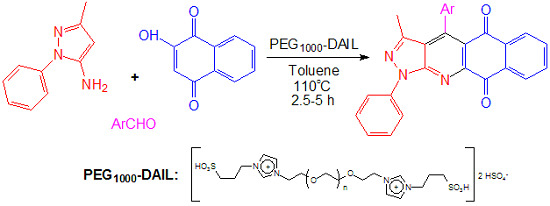
PEG1000-Based Dicationic Acidic Ionic Liquid Catalyzed One-Pot Synthesis of 4-Aryl-3-Methyl-1-Phenyl-1H-Benzo[h]pyrazolo [3,4-b]quinoline-5,10-Diones via Multicomponent Reactions
Abstract
:1. Introduction
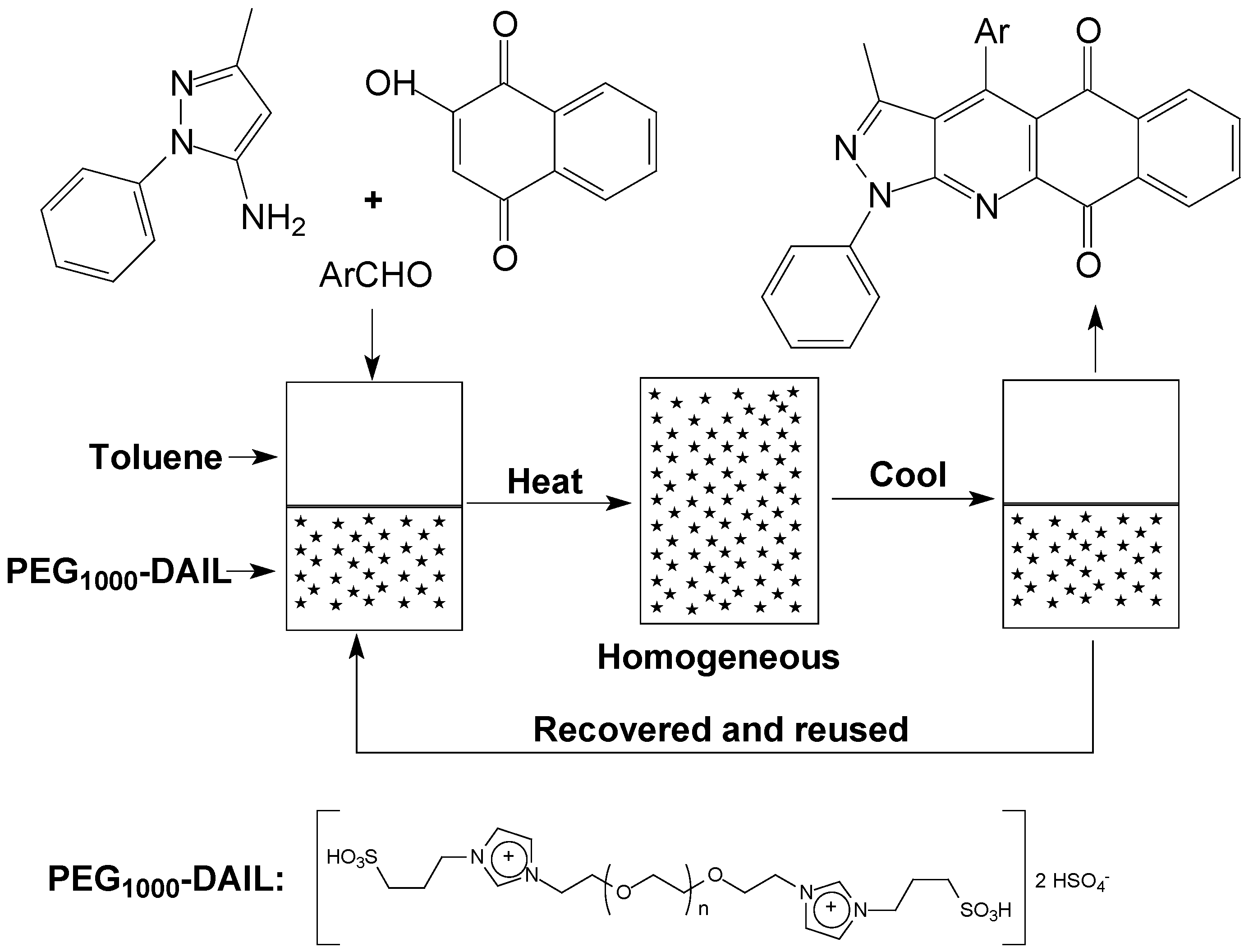
2. Results and Discussion
| Entry | T (°C) | Time (h) | Yield (%) a |
|---|---|---|---|
| 1 | r.t. | 6 | 8 |
| 2 | 40 | 6 | 21 |
| 3 | 50 | 6 | 30 |
| 4 | 60 | 6 | 43 |
| 5 | 70 | 6 | 51 |
| 6 | 80 | 6 | 65 |
| 7 | 90 | 6 | 81 |
| 8 | 100 | 5 | 89 |
| 9 b | 110 | 3 | 89, 89, 87, 86, 86, 84 |
| Entry | Ar | Time (h) | Yield (%) a | mp (°C) | mp (°C) [Ref.] |
|---|---|---|---|---|---|
| 1 | C6H5 | 3 | 89 | 264–266 | 266–267 [8] |
| 2 | 4-ClC6H4 | 3.5 | 91 | 243–245 | 243–245 [8] |
| 3 | 2-ClC6H4 | 4 | 86 | 228–229 | 229–230 [8] |
| 4 | 4-CH3C6H4 | 2.5 | 88 | 276–277 | 276–277 [8] |
| 5 | 4-CH3OC6H4 | 3 | 89 | 275–276 | 274–275 [8] |
| 6 | 4-NO2C6H4 | 4 | 90 | 325–327 | 326–328 [8] |
| 7 | 3-NO2C6H4 | 5 | 85 | 288–289 | 288–289 [8] |
| 8 | 4-FC6H4 | 5 | 91 | 282–284 | 282–283 [8] |
| 9 | 3,4-Cl2C6H3 | 6 | 87 | 268–270 | 269–270 [8] |

3. Experimental Section
3.1. General Procedure
3.2. General Experimental Procedure for the Synthesis of 4-Aryl-3-Methyl-1-Phenyl-1H-Benzo[h]pyrazolo[3,4-b]quinoline-5,10-diones
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ren, Y.M.; Yang, R.C.; Cai, C. Molecular iodine-catalyzed multicomponent reactions: an efficient catalyst for organic synthesis. RSC Adv. 2013, 3, 7182–7204. [Google Scholar] [CrossRef]
- Crenshaw, R.R.; Luke, G.M.; Smirnoff, P. Interferon inducing activities of derivatives of 1,3-dimethyl-4-(3-dimethylaminopropylamino)-1H-pyrazolo[3,4-b]quinoline and related compounds. J. Med. Chem. 1976, 19, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.S.; Ismail, M.M.; Barakat, S.E.S.; Abdul-Rahman, A.A.A.; Bayomi, A.H.; El-Gamal, K.M.A. Synthesis and antimicrobial activity of some new quinoline and 1H-pyrazolo[3,4-b]quinoline derivatives. Bull. Pharm. Sci. Assiut. Univ. 2004, 27, 237–245. [Google Scholar]
- Wolin, R.; Wang, D.; Kelly, J.; Afonso, A.; James, L.; Kirschmeier, P.; McPhail, A.T. Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of oncogenic Ras. Bioorg. Med. Chem. Lett. 1996, 6, 195–200. [Google Scholar] [CrossRef]
- Quiroga, J.; Insuasty, B.; Hormaza, A.; Saitz, C.; Jullian, C. Synthesis of 4-aryl-4,7,8,9-tetrahydro-6H-pyrazolo[3,4-b]quinolin-5-ones. J. Heterocycl. Chem. 1998, 35, 575–578. [Google Scholar] [CrossRef]
- Hua, G.P.; Xu, J.N.; Tu, S.J.; Wang, Q.; Zhang, J.P.; Zhu, X.T.; Li, T.J.; Zhu, S.L.; Zhang, X.J. Facile three component one-pot synthesis of 5-aryl-1,5,6,7,8,9-hexahydro-2H-pyrazolo[5,4-b]quinolin-6-one derivatives under microwave irradiation. Chin. J. Org. Chem. 2005, 25, 1610–1614. [Google Scholar]
- Wu, L.; Ma, S.; Yan, F.; Yang, C. Sulfamic-acid-catalyzed simple and efficient synthesis of 4-aryl-3-methyl-1-phenyl-1H-benzo[g]pyrazolo[3,4-b]quinoline-5,10-diones under solvent-free conditions. Monatsh. Chem. 2010, 141, 565–568. [Google Scholar] [CrossRef]
- Wu, L.; Yang, L.; Yan, F.; Yang, C.; Fang, L. Molecular iodine: a versatile catalyst for the synthesis of 4-aryl-3-methyl-1-phenyl-1H-benzo[h]pyrazolo[3,4-b]quinoline-5,10-diones in water. Bull. Korean. Chem. Soc. 2010, 31, 1051–1054. [Google Scholar] [CrossRef]
- Wu, L.; Dong, R.Y.; Yang, C.G.; Yan, F.L. Synthesis of 4-aryl-3-methyl-1-phenyl-1H-benzo[h]pyrazolo[3,4-b]quinoline-5,10-diones using diammonium hydrogen phosphate as an efficient and versatile catalyst in water. J. Chin. Chem. Soc. 2010, 57, 19–23. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chaudhary, A.; Nand, B.; Lumb, A. Aqua mediated indium(III) chloride catalyzed synthesis of fused pyrimidines and pyrazoles. Tetrahedron Lett. 2012, 53, 3018–3022. [Google Scholar] [CrossRef]
- Yoshioka, K.; Yamada, T.; Ohno, H.; Miyafuji, H. Production of 2-hydroxyacetylfuran from lignocellulosics treated with ionic liquid–water mixtures. RSC Adv. 2015, 5, 72405–72409. [Google Scholar] [CrossRef]
- Ganapathi, P.; Ganesan, K. Synthesis and characterization of 1,2-dimethyl imidazolium ionic liquids and their catalytic activities. Synth. Commun. 2015, 45, 2135–2141. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Recent progress in ionic liquids and their applications in organic synthesis. Org. Prep. Proced. Int. 2015, 47, 249–308. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Acidic bronsted ionic liquids. Org. Prep. Proced. Int. 2010, 42, 285–362. [Google Scholar]
- El-Shamy, A.M.; Zakaria, Kh.; Abbas, M.A.; Zein El Abedin, S. Anti-bacterial and anti-corrosion effects of the ionic liquid 1-butyl-1-methylpyrrolidinium trifluoromethylsulfonate. J. Mol. Liq. 2015, 211, 363–369. [Google Scholar] [CrossRef]
- Pal, A.; Pillania, A. The effect of hydrophilic ionic liquid 1-butyl-2,3-dimethylimidazolium bromide on the aggregation behavior of tetradecyltrimethylammonium bromide in aqueous media. J. Mol. Liq. 2015, 209, 6–13. [Google Scholar] [CrossRef]
- Qiao, Y.; Headley, A.D. Ionic Liquid immobilized organocatalysts for asymmetric reactions in aqueous media. Catalysts 2013, 3, 709–725. [Google Scholar] [CrossRef]
- Ren, Y.M.; Cai, C. A green procedure for the protection of carbonyl compounds catalyzed by iodine in ionic liquid. Tetrahedron Lett. 2008, 49, 7110–7112. [Google Scholar] [CrossRef]
- Ren, Y.M.; Cai, C. Molecular iodine in ionic liquid: A green catalytic system for esterification and transesterification. Synth. Commun. 2010, 40, 1670–1676. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shao, J.J.; Wu, Z.C.; Yang, R.C.; Zhang, Z.; Tao, T.X. PEG1000-based dicationic acidic ionic liquid as an efficient catalyst for mannich-type reaction in water. Synth. Commun. 2014, 44, 2529–2534. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shao, J.J.; Wu, Z.C.; Xu, M.D. PEG1000-based dicationic acidic ionic liquid catalyzed one-pot synthesis of 1,4-dihydropyridines via the Hantzsch reaction. Org. Prep. Proced. Int. 2014, 46, 545–550. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shao, J.J.; Wu, Z.C.; Zhang, S.; Tao, T.X. Facile protection of carbonyl compounds as oxathiolanes and thioacetals promoted by PEG1000-based dicationic acidic ionic liquid as chemoselective and recyclable catalyst. J. Mol. Liq. 2014, 196, 392–394. [Google Scholar] [CrossRef]
- Zhi, H.; Lü, C.; Zhang, Q.; Luo, J. A new PEG-1000-based dicationic ionic liquid exhibiting temperature-dependent phase behavior with toluene and its application in one-pot synthesis of benzopyrans. Chem. Commun. 2009, 2878–2880. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Yang, J.; Jiao, C. Thermal-regulated PEG1000-based ionic liquid/PM for one-pot three-component synthesis of 2,4,5-trisubstituted imidazoles. Catal. Sci. Technol. 2011, 1, 243–245. [Google Scholar] [CrossRef]
- Hu, Y.L.; Jiang, H.; Zhu, J.; Lu, M. Facile and efficient hydrolysis of organic halides, epoxides, and esters with water catalyzed by ferric sulfate in a PEG1000-DAIL[BF4]/toluene temperature-dependent biphasic system. New J. Chem. 2011, 35, 292–298. [Google Scholar] [CrossRef]
- Lu, H.; Sun, L.; Wu, D.; Gao, Y.; Shi, Y.; Xue, Q. Synthesis of 6-amino-purine derivatives catalyzed by polyethylene glycol1000-dicationic acidic ionic liquid. Chin. J. Org. Chem. 2012, 32, 1880–1887. [Google Scholar] [CrossRef]
- Wang, Y.; Zhi, H.; Luo, J. A facile and efficient protocol for esterification and acetalization in a PEG1000-D(A)IL/toluene thermoregulated catalyst-media combinedsystems. J. Mol. Catal. A 2013, 379, 46–52. [Google Scholar] [CrossRef]
- Ren, Y.M.; Cai, C. Convenient and efficient method for synthesis of substituted 2-amino-2-chromenes using catalytic amount of iodine and K2CO3 in aqueous medium. Catal. Commun. 2008, 9, 1017–1020. [Google Scholar] [CrossRef]
- Ren, Y.M.; Cai, C. Three-component condensation catalyzed by molecular iodine for the synthesis of 2,4,6-triarylpyridines and 5-unsubstituted-3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Monatsh. Chem. 2009, 140, 49–52. [Google Scholar] [CrossRef]
- Ren, Y.M.; Cai, C. A solvent-free synthesis of 1,2,4,5-tetrasubstituted imidazoles using molecular iodine as catalyst. J. Chem. Res. 2010, 34, 133–134. [Google Scholar] [CrossRef]
- Zeng, L.Y.; Ren, Y.M.; Cai, C. Iodine-catalyzed, multicomponent, one-pot synthesis of 5-aryl-5,8-dihydrotetrazolo[1,5-a]pyrimidine-7-carboxylic acids. Synth. Commun. 2011, 41, 3635–3643. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.-M.; Jin, S.; Yan, H.-J.; Zhang, Z. PEG1000-Based Dicationic Acidic Ionic Liquid Catalyzed One-Pot Synthesis of 4-Aryl-3-Methyl-1-Phenyl-1H-Benzo[h]pyrazolo [3,4-b]quinoline-5,10-Diones via Multicomponent Reactions. Catalysts 2015, 5, 1649-1656. https://doi.org/10.3390/catal5041649
Ren Y-M, Jin S, Yan H-J, Zhang Z. PEG1000-Based Dicationic Acidic Ionic Liquid Catalyzed One-Pot Synthesis of 4-Aryl-3-Methyl-1-Phenyl-1H-Benzo[h]pyrazolo [3,4-b]quinoline-5,10-Diones via Multicomponent Reactions. Catalysts. 2015; 5(4):1649-1656. https://doi.org/10.3390/catal5041649
Chicago/Turabian StyleRen, Yi-Ming, Shuo Jin, Hai-Jun Yan, and Ze Zhang. 2015. "PEG1000-Based Dicationic Acidic Ionic Liquid Catalyzed One-Pot Synthesis of 4-Aryl-3-Methyl-1-Phenyl-1H-Benzo[h]pyrazolo [3,4-b]quinoline-5,10-Diones via Multicomponent Reactions" Catalysts 5, no. 4: 1649-1656. https://doi.org/10.3390/catal5041649
APA StyleRen, Y.-M., Jin, S., Yan, H.-J., & Zhang, Z. (2015). PEG1000-Based Dicationic Acidic Ionic Liquid Catalyzed One-Pot Synthesis of 4-Aryl-3-Methyl-1-Phenyl-1H-Benzo[h]pyrazolo [3,4-b]quinoline-5,10-Diones via Multicomponent Reactions. Catalysts, 5(4), 1649-1656. https://doi.org/10.3390/catal5041649