The Role of Ozone in the Reaction Mechanism of a Bare Zeolite-Plasma Hybrid System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Role of Zeolites in the Plasma Hybrid System
| Zeolite | C7H8 decomposition (%) Upstream:Downstream | Product selectivity (%) | Carbon balance (%) | Surface area (m2/g) | SiO2/Al2O3 | Channel (Å) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CO2 | HCOOH | ||||||||||||
| None | 15 | 35 | 48 | 17 | 97 | - | - | - | ||||||
| Na-Y | 21 | 52 | 36 | 33 | 56 | 63 | 8 | 4 | 95 | 92 | 750 | 3.1 | 7.4 | |
| H-Y(1) | 18 | 55 | 41 | 45 | 52 | 47 | 7 | 8 | 97 | 99 | 650 | 1.9 | 7.4 | |
| H-Y(2) | 20 | 50 | 47 | 42 | 46 | 51 | 7 | 7 | 98 | 96 | 520 | 5.6 | 7.4 | |
| MOR | 23 | 35 | 46 | 42 | 45 | 52 | 9 | 6 | 97 | 96 | 460 | 15.2 | 6.7 × 7.0 | |
| FER | 19 | 20 | 38 | 33 | 49 | 65 | 13 | 2 | 96 | 99 | 270 | 17.9 | 4.3 × 5.5 | |
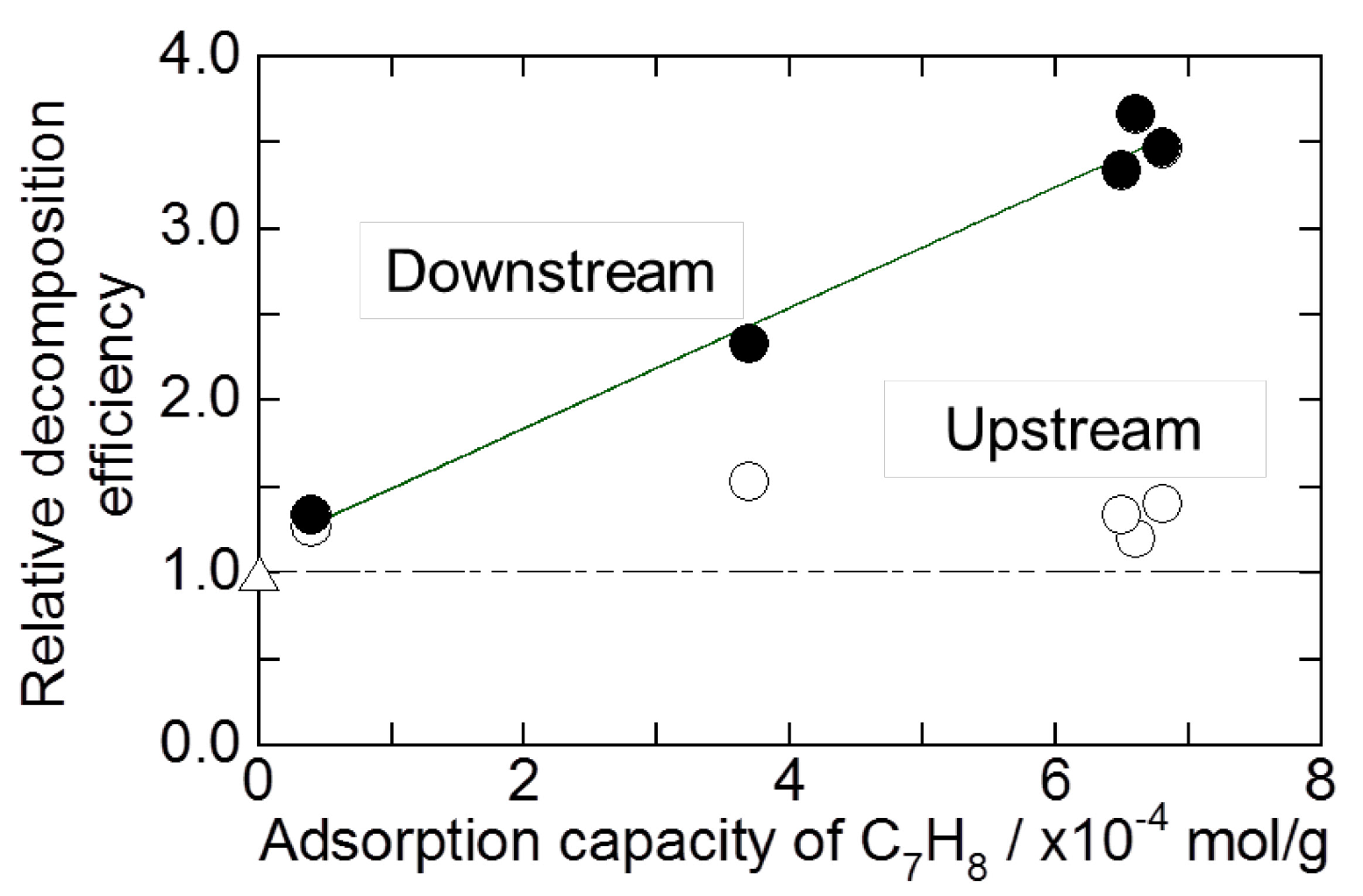

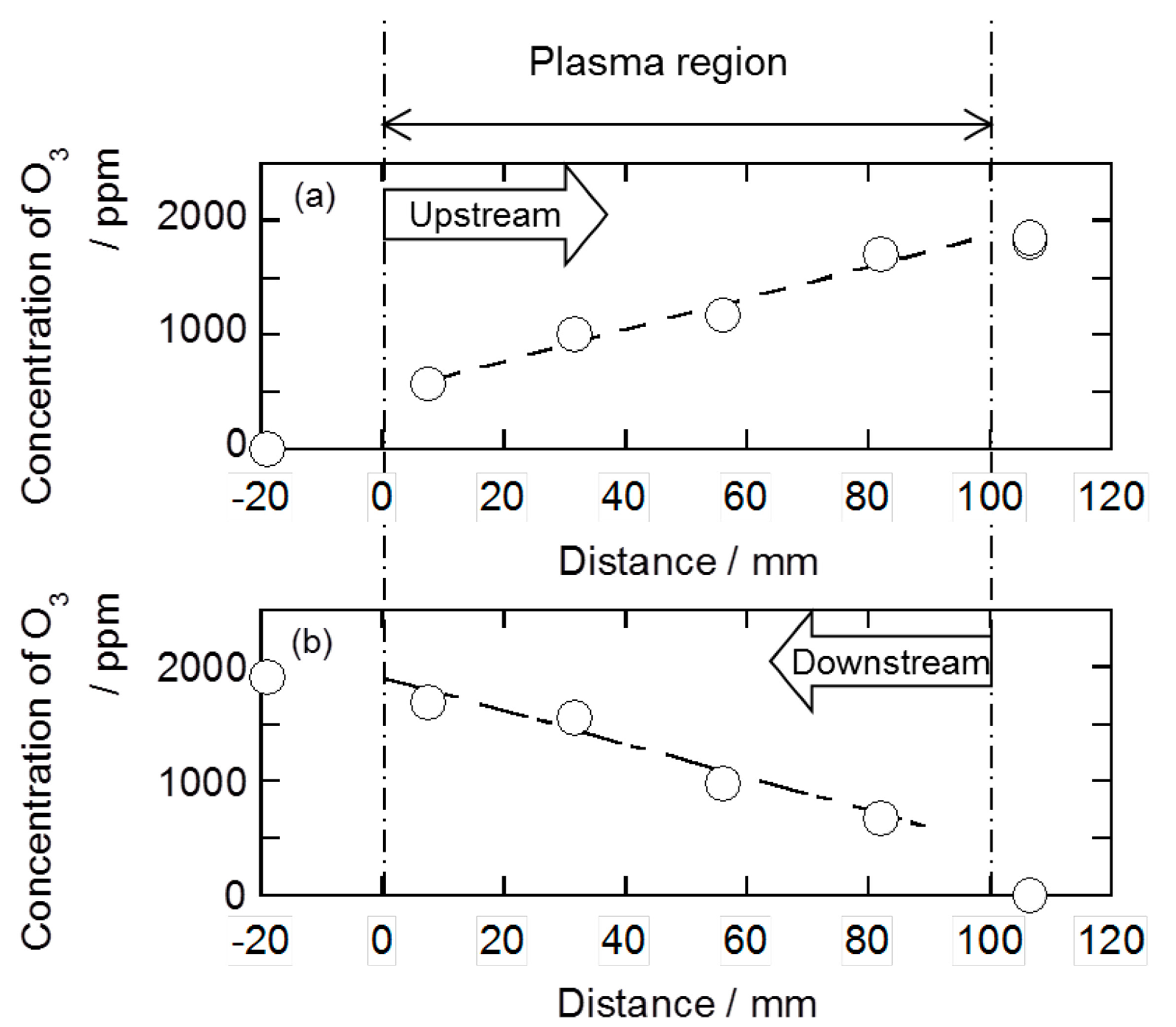

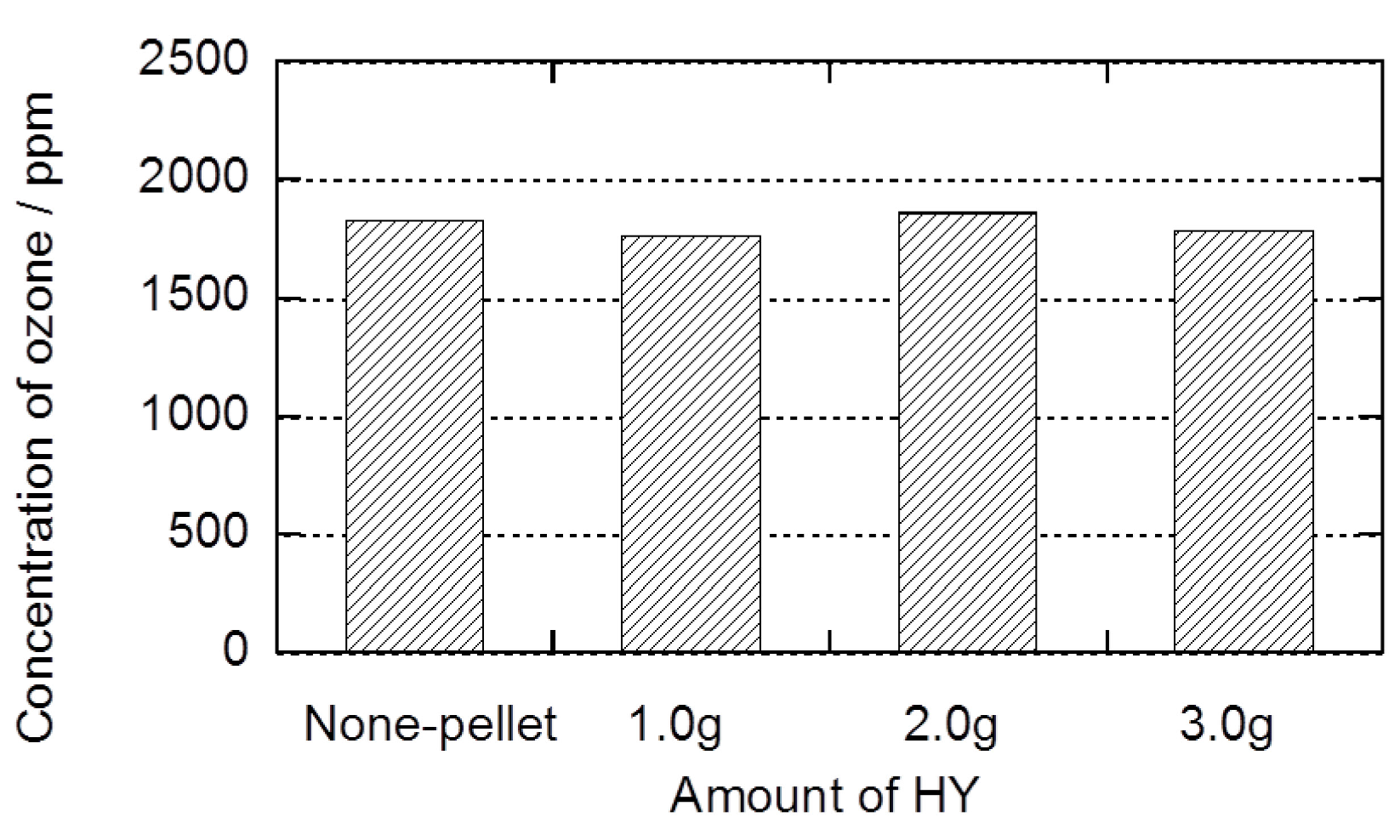
2.2. Decomposition of Toluene Adsorbed onto the Zeolites by O3
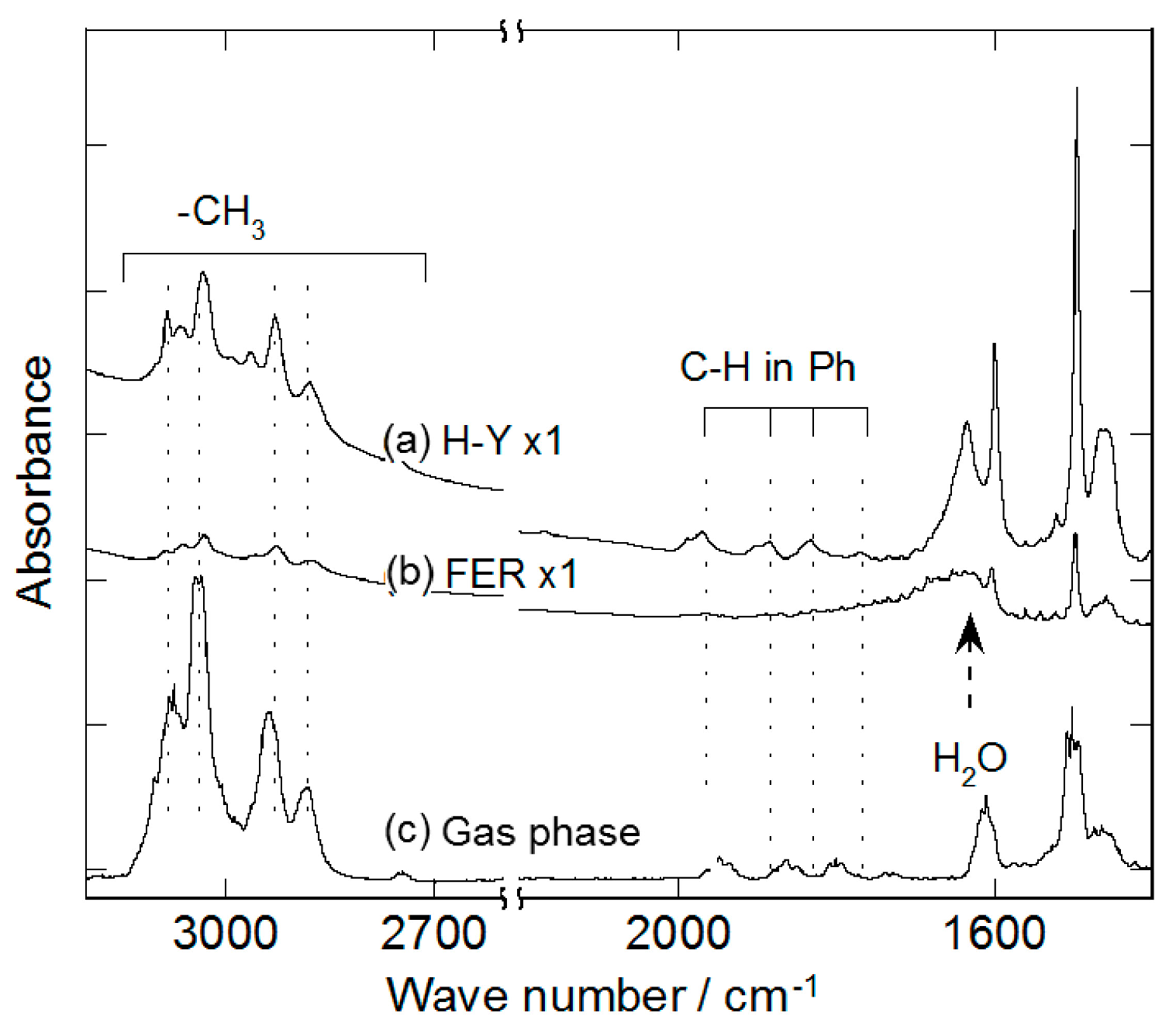
| Bond | Gas phase | Liquid film | on H-Y |
|---|---|---|---|
| C–H in Ph | 3101 | 3087 | 3083 |
| 3076 | 3062 | 3065 | |
| 3037 | 3026 | 3031 | |
| C–H in CH3 | - | 2948 | 2963 |
| 2938 | 2920 | 2928 | |
| 2881 | 2873 | 2879 | |
| C–H in Ph | 1942 | 1942 | 1969 |
| 1856 | 1858 | 1884 | |
| 1801 | 1803 | 1832 | |
| 1732 | - | 1772 | |
| C–C in Ph | 1611 | 1605 | 1599 |
| - | 1524 | - | |
| 1500 | 1496 | 1495 | |
| 1463 | 1451 | 1457 | |
| Infra-red DB | SDBS | this work |
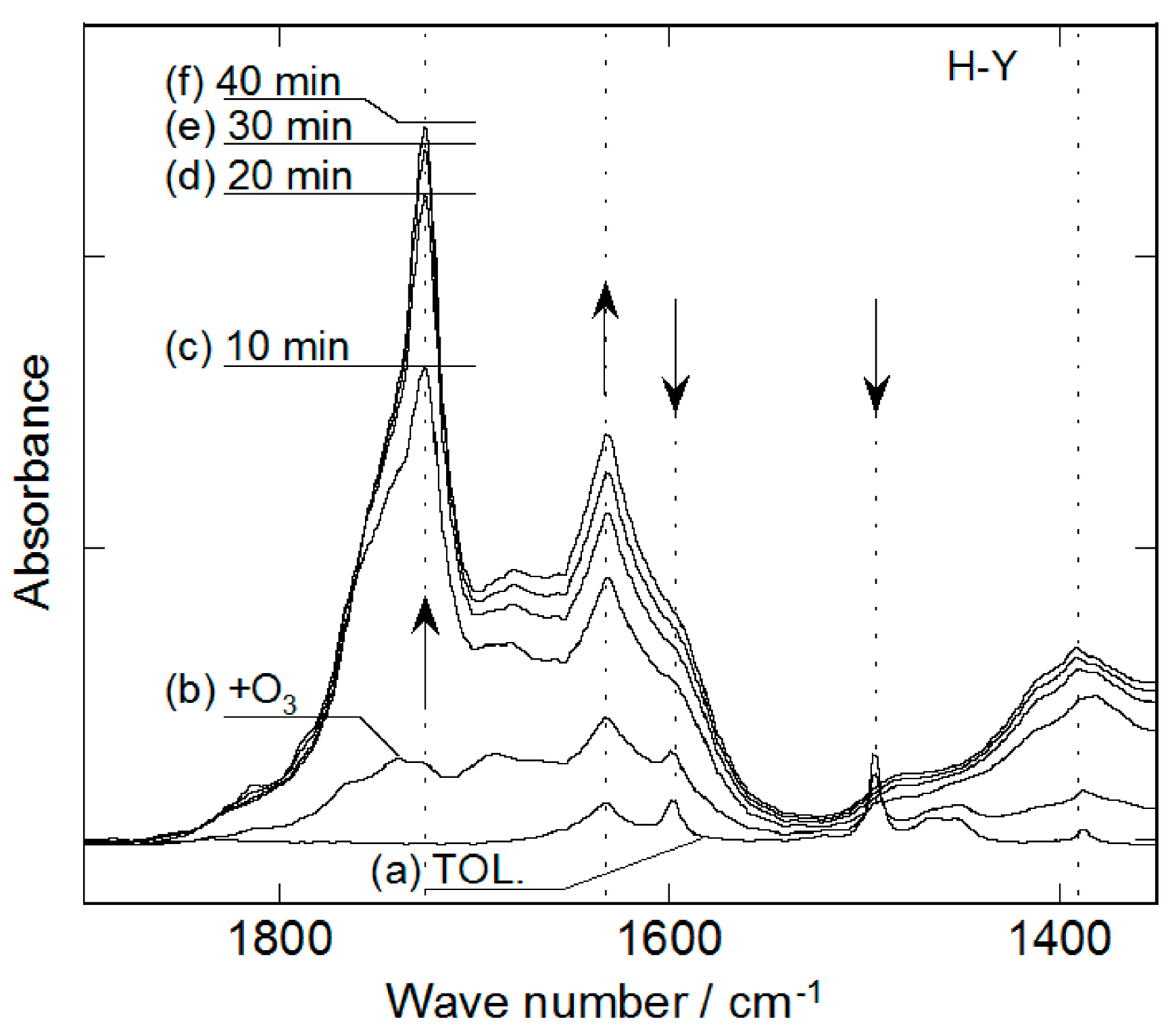
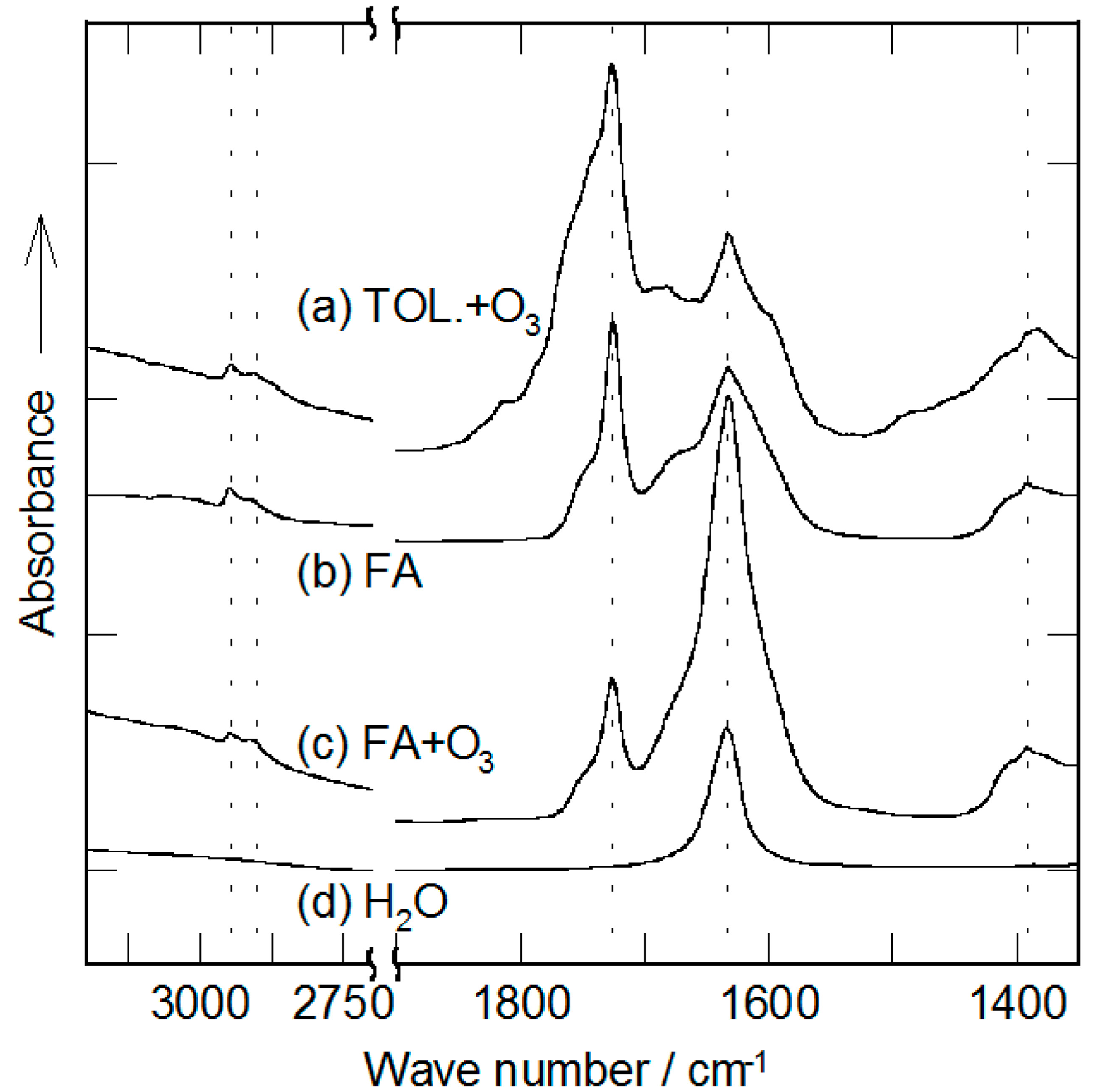
3. Experimental Section
3.1. Decomposition of Toluene (C7H8)
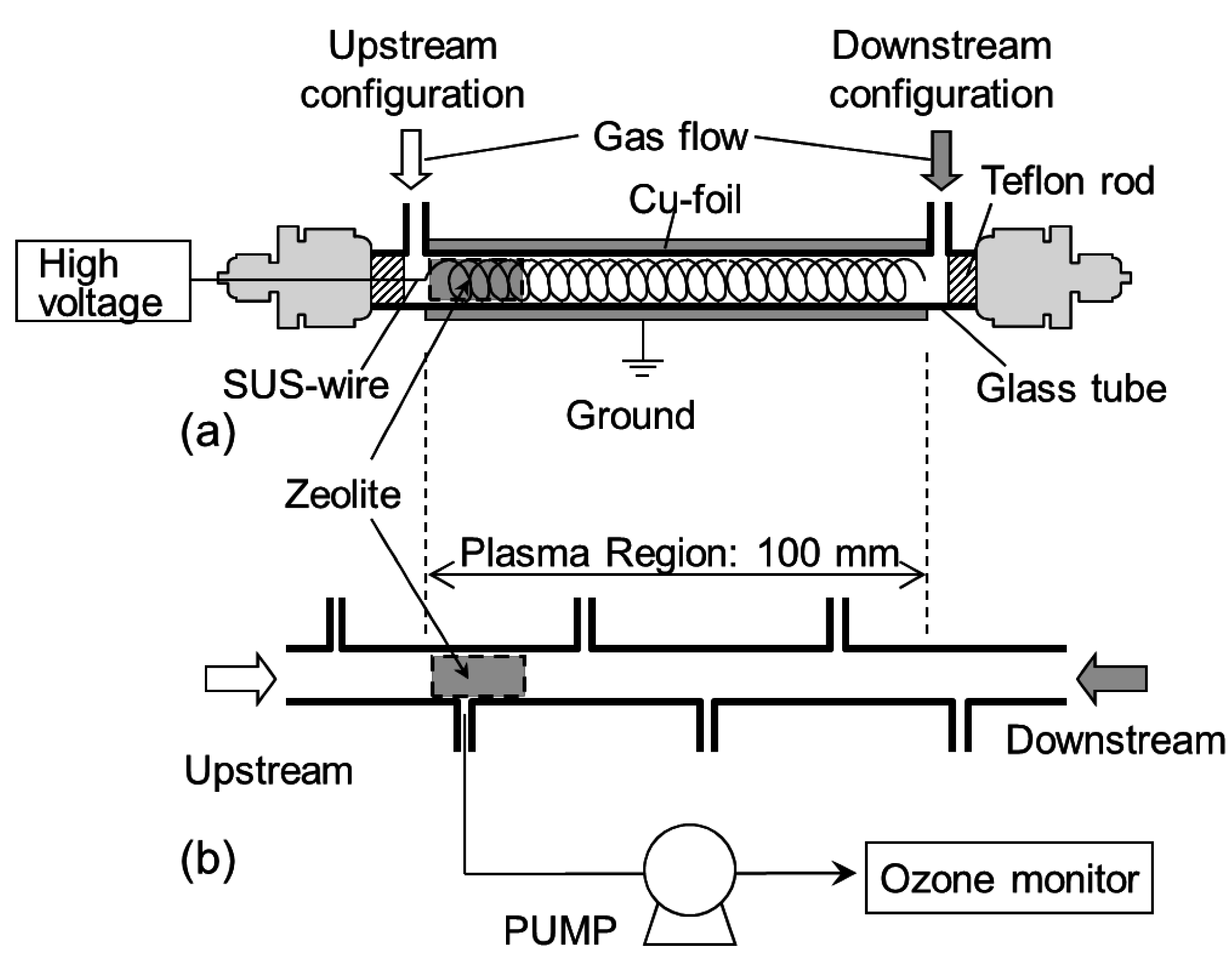
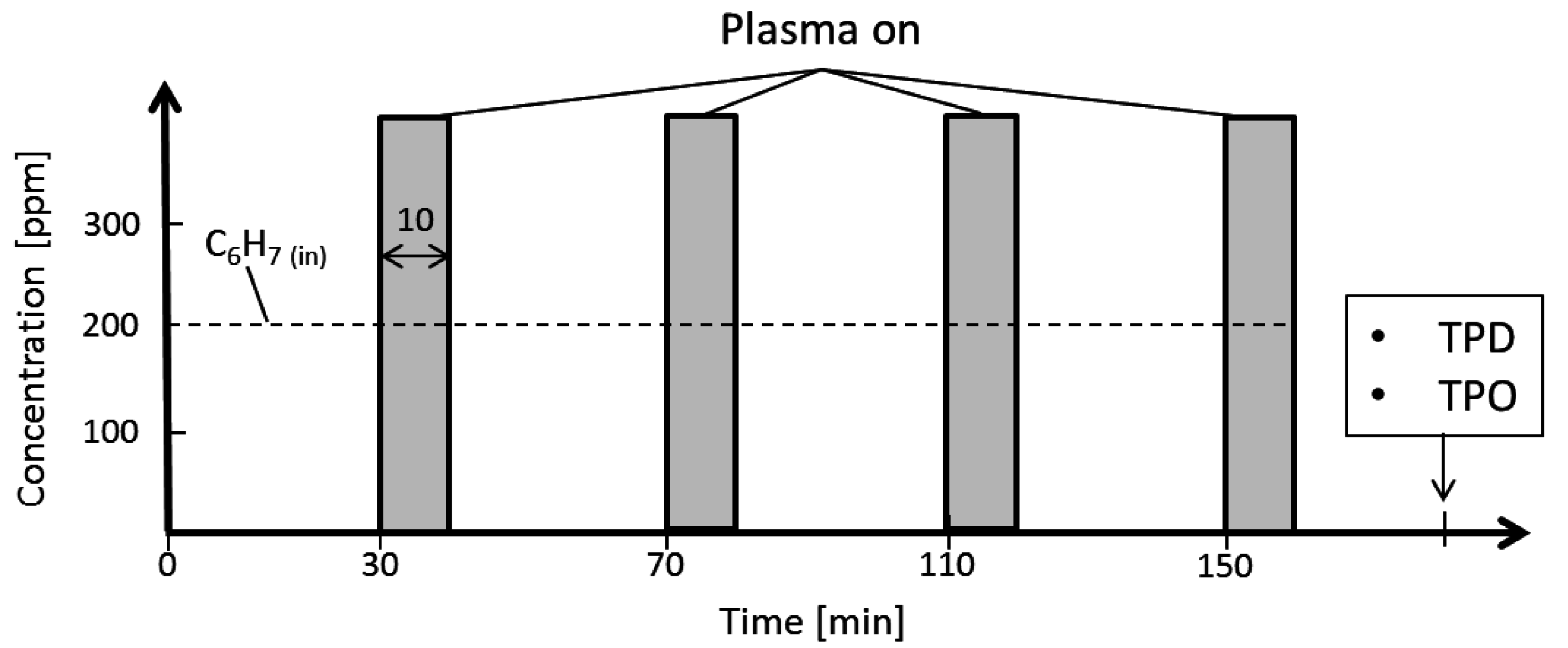
3.2. Measurement of the Ozone Concentration in the Reactor
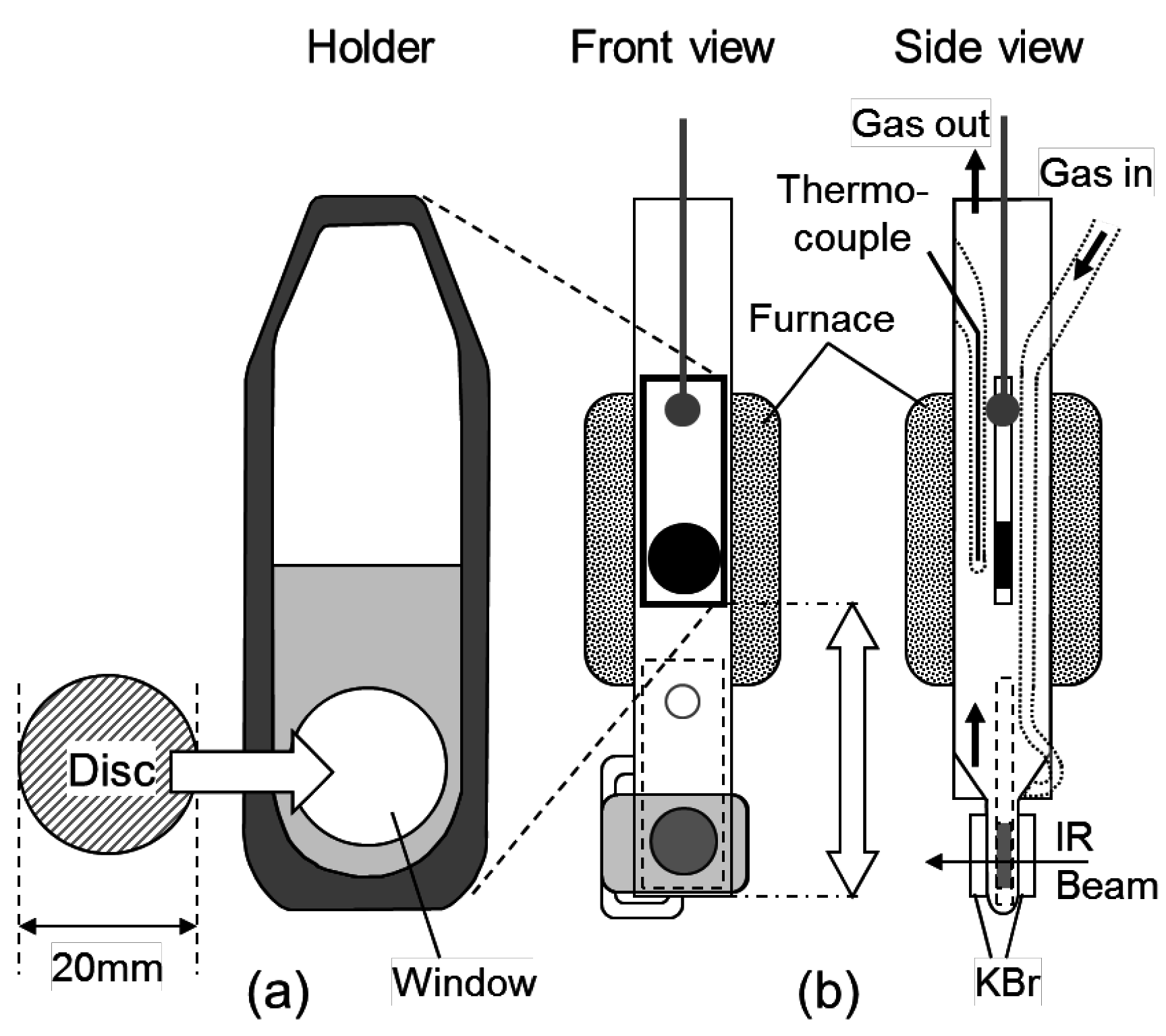
3.3. Infrared Measurement of the Chemical Species Adsorbed onto the Zeolites
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Oda, T. Non-thermal plasma processing for environmental protection: Decomposition of dilute VOCs in air. J. Electrostat. 2003, 57, 293–311. [Google Scholar] [CrossRef]
- Nunez, C.M.; Ramsey, G.H.; Ponder, W.H.; Abbott, J.H.; Hamel, L.E.; Kariher, P.H. Corona destruction—An innovative control technology for VOCs and air toxics. J. Air Waste Manag. 1993, 43, 242–247. [Google Scholar] [CrossRef]
- Yamamoto, T.; Futamura, S. Nonthermal plasma processing for controlling volatile organic compounds. Combust. Sci. Technol. 1998, 133, 117–133. [Google Scholar] [CrossRef]
- Sobacchi, M.G.; Saveliev, A.V.; Fridman, A.A.; Gutsol, A.F.; Kennedy, L.A. Experimental assessment of pulsed corona discharge for treatment of VOC emissions. Plasma Chem. Plasma Process. 2003, 23, 347–370. [Google Scholar] [CrossRef]
- Ogata, A.; Ito, D.; Mizuno, K.; Kushiyama, S.; Gal, A.; Yamamoto, T. Effect of coexisting components on aromatic decomposition in a packed-bed plasma reactor. Appl. Catal. A 2002, 236, 9–15. [Google Scholar] [CrossRef]
- Park, D.W.; Yoon, S.H.; Kim, G.J.; Sekiguchi, H. The effect of catalyst on the decomposition of dilute benzene using dielectric barrier discharge. J. Ind. Eng. Chem. 2002, 8, 393–398. [Google Scholar]
- Oda, T.; Yamashita, R.; Haga, I.; Takahashi, T.; Masuda, S. Decomposition of gaseous organic contaminants by surface discharge induced plasma chemical processing spcp. IEEE Trans. Ind. Appl. 1996, 32, 118–124. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.H.; Ogata, A.; Einaga, H.; Futamura, S.; Park, D.W. Effect of zeolite in surface discharge plasma on the decomposition of toluene. Catal. Lett. 2005, 99, 101–104. [Google Scholar] [CrossRef]
- Kim, H.H.; Oh, S.M.; Ogata, A.; Futamura, S. Decomposition of benzene using Ag/TiO2 packed plasma-driven catalyst reactor: Influence of electrode configuration and Ag-loading amount. Catal. Lett. 2004, 96, 189–194. [Google Scholar] [CrossRef]
- Futamura, S.; Einaga, H.; Kabashima, H.; Lee, Y.H. Synergistic effect of silent discharge plasma and catalysts on benzene decomposition. Catal. Today 2004, 89, 89–95. [Google Scholar] [CrossRef]
- Ogata, A.; Einaga, H.; Kabashima, H.; Futamura, S.; Kushiyama, S.; Kim, H.H. Effective combination of nonthermal plasma and catalysts for decomposition of benzene in air. Appl. Catal. B 2003, 46, 87–95. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I.; Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Improved performance of non-thermal plasma reactor during decomposition of trichloroethylene: Optimization of the reactor geometry and introduction of catalytic electrode. Appl. Catal. B 2007, 74, 270–277. [Google Scholar] [CrossRef]
- Han, S.B.; Oda, T.; Ono, R. Improvement of the energy efficiency in the decomposition of dilute trichloroethylene by the barrier discharge plasma process. IEEE Trans. Ind. Appl. 2005, 41, 1343–1349. [Google Scholar] [CrossRef]
- Hakoda, T.; Matsumoto, K.; Mizuno, A.; Kojima, T.; Hirota, K. Catalytic oxidation of xylene in air using TiO2 under electron beam irradiation. Plasma Chem. Plasma Process. 2008, 28, 25–37. [Google Scholar] [CrossRef]
- Rousseau, A.; Meshchanov, A.V.; Roepcke, J. Evidence of plasma-catalyst synergy in a low-pressure discharge. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.H.; Ogata, A. Microscopic observation of discharge plasma on the surface of zeolites supported metal nanoparticles. J. Phys. D 2009, 42, 135210. [Google Scholar] [CrossRef]
- Ogata, A.; Ito, D.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Removal of dilute benzene using a zeolite-hybrid plasma reactor. IEEE Trans. Ind. Appl. 2001, 37, 959–964. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.H.; Einaga, H.; Ogata, A.; Futamura, S.; Park, D.W. Zeolite-combined plasma reactor for decomposition of toluene. Thin Solid Films 2006, 506, 418–422. [Google Scholar] [CrossRef]
- Sano, T.; Negishi, N.; Sakai, E.; Matsuzawa, S. Contributions of photocatalytic/catalytic activities of TiO2 and g-Al2O3 in nonthermal plasma on oxidation of acetaldehyde and co. J. Mol. Catal. A Chem. 2006, 245, 235–241. [Google Scholar] [CrossRef]
- Bulanin, K.M.; Lavalley, J.C.; Lamotte, J.; Mariey, L.; Tsyganenko, N.M.; Tsyganenko, A.A. Infrared study of ozone adsorption on CeO2. J. Phys. Chem. B 1998, 102, 6809–6816. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S. Catalytic oxidation of benzene with ozone over mn ion-exchanged zeolites. Catal. Commun. 2007, 8, 557–560. [Google Scholar] [CrossRef]
- Teramoto, Y.; Kim, H.H.; Ogata, A.; Negishi, N. Study of plasma-induced surface active oxygen on zeolite-supported silver nanoparticles. Catal. Lett. 2013, 143, 1374–1378. [Google Scholar] [CrossRef]
- Kim, H.H.; Sugasawa, M.; Hirata, H.; Teramoto, Y.; Kosuge, K.; Negishi, N.; Ogata, A. Ozone-assisted catalysis of toluene with layered ZSM-5 and Ag/ZSM-5 zeolites. Plasma Chem. Plasma Process. 2013, 33, 1083–1098. [Google Scholar] [CrossRef]
- Einaga, H.; Ogata, A. Benzene oxidation with ozone over supported manganese oxide catalysts: Effect of catalyst support and reaction conditions. J. Hazard Mater. 2009, 164, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Kwong, C.W.; Chao, C.Y.H.; Hui, K.S.; Wan, M.P. Catalytic ozonation of toluene using zeolite and MCM-41 materials. Environ. Sci. Technol. 2008, 42, 8504–8509. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.H.; Kwong, C.W.; Hui, K.S. Potential use of a combined ozone and zeolite system for gaseous toluene elimination. J. Hazard Mater. 2007, 143, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.C.; Thornberry, T.; Abbatt, J.P.D. Ozone decomposition kinetics on alumina: Effects of ozone partial pressure, relative humidity and repeated oxidation cycles. Atmos. Chem. Phys. 2004, 4, 1301–1310. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. I. In situ raman spectroscopy and ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Li, W.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 2. Steady-state and transient kinetic studies. J. Am. Chem. Soc. 1998, 120, 9047–9052. [Google Scholar] [CrossRef]
- Ogata, A.; Shintani, N.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Decomposition of benzene using a nonthermal plasma reactor packed with ferroelectric pellets. IEEE Trans. Ind. Appl. 1999, 35, 753–759. [Google Scholar] [CrossRef]
- Kuroki, T.; Fujioka, T.; Okubo, M.; Yamamoto, T. Toluene concentration using honeycomb nonthermal plasma desorption. Thin Solid Films 2007, 515, 4272–4277. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teramoto, Y.; Kim, H.-H.; Negishi, N.; Ogata, A. The Role of Ozone in the Reaction Mechanism of a Bare Zeolite-Plasma Hybrid System. Catalysts 2015, 5, 838-850. https://doi.org/10.3390/catal5020838
Teramoto Y, Kim H-H, Negishi N, Ogata A. The Role of Ozone in the Reaction Mechanism of a Bare Zeolite-Plasma Hybrid System. Catalysts. 2015; 5(2):838-850. https://doi.org/10.3390/catal5020838
Chicago/Turabian StyleTeramoto, Yoshiyuki, Hyun-Ha Kim, Nobuaki Negishi, and Atsushi Ogata. 2015. "The Role of Ozone in the Reaction Mechanism of a Bare Zeolite-Plasma Hybrid System" Catalysts 5, no. 2: 838-850. https://doi.org/10.3390/catal5020838
APA StyleTeramoto, Y., Kim, H.-H., Negishi, N., & Ogata, A. (2015). The Role of Ozone in the Reaction Mechanism of a Bare Zeolite-Plasma Hybrid System. Catalysts, 5(2), 838-850. https://doi.org/10.3390/catal5020838






