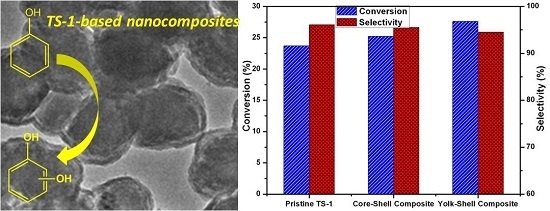
Facile Synthesis of Yolk/Core-Shell Structured TS-1@Mesosilica Composites for Enhanced Hydroxylation of Phenol
Abstract
:1. Introduction
2. Results and Discussion
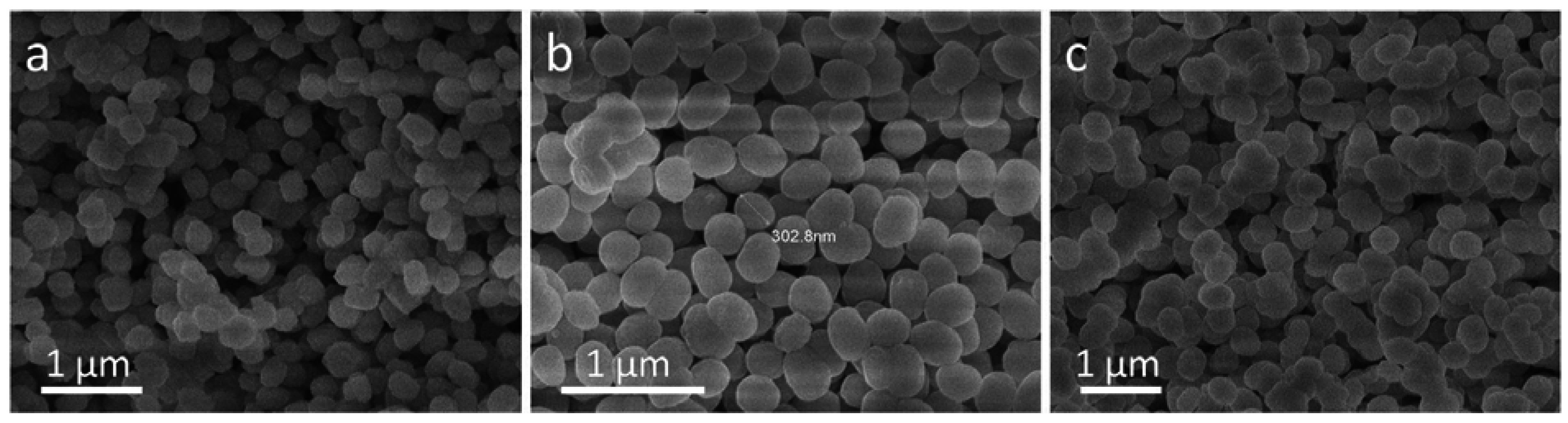
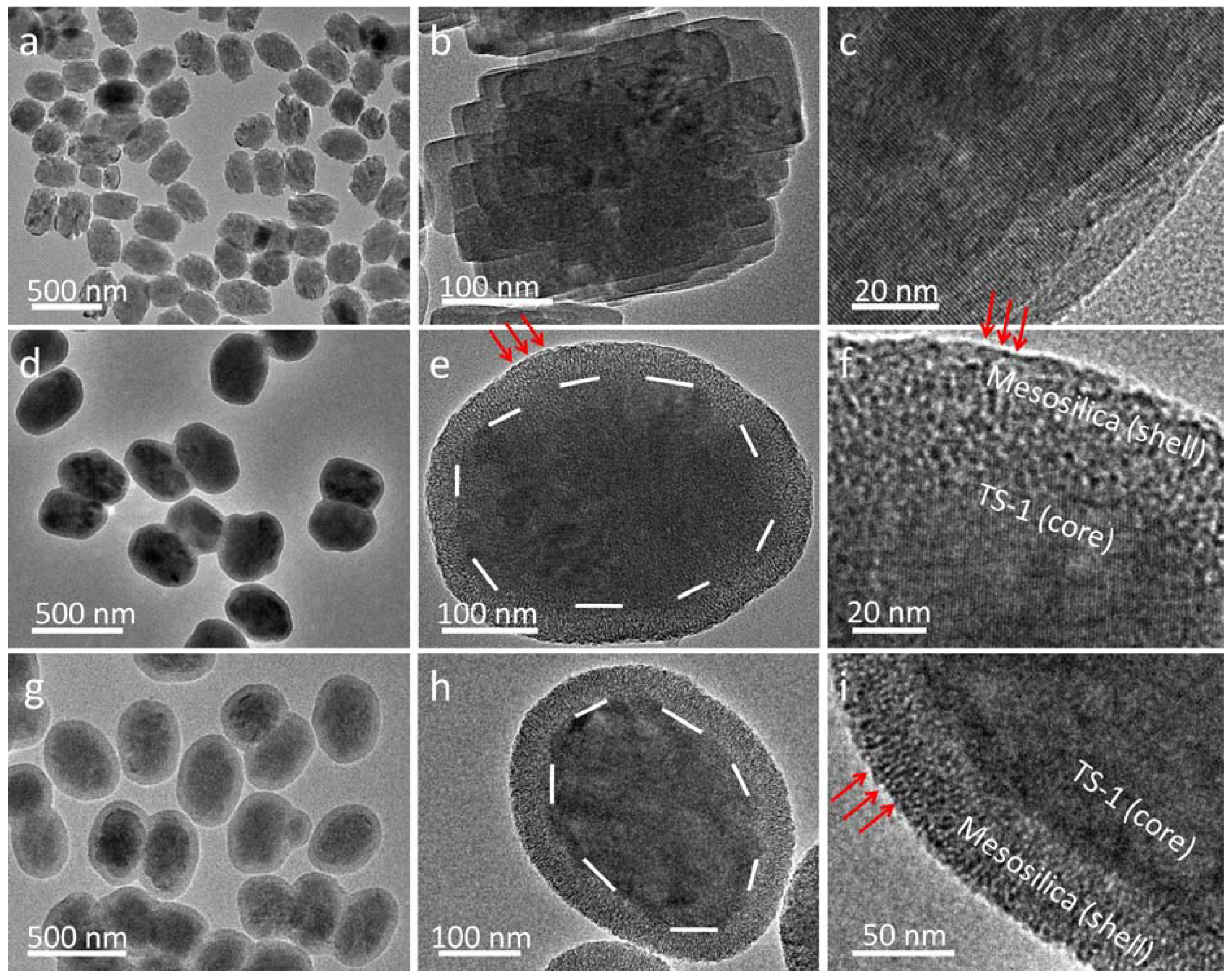
2.1. Synthesis and Characterization of CS-TS-1@mSiO2
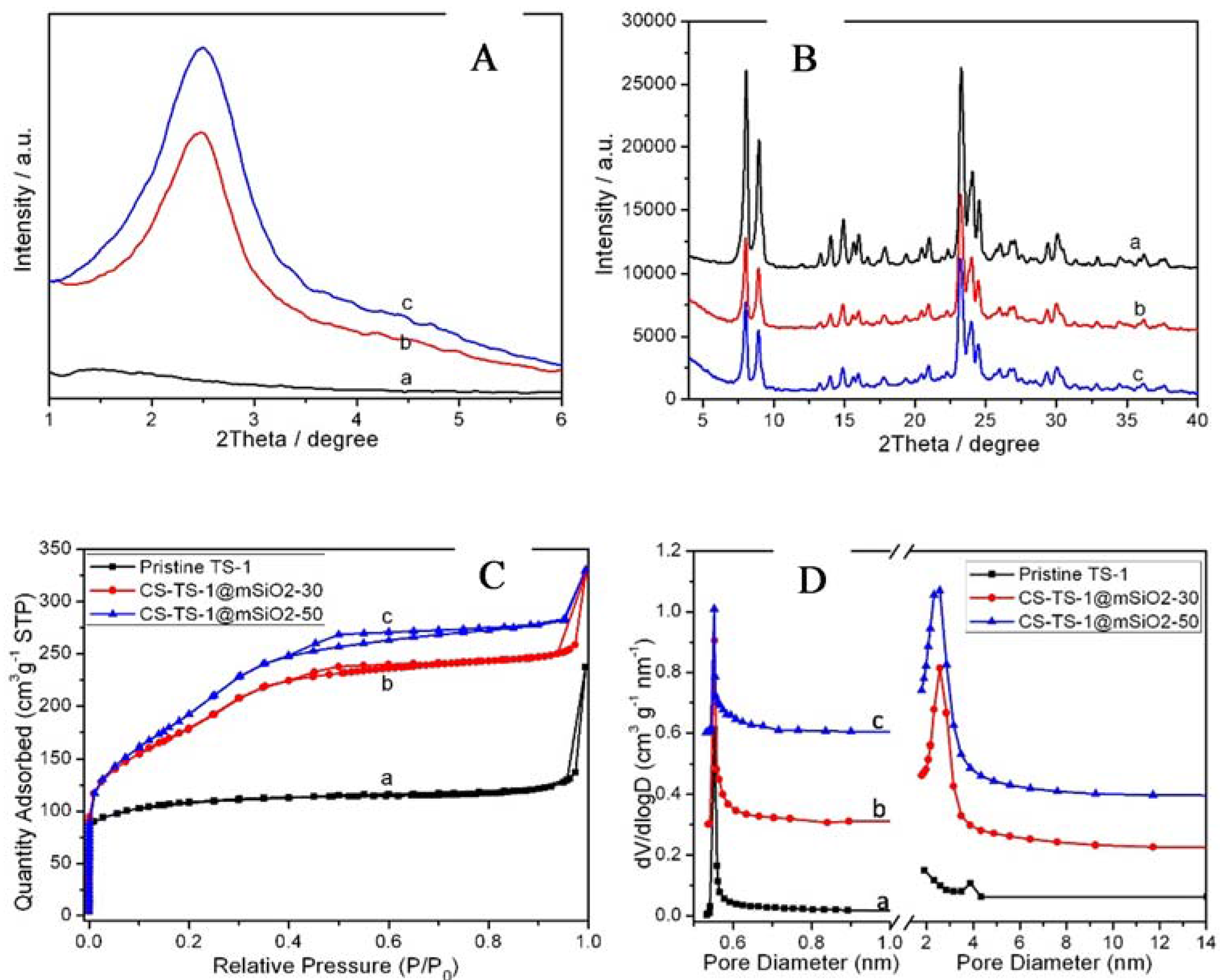
| Sample | SBET [m2 g−1] | Smicro [m2 g−1] | Sext [m2 g−1] | Vmicro [m3 g−1] | Vext [m3 g−1] | Dmicro [nm] | Dmeso [nm] |
|---|---|---|---|---|---|---|---|
| pristine TS-1 | 368.5 | 249.3 | 119.2 | 0.12 | 0.26 | 0.55 | - |
| CS-TS-1@mSiO2-30 | 564.6 | 71.8 | 492.8 | 0.031 | 0.412 | 0.55 | 2.6 |
| CS-TS-1@mSiO2-50 | 635.9 | 53.2 | 582.7 | 0.027 | 0.454 | 0.55 | 2.6 |
| YS-TS-1@mSiO2 | 695.2 | 57.2 | 638.0 | 0.028 | 0.484 | 0.55 | 2.8 |
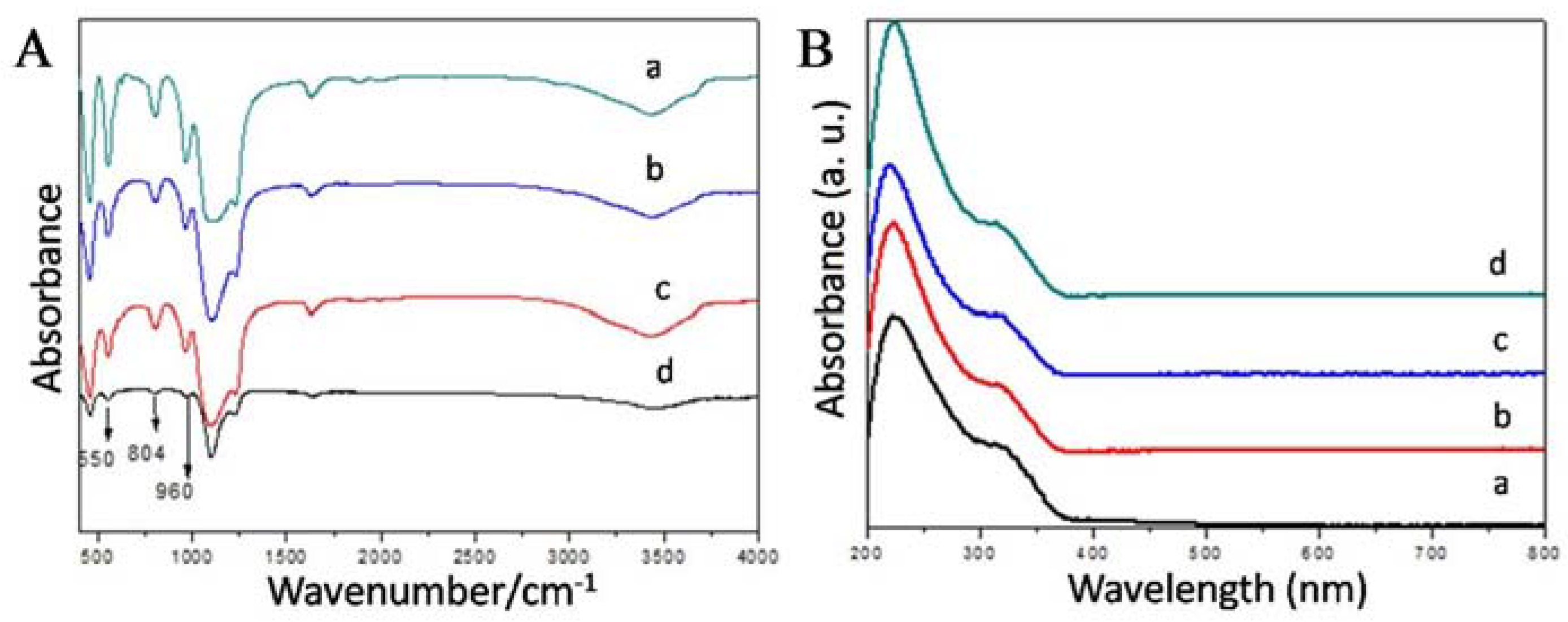
2.2. Synthesis and Characterization of YS-TS-1@mSiO2
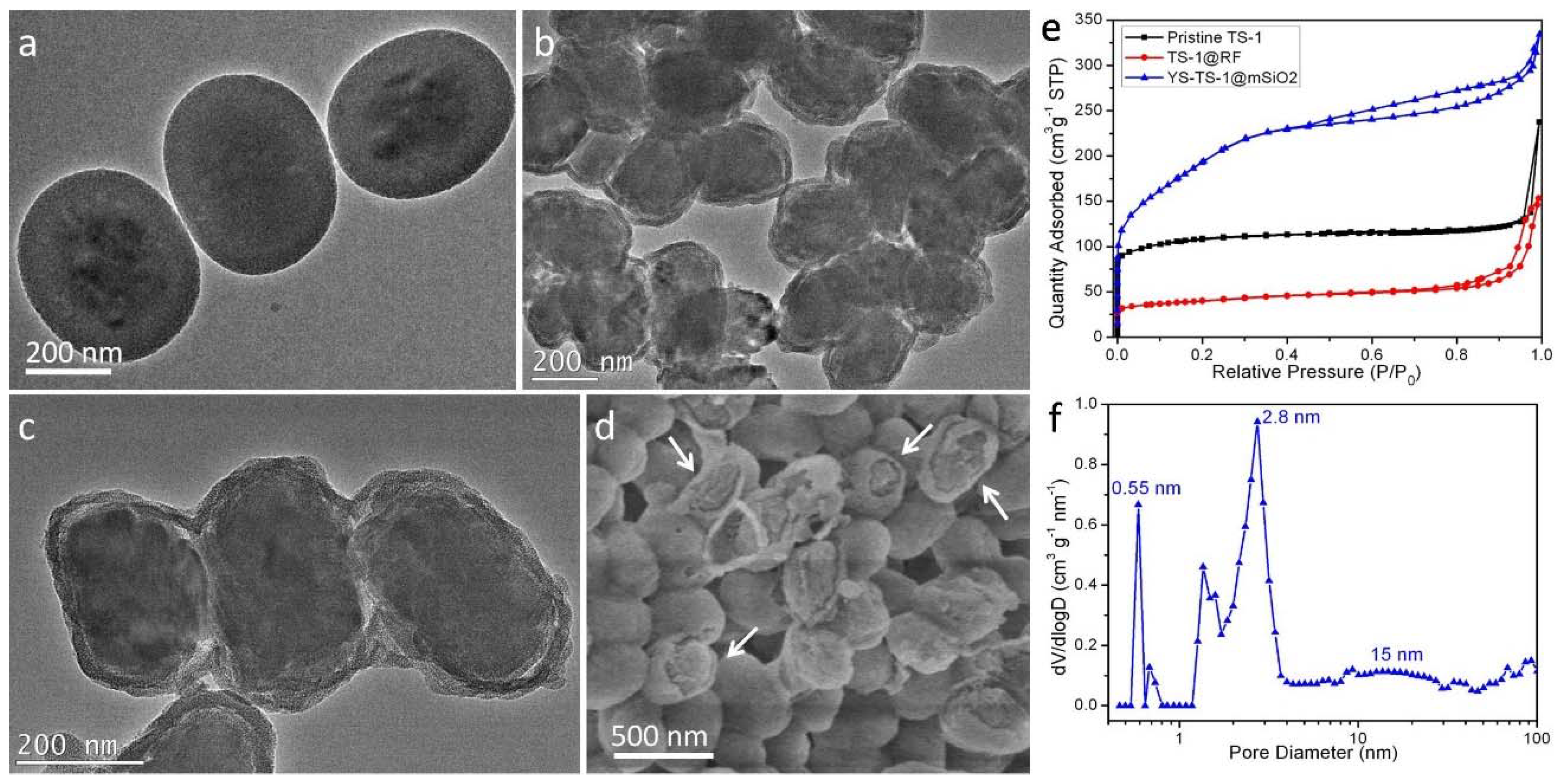
2.3. Catalytic Performance of CS/YS-TS-1@mSiO2 in Hydroxylation of Phenol
| Entry | Catalyst | Reaction Time | Conv. /% | Product Sel. b /% | HQ/CAT | TOF c /h−1 | ||
|---|---|---|---|---|---|---|---|---|
| CAT | HQ | BQ | ||||||
| 1 | Pristine TS-1 | 1 h | 6.2 | 10.3 | 1.5 | 88.2 | 0.15 | 29.6 |
| 2 | 3 h | 16.6 | 60.7 | 25.1 | 14.1 | 0.41 | ||
| 3 | 5 h | 23.7 | 55.1 | 41.0 | 3.9 | 0.74 | ||
| 4 | CS-TS-1@mSiO2 | 1 h | 18.7 | 55.8 | 24.5 | 19.7 | 0.44 | 31.5 |
| 5 | 3 h | 24.3 | 51.2 | 38.1 | 10.7 | 0.74 | ||
| 6 | 5 h | 25.2 | 45.7 | 49.8 | 4.5 | 1.09 | ||
| 7 | YS-TS-1@mSiO2 | 1 h | 20.7 | 49.7 | 24.1 | 26.2 | 0.48 | 34.4 |
| 8 | 3 h | 24.8 | 50.8 | 38.6 | 10.6 | 0.76 | ||
| 9 | 5 h | 27.6 | 47.4 | 47.1 | 5.5 | 0.99 | ||
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of Pristine TS-1 Zeolite
3.3. Synthesis of Core-Shell TS-1@mesosilica Composites
3.4. Synthesis of Yolk-Shell TS-1@Mesosilica Composites
3.5. Catalytic Tests
3.6. Materials Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thangaraj, A.; Kumar, R.; Ratnasamy, P. Catalytic Properties of Crystalline Titanium Silicalites II. Hydroxylation of Phenol with Hydrogen Peroxide over TS-1 Zeolites. J. Catal. 1991, 131, 294–297. [Google Scholar] [CrossRef]
- Martens, J.A.; Buskens, P.; Jacobs, P.A.; van der Pol, A.; van Hooff, J.H.C.; Ferrini, C.; Kouwenhoven, H.W.; Kooyman, P.J.; van Bekkum, H. Hydroxylation of Phenol with Hydrogen Peroxide on EUROTS-1 Catalyst. Appl. Catal. A 1993, 99, 71–84. [Google Scholar] [CrossRef]
- Hamilton, G.; Friedman, J.; Campbell, P. The Hydroxylation of Anisole by Hydrogen Peroxide in the Presence of Catalytic Amounts of Ferric Ion and Catechol. Scope, Requirements, and Kinetic Studies. J. Am. Chem. Soc. 1966, 88, 5266–5268. [Google Scholar] [CrossRef]
- Villa, A.; Caro, C.; Correa, C. Cu- and Fe-ZSM-5 as Catalysts for Phenol Hydroxylation. J. Mol. Catal. A 2005, 228, 233–240. [Google Scholar] [CrossRef]
- Li, B.; Xu, J.; Liu, J.; Zuo, S.; Pan, Z.; Wu, Z. Preparation of Mesoporous Ferrisilicate with High Content of Framework Iron by pH-Modification Method and Its Catalytic Performance. J. Colloid Interface Sci. 2012, 366, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Yoon, S.-S.; Jang, S.-H.; Ahn, W.-S. Phenol Hydroxylation Using Fe-MCM-41 Catalysts. Catal. Today 2006, 111, 280–287. [Google Scholar] [CrossRef]
- Liu, H.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J. Study on the Synthesis and the Catalytic Properties of Fe-HMS Materials in the Hydroxylation of Phenol. Microporous Mesoporous Mater. 2008, 108, 56–64. [Google Scholar] [CrossRef]
- Fu, W.; Wang, R.; Wu, L.; Wang, H.; Wang, X.; Wang, A.; Zhang, Z.; Qiu, S. Synthesis of Cu2(OH)PO4 Crystals with Various Morphologies and Their Catalytic Activity in Hydroxylation of Phenol. Chem. Lett. 2013, 42, 772–774. [Google Scholar] [CrossRef]
- Clerici, M.G. Oxidation of Saturated Hydrocarbons with Hydrogen Peroxide Catalysed by Titanium Silicalite. Appl. Catal. 1991, 68, 249–261. [Google Scholar] [CrossRef]
- Xiao, F.; Han, Y.; Yu, Y.; Meng, X.; Yang, M.; Wu, S. Hydrothermally Stable Ordered Mesoporous Titanosilicates with Highly Active Catalytic Sites. J. Am. Chem. Soc. 2002, 133, 888–889. [Google Scholar] [CrossRef]
- Lin, K.; Wang, L.; Meng, F.; Sun, Z.; Yang, Q.; Cui, Y.; Jiang, D.; Xiao, F.S. Formation of Better Catalytically Active Titanium Species in Ti-MCM-41 by Vapor-phase Silylation. J. Catal. 2005, 235, 423–427. [Google Scholar] [CrossRef]
- Tsai, S.-T.; Chao, P.-Y.; Tsai, T.-C.; Wang, I.; Liu, X.; Guo, X.-W. Effects of Pore Structure of Post-treated TS-1 on Phenol Hydroxylation. Catal. Today 2009, 148, 174–178. [Google Scholar] [CrossRef]
- Zhu, G.; Ni, L.; Qi, W.; Ding, S.; Li, X.; Wang, R. Synthesis and Morphology Research of Framework Ti-rich TS-1 Containing No Extraframework Ti Species in the Presence of CO2. Inorg. Chem. Commun. 2014, 40, 129–132. [Google Scholar] [CrossRef]
- Shi, C.; Chu, B.; Lin, M.; Long, J.; Wang, R. Cyclohexane Mild Oxidation Catalyzed by New Titanosilicate with Hollow Structure. Catal. Today 2011, 175, 398–403. [Google Scholar] [CrossRef]
- Cheneviere, Y.; Chieux, F.; Caps, V.; Tuel, A. Synthesis and Catalytic Properties of TS-1 with Mesoporous/microporous Hierarchical Structures Obtained in the Presence of Amphiphilic Organosilanes. J. Catal. 2010, 269, 161–168. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. Advances in the synthesis of titanosilicates: From the medium pore TS-1 zeolite to highly-accessible ordered materials. Micropor. Mesopor. Mater. 2014, 189, 31–40. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Tian, G.; Li, Y.; Rooke, J.; Zhu, G.; Qiu, S.; Yang, X.; Su, B. Highly Stable and Reusable Multimodal Zeolite TS-1 Based Catalysts with Hierarchically Interconnected Three-Level Micro-Meso-Macroporous Structure. Angew. Chem. Int. Ed. 2011, 50, 11156–11161. [Google Scholar] [CrossRef]
- Xin, H.; Zhao, J.; Xu, S.; Li, J.; Zhang, W.; Guo, X.; Hensen, E.; Li, C. Enhanced Catalytic Oxidation by Hierarchically Structured TS-1 Zeolite. J. Phys. Chem. C 2010, 114, 6553–6559. [Google Scholar] [CrossRef]
- Kang, Z.; Fang, G.; Ke, Q.; Hu, J.; Tang, T. Superior Catalytic Performance of Mesoporous Zeolite TS-1 for the Oxidation of Bulky Organic Sulfides. ChemCatChem 2013, 5, 2191–2194. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Ahn, W.; Ryoo, R. MFI Titanosilicate Nanosheets with Single-unit-cell Thickness as An Oxidation Catalyst Using Peroxides. ACS Catal. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Ding, S.; Zhang, Z.; Li, J.; Qiu, S. Mesoporous MFI Zeolites with Self-stacked Morphology Templated by Cationic Polymer by Cationic Polymer. Chem. Commun. 2010, 46, 7418–7420. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Du, J.; Li, B.; Si, M.; Yang, Y.; Hu, Y.; Niu, G.; Zhang, Y.; Xu, H.; Tu, B.; et al. Controllable Fabrication of Uniform Core-Shell Structured Zeolite@SBA-15 Composites. Chem. Sci. 2011, 2, 2006–2016. [Google Scholar] [CrossRef]
- Xu, L.; Ren, Y.; Wu, H.; Liu, Y.; Wang, Z.; Zhang, Y.; Xu, J.; Peng, H.; Wu, P. Core/shell-Structured TS-1@mesoporous Silica-supported Au Nanoparticles for Selective Epoxidation of Propylene with H2 and O2. J. Mater. Chem. 2011, 21, 10852–10858. [Google Scholar] [CrossRef]
- Xu, L.; Peng, H.; Zhang, K.; Wu, H.; Chen, L.; Liu, Y.; Wu, P. Core-Shell-Structured Titanosilicate As A Robust Catalyst for Cyclohexanone Ammoximation. ACS Catal. 2012, 3, 103–110. [Google Scholar] [CrossRef]
- Qian, X.; Li, B.; Hu, Y.; Niu, G.; Zhang, D.; Che, R.; Tang, Y.; Su, D.; Asiri, A.; Zhao, D. Exploring Meso-/Microporous Composite Molecular Sieves with Core-Shell Structures. Chem. Eur. J. 2012, 18, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Qian, X.; Tu, B.; Zhao, D. Generalized Synthesis of Core-Shell Structured Nano-zeolite@ordered mesoporous Silica Composites. Catal. Today 2013, 204, 2–7. [Google Scholar] [CrossRef]
- Li, C.; Lu, Y.; Wu, H.; Wu, P.; He, M. A Hierarchically Core/Shell-Structured Titanosilicate with Multiple Mesopore Systems for Highly Efficient Epoxidation of Alkenes. Chem. Commun. 2015, 51, 14905–14908. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xu, L.; Wu, H.; Zhang, K.; Wu, P. One-pot Synthesis of Benzamide Over A Robust Tandem Catalyst Based on Center Radially Fibrous Silica Encapsulated TS-1. Chem. Commun. 2013, 49, 2709–2711. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, R.; Li, X.; Wang, X.; Zeng, S.; Ding, S.; Li, L.; Zhang, Z.; Qiu, S. An Organosilane-directed Growth-induced Etching Strategy for Preparing Hollow/Yolk-shell Mesoporous Organosilica Nanospheres with Perpendicular Mesochannels and Amphiphilic Frameworks. J. Mater. Chem. A 2014, 2, 12403–12412. [Google Scholar] [CrossRef]
- Zou, H.; Wang, R.; Dai, J.; Wang, Y.; Wang, X.; Zhang, Z.; Qiu, S. Amphiphilic Hollow Porous Shell Encapsulated Au@Pd Bimetal Nanoparticles for Aerobic Oxidation of Alcohols in Water. Chem. Commun. 2015, 51, 14601–14604. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Wang, X.; Xiao, Y.; Liu, Y.; Huo, Q. A Versatile Cooperative Template-directed Coating Method to Construct Uniform Microporous Carbon Shells for Multifunctional Core-Shell Nanocomposites. Nanoscale 2013, 5, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Sun, Q.; Fan, D.; Fu, W.; Liu, L.; Wang, R. Facile Synthesis of Yolk/Core-Shell Structured TS-1@Mesosilica Composites for Enhanced Hydroxylation of Phenol. Catalysts 2015, 5, 2134-2146. https://doi.org/10.3390/catal5042134
Zou H, Sun Q, Fan D, Fu W, Liu L, Wang R. Facile Synthesis of Yolk/Core-Shell Structured TS-1@Mesosilica Composites for Enhanced Hydroxylation of Phenol. Catalysts. 2015; 5(4):2134-2146. https://doi.org/10.3390/catal5042134
Chicago/Turabian StyleZou, Houbing, Qingli Sun, Dongyu Fan, Weiwei Fu, Lijia Liu, and Runwei Wang. 2015. "Facile Synthesis of Yolk/Core-Shell Structured TS-1@Mesosilica Composites for Enhanced Hydroxylation of Phenol" Catalysts 5, no. 4: 2134-2146. https://doi.org/10.3390/catal5042134
APA StyleZou, H., Sun, Q., Fan, D., Fu, W., Liu, L., & Wang, R. (2015). Facile Synthesis of Yolk/Core-Shell Structured TS-1@Mesosilica Composites for Enhanced Hydroxylation of Phenol. Catalysts, 5(4), 2134-2146. https://doi.org/10.3390/catal5042134