Natural Hematite as a Low-Cost and Earth-Abundant Cathode Material for Performance Improvement of Microbial Fuel Cells
Abstract
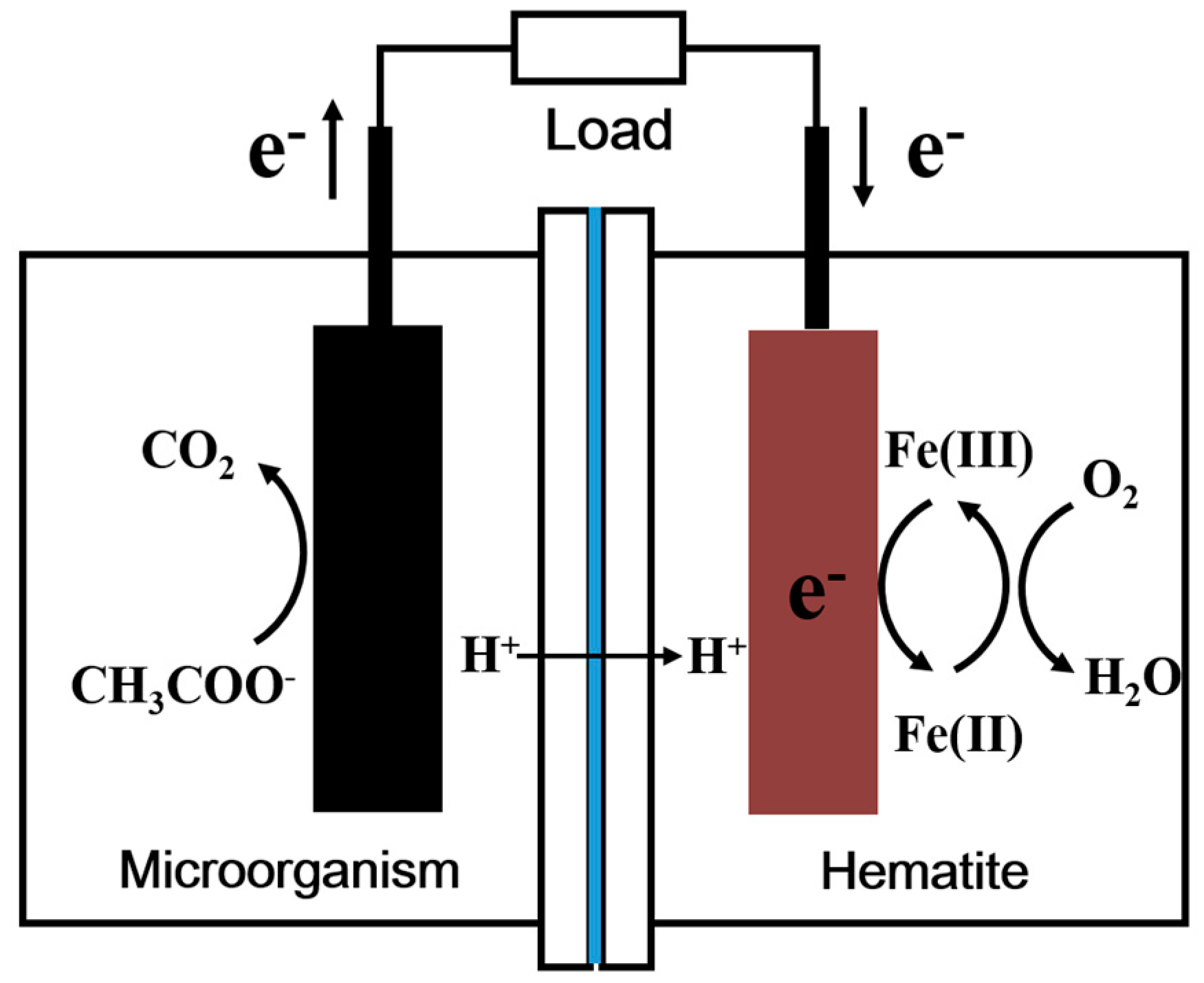
:1. Introduction
2. Results and Discussion
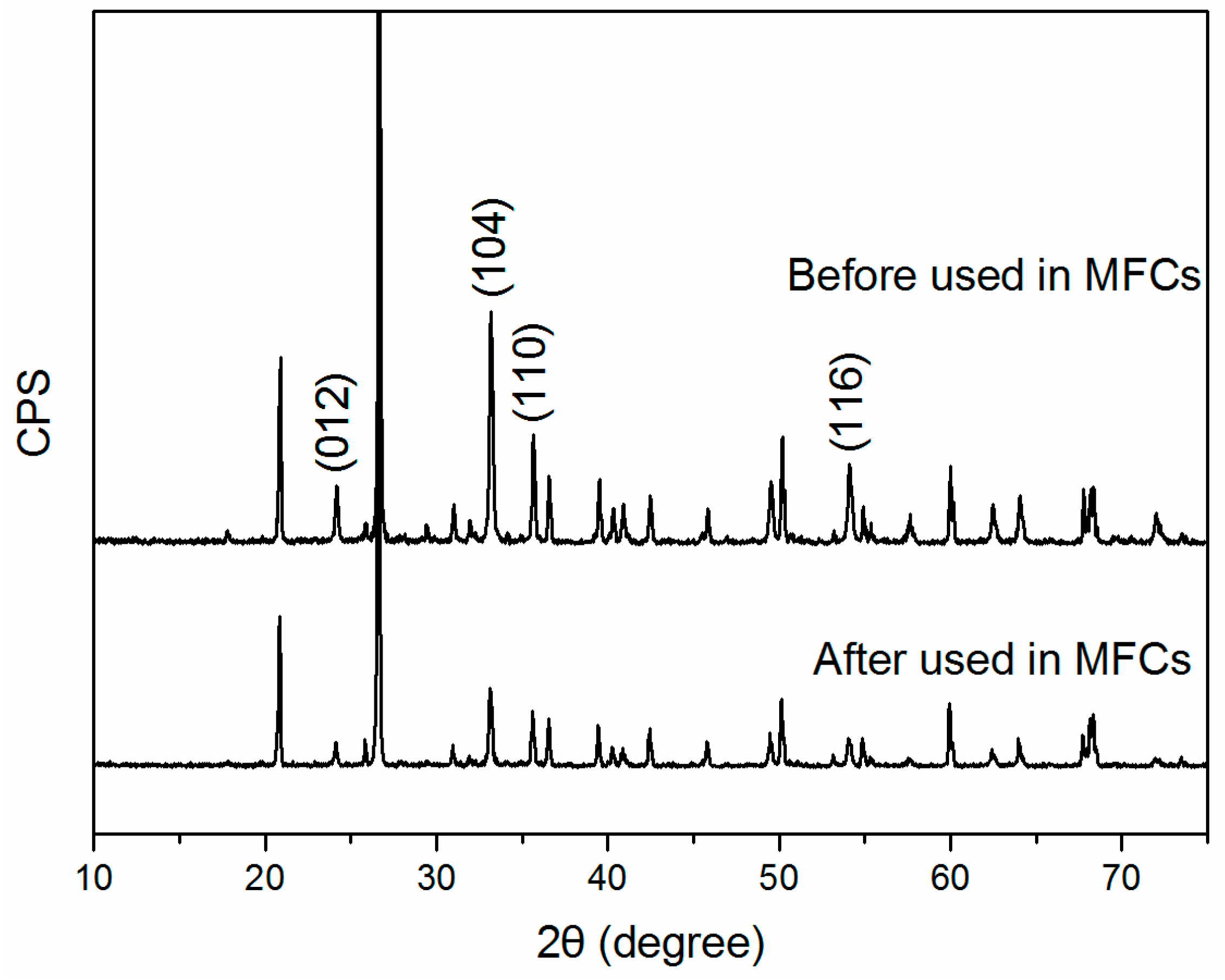
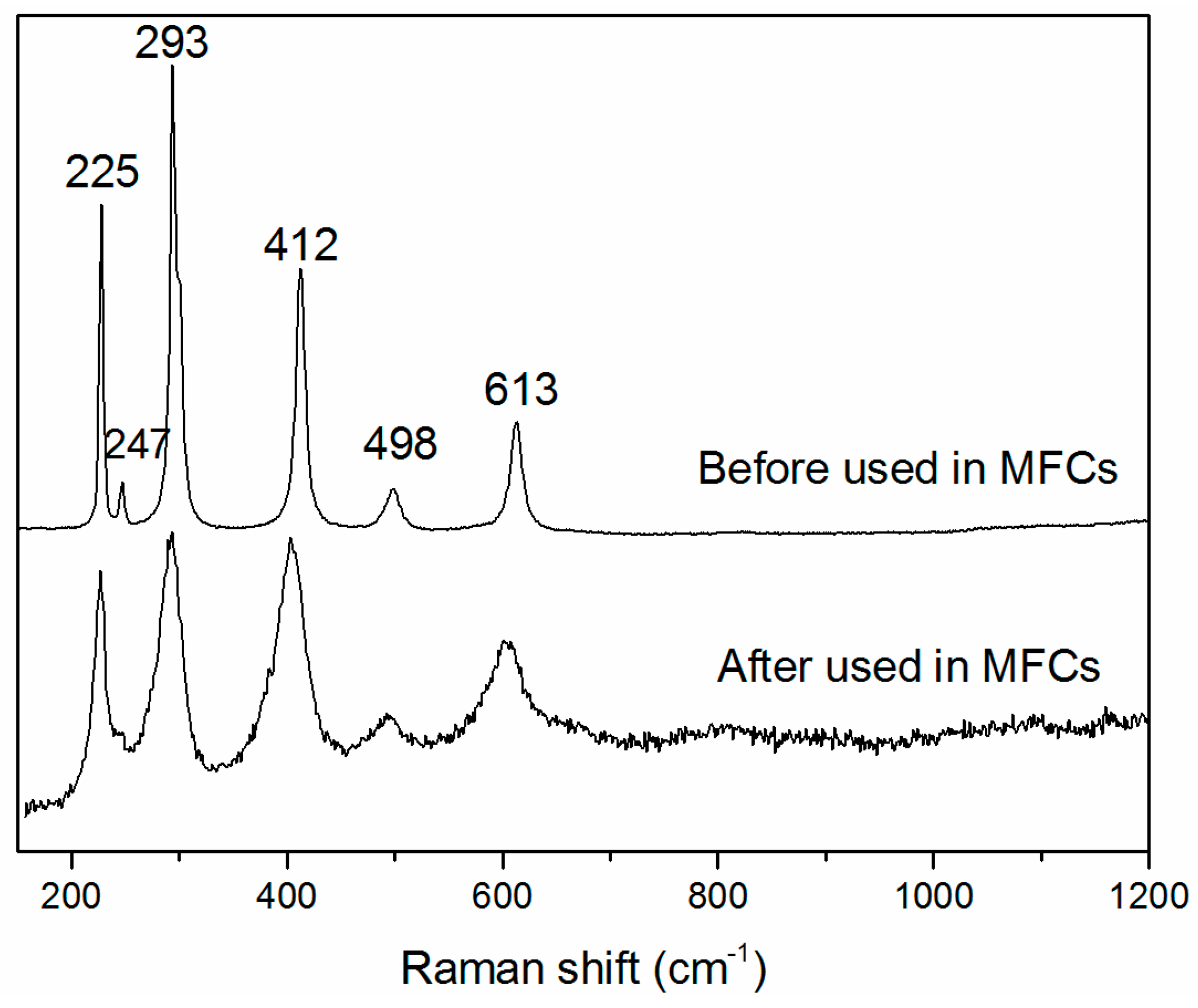
2.1. XRD and Raman Characterization of Hematite
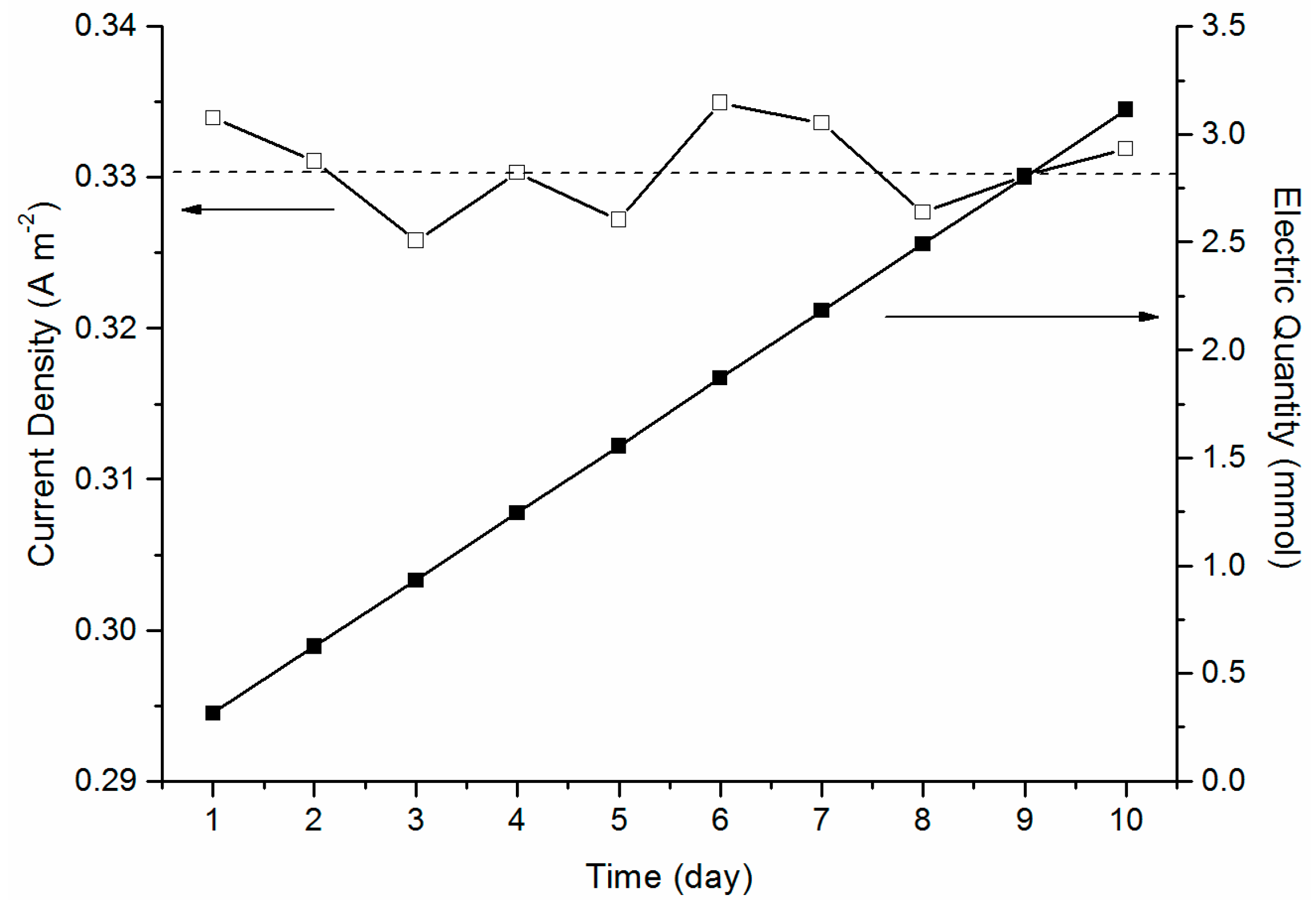
2.2. MFC Performance
2.3. Electrochemical Characterization
3. Materials and Methods
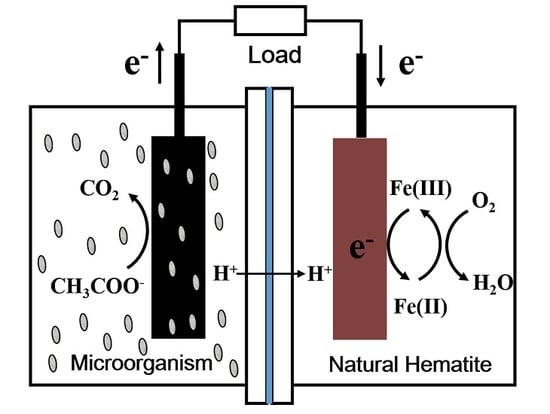
3.1. MFC Configuration
3.2. Natural Hematite Characterization
3.3. Hematite Electrode Preparation and Iron Determination
3.4. Electricity and Electrochemical Measurement Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Tender, L.M.; Reimers, C.E.; Stecher, H.A.; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 2002, 20, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Min, B.; Logan, B.E. Cathode performance as a factor in electricity generation in microbial fuel cells. Environ. Sci. Technol. 2004, 38, 4900–4904. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.; Zuo, J.; Ling, A.; Logan, B.E. Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. J. Power Sources 2010, 195, 1130–1135. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, M.; Liu, C.; Liu, L.; Liu, C.; Dong, S. Development of high performance of Co/Fe/N/CNT nanocatalyst for oxygen reduction in microbial fuel cells. Talanta 2010, 81, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Hijazi, I.; Bourgeteau, T.; Cornut, R.; Morozan, A.; Filoramo, A.; Leroy, J.; Derycke, V.; Jousselme, B.; Campidelli, S. Carbon nanotube-templated synthesis of covalent porphyrin network for oxygen reduction reaction. J. Am. Chem. Soc. 2014, 136, 6348–6354. [Google Scholar] [CrossRef] [PubMed]
- Birry, L.; Mehta, P.; Jaouen, F.; Dodelet, J.P.; Guiot, S.R.; Tartakovsky, B. Application of iron-based cathode catalysts in a microbial fuel cell. Electrochim. Acta 2011, 56, 1505–1511. [Google Scholar] [CrossRef]
- HaoYu, E.; Cheng, S.; Scott, K.; Logan, B.E. Microbial fuel cell performance with non-Pt cathode catalysts. J. Power Sources 2007, 171, 275–281. [Google Scholar] [CrossRef]
- Cheng, S.A.; Liu, H.; Logan, B.E. Cathode materials and polymer binders as factors that affect power densities in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Harnisch, F.; Schröder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Application of pyrolysed iron(II) phthalocyanine and CoTMPP based oxygen reduction catalysts as cathode materials in microbial fuel cells. Electrochem. Commun. 2005, 7, 1405–1410. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, J.Y.; Han, S.B.; Park, K.W.; Saratale, G.D.; Oh, S.E. Application of Co-naphthalocyanine (CoNPc) as alternative cathode catalyst and support structure for microbial fuel cells. Bioresour. Technol. 2011, 102, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhu, Y.; Xu, K.; Jin, Y.; Ren, Z.J.; Liu, Z.; Zhang, W. Metallated porphyrin based porous organic polymers as efficient electrocatalysts. Nanoscale 2015, 7, 18271–18277. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Yang, H.; Zhu, Y.; Huggins, T.; Ren, Z.J.; Liu, Z.; Zhang, W. Synthesis of a conjugated porous Co (II) porphyrinylene–ethynylene framework through alkyne metathesis and its catalytic activity study. J. Mater. Chem. A 2015, 3, 4954–4959. [Google Scholar] [CrossRef]
- Lu, G.; Zhu, Y.; Lu, L.; Xu, K.; Wang, H.; Jin, Y.; Ren, Z.J.; Liu, Z.; Zhang, W. Iron-rich nanoparticle encapsulated, nitrogen doped porous carbon materials as efficient cathode electrocatalyst for microbial fuel cells. J. Power Sources 2016, 315, 302–307. [Google Scholar] [CrossRef]
- Ahmed, J.; Yuan, Y.; Zhou, L.; Kim, S. Carbon supported cobalt oxide nanoparticles-iron phthalocyanine as alternative cathode catalyst for oxygen reduction in microbial fuel cells. J. Power Sources 2012, 208, 170–175. [Google Scholar] [CrossRef]
- Freguia, S.; Rabaey, K.; Yuan, Z.; Keller, J. Non-catalyst cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochim. Acta 2007, 53, 598–603. [Google Scholar] [CrossRef]
- He, Z.; Angenent, L.T. Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 2006, 18, 2009–2015. [Google Scholar] [CrossRef]
- Clauwaert, P.; Van der Ha, D.; Boon, N.; Verbeken, K.; Verhaege, M.; Rabaey, K.; Verstraete, W. Open air biocathode enables effective electricity generation with microbial fuel cells. Environ. Sci. Technol. 2007, 41, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, A.; Beyenal, H.; Lewandowski, Z. Microbial fuel cell using anaerobic respiration as an anodic reaction and biomineralized manganese as a cathodic reactant. Environ. Sci. Technol. 2005, 39, 4666–4671. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lee, J.H.D.; Xia, Q.; Ma, Y.; Yu, Y.; Yung, L.Y.L.; Zhou, Z. A graphene-based electrochemical filter for water purification. J. Mater. Chem. A 2014, 2, 16554–16562. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Ong, C.N.; Vecitis, C.D.; Zhou, Z. Electrochemical wastewater treatment with carbon nanotube filters coupled with in situ generated H2O2. Environ. Sci. Water Res. Technol. 2015, 1, 769–778. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.C.; Jung, J.H.; Kim, H.J. Pore characterization of multi-walled carbon nanotubes modified by KOH. Chem. Phys. Lett. 2005, 416, 251–255. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Wang, C.; Hou, S.X.; Yang, N. Sustainable energy recovery in wastewater treatment by microbial fuel cells: Stable power generation with nitrogen-doped graphene cathode. Environ. Sci. Technol. 2013, 47, 13889–13895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wen, Z.; Ci, S.; Mao, S.; Chen, J.; He, Z. Synthesizing nitrogen-doped activated carbon and probing its active sites for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 2014, 6, 7464–7470. [Google Scholar] [CrossRef] [PubMed]
- Roche, I.; Katuri, K.; Scott, K. A microbial fuel cell using manganese oxide oxygen reduction catalysts. J. Appl. Electrochem. 2010, 40, 13–21. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.; Suib, S.; Lei, Y.; Li, B. Manganese dioxide as a new cathode catalyst in microbial fuel cells. J. Power Sources 2010, 195, 2586–2591. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Wang, J.; Zhu, C.; Urynowicz, M.A. Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem. Commun. 2007, 9, 1730–1734. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Jin, S.; Ding, H.; Zeng, C.; Wang, X.; Wang, C. Microbial fuel cell equipped with a photocatalytic rutile-coated cathode. Energy Fuels 2009, 24, 1184–1190. [Google Scholar] [CrossRef]
- USGS Mineral Resource Program. Available online: http://minerals.usgs.gov (accessed on 31 August 2016).
- Manufacturers, Suppliers, Exporters & Importers from the World’s Largest Online B2B Marketplace—Alibaba.com. Available online: http://www.alibaba.com (accessed on 31 August 2016).
- Pandit, S.; Khilari, S.; Roy, S.; Pradhan, D.; Das, D. Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: Effect of different anodic operating conditions. Bioresour. Technol. 2014, 166, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jia, Y.; Wu, W.; Bai, L.; Ge, L.; Gu, Z. A layer-by-layer self-assembled Fe2O3 nanorod-based composite multilayer film on ITO anode in microbial fuel cell. Colloids Surf. A 2011, 390, 56–61. [Google Scholar] [CrossRef]
- Nakamura, R.; Kai, F.; Okamoto, A.; Newton, G.J.; Hashimoto, K. Self-constructed electrically conductive bacterial networks. Angew. Chem. Int. Ed. 2009, 48, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wang, H.; Ling, Y.; Wang, G.; Thelen, M.P.; Li, Y. Photoenhanced electrochemical interaction between Shewanella and a hematite nanowire photoanode. Nano Lett. 2014, 14, 3688–3693. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B.; Cheng, Y.Y.; Li, L.L.; Li, W.W.; Huang, Y.X.; Pei, D.N.; Tong, Z.H.; Mu, Y.; Yu, H.Q. Light-driven microbial dissimilatory electron transfer to hematite. Phys. Chem. Chem. Phys. 2014, 16, 23003–23011. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Seo, S.J.; Maitlo, H.A.; Park, J.Y. The enhancement of ammonium removal from ethanolamine wastewater using air-cathode microbial fuel cells coupled to ferric reduction. Bioresour. Technol. 2015, 190, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, N.F.; Jalil, A.A.; Triwahyono, S.; Muhid, M.N.M.; Sapawe, N.; Satar, M.A.H.; Asaari, H. Photodecolorization of methyl orange over α-Fe2O3-supported HY catalysts: The effects of catalyst preparation and dealumination. Chem. Eng. J. 2012, 191, 112–122. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Mshoperi, E.; Fogel, R.; Limson, J. Application of carbon black and iron phthalocyanine composites in bioelectricity production at a brewery wastewater fed microbial fuel cell. Electrochim. Acta 2014, 128, 311–317. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Mecheri, B.; D’Epifanio, A.; Sciarria, T.P.; Adani, F.; Licoccia, S. Iron chelates as low-cost and effective electrocatalyst for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrog. Energy 2014, 39, 6462–6469. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Ho, G.; Cord-Ruwisch, R. Affinity of microbial fuel cell biofilm for the anodic potential. Environ. Sci. Technol. 2008, 42, 3828–3834. [Google Scholar] [CrossRef] [PubMed]
- Straub, K.L.; Benz, M.; Schink, B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001, 34, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Schaetzle, O.; Barrière, F.; Baronian, K. Bacteria and yeasts as catalysts in microbial fuel cells: Electron transfer from micro-organisms to electrodes for green electricity. Energy Environ. Sci. 2008, 1, 607–620. [Google Scholar] [CrossRef]
- Richter, H.; Nevin, K.P.; Jia, H.; Lowy, D.A.; Lovley, D.R.; Tender, L.M. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2009, 2, 506–516. [Google Scholar] [CrossRef]
- Zhao, F.; Harnisch, F.; Schröder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5193–5199. [Google Scholar] [CrossRef] [PubMed]
- Ghahremaninezhad, A.; Asselin, E.; Dixon, D.G. In situ electrochemical analysis of surface layers on a pyrrhotite electrode in hydrochloric acid solution. J. Electrochem. Soc. 2010, 157, C248–C257. [Google Scholar] [CrossRef]
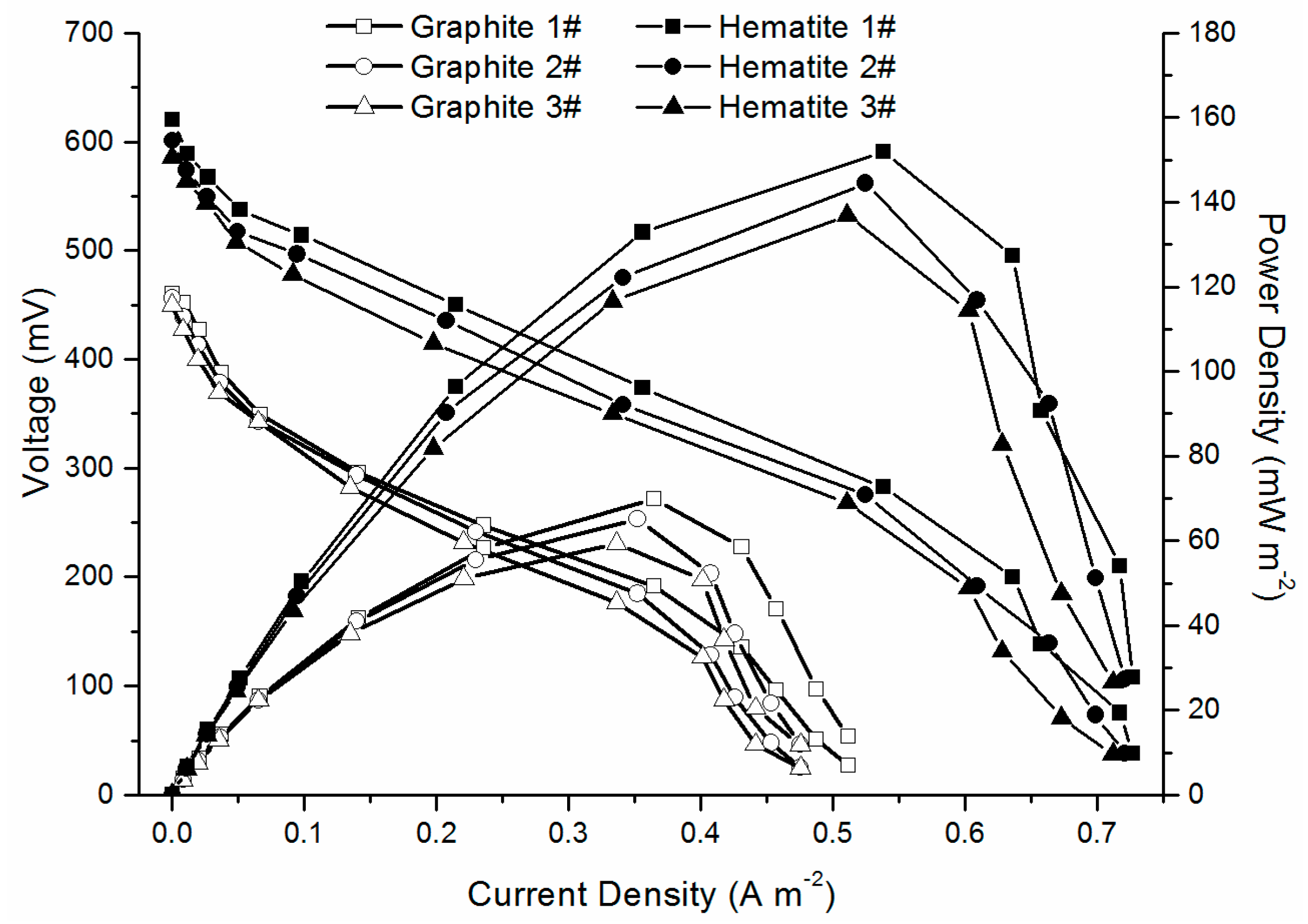
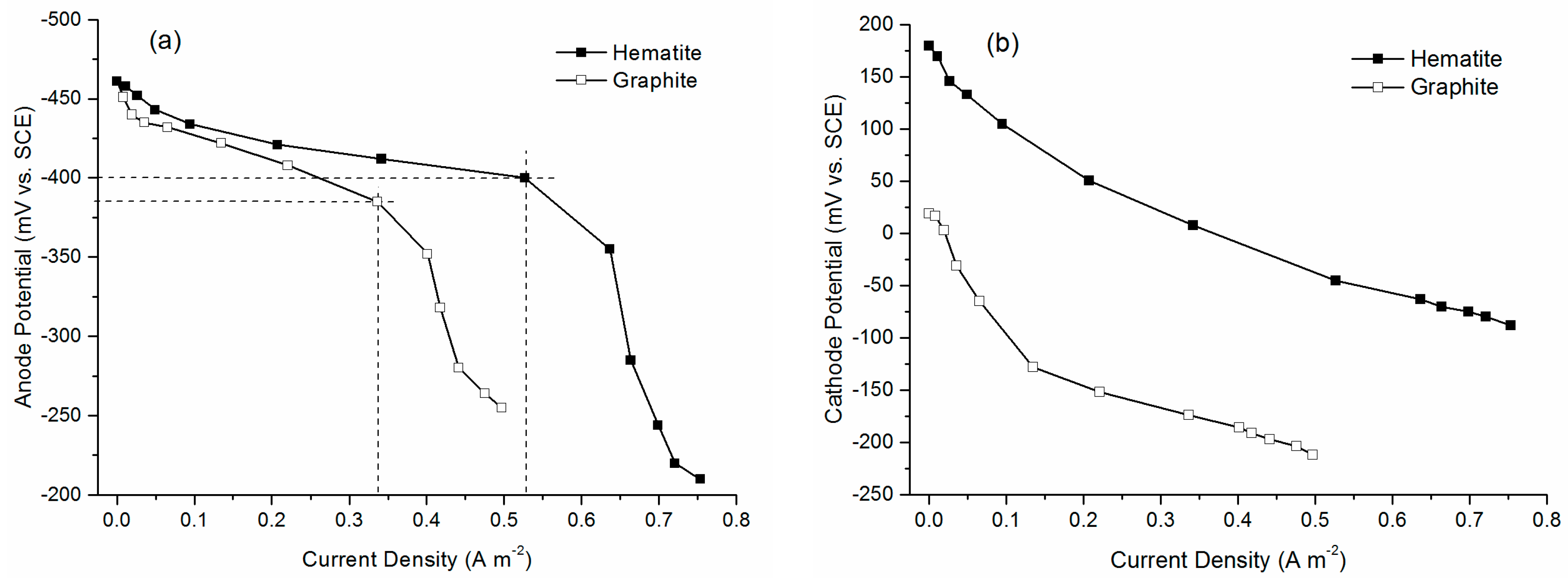
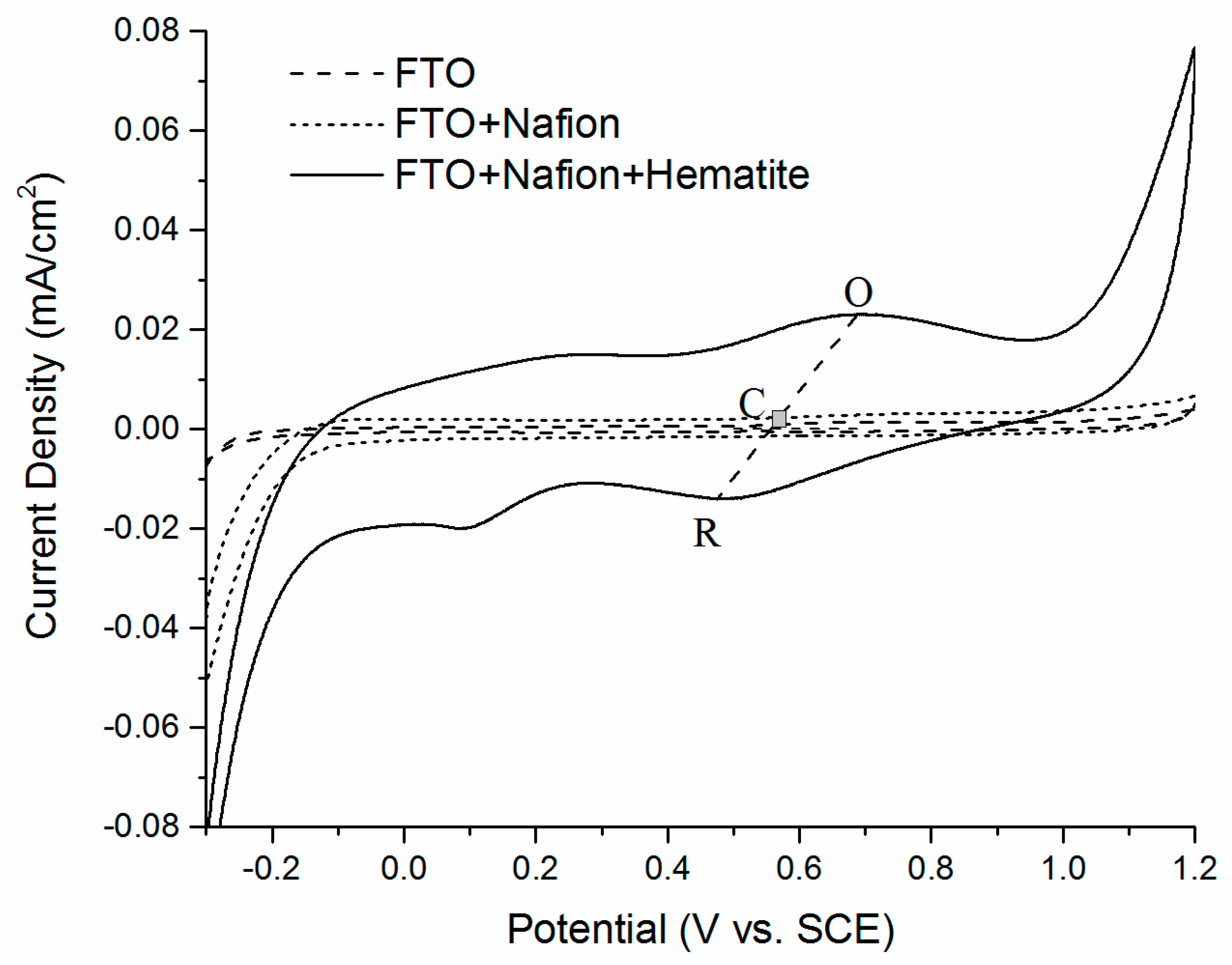
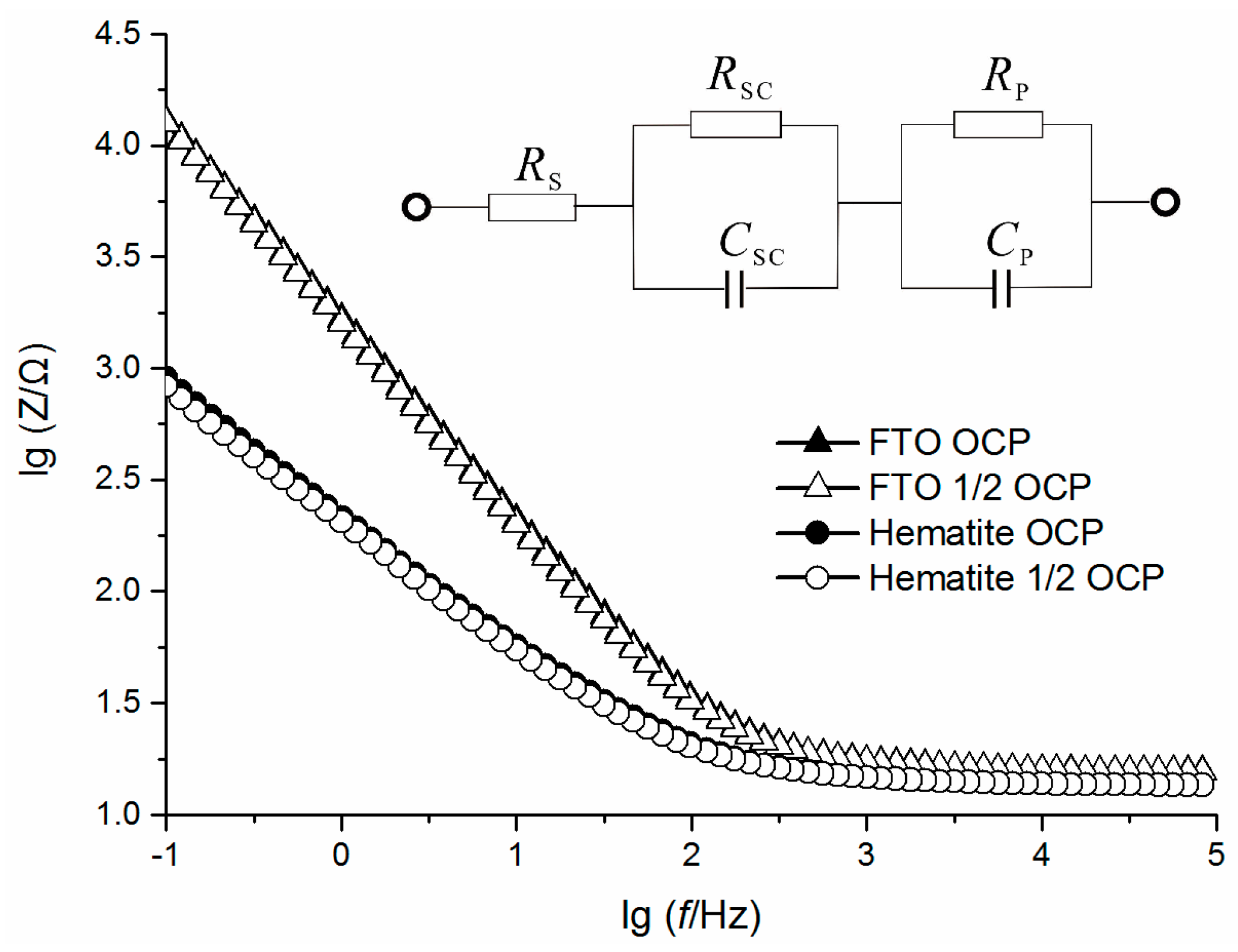
| Cathode Material | Open Circuit Voltage (mV) | Maximum Output Power (mW/m2) | System Resistance (Ω) |
|---|---|---|---|
| Graphite | 455.4 ± 5.4 | 64.8 ± 5.2 | 569.8 ± 10.9 |
| Hematite | 602.3 ± 17.4 | 144.4 ± 7.5 | 549.5 ± 12.2 |
| Relative percentage | 132% | 223% | 96.4% |
| Cathode Material | Rs (Ω) | Rsc (Ω) | Rp (Ω) | Rsc + Rp (Ω) |
|---|---|---|---|---|
| FTO | 16.47 | 57,250 | 111.9 | 57,361.9 |
| Hematite-FTO | 14.71 | 1334 | 69.3 | 1403.3 |
| Relative percentage | 89.3% | 2.33% | 61.9% | 2.446% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Ding, H.; Li, Y.; Lu, A. Natural Hematite as a Low-Cost and Earth-Abundant Cathode Material for Performance Improvement of Microbial Fuel Cells. Catalysts 2016, 6, 157. https://doi.org/10.3390/catal6100157
Ren G, Ding H, Li Y, Lu A. Natural Hematite as a Low-Cost and Earth-Abundant Cathode Material for Performance Improvement of Microbial Fuel Cells. Catalysts. 2016; 6(10):157. https://doi.org/10.3390/catal6100157
Chicago/Turabian StyleRen, Guiping, Hongrui Ding, Yan Li, and Anhuai Lu. 2016. "Natural Hematite as a Low-Cost and Earth-Abundant Cathode Material for Performance Improvement of Microbial Fuel Cells" Catalysts 6, no. 10: 157. https://doi.org/10.3390/catal6100157
APA StyleRen, G., Ding, H., Li, Y., & Lu, A. (2016). Natural Hematite as a Low-Cost and Earth-Abundant Cathode Material for Performance Improvement of Microbial Fuel Cells. Catalysts, 6(10), 157. https://doi.org/10.3390/catal6100157