Characterization and Catalytic Activity of Mn-Co/TiO2 Catalysts for NO Oxidation to NO2 at Low Temperature
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activity of NO Oxidation to NO2
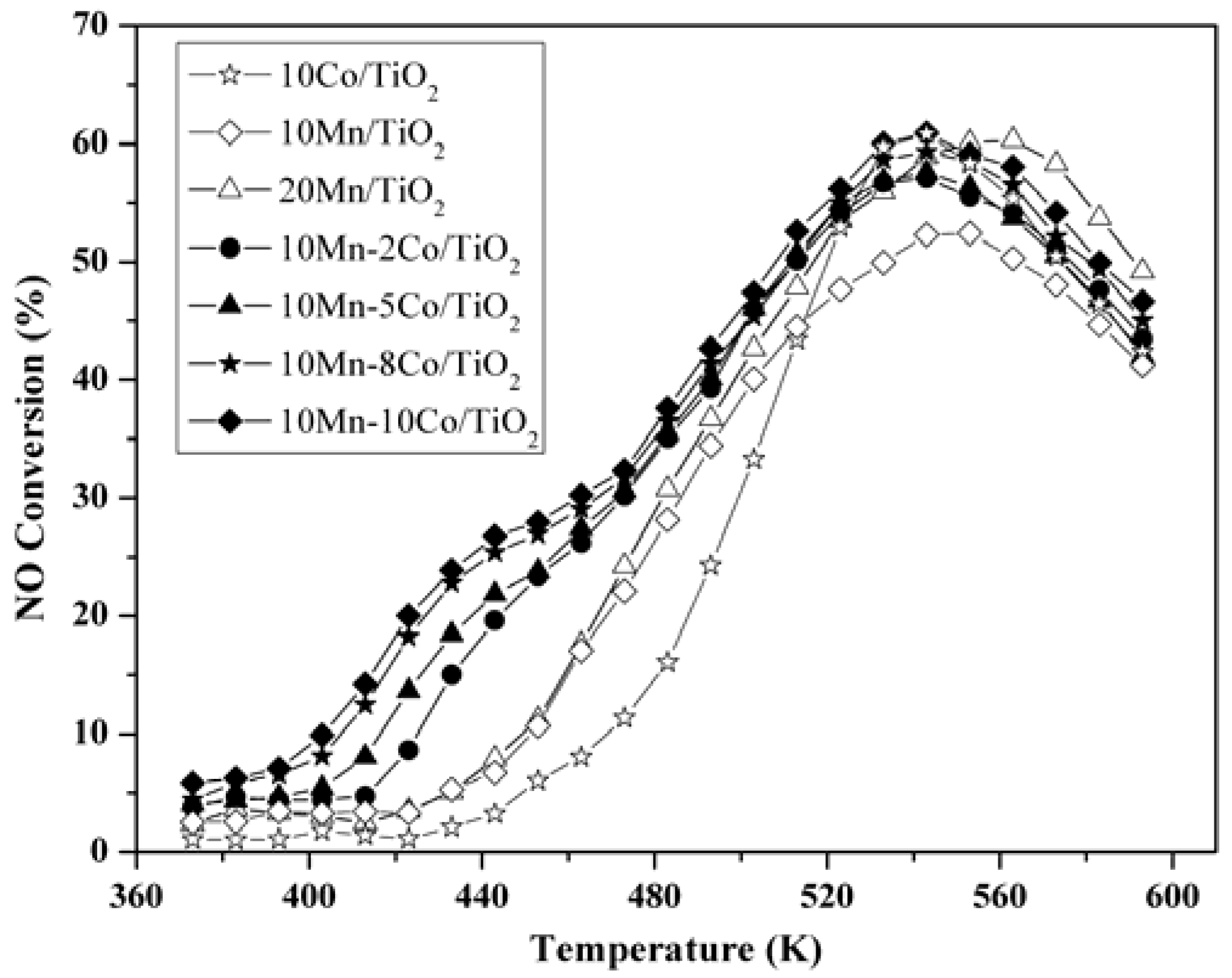
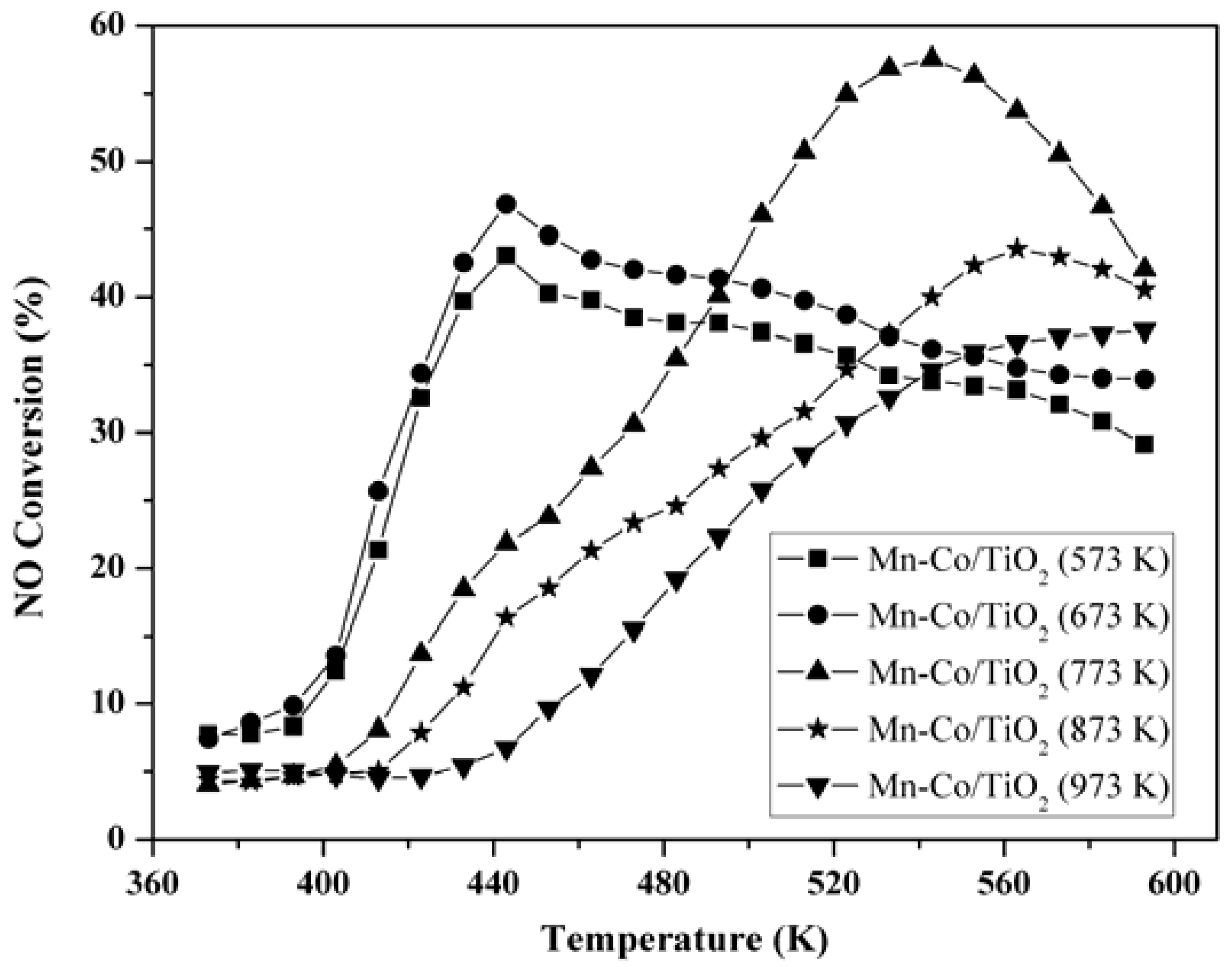
2.2. Nitrogen Adsorption-Desorption Characterization
| Catalyst | SBET (cm2·g−1) | Average Pore Diameter (nm) |
|---|---|---|
| 10Mn/TiO2 (773 K) | 48.35 | 8.67 |
| 10Mn-5Co/TiO2 (573 K) | 50.57 | 7.21 |
| 10Mn-5Co/TiO2 (673 K) | 50.38 | 6.86 |
| 10Mn-5Co/TiO2 (773 K) | 45.79 | 8.52 |
| 10Mn-5Co/TiO2 (873 K) | 30.51 | 8.38 |
| 10Mn-5Co/TiO2 (973 K) | 7.01 | 6.27 |
| 10Mn-10Co/TiO2 (773 K) | 42.13 | 8.02 |
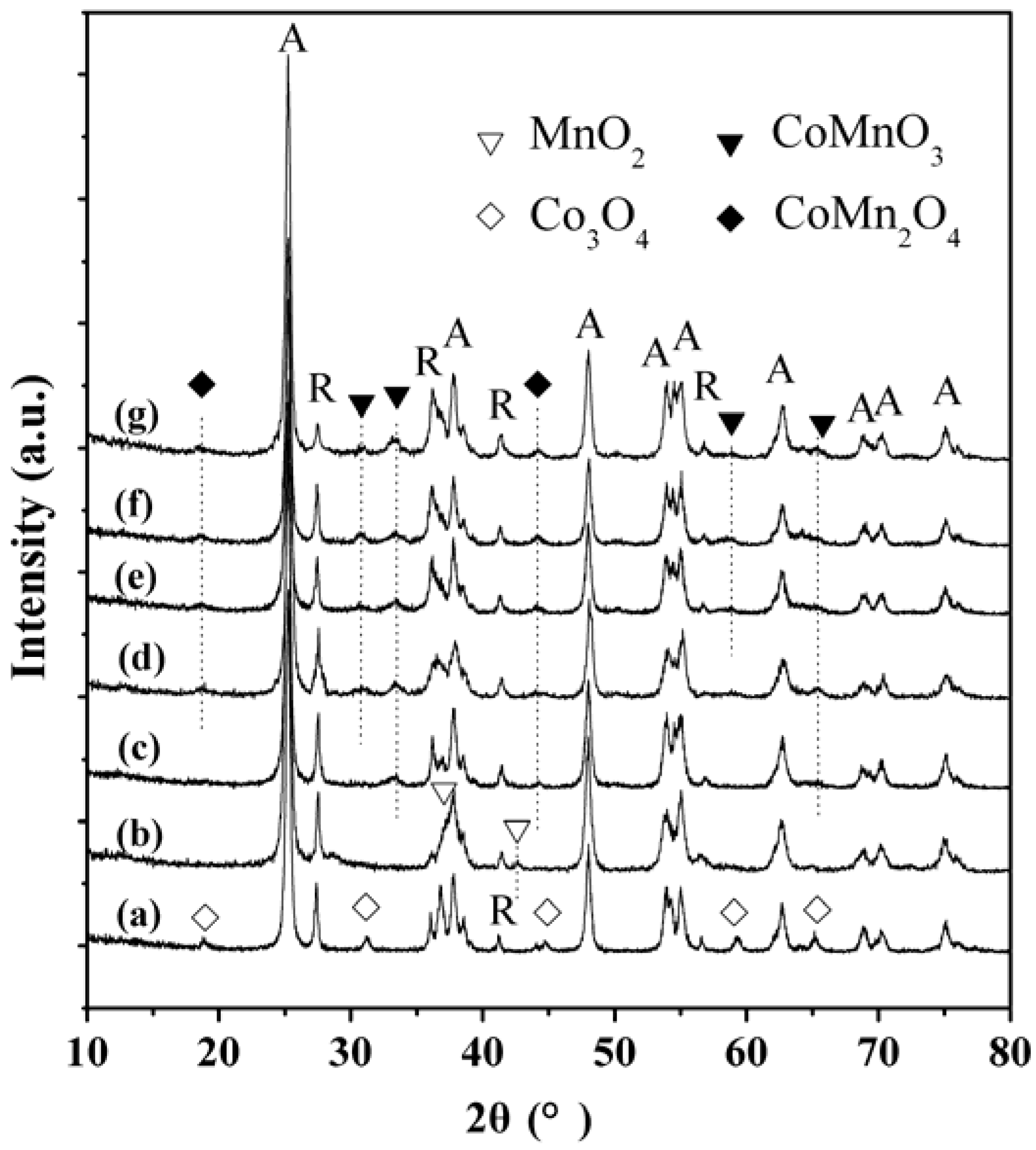
2.3. XRD Characterization
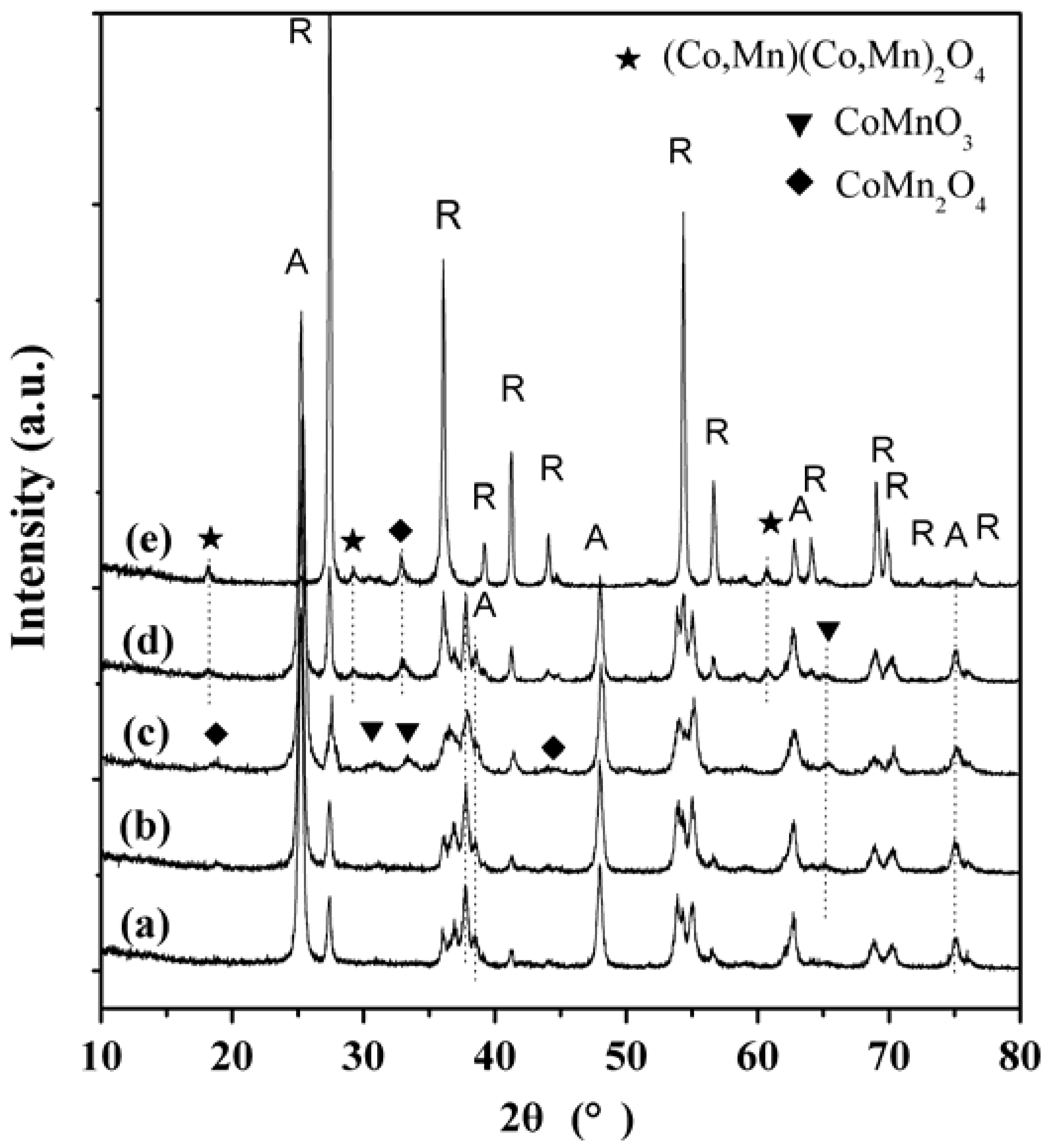
2.4. TEM Characterization
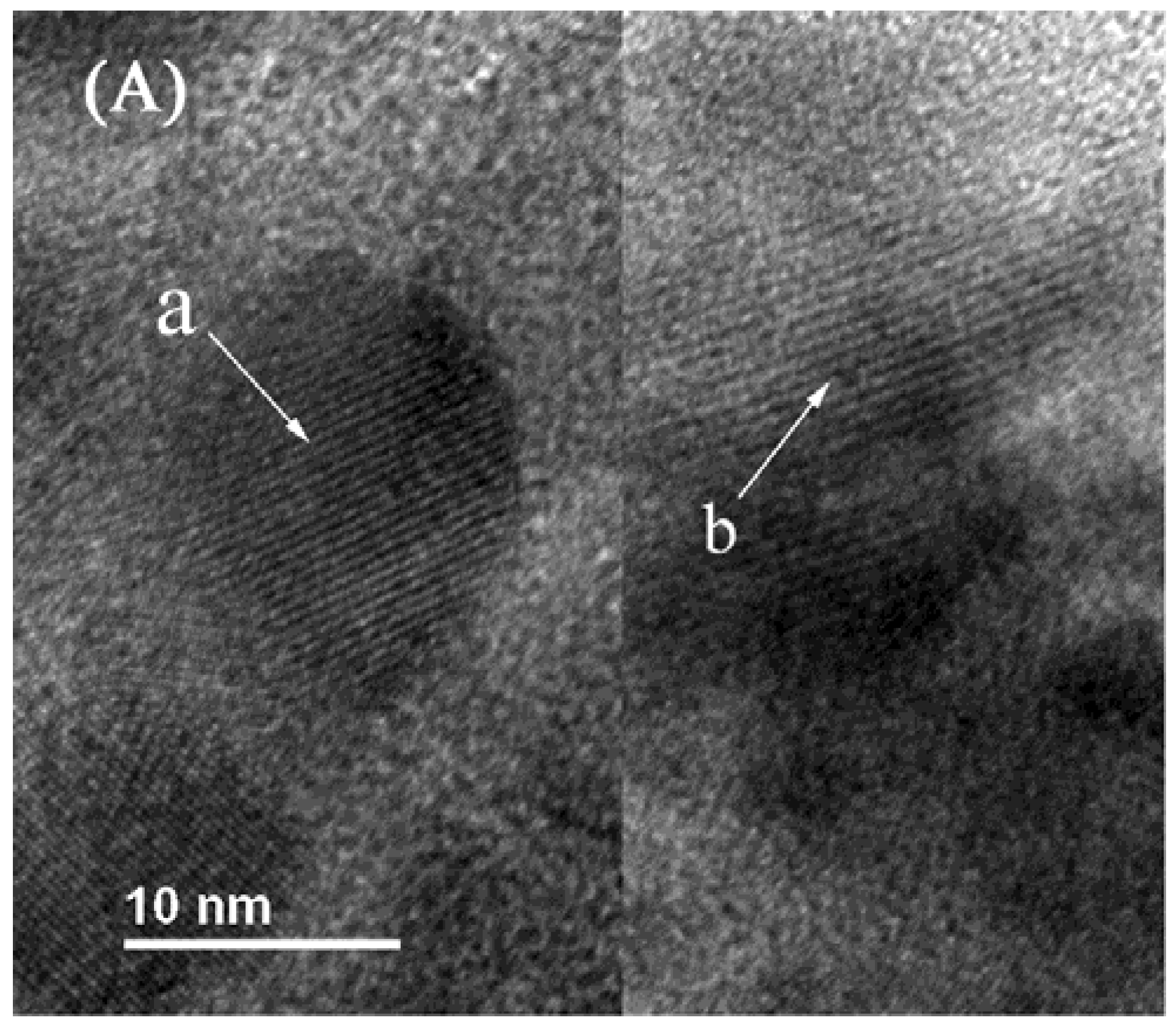
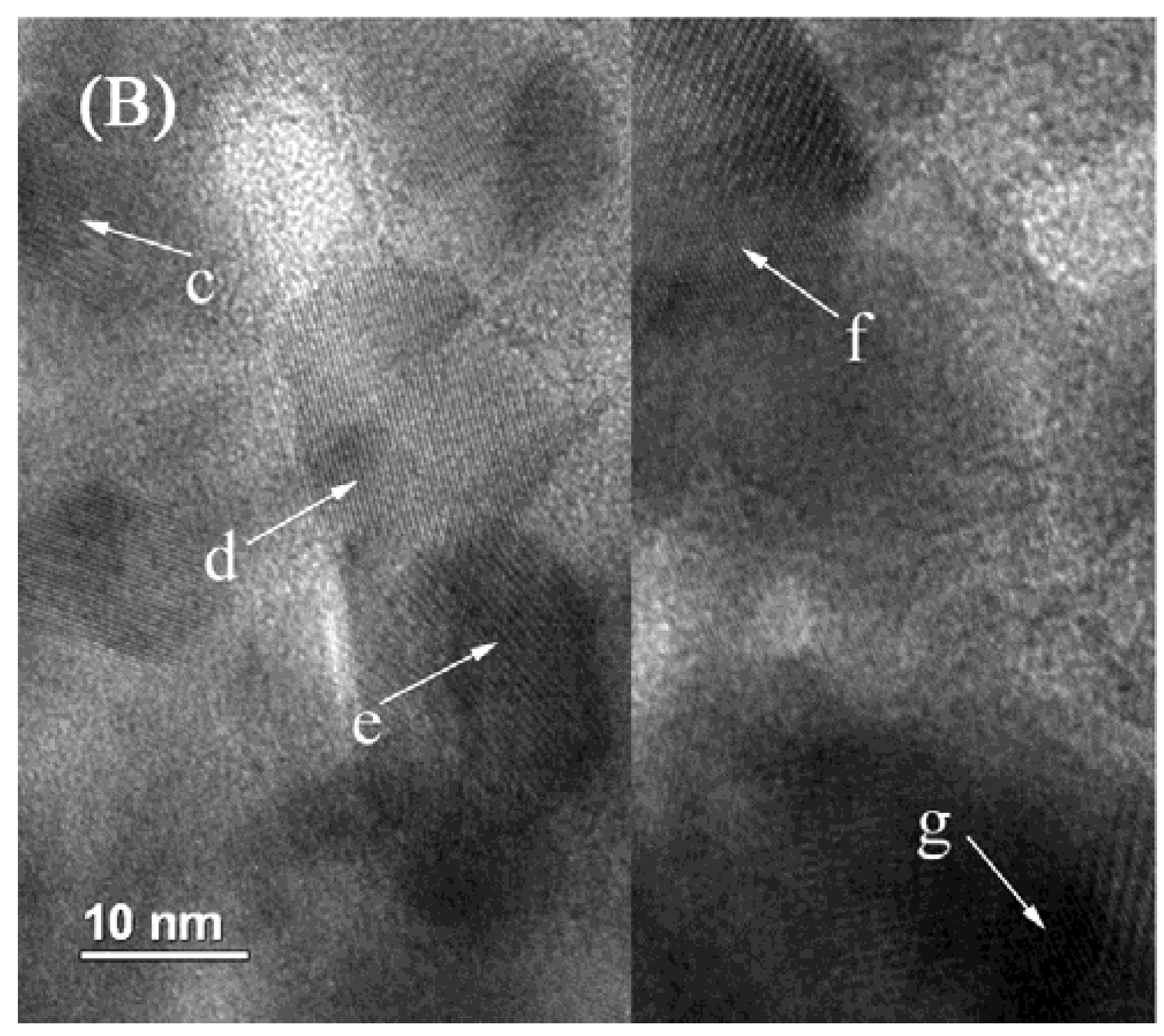
2.5. XPS Characterization
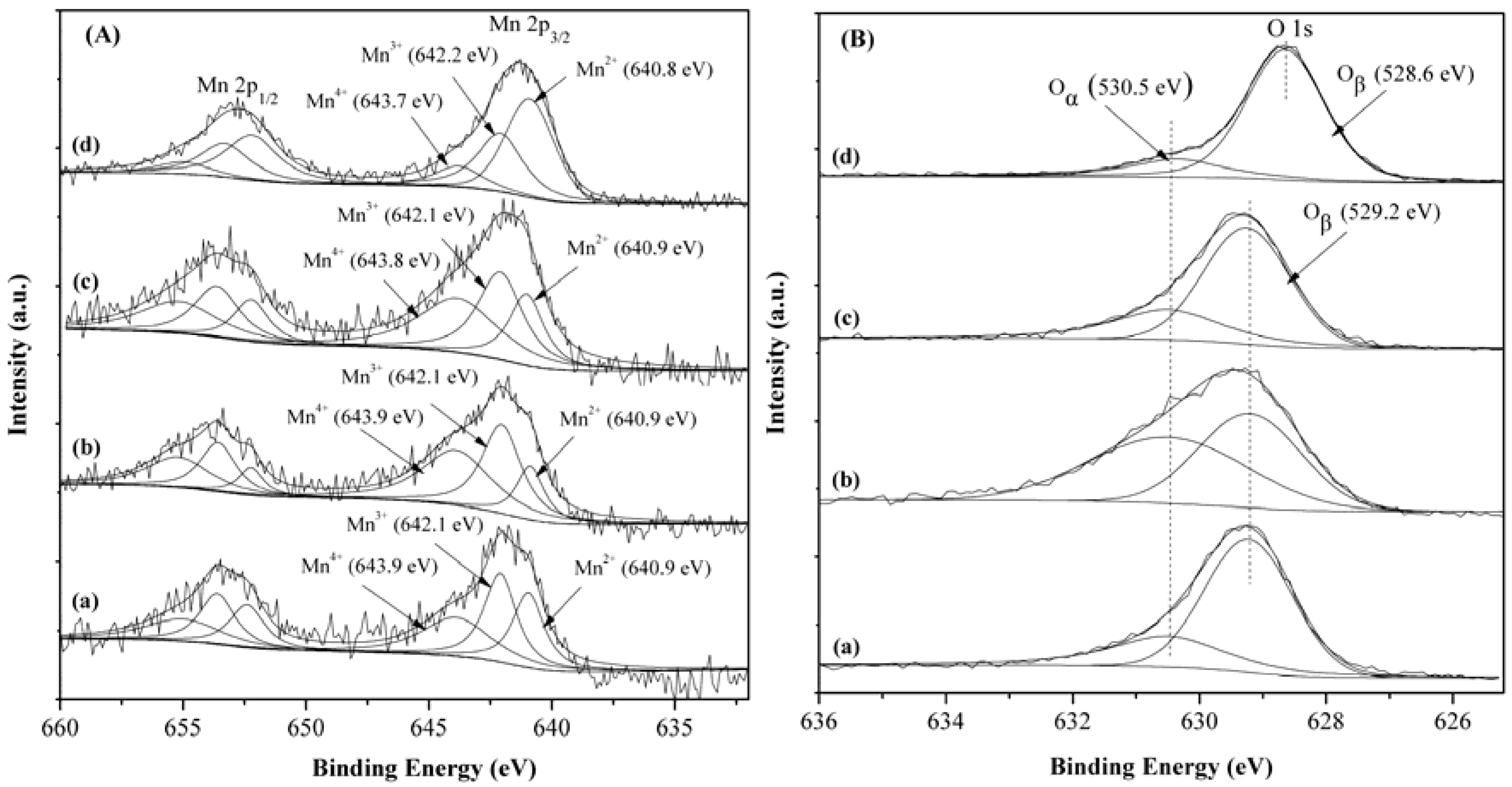
| Catalyst | Mn4+:Mn3+:Mn2+ | Oα:Oβ | Mn at. % | Co at. % |
|---|---|---|---|---|
| 10Mn/TiO2 (773 K) | 34:36:30 | 30:70 | 6.48 | - |
| 10Mn-5Co/TiO2 (673 K) | 43:39:17 | 54:46 | 5.47 | 2.32 |
| 10Mn-5Co/TiO2 (773 K) | 40:37:23 | 32:68 | 6.19 | 3.73 |
| 10Mn-5Co/TiO2 (973 K) | 14:36:50 | 17:83 | 6.73 | 1.54 |
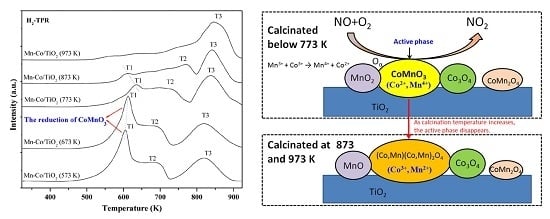
2.6. H2-TPR Characterization
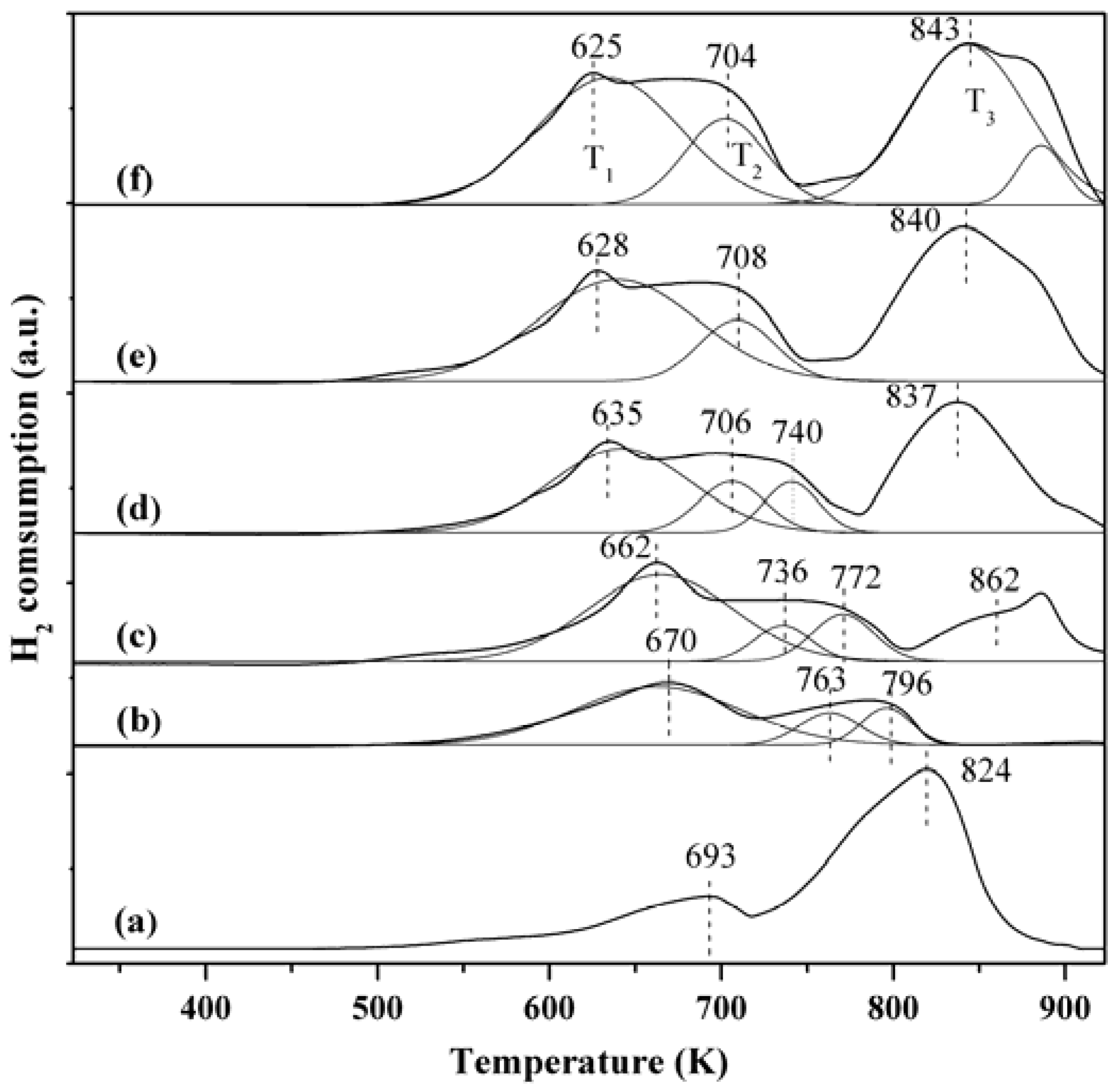
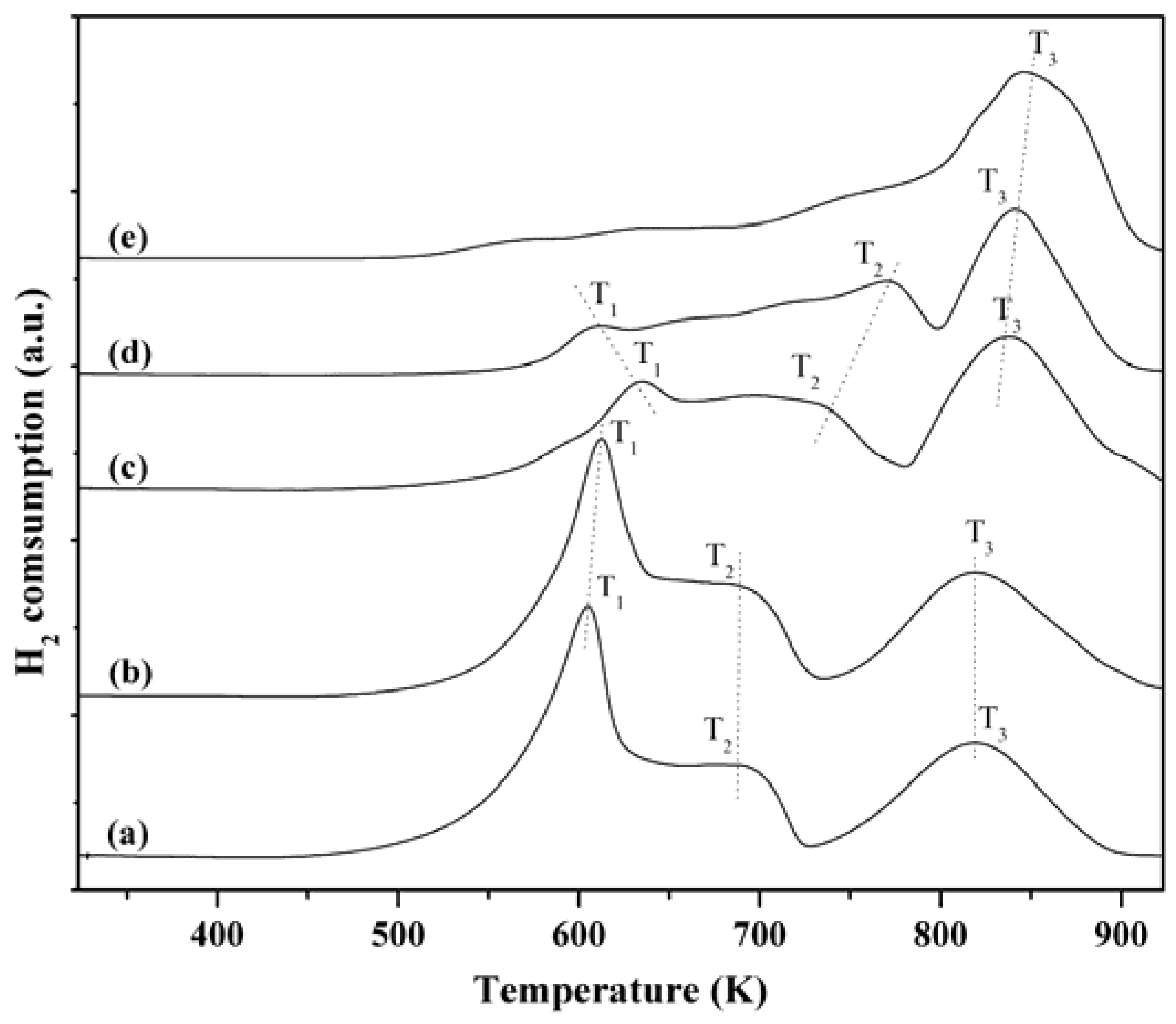
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Evaluation
3.3. Catalyst Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. Sci. Eng. 2004, 46, 163–245. [Google Scholar]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. NH3-NO/NO2 chemistry over V-based catalysts and its role in the mechanism of the Fast SCR reaction. Catal. Today 2006, 114, 3–12. [Google Scholar] [CrossRef]
- Amberntsson, A.; Fridell, E.; Skoglundh, M. Influence of platinum and rhodium composition on the NOx storage and sulphur tolerance of a barium based NOx storage catalyst. Appl. Catal. B 2003, 46, 429–439. [Google Scholar] [CrossRef]
- Li, L.; Qu, L.; Cheng, J.; Li, J.; Hao, Z. Oxidation of nitric oxide to nitrogen dioxide over Ru catalysts. Appl. Catal. B 2009, 88, 224–231. [Google Scholar] [CrossRef]
- Salasc, S.; Skoglundh, M.; Fridell, E. A comparison between Pt and Pd in NOx storage catalysts. Appl. Catal. B 2002, 36, 145–160. [Google Scholar] [CrossRef]
- Weiss, B.M.; Iglesia, E. Mechanism and site requirements for NO oxidation on Pd catalysts. J. Catal. 2010, 272, 74–81. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Atribak, I.; Bueno-López, A.; García-García, A. Influence of the cerium precursor on the physico-chemical features and NO to NO2 oxidation activity of ceria and ceria-zirconia catalysts. J. Mol. Catal. A 2010, 323, 52–58. [Google Scholar] [CrossRef]
- Metkar, P.S.; Balakotaiah, V.; Harold, M.P. Experimental and kinetic modeling study of NO oxidation: Comparison of Fe and Cu-zeolite catalysts. Catal. Today 2012, 184, 115–128. [Google Scholar] [CrossRef]
- Vijay, R.; Hendershot, R.J.; Rivera-Jiménez, S.M.; Rogers, W.B.; Feist, B.J.; Snively, C.M.; Lauterbach, J. Noble metal free NOx storage catalysts using cobalt discovered via high-throughput experimentation. Catal. Commun. 2005, 6, 167–171. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Wu, Z.; Liu, Y. NO Catalytic Oxidation Behaviors over CoOx/TiO2 Catalysts Synthesized by Sol-Gel Method. Catal. Lett. 2010, 134, 295–302. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, N.; Xiao, L.; Liu, Y.; Wang, H. MnOx/TiO2 composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation. J. Colloid Interface Sci. 2010, 352, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Brik, Y.; Kacimi, M.; Ziyad, M.; Bozon-Verduraz, F. Titania-Supported Cobalt and Cobalt-Phosphorus Catalysts: Characterization and Performances in Ethane Oxidative Dehydrogenation. J. Catal. 2001, 202, 118–128. [Google Scholar] [CrossRef]
- Yang, W.-H.; Kim, M.H.; Ham, S.-W. Effect of calcination temperature on the low-temperature oxidation of CO over CoOx/TiO2 catalysts. Catal. Today 2007, 123, 94–103. [Google Scholar] [CrossRef]
- Zafeiratos, S.; Dintzer, T.; Teschner, D.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Methanol oxidation over model cobalt catalysts: Influence of the cobalt oxidation state on the reactivity. J. Catal. 2010, 269, 309–317. [Google Scholar] [CrossRef]
- Petitto, S.C.; Marsh, E.M.; Carson, G.A.; Langell, M.A. Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water. J. Mol. Catal. A 2008, 281, 49–58. [Google Scholar] [CrossRef]
- Irfan, M.F.; Goo, J.H.; Kim, S.D. Co3O4 based catalysts for NO oxidation and NOx reduction in fast SCR process. Appl. Catal. B 2008, 78, 267–274. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) formulations for low-temperature selective catalytic reduction of NOx with NH3: Surface properties and key components in relation to the activity of NOx reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Kim, S.S.; Kwon, D.W.; Hong, S.C. Effect of the Mn oxidation state and lattice oxygen in Mn-based TiO2 catalysts on the low-temperature selective catalytic reduction of NO by NH3. J. Air Waste Manag. 2012, 62, 1085–1092. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Meng, B.; Zhao, Z.; Chen, Y.; Wang, X.; Li, Y.; Qiu, J. Low-temperature synthesis of Mn-based mixed metal oxides with novel fluffy structures as efficient catalysts for selective reduction of nitrogen oxides by ammonia. Chem. Commun. 2014, 50, 12396–12399. [Google Scholar] [CrossRef] [PubMed]
- Venkataswamy, P.; Jampaiah, D.; Lin, F.; Alxneit, I.; Reddy, B.M. Structural properties of alumina supported Ce-Mn solid solutions and their markedly enhanced catalytic activity for CO oxidation. Appl. Surf. Sci. 2015, 349, 299–309. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Wu, G.; Wei, F.; Li, X.; Chen, H.; Wang, S. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides CoxMn3−xO4 for Fenton-Like reaction in water. J. Hazard. Mater. 2015, 296, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Rida, K.; Benabbas, A.; Bouremmad, F.; Peña, M.A.; Martínez-Arias, A. Surface properties and catalytic performance of La1−xSrxCrO3 perovskite-type oxides for CO and C3H6 combustion. Catal. Commun. 2006, 7, 963–968. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrog. Energ. 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadús, L.E. La1−xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Petitto, S.C.; Langell, M.A. Surface composition and structure of Co3O4(110) and the effect of impurity segregation. J. Vac. Sci. Technol. A 2004, 22, 1690–1696. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yin, J.S.; Mo, W.D.; Zhang, Z.J. In-Situ Analysis of Valence Conversion in Transition Metal Oxides Using Electron Energy-Loss Spectroscopy. J. Phys. Chem. B 1997, 101, 6794–6798. [Google Scholar] [CrossRef]
- Sreekanth, P.M.; Pena, D.A.; Smirniotis, P.G. Titania Supported Bimetallic Transition Metal Oxides for low-temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6444–6449. [Google Scholar] [CrossRef]
- Standard Electrode Potential (Data Page). Available online: https://en.wikipedia.org/wiki/Standard_electrode_potential_(data_page) (accessed on 19 October 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Wang, Y.; Pang, D.; Ouyang, F.; Zhang, C.; Cao, G. Characterization and Catalytic Activity of Mn-Co/TiO2 Catalysts for NO Oxidation to NO2 at Low Temperature. Catalysts 2016, 6, 9. https://doi.org/10.3390/catal6010009
Qiu L, Wang Y, Pang D, Ouyang F, Zhang C, Cao G. Characterization and Catalytic Activity of Mn-Co/TiO2 Catalysts for NO Oxidation to NO2 at Low Temperature. Catalysts. 2016; 6(1):9. https://doi.org/10.3390/catal6010009
Chicago/Turabian StyleQiu, Lu, Yun Wang, Dandan Pang, Feng Ouyang, Changliang Zhang, and Gang Cao. 2016. "Characterization and Catalytic Activity of Mn-Co/TiO2 Catalysts for NO Oxidation to NO2 at Low Temperature" Catalysts 6, no. 1: 9. https://doi.org/10.3390/catal6010009
APA StyleQiu, L., Wang, Y., Pang, D., Ouyang, F., Zhang, C., & Cao, G. (2016). Characterization and Catalytic Activity of Mn-Co/TiO2 Catalysts for NO Oxidation to NO2 at Low Temperature. Catalysts, 6(1), 9. https://doi.org/10.3390/catal6010009