Removal of Toluene over NaX Zeolite Exchanged with Cu2+
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Materials
2.1.1. Redox Properties
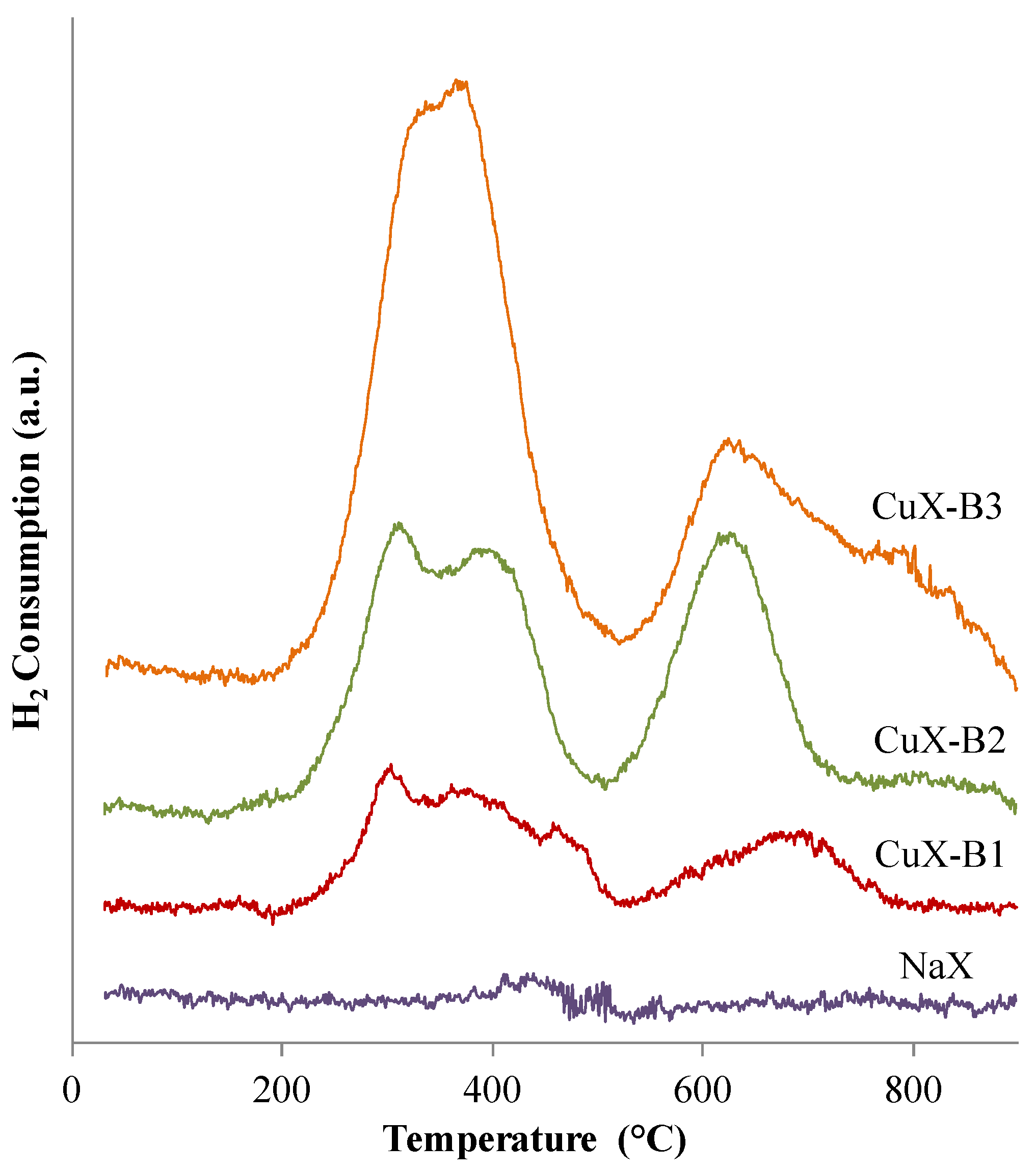
- (i)
- NaX sample is irreducible;
- (ii)
- All the copper species are present as CuO and Cu2+ species in the copper-exchanged samples;
- (iii)
- All the copper species are reduced by H2 in the copper-exchanged samples into Cu0 species.
| Sample | Consumed H2 (mmol/g) | LTR Consumed H2 (mmol/g) | HTR Consumed H2 (mmol/g) | Copper Content (wt. %) * | Molar Ratio Cu2+/Cutotal | Degree of Exchange |
|---|---|---|---|---|---|---|
| NaX | 0.02 | - | - | - | - | - |
| CuX-B1 | 0.17 | 0.12 | 0.05 | 1.1 | 0.6 | 2.0 |
| CuX-B2 | 0.36 | 0.23 | 0.13 | 2.3 | 0.7 | 5.3 |
| CuX-B3 | 0.62 | 0.41 | 0.21 | 4.0 | 0.7 | 8.5 |
2.1.2. Bulk Structure
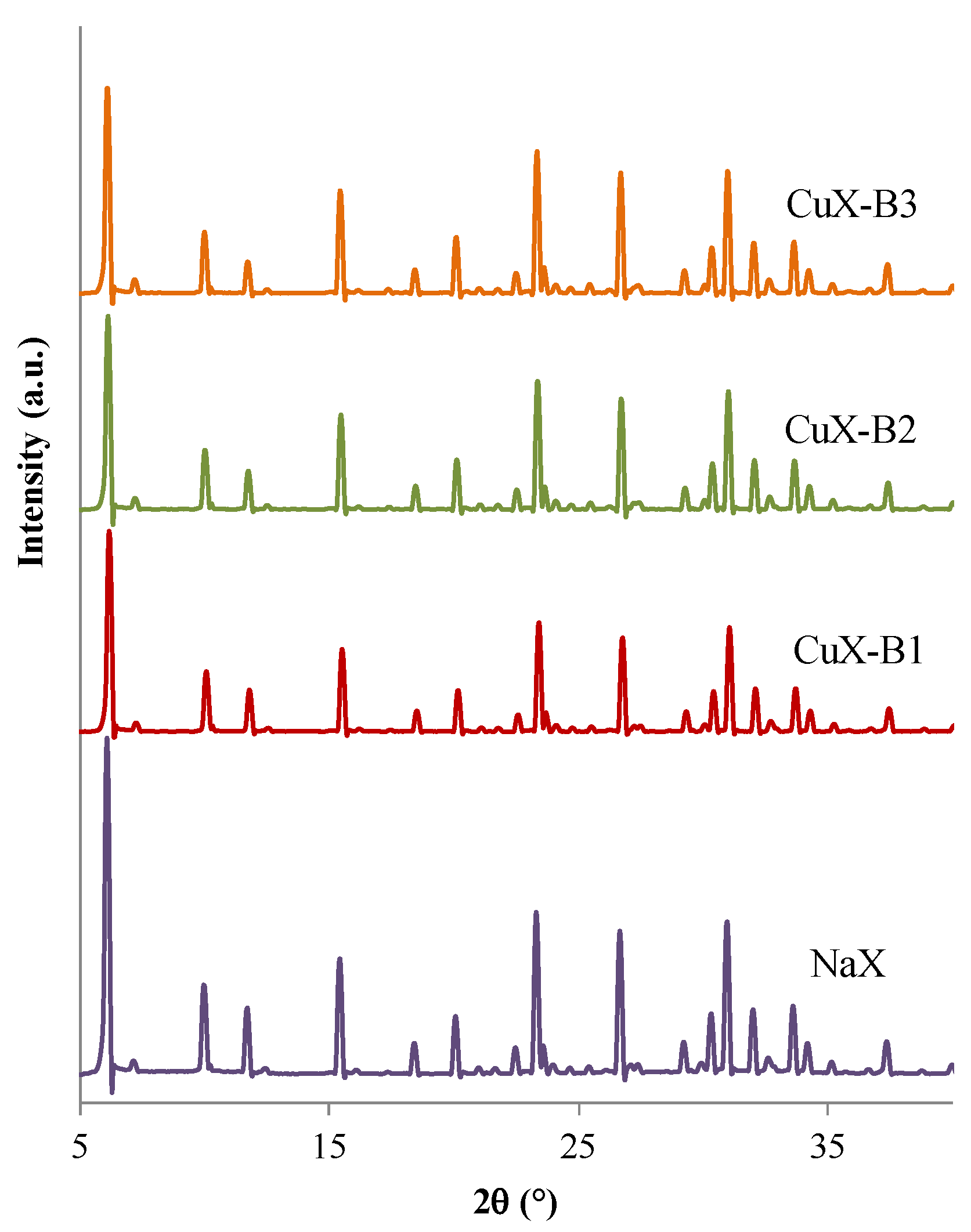
2.1.3. Specific Surface Area
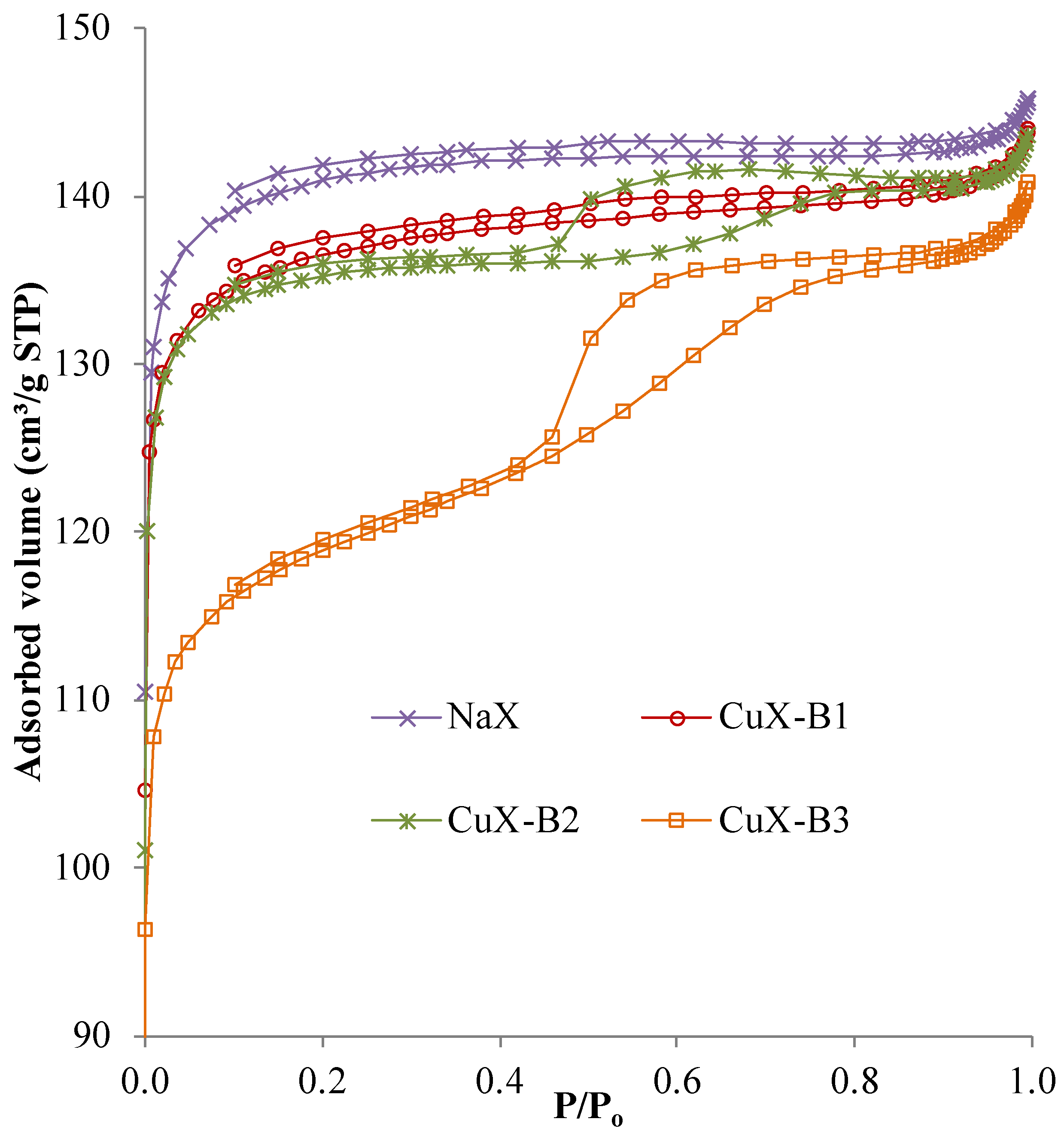
| Sample | Specific Surface Area (m2/g) | Pore Volume * (cm3/g) | Micropore Volume ** (cm3/g) |
|---|---|---|---|
| NaX | 451 | 0.226 | 0.218 |
| CuX-B1 | 434 | 0.223 | 0.211 |
| CuX-B2 | 432 | 0.222 | 0.201 |
| CuX-B3 | 384 | 0.218 | 0.185 |
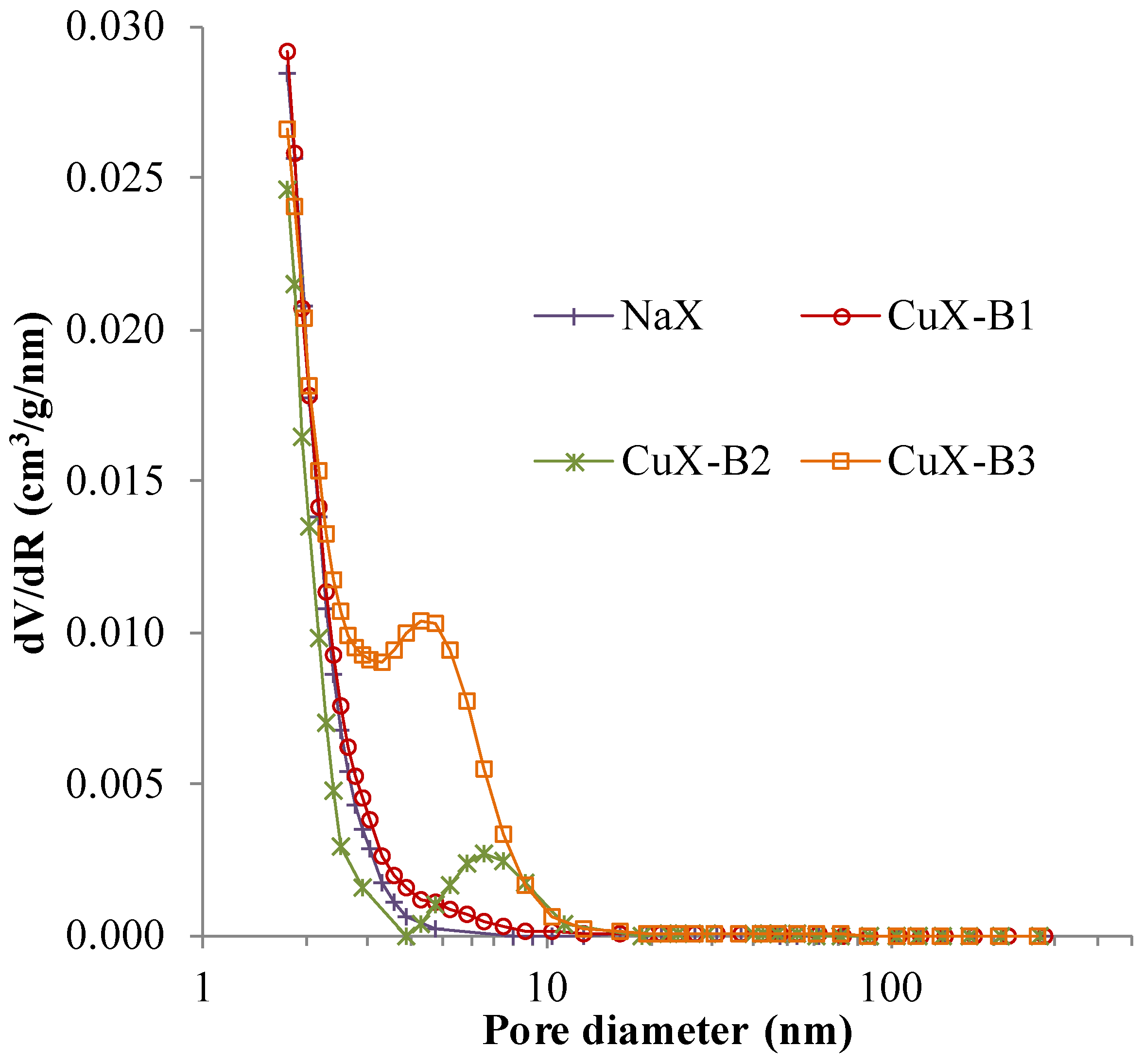
2.1.4. XPS Analyses
| Sample | Si (at. %) | Al (at. %) | O (at. %) | Na (at. %) | Cu (at. %) | Cu 2p3/2 (eV) | Sat Cu 2p3/2 (eV) |
|---|---|---|---|---|---|---|---|
| NaX | 13.4 | 9.6 | 61.4 | 15.6 | - | - | - |
| CuX-B1 | 14.3 | 10.8 | 61.6 | 12.5 | 0.8 | 934.9 | 944.5 |
| CuX-B2 | 15.1 | 10.7 | 63.9 | 9.2 | 1.1 | 934.6 | 944.3 |
| CuX-B3 | 15.0 | 12.0 | 63.3 | 8.0 | 1.7 | 934.6 | 944.7 |
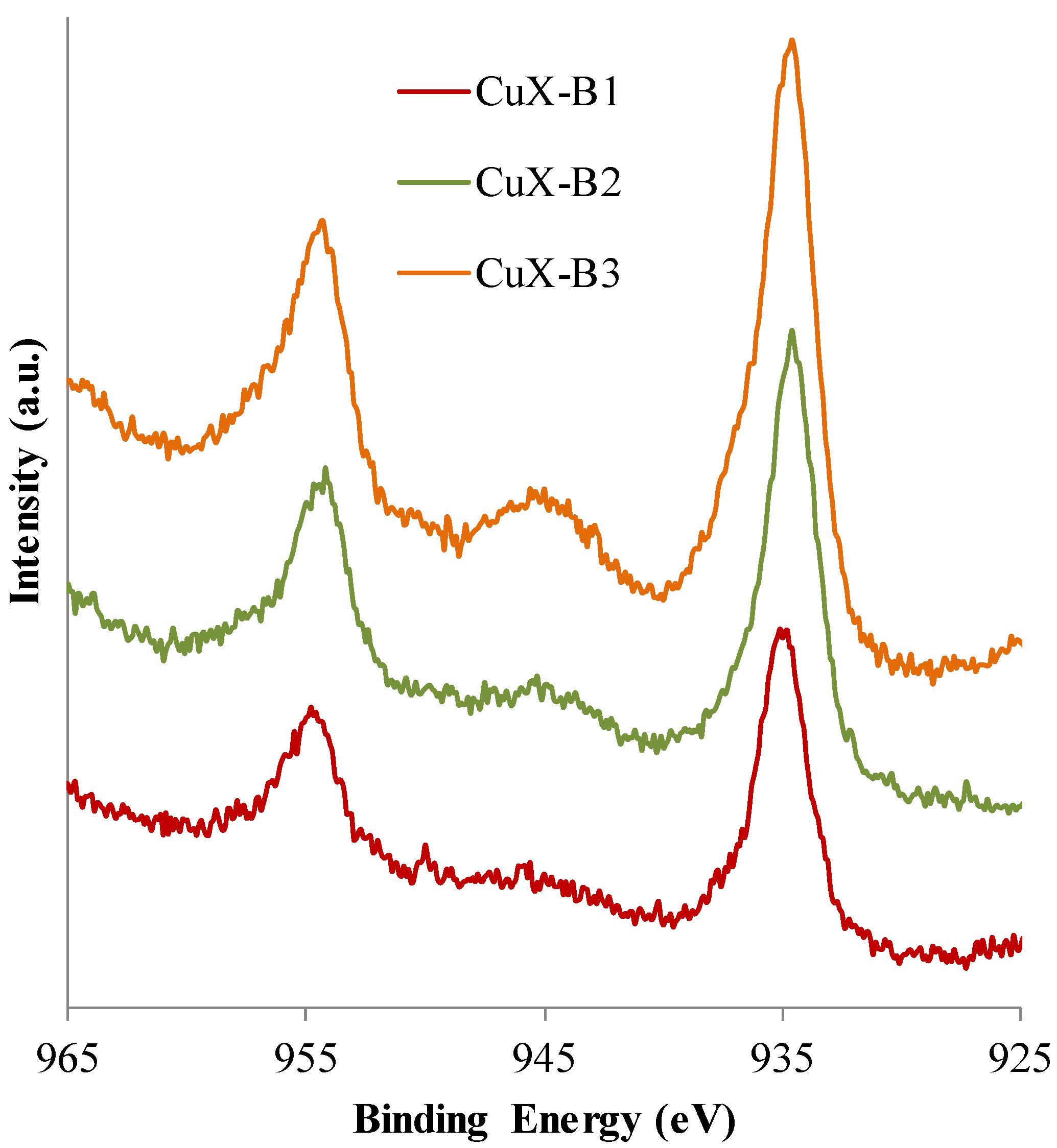
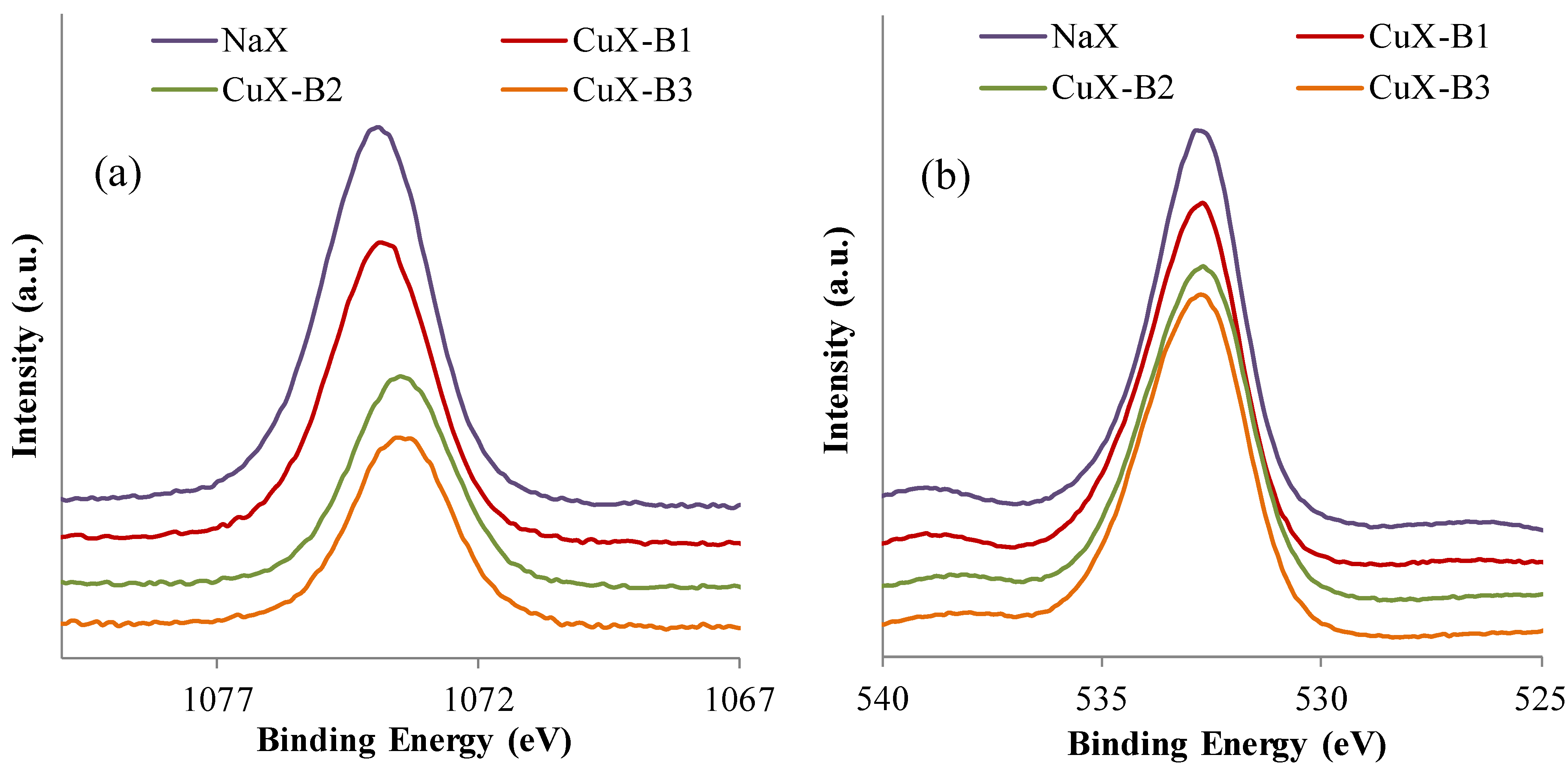
2.2. Adsorption Tests
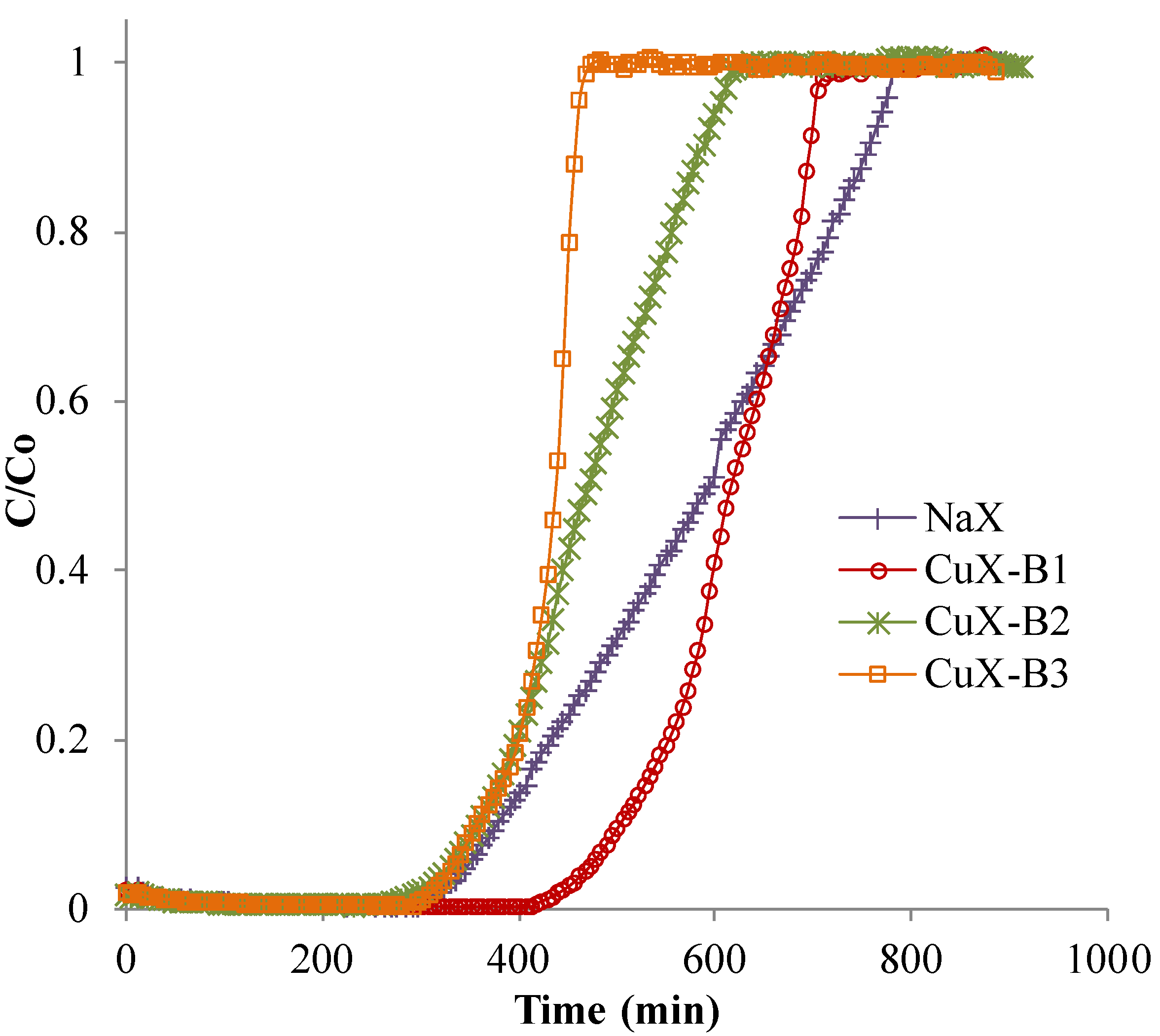
| Sample | Adsorption Capacity (g/100 g Solid) | t 5% (min) | t 50% (min) | t 95% (min) | PUB (%) |
|---|---|---|---|---|---|
| NaX | 20.1 | 347 | 594 | 770 | 40 |
| CuX-B1 | 22.2 | 473 | 616 | 704 | 23 |
| CuX-B2 | 16.7 | 330 | 473 | 605 | 31 |
| CuX-B3 | 15.1 | 336 | 440 | 462 | 21 |
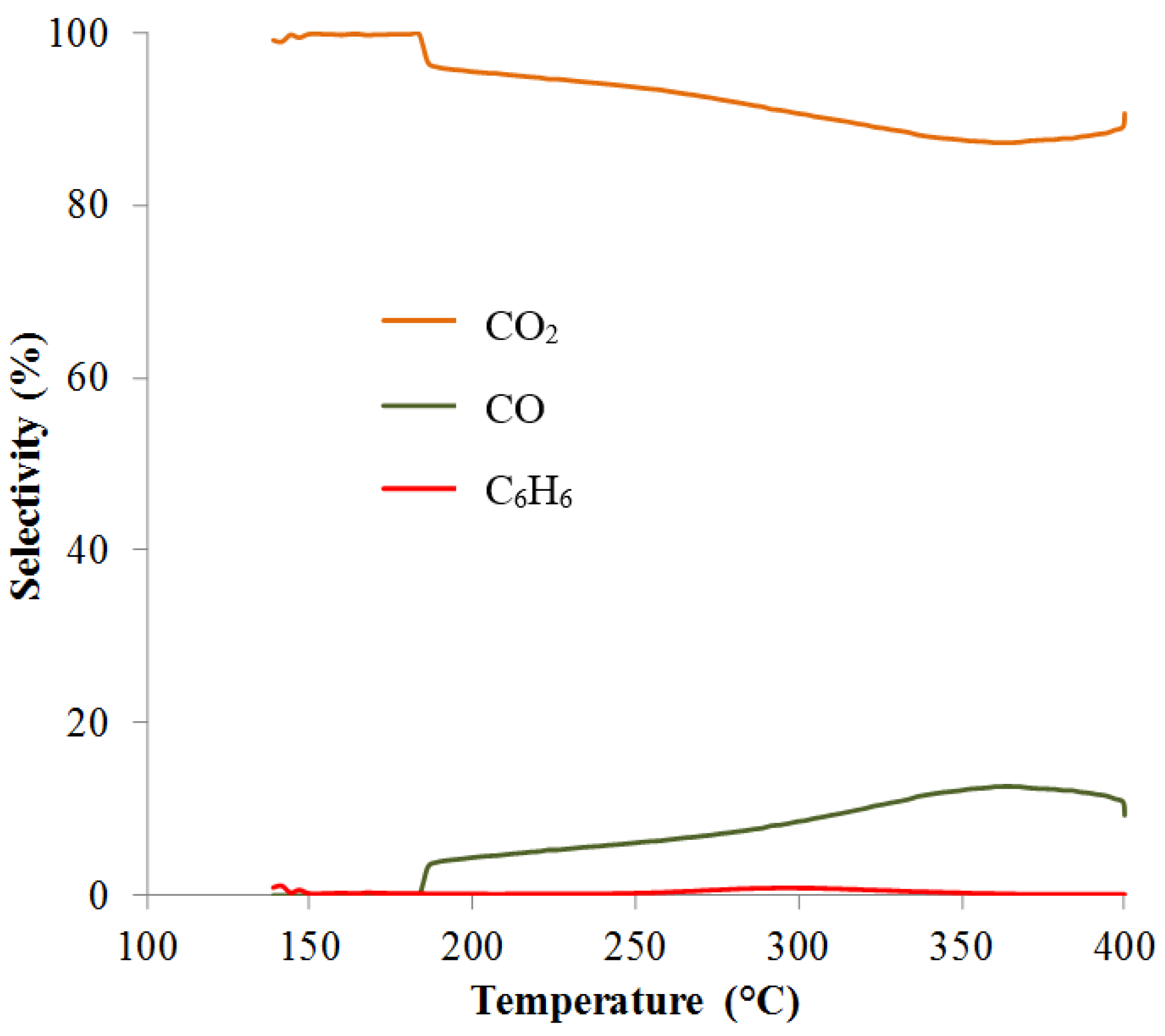
2.3. Catalytic Tests
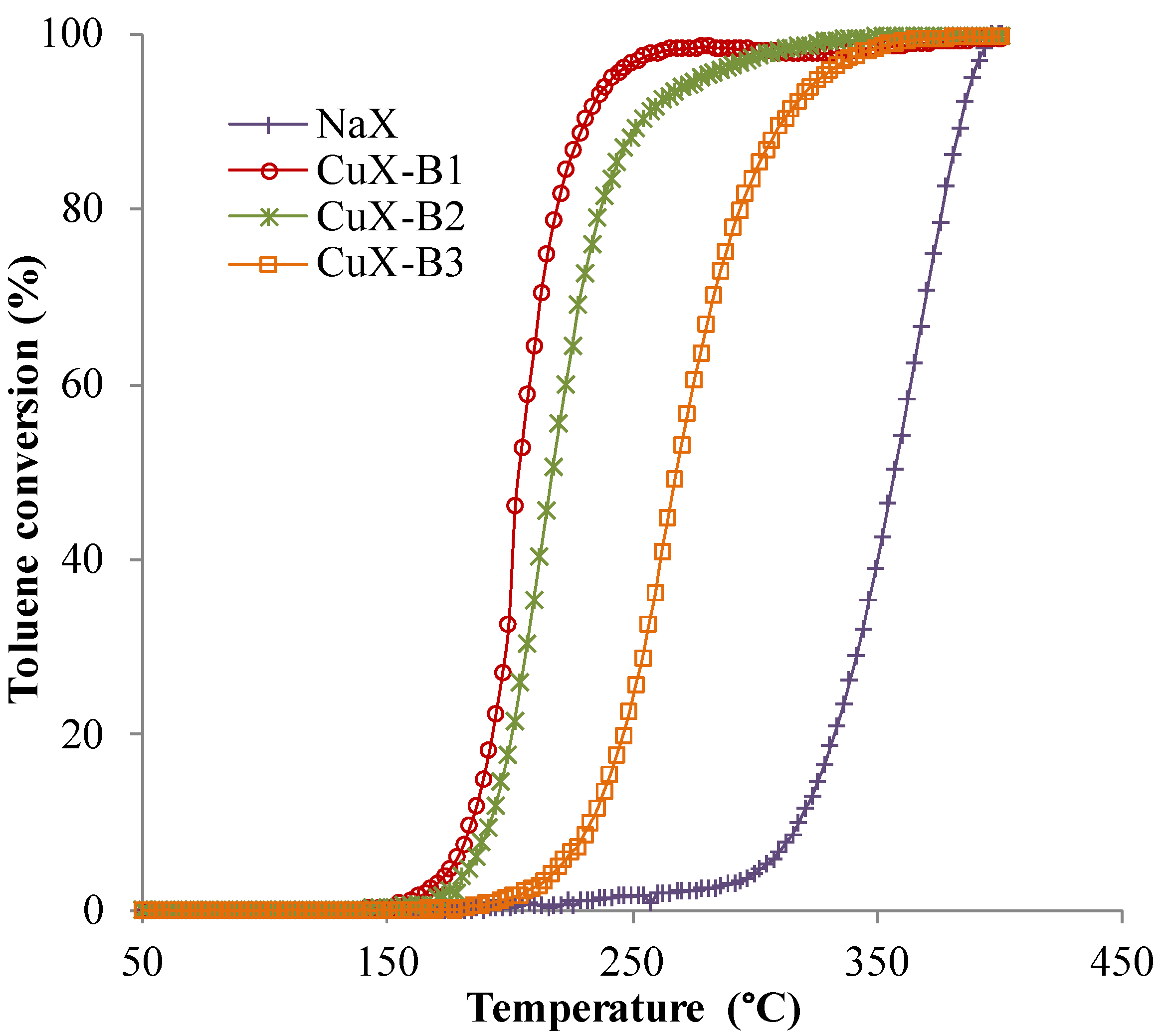
| Sample | T50% (°C) | T98% (°C) |
|---|---|---|
| NaX | 357 | 394 |
| CuX-B1 | 203 | 263 |
| CuX-B2 | 217 | 320 |
| CuX-B3 | 270 | 346 |
3. Experimental Section
3.1. Materials Synthesis
3.2. Characterization Techniques
- Degassing treatment to remove the adsorbed species: The sample was heated from room temperature to 200 °C (10 °C/min) under an argon flow (50 mL/min). The sample was then maintained at 200 °C for 1 h and cooled to room temperature under argon atmosphere.
- Reduction process: The reduction mixture containing 5% v/v H2 in argon was send to the sample at room temperature, the flow was 50 mL/min. After a short stabilization process the temperature was increased from room temperature to 900 °C (with 10 °C/min heating rate). Finally, the sample was cooled to room temperature under argon.
3.3. Toluene Catalytic Oxidation Test
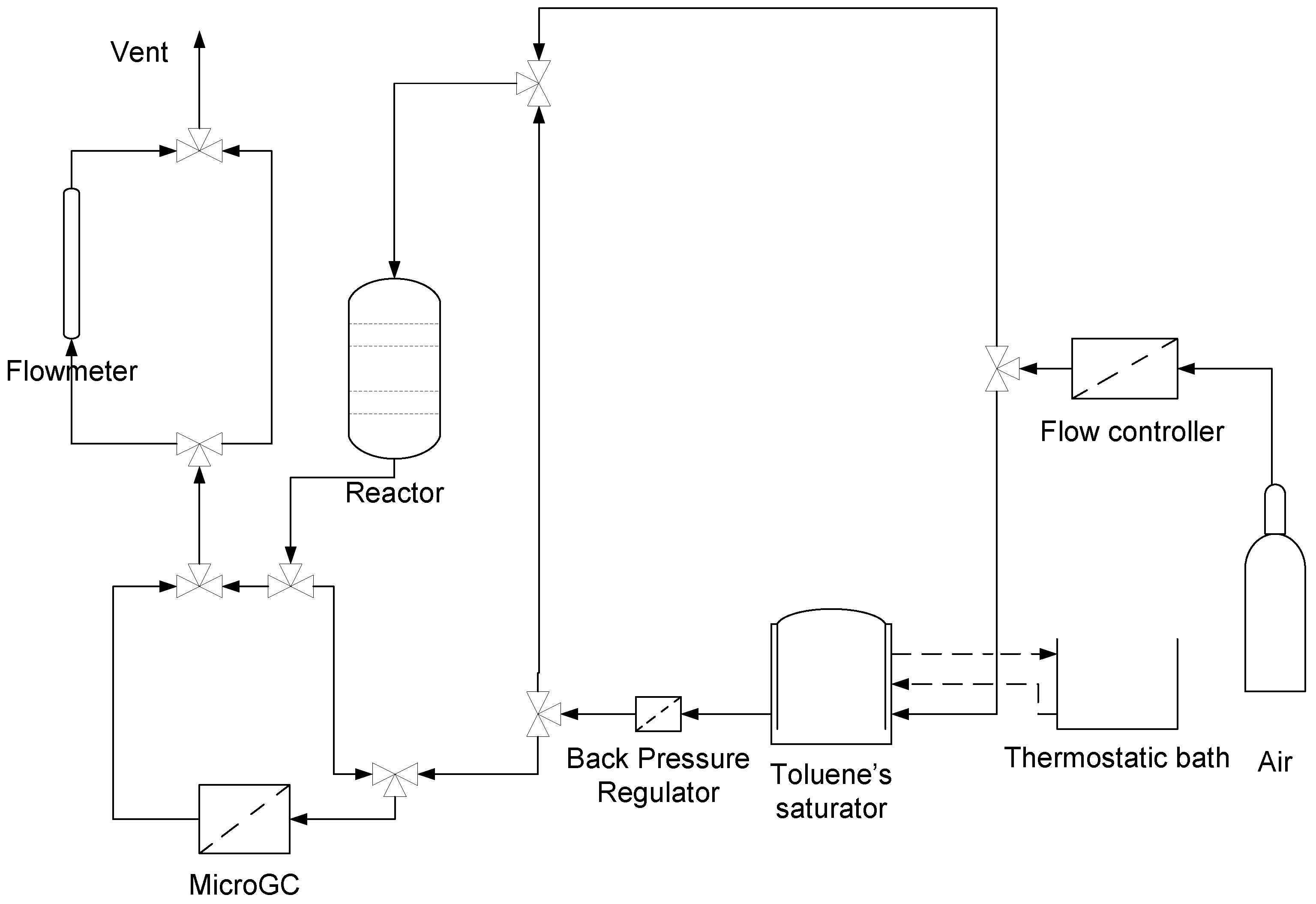
- Step 1: Catalyst activation is the first step of the catalyst test. The conditions employed are an air stream with a flow of 70 mL/min. The aim of this activation process is to clean the catalyst surface and remove physisorbed water and surface impurities that can eventually be present on the catalyst.
- Step 2: The toluene catalytic oxidation start, the temperature is increased and the effluent gas from the reactor is analyzed using an on-line microGC analyzer; injections were made every five minutes.
3.4. Toluene Adsorption Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kostiainen, R. Volatile organic compounds in the indoor air of normal and sick houses. Atmos. Environ. 1995, 29, 693–702. [Google Scholar] [CrossRef]
- Soylu, G.; Özcelik, Z.; Boz, I. Total oxidation of toluene over metal oxides supported on a natural clinoptilolite-type zeolite. Chem. Eng. J. 2010, 162, 380–387. [Google Scholar] [CrossRef]
- Kima, H.; Kimb, T.; Koh, H.; Lee, S.; Mina, B. Complete benzene oxidation over Pt-Pd bimetal catalyst supported on γ-alumina: Influence of Pt-Pd ratio on the catalytic activity. Appl. Catal. A 2005, 280, 125–131. [Google Scholar] [CrossRef]
- Antunes, A.; Ribeiro, M.; Silva, J.; Ribeiro, F.; Magnoux, P.; Guisnet, M. Catalytic oxidation of toluene over CuNaHY zeolites, Coke formation and removal. Appl. Catal. B 2001, 33, 149–164. [Google Scholar] [CrossRef]
- Tidahy, H.; Siffert, S.; Wyrwalski, F.; Lamonier, J.-F.; Aboukais, A. Catalytic activity of copper and palladium based catalysts for toluene total oxidation. Catal. Today 2007, 119, 317–320. [Google Scholar] [CrossRef]
- Carpentier, J.; Lamonier, J.-F.; Siffert, S.; Zhilinskaya, E.; Aboukais, A. Characterisation of Mg/Al hydrotalcite with interlayer palladium complex for catalytic oxidation of toluene. Appl. Catal. A 2002, 234, 91–101. [Google Scholar] [CrossRef]
- Torres, J.Q.; Royer, S.; Bellat, J.-P.; Giraudon, J.-M.; Lamonier, J.-F. Formaldehyde: Catalytic Oxidation as a Promising Soft Way of Elimination. ChemSusChem 2013, 6, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Averlant, R.; Royer, S.; Giraudon, J.-M.; Bellat, J.-P.; Bezverkhyy, I.; Weber, G.; Lamonier, J.-F. Mesoporous silica confined MnO2 nanoparticles as highly efficient catalyst for low temperature elimination of formaldehyde. ChemCatChem 2014, 6, 152–161. [Google Scholar] [CrossRef]
- Bai, L.; Wyrwalski, F.; Lamonier, J.-F.; Khodakov, A.Y.; Monflier, E.; Ponchel, A. Effects of β-cyclodextrin introduction to zirconia supported-cobalt oxide catalysts: From molecule-ion associations to complete oxidation of formaldehyde. Appl. Catal. B 2013, 138–139, 381–390. [Google Scholar] [CrossRef]
- Torres, J.Q.; Giraudon, J.-M.; Gervasini, A.; Dujardin, C.; Lancelot, C.; Trentesaux, M.; Lamonier, J.-F. Total Oxidation of Formaldehyde over MnOx–CeO2 Catalysts: The Effect of Acid Treatment. ACS Catal. 2015, 5, 2260–2269. [Google Scholar]
- Zimowska, M.; Michalik-Zym, A.; Janik, R.; Machej, T.; Gurgul, J.; Socha, R.; Podobinski, J.; Serwicka, E. Catalytic combustion of toluene over mixed Cu–Mn oxides. Catal. Today 2007, 119, 321–326. [Google Scholar] [CrossRef]
- Lee, J.; Park, N.; Ryu, S.; Kim, K.; Lee, T. Self-oscillatory behavior in toluene oxidation on zeolite-NaX. Appl. Catal. A Gen. 2004, 275, 79–86. [Google Scholar] [CrossRef]
- Ribeiro, M.; Silva, J.; Brimaud, S.; Antunes, A.; Silva, E.; Fernandes, A.; Magnoux, P.; Murphy, D. Improvement of toluene catalytic combustion by addition of cesium in copper exchanged zeolites. Appl. Catal. B Environ. 2007, 70, 384–392. [Google Scholar] [CrossRef]
- Weber, G.; Bertrand, O.; Fromont, E.; Bourg, S.; Bouvier, F.; Bissinger, D.; Simmonot-Grange, M.H. TCE Adsorption on Hydrophobic Y and MFI Zeolites. J. Chim. Phys. 1996, 93, 1412–1425. [Google Scholar]
- Fajula, F.; Plee, D. Application of Molecular Sieves in View of Cleaner Technology. Stud. Surf. Sci. Catal. 1994, 85, 633–651. [Google Scholar]
- Benmaamar, Z.; Bengueddach, A. Correlation with different models for adsorption isotherms of m-xylene and toluene on zeolites. J. Appl. Sci. Environ. Sanit. 2007, 2, 43–56. [Google Scholar]
- Jacobs, P.; Linarth, J.; Nijs, H.; Uytterhoeven, J. Redox Behaviour of Transition Metal Ions in Zeolites, Part 5: Method of Quantitative Determination of Bidisperse Distributions of Metal Particle Sizes in Zeolites. J. Chem. Soc. Faraday Trans. 1977, 73, 1745–1754. [Google Scholar] [CrossRef]
- Kieger, S.; Delahay, G.; Coq, B. Influence of co-cations in the selective catalytic reduction of NO by NH3 over copper exchanged faujasite zeolites. Appl. Catal. B Environ. 2000, 25, 1–9. [Google Scholar] [CrossRef]
- Torre-Abreu, C.; Henriques, C.; Ribeiro, F.; Delahay, G.; Ribeiro, M. Selective catalytic reduction of NO on copper-exchanged zeolites: The role of the structure of the zeolite in the nature of copper-active sites. Catal. Today 1999, 54, 407–418. [Google Scholar] [CrossRef]
- Bulánek, R.; Wichterlová, B.; Sobalík, Z.; Tichý, J. Reducibility and oxidation activity of Cu ions in zeolites: Effect of Cu ion coordination and zeolite framework composition. Appl. Catal. B Environ. 2001, 31, 13–25. [Google Scholar] [CrossRef]
- Vijaikumar, S.; Subramanian, T.; Pitchumani, K. Zeolite Encapsulated Nanocrystalline CuO: A Redox Catalyst for the Oxidation of Secondary Alcohols. J. Nanomater. 2008, 2008, 1–7. [Google Scholar] [CrossRef]
- Abu-Zied, B. Cu2+-acetate exchanged X zeolites: Preparation, characterization and N2O decomposition activity. Microporous Mesoporous Mater. 2011, 139, 59–66. [Google Scholar] [CrossRef]
- Benaliouche, F.; Boucheffa, Y.; Ayrault, P.; Mignard, S.; Magnoux, P. NH3-TPD and FTIR spectroscopy of pyridine adsorption studies for characterization of Ag- and Cu-exchanged X zeolites. Microporous Mesoporous Mater. 2008, 111, 80–88. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Stoeckli, H.F. Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Interface Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef]
- Díaz, E.; Ordóñez, S.; Vega, A.; Coca, J. Catalytic combustion of hexane over transition metal modified zeolites NaX and CaA. Appl. Catal. B Environ. 2005, 56, 313–322. [Google Scholar] [CrossRef]
- Hammoudi, H.; Bendenia, S.; Marouf-Khelifa, K.; Marouf, R.; Schott, J.; Khelifa, A. Effect of the binary and ternary exchanges on crystallinity and textural properties of X zeolites. Microporous Mesoporous Mater. 2008, 113, 343–351. [Google Scholar] [CrossRef]
- Shpiro, E.; Grünert, W.; Joyner, R.; Baeva, G. Nature, distribution and reactivity of copper species in over-exchanged Cu-ZSM-5 catalysts: An XPS/XAES study. Catal. Lett. 1994, 24, 159–169. [Google Scholar] [CrossRef]
- Ning, P.; Qiu, J.; Wang, X.; Liu, W.; Chen, W. Metal loaded zeolite adsorbents for hydrogen cyanide removal. J. Environ. Sci. 2013, 25, 808–814. [Google Scholar] [CrossRef]
- Morales, J.; Sánchez, L.; Martín, F.; Ramos-Barrado, J.; Sánchez, M. Use of low-temperature nanostructured CuO thin films deposited by spray-pyrolysis in lithium cells. Thin Solid Films 2005, 474, 133–140. [Google Scholar] [CrossRef]
- Meda, L.; Ranghino, G.; Moretti, G.; Cerofolini, G. XPS detection of some redox phenomena in Cu-zeolites. Surf. Interface Anal. 2002, 33, 516–521. [Google Scholar] [CrossRef]
- Gruenert, W.; Sauerlandt, U.; Schloegl, R.; Karge, H. XPS investigations of lanthanum in faujasite-type zeolites. J. Phys. Chem. 1993, 97, 1413–1419. [Google Scholar] [CrossRef]
- Almeida, K.; Landers, R.; Cardoso, D. Properties of faujasite zeolites containing methyl-substituted ammonium cations. J. Catal. 2012, 294, 151–160. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Archipov, T.; Santra, S.; Ene, A.; Stoll, H.; Rauhut, G.; Roduner, E. Adsorption of Benzene to Copper in CuHY Zeolite. J. Phys. Chem. C 2009, 113, 4107–4116. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, Z.; Li, X.; Wang, Y.; Ma, D.; Wu, J. Comparison of dynamic adsorption/desorption characteristics of toluene on different porous materials. J. Environ. Sci. 2012, 24, 520–528. [Google Scholar] [CrossRef]
- Boikov, E.; Vishnetskaya, M.; Emel’yanov, A.; Tomskii, I.; Shcherbakov, N. The Selective Catalytic Oxidation of Toluene. Russ. J. Phys. Chem. A 2008, 82, 2233–2237. [Google Scholar] [CrossRef]
- Kowenje, C.; Jones, B.; Doetschman, D.; Yang, S.; Kanyi, C. Effects of cation siting and spin-spin interactions on the electron paramagnetic resonance (EPR) of Cu2+ exchanged X Faujasite zeolite. Chem. Phys. 2006, 330, 401–409. [Google Scholar] [CrossRef]
- Duprat, F. Light-off curve of catalytic reaction and kinetics. Chem. Eng. Sci. 2002, 57, 901–911. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, D.; Chlala, D.; Labaki, M.; Royer, S.; Bellat, J.-P.; Bezverkhyy, I.; Giraudon, J.-M.; Lamonier, J.-F. Removal of Toluene over NaX Zeolite Exchanged with Cu2+. Catalysts 2015, 5, 1479-1497. https://doi.org/10.3390/catal5031479
Romero D, Chlala D, Labaki M, Royer S, Bellat J-P, Bezverkhyy I, Giraudon J-M, Lamonier J-F. Removal of Toluene over NaX Zeolite Exchanged with Cu2+. Catalysts. 2015; 5(3):1479-1497. https://doi.org/10.3390/catal5031479
Chicago/Turabian StyleRomero, Douglas, Dayan Chlala, Madona Labaki, Sébastien Royer, Jean-Pierre Bellat, Igor Bezverkhyy, Jean-Marc Giraudon, and Jean-François Lamonier. 2015. "Removal of Toluene over NaX Zeolite Exchanged with Cu2+" Catalysts 5, no. 3: 1479-1497. https://doi.org/10.3390/catal5031479
APA StyleRomero, D., Chlala, D., Labaki, M., Royer, S., Bellat, J.-P., Bezverkhyy, I., Giraudon, J.-M., & Lamonier, J.-F. (2015). Removal of Toluene over NaX Zeolite Exchanged with Cu2+. Catalysts, 5(3), 1479-1497. https://doi.org/10.3390/catal5031479







