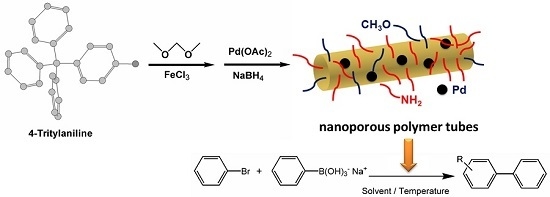
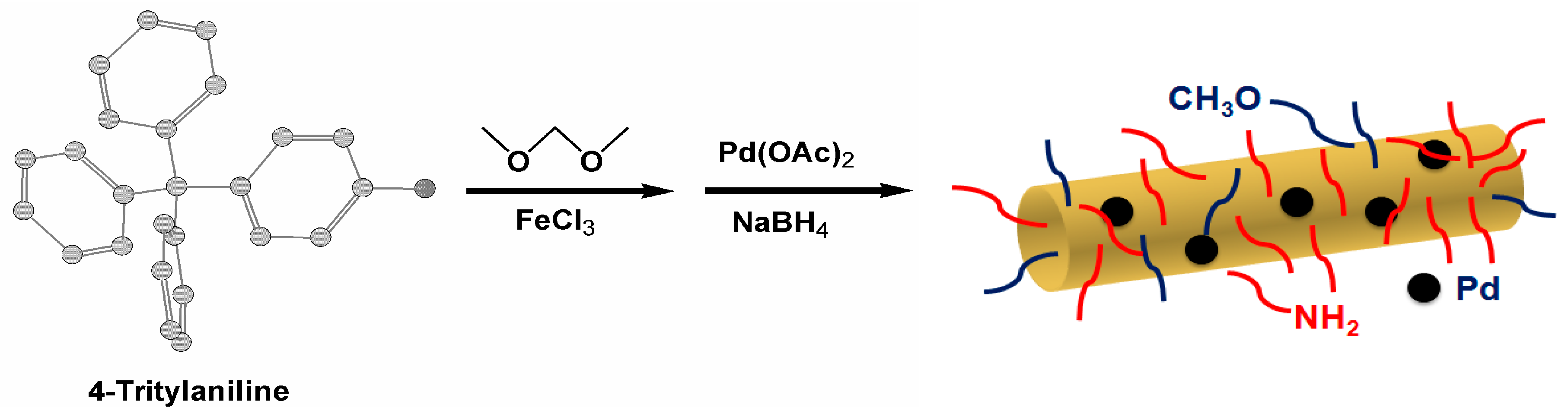
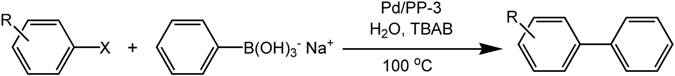Palladium Nanoparticles Tethered in Amine-Functionalized Hypercrosslinked Organic Tubes as an Efficient Catalyst for Suzuki Coupling in Water
Abstract
:1. Introduction
2. Results and Discussion
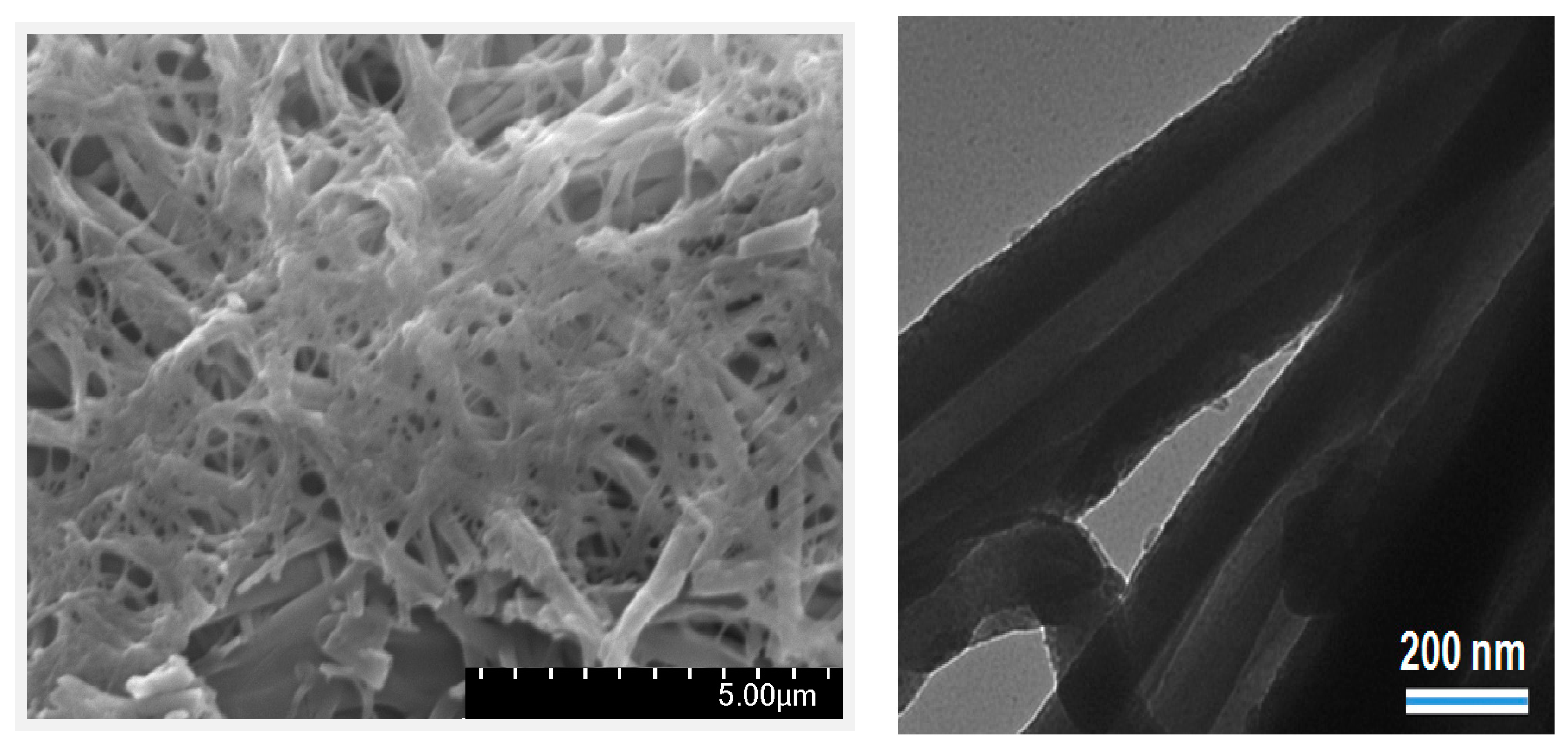
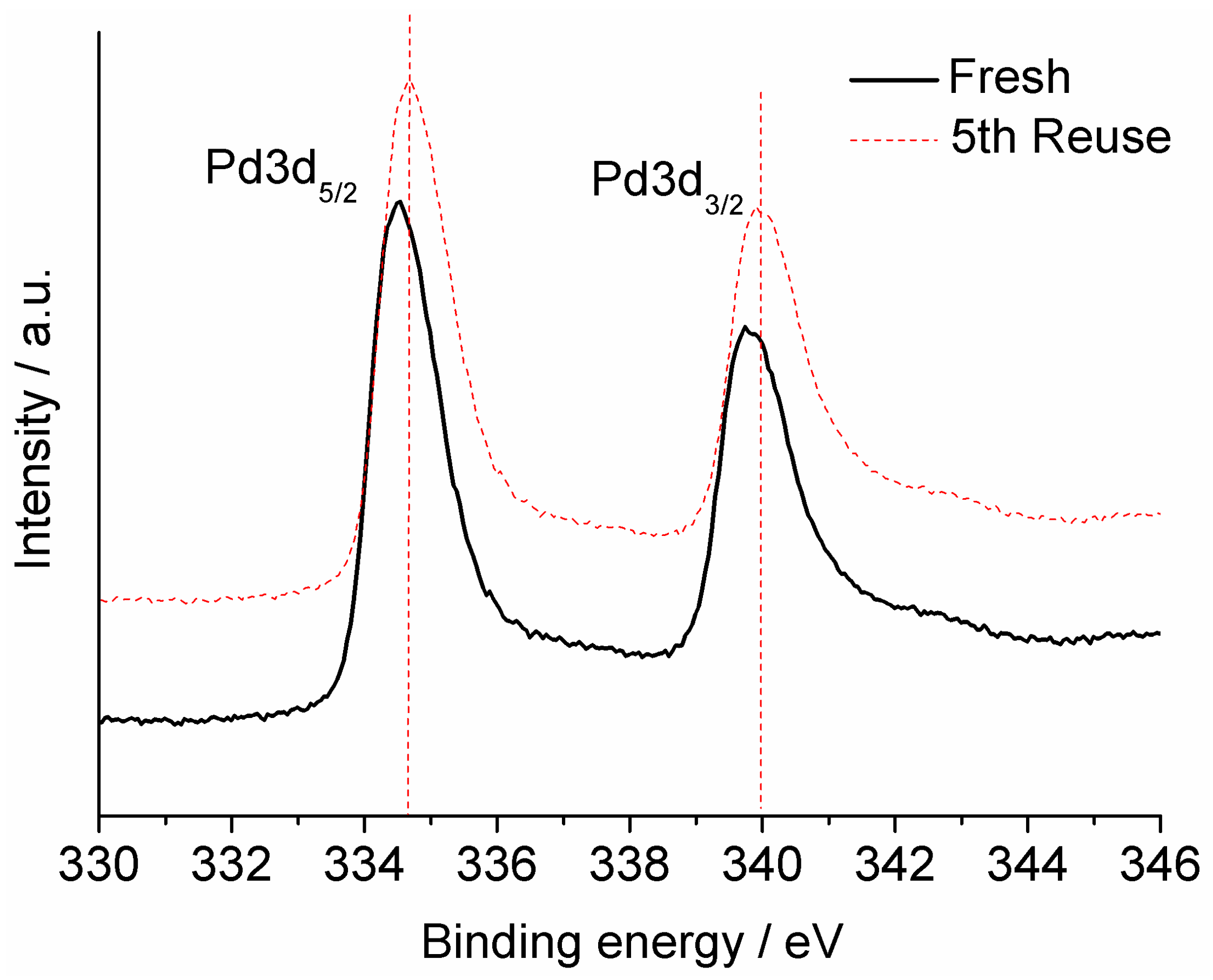
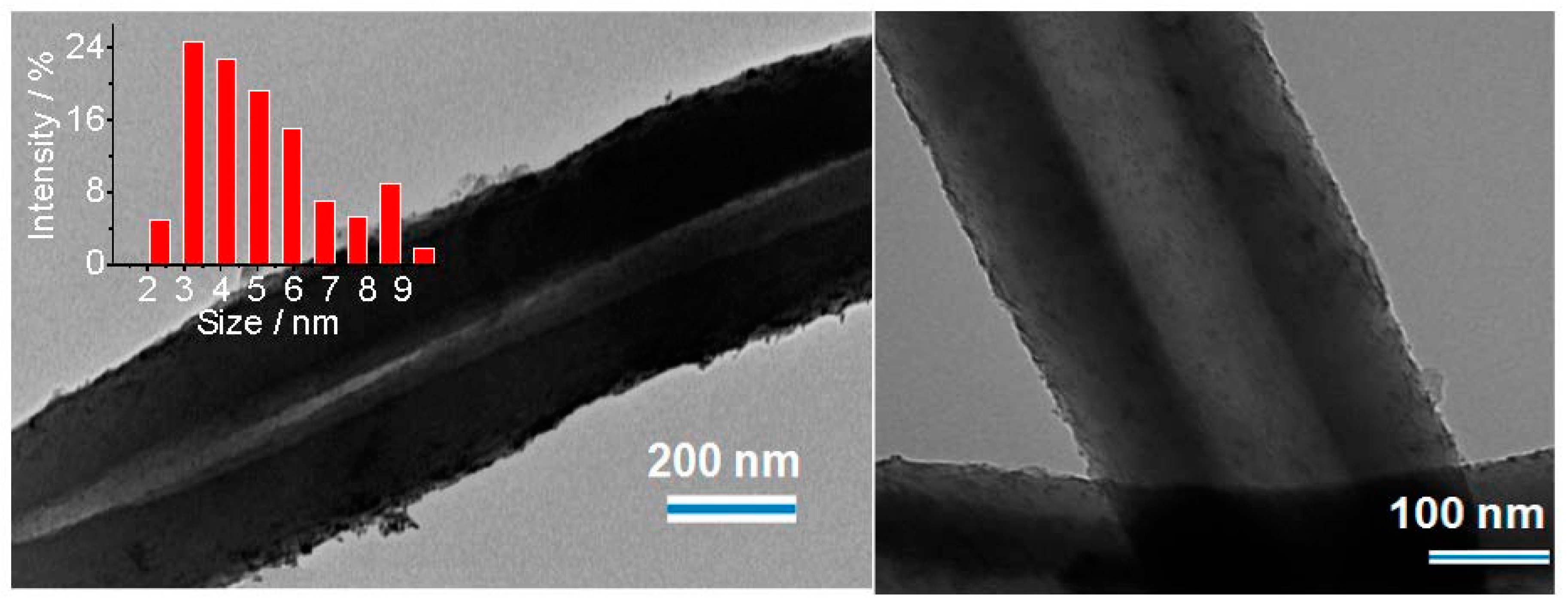
2.1. Synthesis and Characterization of Pd/PP-3 Tubes
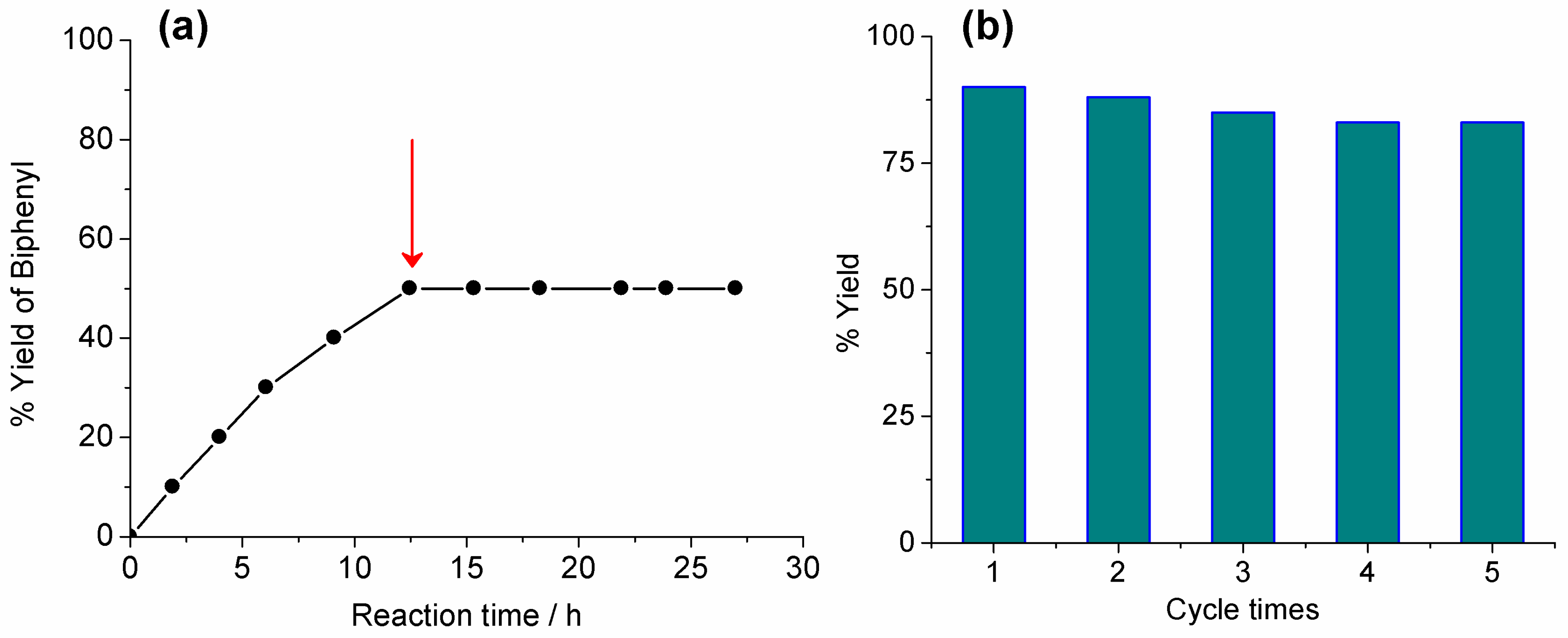
2.2. Heterogeneous Catalysis for Suzuki–Miyaura Cross Coupling Reaction in Water
3. Materials and Methods
3.1. Instrumentation
3.2. Methods
3.3. General Procedure for Suzuki–Miyaura Coupling Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catal. 2011, 1, 819–835. [Google Scholar] [CrossRef]
- Modak, A.; Pramanik, M.; Inagaki, S.; Bhaumik, A. A triazine functionalized porous organic polymer: excellent CO2 storage material and support for designing Pd nanocatalyst for C–C cross-coupling reactions. J. Mater. Chem. A 2014, 2, 11642–11650. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Wang, L.; Xiao, F.S. Task-Specific Design of Porous Polymer Heterogeneous Catalysts beyond Homogeneous Counterparts. ACS Catal. 2015, 5, 4556–4567. [Google Scholar] [CrossRef]
- Zhang, P.; Weng, Z.; Guo, J.; Wang, C. Solution-Dispersible, Colloidal, Conjugated Porous Polymer Networks with Entrapped Palladium Nanocrystals for Heterogeneous Catalysis of the Suzuki–Miyaura Coupling Reaction. Chem. Mater. 2011, 23, 5243–5249. [Google Scholar] [CrossRef]
- Modak, A.; Mondal, J.; Bhaumik, A. Highly Porous Organic Polymer containing Free -CO2H Groups: A Convenient Carbocatalyst for Indole C-H Activation at Room Temperature. ChemCatChem 2013, 5, 1749–1753. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Sprick, R.S.; Jiang, J.X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable Organic Photocatalysts for Visible-Light-Driven Hydrogen Evolution. J. Am. Chem. Soc. 2015, 137, 3265. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.; Yamanaka, K.I.; Goto, Y.; Inagaki, S. Photocatalytic H2 Evolution by Pt-Loaded 9,9’-Spirobifluorene-Based Conjugated Microporous Polymers under Visible-Light Irradiation. Bull. Chem. Soc. Jpn. 2016, 89, 887–891. [Google Scholar] [CrossRef]
- Liras, M.; Marta Iglesias, M.; Félix Sánchez, F. Conjugated Microporous Polymers Incorporating BODIPY Moieties as Light-Emitting Materials and Recyclable Visible-Light Photocatalysts. Macromolecules 2016, 49, 1666–1673. [Google Scholar] [CrossRef]
- Zhou, H.C.J.; Kitagawa, S. Metal-Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Maegawa, Y.; Goto, Y.; Hara, K.; Inagaki, S. Heterogeneous Catalysis for Water Oxidation by an Iridium Complex Immobilized on Bipyridine-Periodic Mesoporous Organosilica. Angew. Chem. Int. Ed. 2016, 55, 7943–7947. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.; Mondal, J.; Aswal, V.K.; Bhaumik, A. A new periodic mesoporous organosilica containing diimine-phloroglucinol, Pd(II)-grafting and its excellent catalytic activity and trans-selectivity in C–C coupling reactions. J. Mater. Chem. 2010, 20, 8099–8106. [Google Scholar] [CrossRef]
- Jagtap, S.; Deshpande, R. True water soluble palladium-catalyzed Heck reactions in aqueous–organic biphasic media. Tetrahedron Lett. 2013, 54, 2733–2736. [Google Scholar] [CrossRef]
- Gilbert, L.; Mercier, C. Solvent effects in heterogeneous catalysis: Application to the synthesis of fine chemicals. Stud. Surface Sci. Catal. 1993, 78, 51–66. [Google Scholar]
- Shaughnessy, K.H.; DeVasher, R.B. Palladium-Catalyzed Cross-Coupling in Aqueous Media: Recent Progress and Current Applications. Curr. Org. Chem. 2005, 9, 595. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by Palladium Pincer Complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.M.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Masjedi, M.; Demiralp, T.; Özkar, S. Testing catalytic activity of ruthenium(III) acetylacetonate in the presence of trialkylphosphite or trialkylphosphine in hydrogen generation from the hydrolysis of sodium borohydride. J. Mol. Catal. A Chem. 2009, 310, 59–63. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Z.; Li, Y. Immobilization of Pd(II) on MOFs as a highly active heterogeneous catalyst for Suzuki–Miyaura and Ullmann-type coupling reactions. Catal. Today 2015, 245, 122–128. [Google Scholar] [CrossRef]
- Li, B.; Guan, Z.; Wang, W.; Yang, X.; Hu, J.; Tan, B.; Li, T. Highly Dispersed Pd Catalyst Locked in Knitting Aryl Network Polymers for Suzuki–Miyaura Coupling Reactions of Aryl Chlorides in Aqueous Media. Adv. Mater. 2012, 24, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, A.; Kamble, S.; Mane, A.; Jha, R.; Salunkhe, R. Modified zeolite immobilized palladium for ligand-free Suzuki–Miyaura cross-coupling reaction. J. Organomet. Chem. 2013, 738, 29–34. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Prez, F.E.D. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Feng, X.; Liang, Y.; Zhi, L.; Thomas, A.; Wu, D.; Lieberwirth, I.; Kolb, U.; Mullen, K. Synthesis of Microporous Carbon Nanofibers and Nanotubes from Conjugated Polymer Network and Evaluation in Electrochemical Capacitor. Adv. Funct. Mater. 2009, 19, 2125–2129. [Google Scholar] [CrossRef]
- Modak, A.; Bhaumik, A. High-throughput Acid-Base Tandem Organocatalysis over Hollow Tube-Shaped Porous Polymers and Carbons. ChemistrySelect 2016, 6, 1192–1200. [Google Scholar] [CrossRef]
- Kang, N.; Hon Park, J.; Choi, J.; Jin, J.; Chun, J.; Jung, I.G.; Jeong, J.; Geun Park, J.; Lee, S.M.; Kim, H.J.; et al. Nanoparticulate Iron Oxide Tubes from Microporous Organic Nanotubes as Stable Anode Materials for Lithium Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 6626–6630. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, K.; Phadtare, S.; Vinod, V.P.; Kumar, A.; Rao, M.; Chaudhari, R.V.; Sastry, M. Gold Nanoparticles Assembled on Amine-Functionalized Na-Y Zeolite: A Biocompatible Surface for Enzyme immobilizaiton. Langmuir 2003, 19, 3858–3863. [Google Scholar] [CrossRef]
- Mandal, S.; Roy, D.; Chaudhari, R.V.; Sastry, M. Pt and Pd Nanoparticles Immobilized on Amine-Functionalized Zeolite: Excellent Catalysts for Hydrogenation and Heck Reactions. Chem. Mater. 2004, 16, 3714–3724. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Z.; Liu, T.; Lü, J.; Lin, Z.; Li, H.; Cao, R. Palladium nanoparticles supported on amino functionalized metal-organic frameworks as highly active catalysts for the Suzuki–Miyaura cross coupling reactions. Catal. Commun. 2011, 14, 27–31. [Google Scholar] [CrossRef]
- Dai, B.; Wen, B.; Zhu, M.; Kanga, L.; Yu, F. Nickel catalysts supported on amino functionalized MCM-41 for syngas methanation. RSC Adv. 2016, 6, 66957–66962. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Tsumori, N.; Xu, Q. Immobilizing Extremely Catalytically Active Palladium Nanoparticles to Carbon Nanospheres: A Weakly-Capping Growth Approach. J. Am. Chem. Soc. 2015, 137, 11743–11748. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Xu, Y.; Jiang, D. High-performance heterogeneous catalysis with surface-exposed stable metal nanoparticles. Sci. Rep. 2014, 4, 7228. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, A.; Okagaki, T.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; Nomura, R. Application of “Boomerang” Linear Polystyrene-Stabilized Pd Nanoparticles to a Series of C–C Coupling Reactions in Water. Catalysts 2015, 5, 106–118. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Yang, W.; Ren, L.; Zhang, Y.; Jia, X.; Zhang, L.; Li, Y.F. PdO nanoparticles enhancing the catalytic activity of Pd/carbon nanotubes for 4-nitrophenol reduction. RSC Adv. 2015, 5, 27526–27532. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Goddard, V.H.M.; Gopee, H.; Harrison, N.L.; Hughes, D.L.; Schubert, C.J.; Sutton, B.M.; Watts, G.L.; Whitehead, A.J. Aryl Trihydroxyborates: Easily Isolated Discrete Species Convenient for Direct Application in Coupling Reactions. Org. Lett. 2006, 8, 4071. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609. [Google Scholar] [CrossRef]
- Rostamnia, S.; Alamgholiloo, H.; Liu, X. Pd-grafted open metal site copper-benzene-1,4-dicarboxylate metal organic frameworks (Cu-BDC MOF’s) as promising interfacial catalysts for sustainable Suzuki coupling. J. Colloid Interface Sci. 2016, 469, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.Y.; Zhang, Q.F.; Yang, H.F.; Li, X.N.; Wang, J.G. Role of Phenolic Groups in the Stabilization of Palladium Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 9783–9789. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Hamada, S.; Watanabe, T.; Hirao, S.; Jun Sawayama, J.; Asahara, H.; Saigo, K.; Kamata, T.; Funabashi, M. Development of a new palladium catalyst supported on phenolic resin. RSC Adv. 2015, 5, 4463–4467. [Google Scholar] [CrossRef]
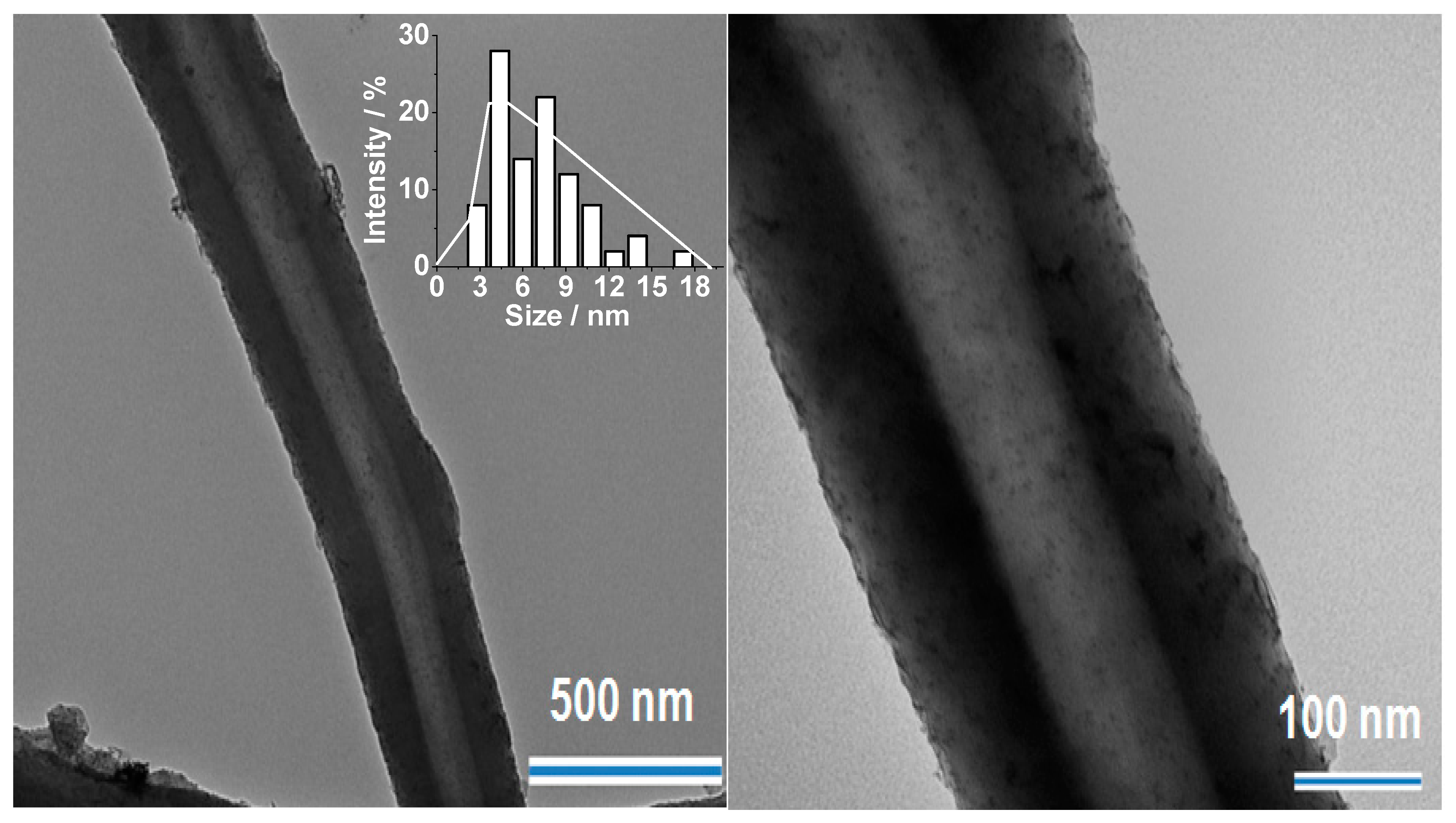
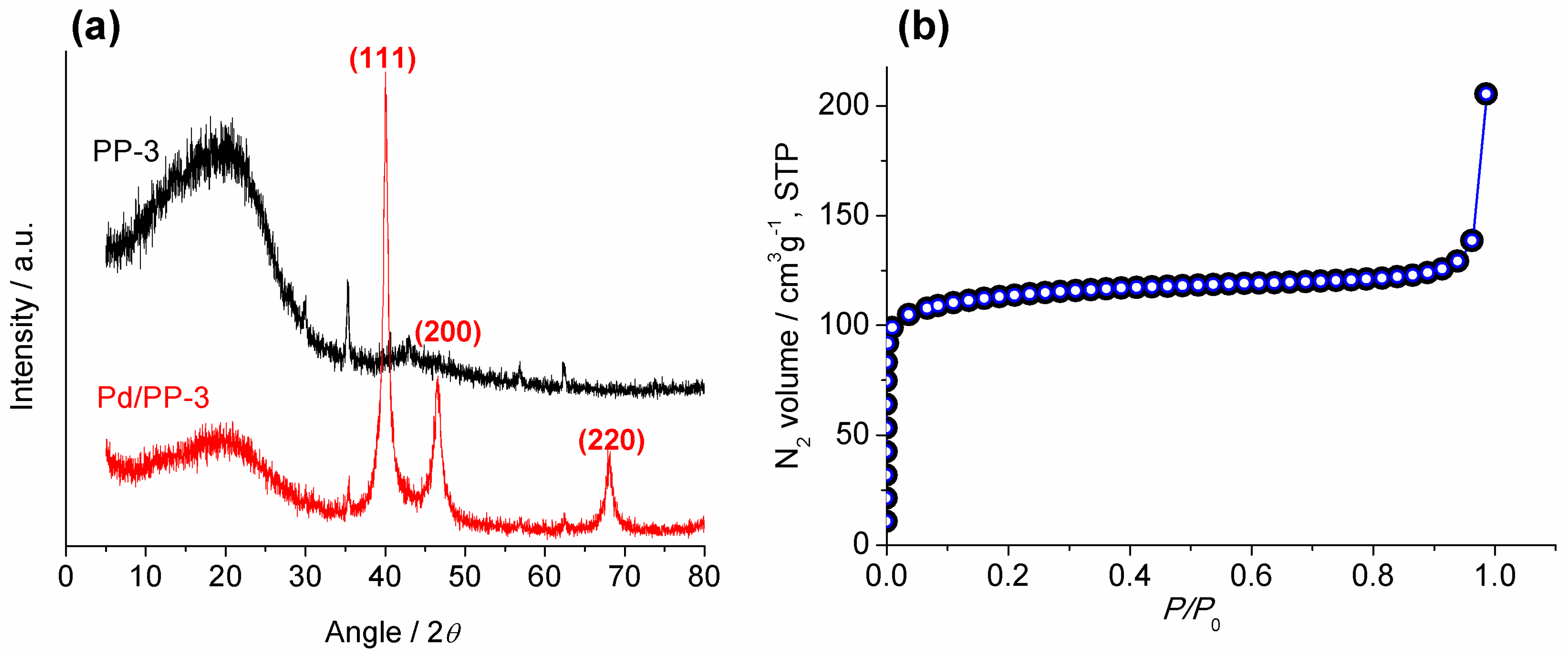
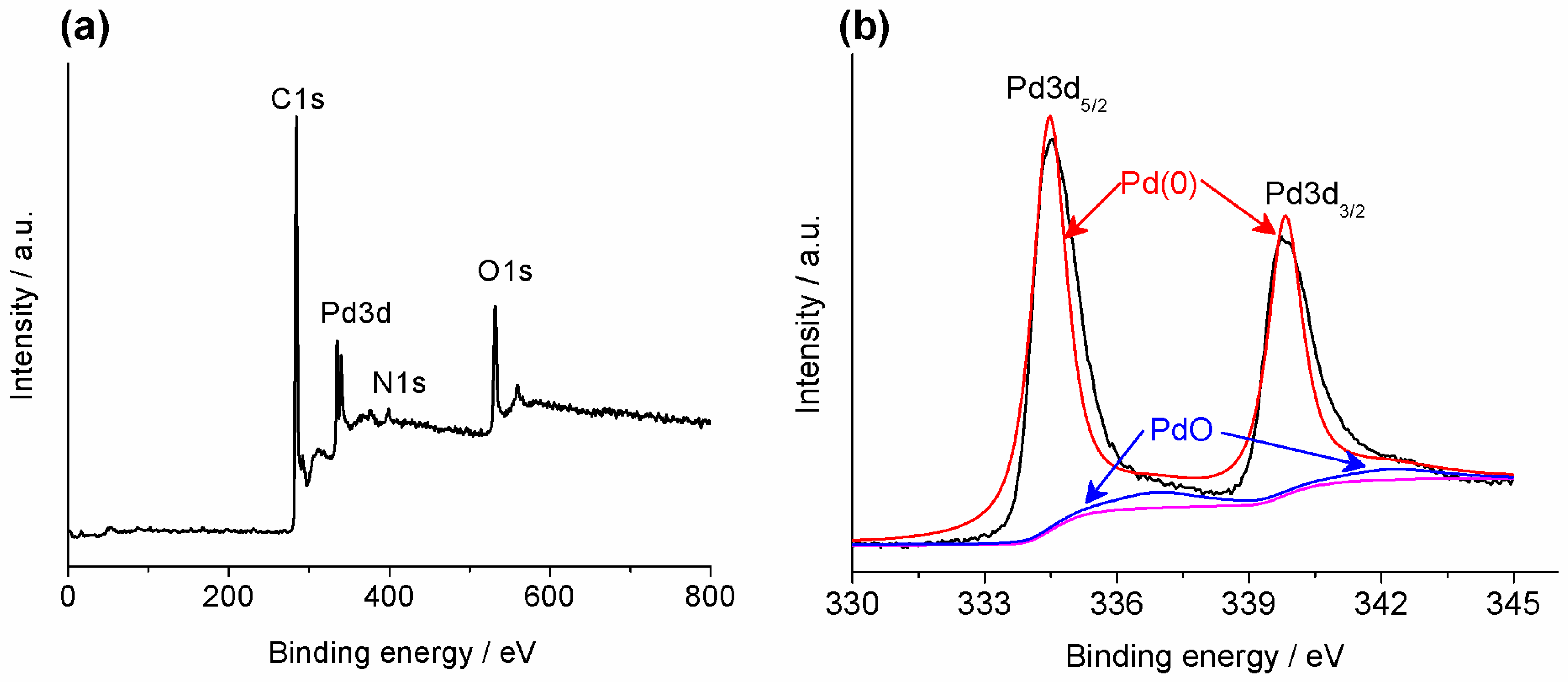
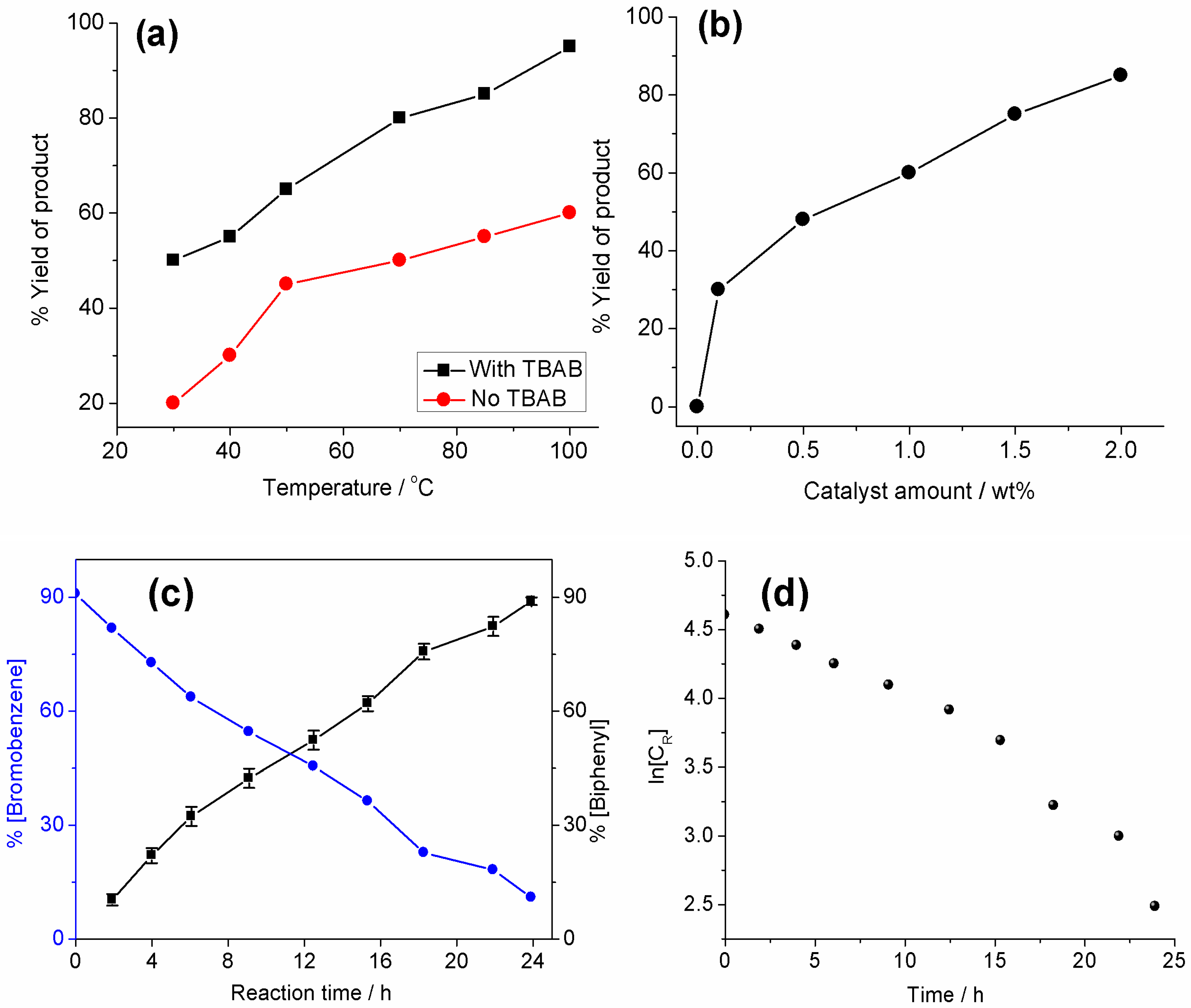
| Entry | Solvent | Temperature/°C | Time/h | Yield a/% |
|---|---|---|---|---|
| 1 | Toluene | 100 | 24 | Traces |
| 2 | 1,4-dioxane | 20 | 24 | 35 |
| 3 | DMF | 100 | 24 | >90 |
| 4 | Dichloromethane | 20 | 24 | <10 |
| 5 | Acetone | 20 | 24 | 50 |
| 6 | Water/DMF | 25 | 24 | >90 |
| 7 | Water b | 100 | 24 | 60 |
| 8 | Water c | 100 | 24 | >90 |
| 9 | Water d | 25 | 24 | 50 |
| Entry | Ar-X | Time/h | % Conv. of Ar-X b | % Selectivity | Rate c/h−1 |
|---|---|---|---|---|---|
| 1 | Iodo benzene | 20 | 90 | >99 | 12.5 |
| 2 | Bromobenzene | 24 | 86 | >99 | 11 |
| 3 | Chlorobenzene | 24 | 48 | >80 | 6 |
| 4 | 4-bromo nitrobenzene | 18 | 88 | >95 | 13.8 |
| 5 | 4-chloro nitrobenzene | 24 | 40 | >78 | 8 |
| 6 | 4-bromo benzaldehyde | 24 | 75 | - | 5.3 |
| 7 | 4-bromo acetophenone | 20 | 76 | >80 | 12.5 |
| 8 | 4-iodo acetophenone | 15 | 88 | >98 | 16.6 |
| 9 | 4-iodo anisole | 19 | 85 | >98 | 13 |
| 10 | 4-bromo anisole | 24 | 79 | >90 | 10 |
| 11 | 4-bromo benzonitrile | 24 | 70 | >90 | 9.2 |
| 12 | 4-chloro benzonitrile | 24 | 30 | >70 | 4.7 |
| 13 | 4-bromo toluene | 20 | 70 | >85 | 11 |
| 14 | 4-bromobenzoic acid | 18 | 60 | >80 | 12.8 |
| 15 | Brombenzene d, e | 24 | 70 d, 55 e | >90 d, >86 e | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modak, A.; Sun, J.; Qiu, W.; Liu, X. Palladium Nanoparticles Tethered in Amine-Functionalized Hypercrosslinked Organic Tubes as an Efficient Catalyst for Suzuki Coupling in Water. Catalysts 2016, 6, 161. https://doi.org/10.3390/catal6100161
Modak A, Sun J, Qiu W, Liu X. Palladium Nanoparticles Tethered in Amine-Functionalized Hypercrosslinked Organic Tubes as an Efficient Catalyst for Suzuki Coupling in Water. Catalysts. 2016; 6(10):161. https://doi.org/10.3390/catal6100161
Chicago/Turabian StyleModak, Arindam, Jing Sun, Wenjun Qiu, and Xiao Liu. 2016. "Palladium Nanoparticles Tethered in Amine-Functionalized Hypercrosslinked Organic Tubes as an Efficient Catalyst for Suzuki Coupling in Water" Catalysts 6, no. 10: 161. https://doi.org/10.3390/catal6100161
APA StyleModak, A., Sun, J., Qiu, W., & Liu, X. (2016). Palladium Nanoparticles Tethered in Amine-Functionalized Hypercrosslinked Organic Tubes as an Efficient Catalyst for Suzuki Coupling in Water. Catalysts, 6(10), 161. https://doi.org/10.3390/catal6100161