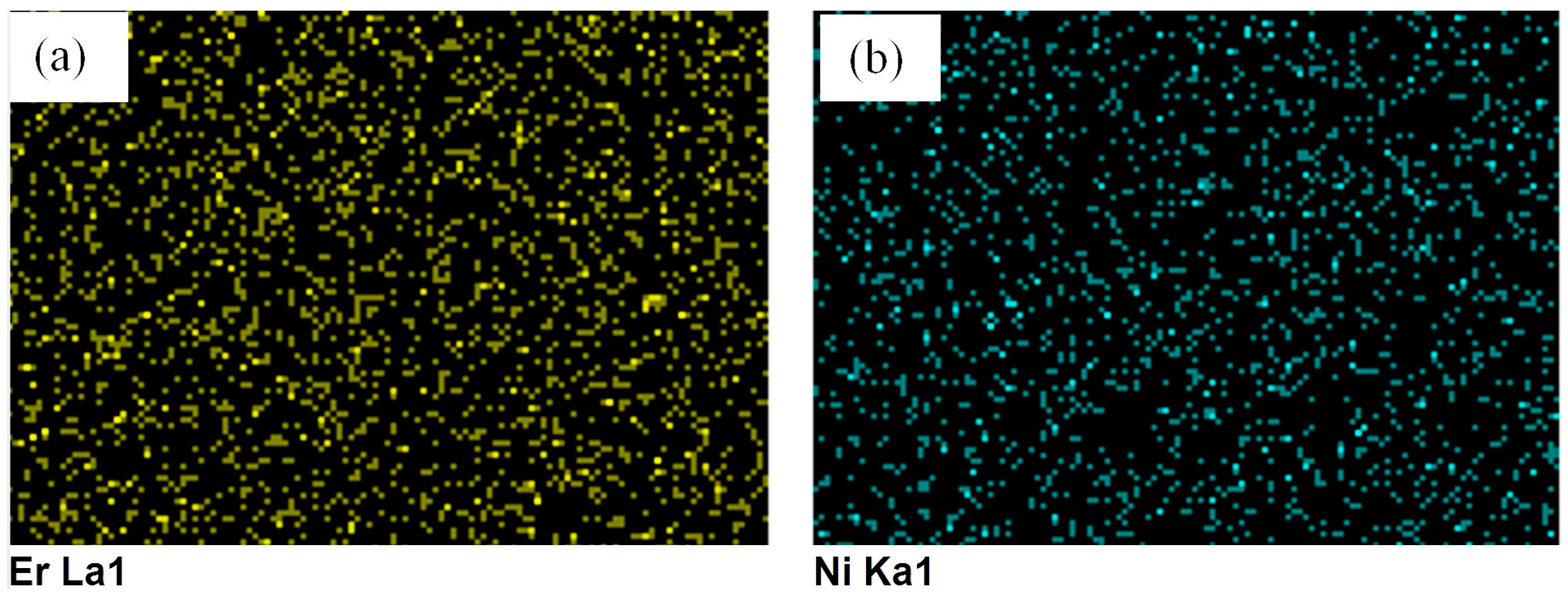
2.2. Catalyst Surface Morphology of TiO2, Er3+/TiO2 and Ni2+/TiO2 NPs
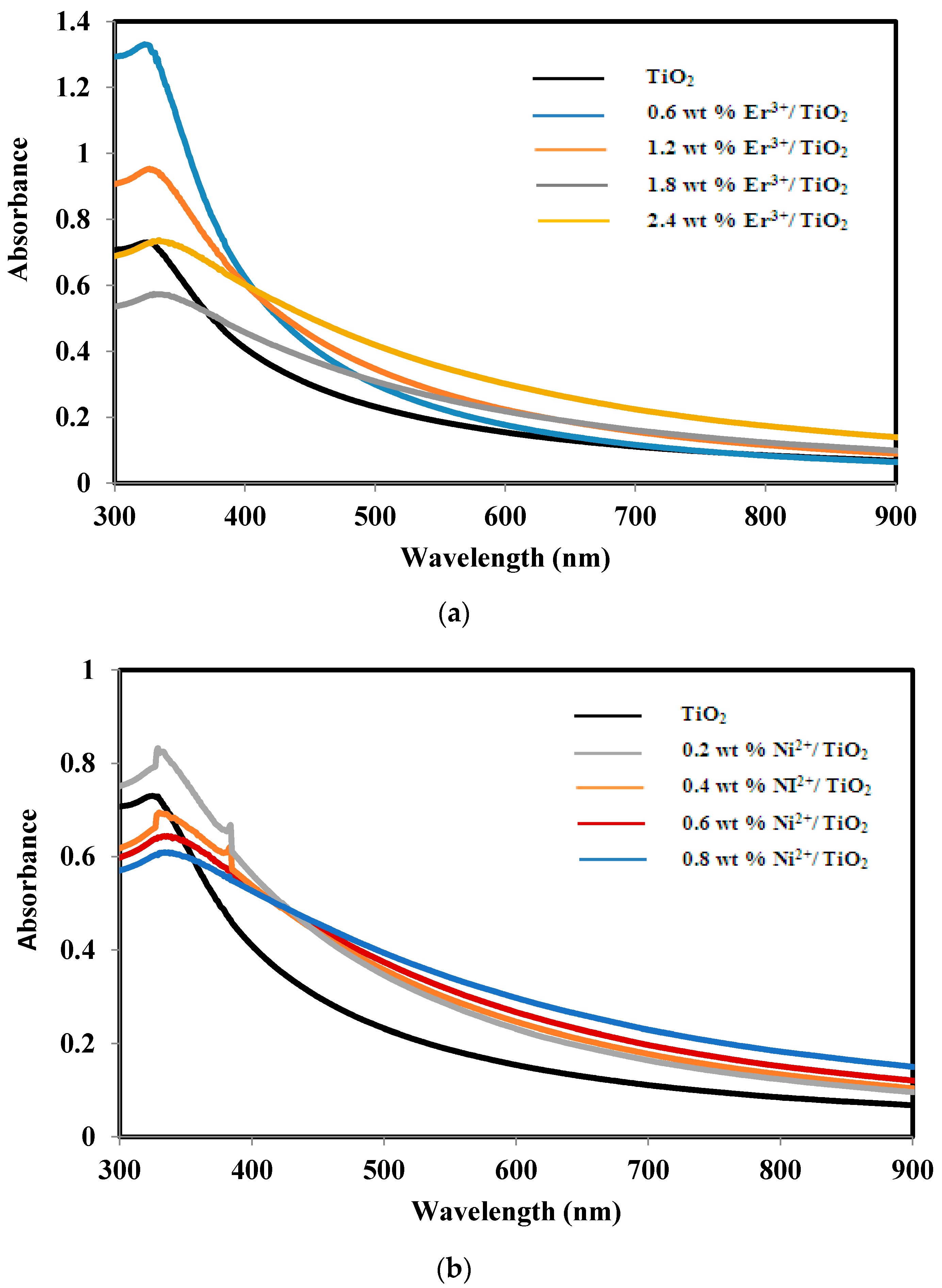
2.3. Absorbance and Band Gap of TiO2, Er3+/TiO2 and Ni2+/TiO2 NPs
2.4. Experimental Design
2.4.1. Response of Solar Photocatalytic Degradation Experiments and Modeling
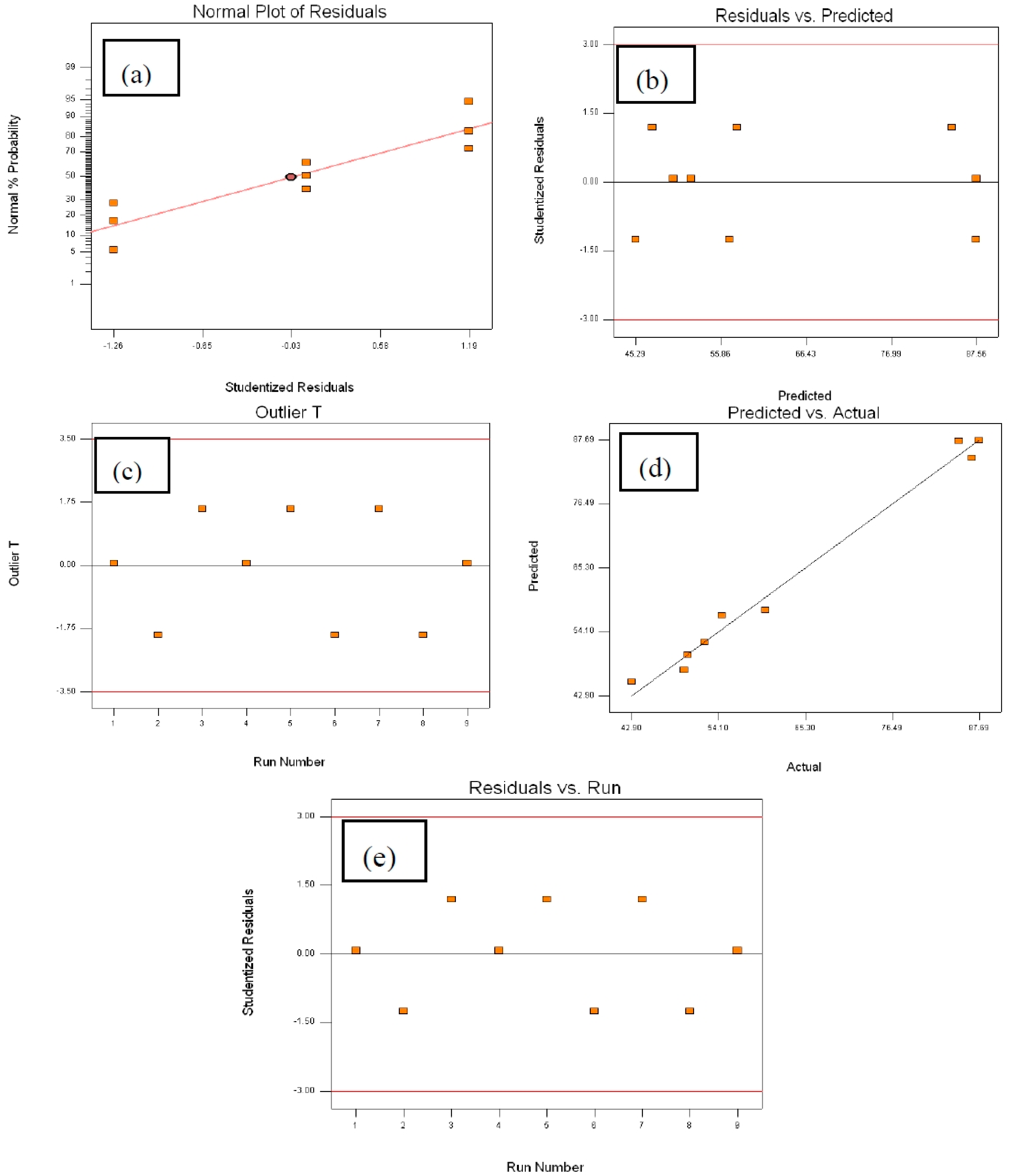
2.4.2. Analysis of Variance (ANOVA)
2.4.3. Optimal Conditions for Maximum DE% and Model Validation
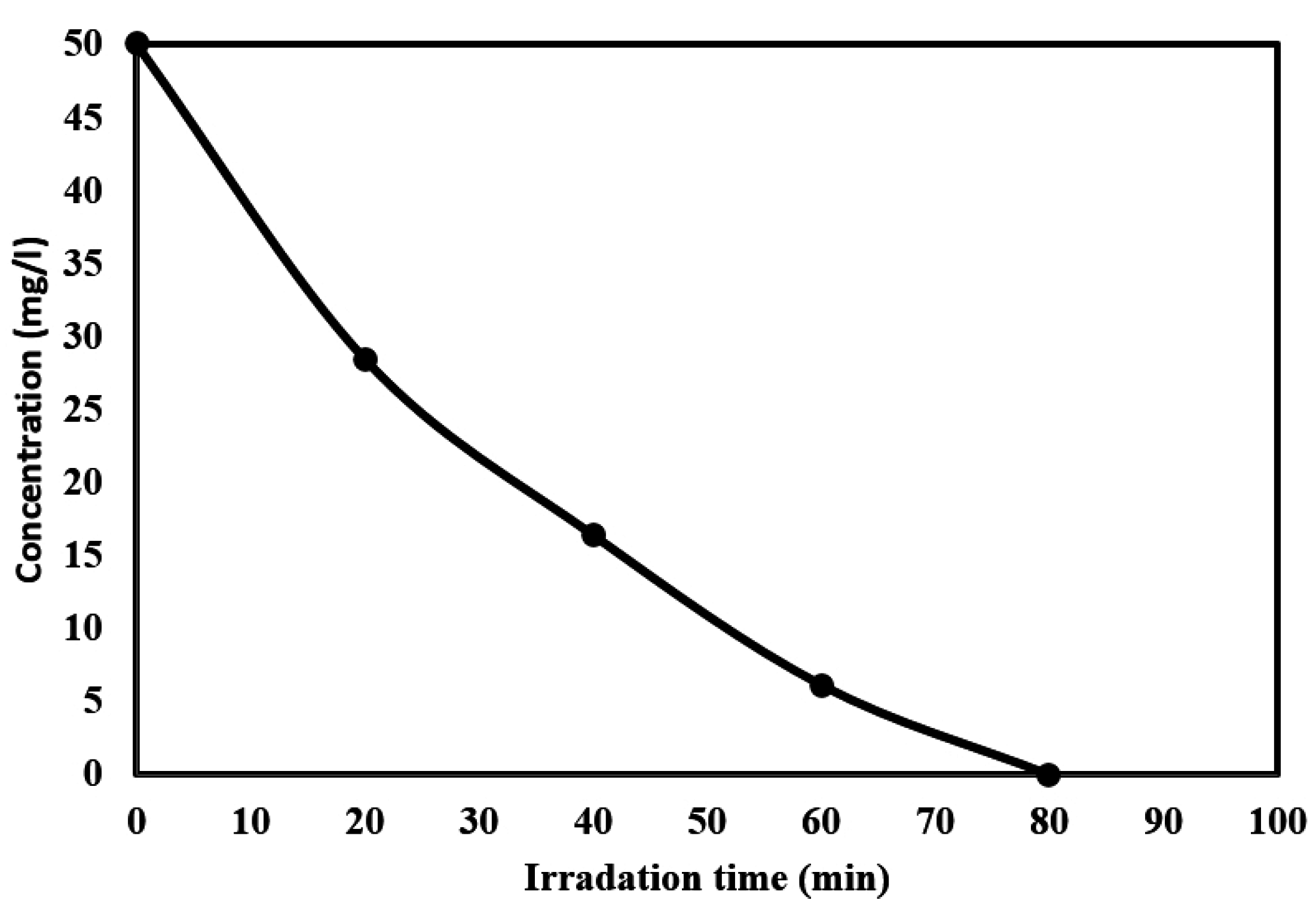
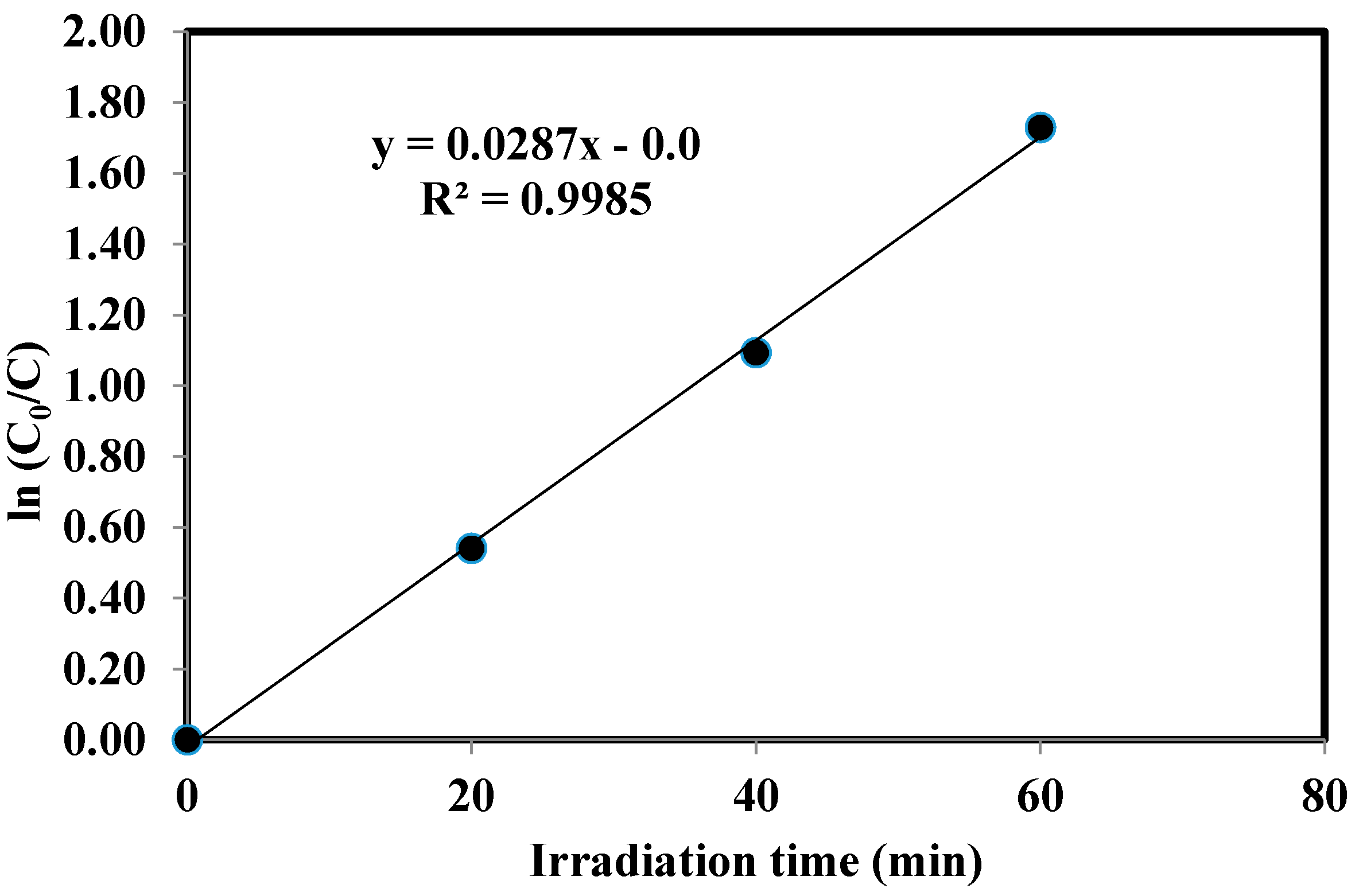
2.5. Kinetics of the Photocatalytic Degradation of Chloroxylenol Using TiO2 NPs
Application of Er3+/TiO2 and Ni2+/TiO2 NPs in the Photocatalytic Degradation of Chloroxylenol
3. Materials and Methods
3.1. Materials
3.2. Preparation of Er3+/TiO2 and Ni2+/TiO2 NPs
3.3. Characterization of Er3+/TiO2 and Ni2+/TiO2 NPs
3.4. Design of Experiment Using Taguchi Orthogonal Array L9 (34)
3.5. Photocatalytic Reactor and Experimental Procedures
3.6. Analytical Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruch, M.K. Chloroxylenol: An old–new antimicrobial. In Handbook of Disinfectants and Antiseptics; Marcel Dekker: New York, NY, USA, 1996; pp. 265–294. [Google Scholar]
- Wilson, M.; Mowad, C. Chloroxylenol. Dermatitis 2007, 18, 120–121. [Google Scholar] [PubMed]
- Kim, B.; Anderson, J.; Mueller, S.; Gaines, W.; Kendall, A. Literature review-efficacy of various disinfectants against Legionella in water systems. Water Res. 2002, 36, 4433–4444. [Google Scholar] [CrossRef]
- Yamano, T.; Shimizu, M.; Noda, T. Allergenicity evaluation of Bioban CS-1135 in experimental animals. Contact Dermat. 2004, 50, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.; Plagellat, C.; Brändli, R.; de Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Fate and removal of polycyclic musks, UV filters and biocides during wastewater treatment. Water Res. 2006, 40, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Song, L.; Hu, J.; Ong, S.; Ng, W. Post-treatment of banknote printing works wastewater ultrafiltration concentrate. Water Res. 2004, 38, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Kestioglu, K.; Yonar, T.; Azbar, N. Feasibility of physico-chemical treatment and advanced oxidation processes (AOPs) as a means of pretreatment of olive mill effluent (OME). Process Biochem. 2005, 40, 2409–2416. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; He, Z.; Li, Y.; Chen, J.; Li, C. Degradation of the biocide 4-chloro-3, 5-dimethylphenol in aqueous medium with ozone in combination with ultraviolet irradiation: Operating conditions influence and mechanism. Chemosphere 2009, 77, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, K.M.; Al-Fassi, F.A.; Al-Hazmi, N.M. Optimization of chloroxylenol degradation by Aspergillus niger using Plackett-Burman design and response surface methodology. Rom. Biotechnol. Lett. 2013, 18, 7983–7994. [Google Scholar]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Estrellan, C.R.; Salim, C.; Hinode, H. Photocatalytic activity of sol–gel derived TiO2 Co-doped with iron and niobium. React. Kinet. Catal. Lett. 2009, 98, 187–192. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, K.; Yu, J.; Peng, P.; Cao, F.; Li, X.; Zhou, X.C. Effects of rare earth Ce doping on the structure and photocatalytic performance of ZnO. Acta Phys. Chim. Sin. 2011, 27, 505–512. [Google Scholar]
- Minero, C.; Pelizzatti, E.; Sega, M.; Friberg, S.E.; Sjoblom, J. The role of humic substances in photocatalytic degradation of water contaminants. J. Dispers. Sci. Technol. 1999, 20, 643–661. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Optimization of process parameters using D-optimal design for synthesis of ZnO nanoparticles via sol–gel technique. J. Ind. Eng. Chem. 2013, 19, 99–105. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Chai, P.V.; Takriff, M.S.; Benamor, A.; Mohammad, A.W. Optimization of nickel oxide nanoparticle synthesis through the sol–gel method using Box–Behnken design. Mater. Des. 2015, 86, 948–956. [Google Scholar] [CrossRef]
- Chung, T.; Ba-Abbad, M.M.; Mohammad, A.W.; Hairom, N.H.; Benamor, A. Synthesis of minimal-size ZnO nanoparticles through sol–gel method: Taguchi design optimization. Mater. Des. 2015, 87, 780–787. [Google Scholar] [CrossRef]
- Hong, G.B.; Ma, C.M. Photocatalytic degradation of indoor air pollutants by Pt-TiO2. J. Nanomater. 2012, 2012, 88–93. [Google Scholar] [CrossRef]
- Chong, M.N.; Lei, S.; Jin, B.; Saint, C.; Chow, C.W. Optimisation of an annular photoreactor process for degradation of Congo Red using a newly synthesized titania impregnated kaolinite nano-photocatalyst. Sep. Purif. Technol. 2009, 67, 355–363. [Google Scholar] [CrossRef]
- Nikazar, M.; Farahani, S.J.; Soltani, M.R. Photocatalytic degradation of ethylene dichloride in water using nano TiO2 supported on clinoptilolite as a photocatalyst. Adv. Mater. Phys. Chem. 2013, 2, 274–276. [Google Scholar] [CrossRef]
- Marandi, R.; Sharif, A.A.M.; Olya, M.E.; Moradi, R. Preparation, characterization of NiFe2O4 nanoparticles supported on clinoptilolite and optimization of photocatalytic degradation of pollutants in textile wastewater by Taguchi experimental design. J. Basic. Appl. Sci. Res. 2013, 3, 443–456. [Google Scholar]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. A comparison of central composite design and Taguchi method for optimizing fenton process. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Mohammed, W.; Marzoog, T.R.; Al-Amiery, A.; Kadhumc, A.; Mohamad, A. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. [Google Scholar]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.K.; Nassir, M.H.; Al-Amiery, A.A. Impact of sulfuric acid treatment of halloysite on physico-chemic property modification. Materials 2016, 9, 620. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed]
- Wasmi, W.; Al-Amiery, A.; Kadhum, A.; Takriff, M.; Mohamad, A. Synthesis of vanadium pentoxide nanoparticles as catalysts for the ozonation of palm oil. Ozone Sci. Eng. 2016, 38, 36–41. [Google Scholar] [CrossRef]
- Obayes, H.; Al-Gebori, A.; Khazaal, S.; Jarad, A.; Alwan, G.; Al-Amiery, A. Hypothetical design of carbon nanotube materials based on [8] circulene. J. Nanoelectron. Optoelectron. 2015, 10, 711–716. [Google Scholar] [CrossRef]
- Obayes, H.; Al-Amiery, A.; Jaffar, H.; Musa, A.; Kadhum, A.; Mohamad, A. Theoretical study for the preparation of sub-carbon nano tubes from the cyclic polymerization reaction of two molecules from corannulene, coronene and circulene aromatic compounds. J. Comput. Theor. Nanosci. 2013, 10, 2453–2457. [Google Scholar] [CrossRef]
- Wasmi, W.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Novel approach: Tungsten oxide nanoparticle as a catalyst for malonic acid ester synthesis via ozonolysis. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Box, J.G.E.P.; Hunter, S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Schleife, A.; Rinke, P.; Bechstedt, F.; Walle, C. Enhanced Optical Absorption Due to Symmetry Breaking in TiO2(1−x) S2x Alloys. J. Phys. Chem. C 2013, 117, 4189–4193. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R. Semiconductor-catalyzed degradation of phenols with sunlight. Sol. Energy Mater. Sol. Cells 2008, 92, 1315–1321. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ahmed, A.S.; Chaman, M.; Khan, W.; Naqvi, A.H.; Azam, A. Structural and frequency dependent dielectric properties of Fe3+ doped ZnO nanoparticles. Mater. Res. Bull. 2012, 47, 3952–3958. [Google Scholar] [CrossRef]
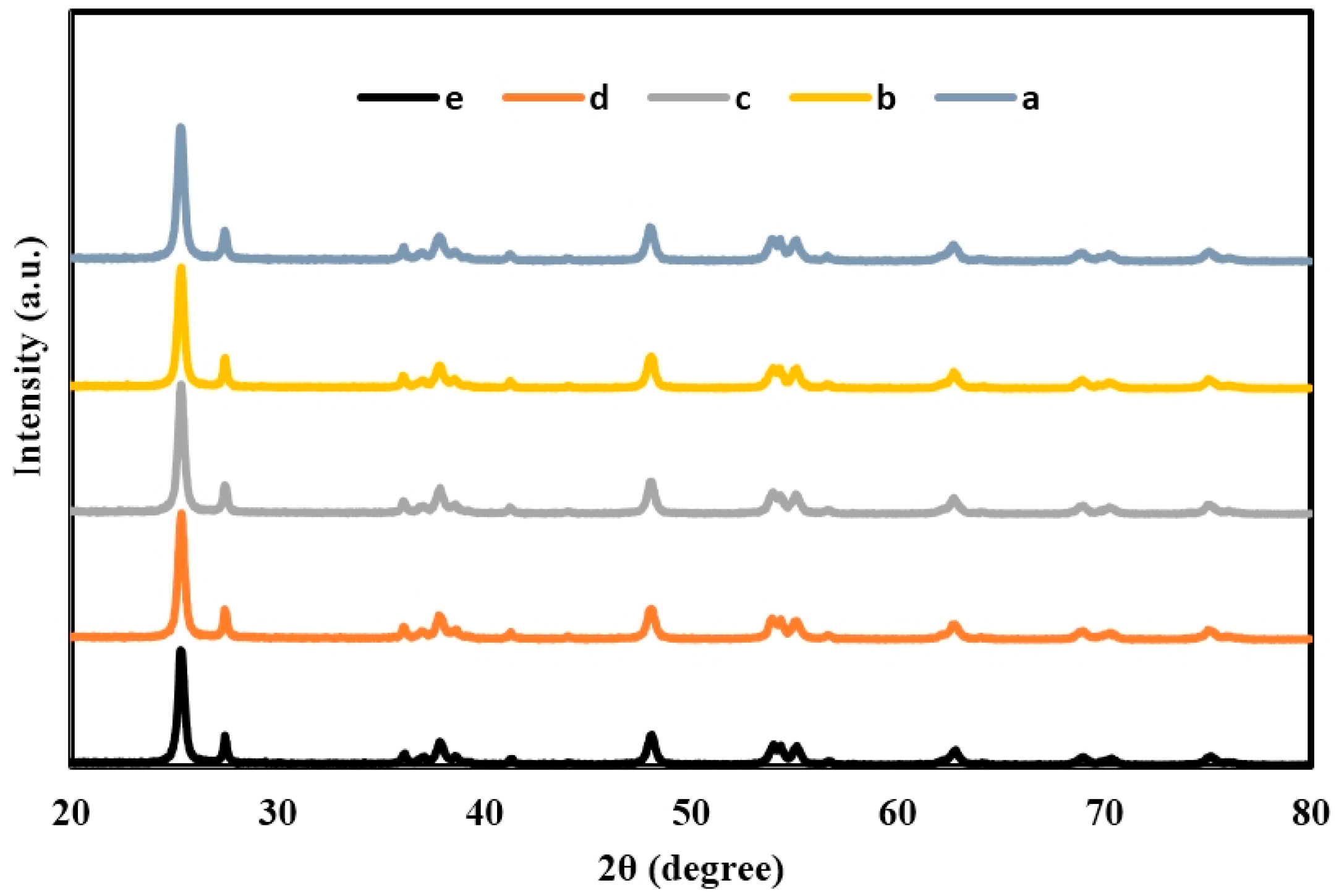
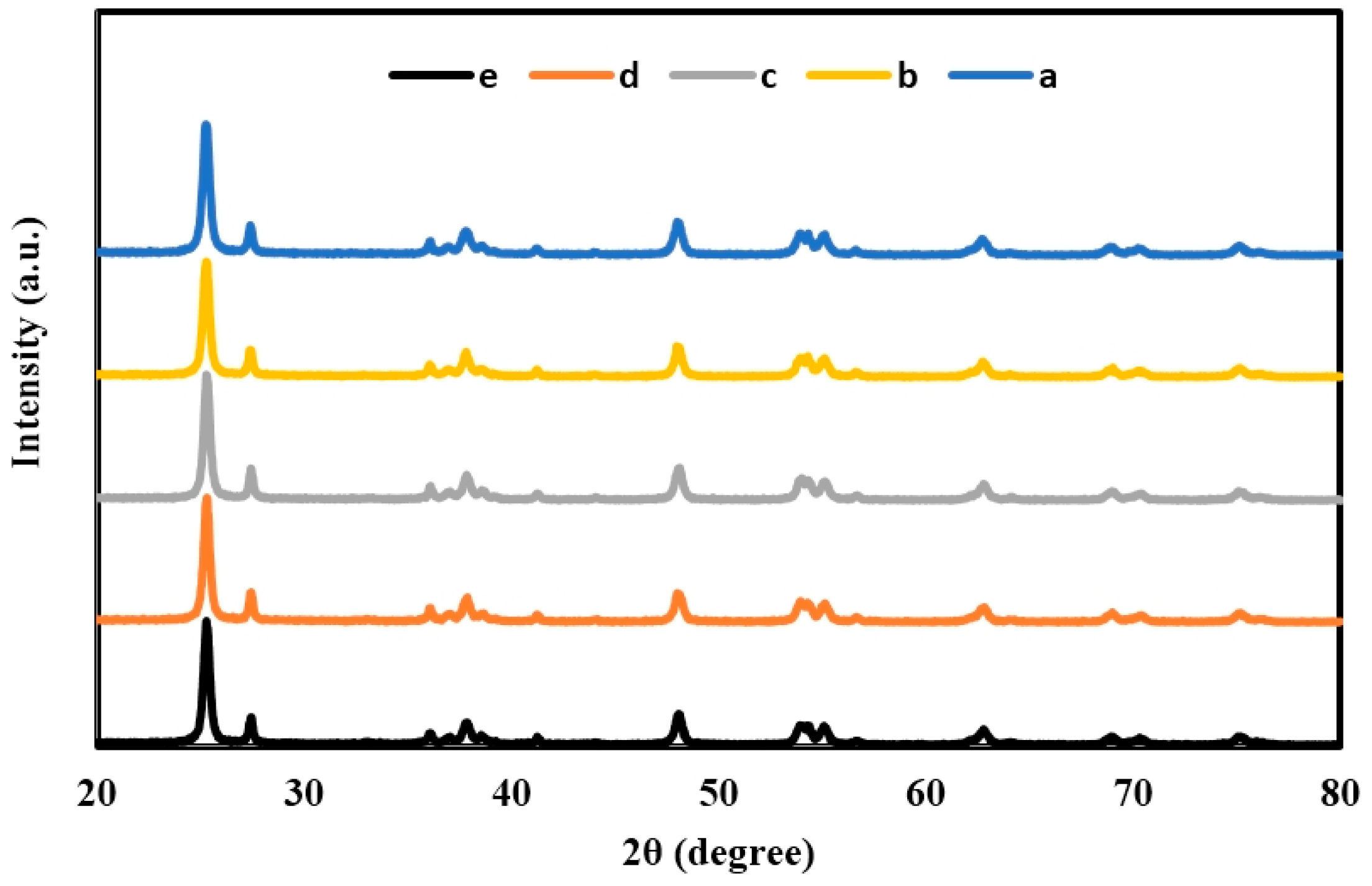
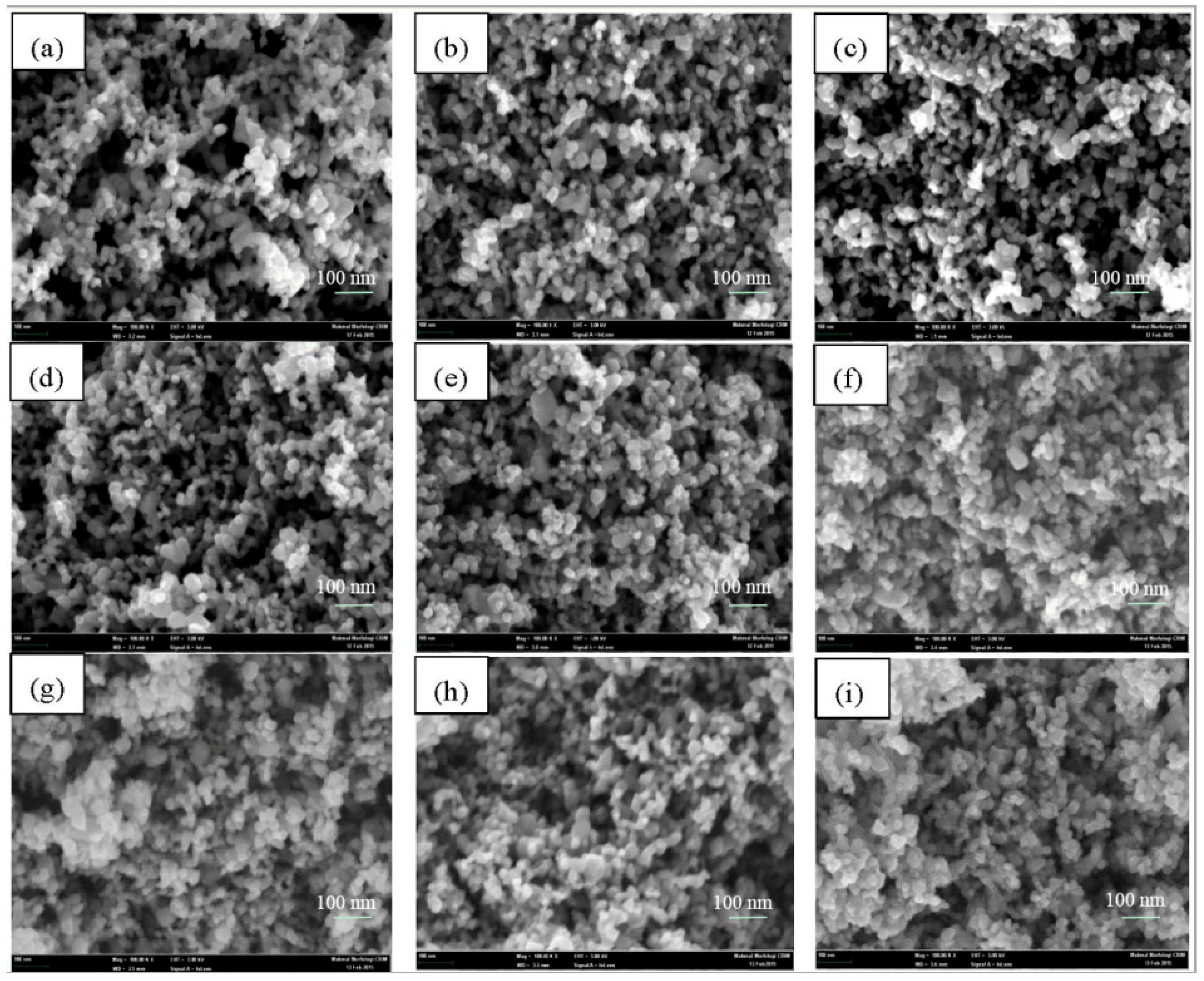
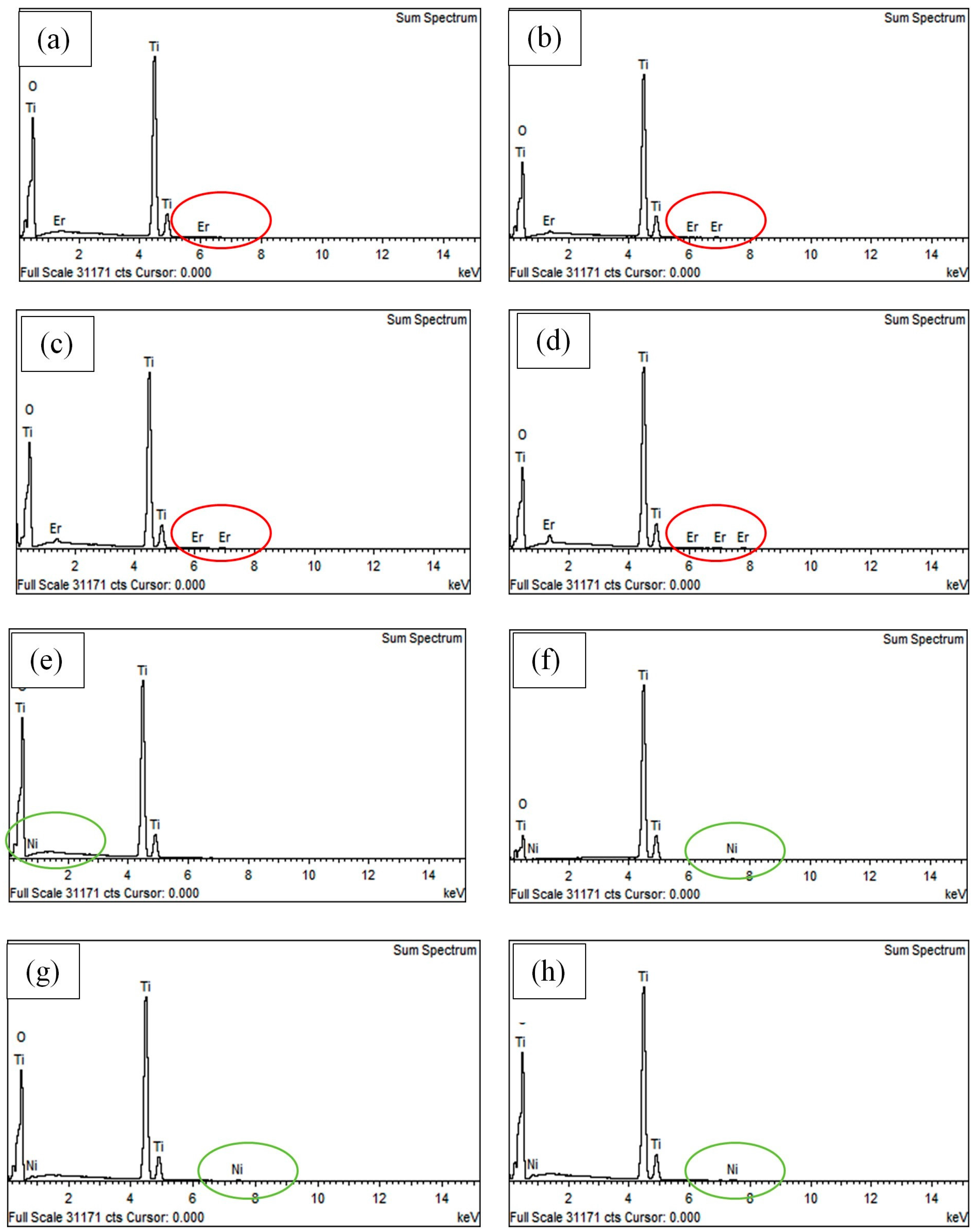



| 2θ | Intensity (Arbitrary Unit, a.u.) | ||||
|---|---|---|---|---|---|
| TiO2 | 0.6 wt % Er/TiO2 | 1.2 wt % Er/TiO2 | 1.8 wt % Er/TiO2 | 2.4 wt % Er/TiO2 | |
| 25.2712 | 2158 | 1984 | 1911 | 1879 | 1855 |
| 27.4136 | 515 | 498 | 493 | 481 | 474 |
| 37.3038 | 127 | 105 | 100 | 90 | 90 |
| 48.0908 | 555 | 536 | 481 | 478 | 473 |
| 53.8206 | 311 | 310 | 288 | 262 | 230 |
| 53.97 | 379 | 368 | 351 | 346 | 336 |
| 55.116 | 367 | 340 | 313 | 291 | 283 |
| 62.7142 | 292 | 285 | 272 | 249 | 241 |
| 68.8675 | 165 | 149 | 143 | 131 | 120 |
| 70.2128 | 145 | 114 | 109 | 108 | 104 |
| 75.0956 | 185 | 164 | 163 | 151 | 147 |
| 2θ | Intensity (Arbitrary Unit, a.u.) | ||||
|---|---|---|---|---|---|
| TiO2 | 0.2 wt % Ni/TiO2 | 0.4 wt % Ni/TiO2 | 0.6 wt % Ni/TiO2 | 0.8 wt % Ni/TiO2 | |
| 25.2712 | 2158 | 1964 | 1922 | 1866 | 1834 |
| 27.4136 | 515 | 504 | 464 | 440 | 410 |
| 37.3038 | 127 | 110 | 100 | 108 | 108 |
| 48.0908 | 555 | 536 | 514 | 509 | 483 |
| 53.8206 | 311 | 284 | 284 | 282 | 270 |
| 53.9700 | 379 | 348 | 347 | 343 | 316 |
| 55.1160 | 367 | 334 | 329 | 319 | 294 |
| 62.7142 | 292 | 254 | 254 | 253 | 242 |
| 68.8675 | 165 | 144 | 138 | 133 | 108 |
| 70.2128 | 145 | 122 | 113 | 111 | 106 |
| 75.0956 | 185 | 178 | 160 | 156 | 141 |
| Sample | Crystallite (nm) |
|---|---|
| TiO2 | 28.72 |
| 0.6 wt % Er3+/TiO2 | 28.84 |
| 1.2 wt % Er3+/TiO2 | 31.53 |
| 1.8 wt % Er3+/TiO2 | 33.93 |
| 2.4 wt % Er3+/TiO2 | 35.93 |
| 0.2 wt % Ni2+/TiO2 | 28.98 |
| 0.4 wt % Ni2+/TiO2 | 30.84 |
| 0.6 wt % Ni2+/TiO2 | 31.10 |
| 0.8 wt % Ni2+/TiO2 | 31.51 |
| Sample | Calculated Metal (wt %) | Actual Metal (wt %) by EDX |
|---|---|---|
| 0.6 wt % Er3+/TiO2 | 0.6 | 0.72 |
| 1.2 wt % Er3+/TiO2 | 1.2 | 1.31 |
| 1.8 wt % Er3+/TiO2 | 1.8 | 2.06 |
| 2.4 wt % Er3+/TiO2 | 2.4 | 2.45 |
| 0.2 wt % Ni2+/TiO2 | 0.2 | 0.16 |
| 0.4 wt % Ni2+/TiO2 | 0.4 | 0.31 |
| 0.6 wt % Ni2+/TiO2 | 0.6 | 0.59 |
| 0.8 wt % Ni2+/TiO2 | 0.8 | 0.74 |
| Sample | Band Gap Energy (eV) |
|---|---|
| TiO2 | 3.13 ± 0.02 |
| 0.6 wt % Er3+/TiO2 | 3.20 ± 0.02 |
| 1.2 wt % Er3+/TiO2 | 3.00 ± 0.02 |
| 1.8 wt % Er3+/TiO2 | 2.63 ± 0.02 |
| 2.4 wt % Er3+/TiO2 | 2.70 ± 0.02 |
| 0.2 wt % Ni2+/TiO2 | 2.60 ± 0.02 |
| 0.4 wt % Ni2+/TiO2 | 2.50 ± 0.02 |
| 0.6 wt % Ni2+/TiO2 | 2.47 ± 0.02 |
| 0.8 wt % Ni2+/TiO2 | 2.61 ± 0.02 |
| Standard | Run Number | A: Catalyst Loading (g/L) | B: pH | C: Concentartion (mg/L) | DE% (Actual) | DE% (Predicted) |
|---|---|---|---|---|---|---|
| 1 | 8 | 1 | 4 | 50 | 86.75 | 84.48 |
| 2 | 9 | 1 | 6 | 100 | 50.12 | 49.99 |
| 3 | 3 | 1 | 8 | 150 | 42.9 | 45.29 |
| 4 | 2 | 2 | 4 | 100 | 54.55 | 56.94 |
| 5 | 6 | 2 | 6 | 150 | 49.65 | 47.38 |
| 6 | 5 | 2 | 8 | 50 | 87.69 | 87.56 |
| 7 | 4 | 3 | 4 | 150 | 52.31 | 52.18 |
| 8 | 7 | 3 | 6 | 50 | 85.1 | 87.49 |
| 9 | 1 | 3 | 8 | 100 | 60.13 | 57.86 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2570.93 | 6 | 428.49 | 26.25 | 0.0371 |
| A | 54.95 | 2 | 27.48 | 1.68 | 0.3722 |
| B | 13.22 | 2 | 6.61 | 0.40 | 0.7120 |
| C | 2502.75 | 2 | 1251.38 | 76.66 | 0.0129 |
| Residual | 32.65 | 2 | 16.32 | – | – |
| Number of Experiment | Catalyst Loading (g/L) | pH | Concentration (mg/L) | DE% |
|---|---|---|---|---|
| 1 | 3 | 4 | 50 | 89.57 |
| 2 | 3 | 4 | 50 | 88.21 |
| 3 | 3 | 4 | 50 | 87.67 |
| Independent Variable | Factor | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Catalyst loading | A | 1 | 2 | 3 |
| pH | B | 4 | 6 | 8 |
| Initial concentration | C | 50 | 100 | 150 |
| Standard | Run Number | A: Catalyst Loading (g/L) | B: pH | C: Concentration (mg/L) |
|---|---|---|---|---|
| 1 | 8 | 1 | 4 | 50 |
| 2 | 9 | 1 | 6 | 100 |
| 3 | 3 | 1 | 8 | 150 |
| 4 | 2 | 2 | 4 | 100 |
| 5 | 6 | 2 | 6 | 150 |
| 6 | 5 | 2 | 8 | 50 |
| 7 | 4 | 3 | 4 | 150 |
| 8 | 7 | 3 | 6 | 50 |
| 9 | 1 | 3 | 8 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.J.; Kadhum, A.A.H.; Ba-Abbad, M.M.; Al-Amiery, A.A. Optimization of Solar Photocatalytic Degradation of Chloroxylenol Using TiO2, Er3+/TiO2, and Ni2+/TiO2 via the Taguchi Orthogonal Array Technique. Catalysts 2016, 6, 163. https://doi.org/10.3390/catal6100163
Mohammed AJ, Kadhum AAH, Ba-Abbad MM, Al-Amiery AA. Optimization of Solar Photocatalytic Degradation of Chloroxylenol Using TiO2, Er3+/TiO2, and Ni2+/TiO2 via the Taguchi Orthogonal Array Technique. Catalysts. 2016; 6(10):163. https://doi.org/10.3390/catal6100163
Chicago/Turabian StyleMohammed, Ahmed J., Abdul Amir H. Kadhum, Muneer M. Ba-Abbad, and Ahmed A. Al-Amiery. 2016. "Optimization of Solar Photocatalytic Degradation of Chloroxylenol Using TiO2, Er3+/TiO2, and Ni2+/TiO2 via the Taguchi Orthogonal Array Technique" Catalysts 6, no. 10: 163. https://doi.org/10.3390/catal6100163
APA StyleMohammed, A. J., Kadhum, A. A. H., Ba-Abbad, M. M., & Al-Amiery, A. A. (2016). Optimization of Solar Photocatalytic Degradation of Chloroxylenol Using TiO2, Er3+/TiO2, and Ni2+/TiO2 via the Taguchi Orthogonal Array Technique. Catalysts, 6(10), 163. https://doi.org/10.3390/catal6100163






