Effect of Phosphine Doping and the Surface Metal State of Ni on the Catalytic Performance of Ni/Al2O3 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of P Doping on the Catalytic Performance
| Entry | Catalyst | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
| 1,2-PDO a | EG a | Others b | |||
| 1 | 17.5% Ni/Al2O3-500 | 42.4 | 66.5 | 21.6 | 11.9 |
| 2 | 17.5% Ni-1.7% P/Al2O3-500 | 42.6 | 77.5 | 11.9 | 10.6 |
| 3 | 17.5% Ni-2.9% P/Al2O3-500 | 42.7 | 85.7 | 6.3 | 8.0 |
| 4 | 17.5% Ni-4.4% P/Al2O3-500 | 25.8 | 91.1 | 3.2 | 5.7 |

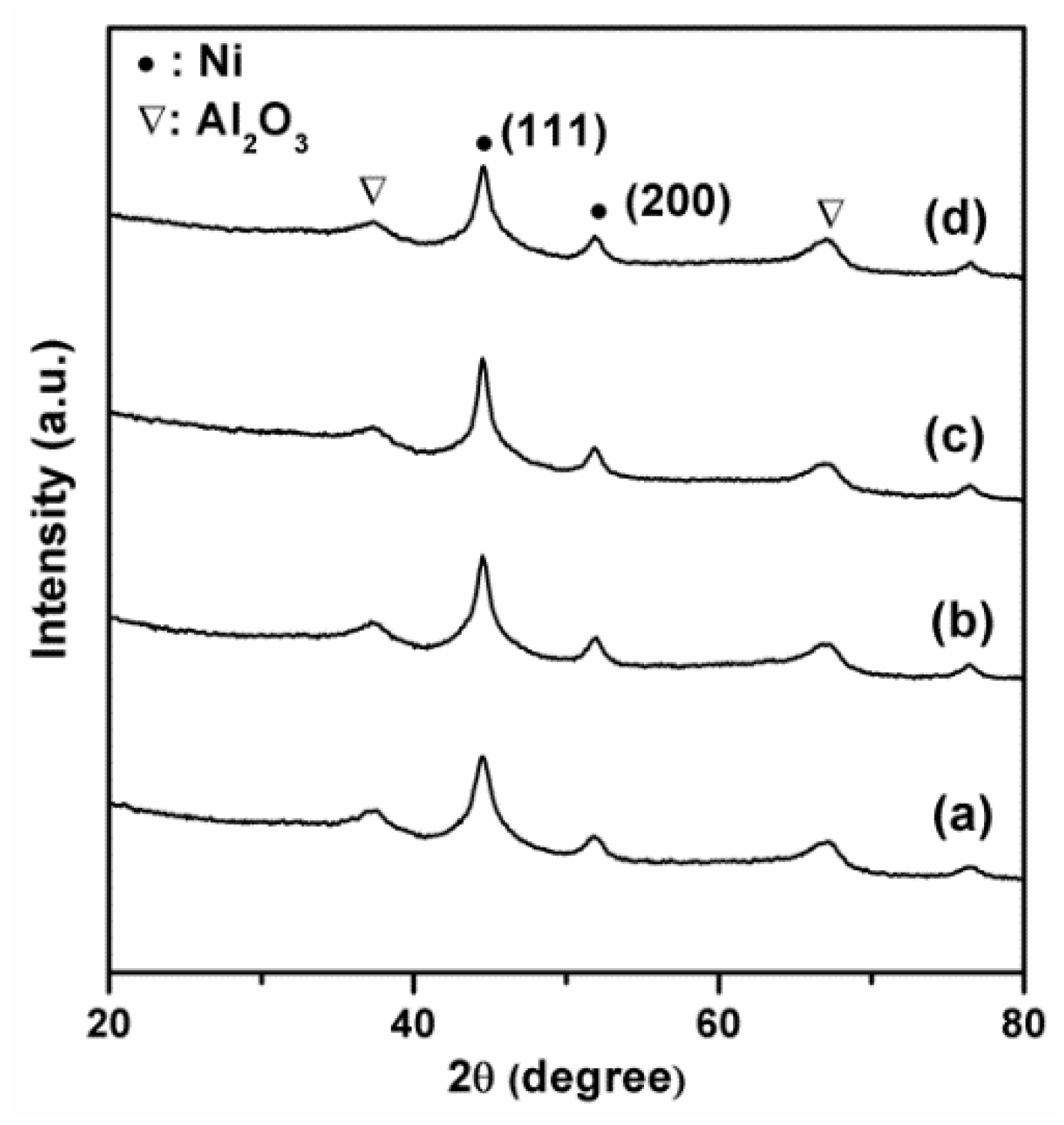
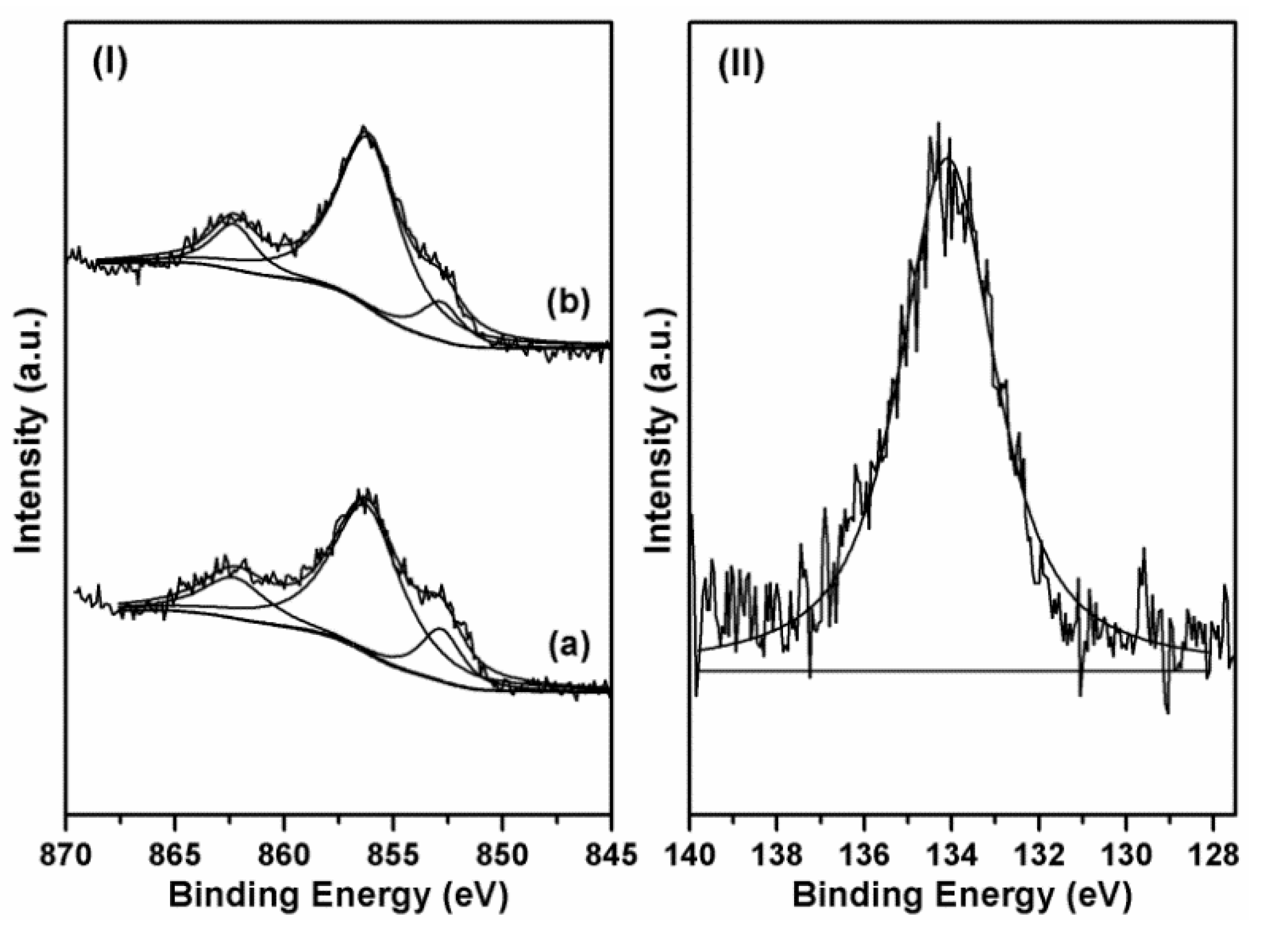
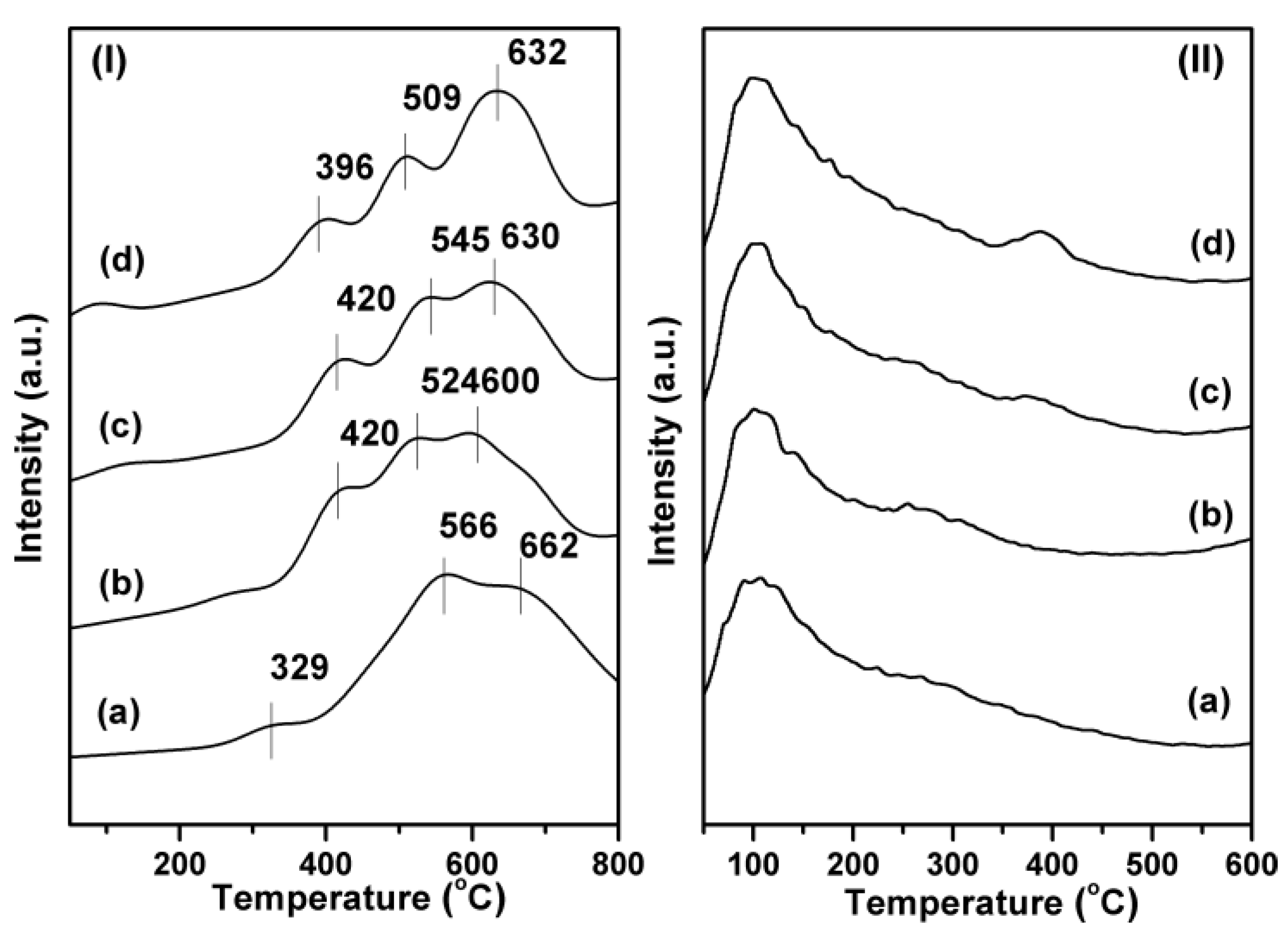
2.2. Influence of Ni Metal State on the Catalytic Performance
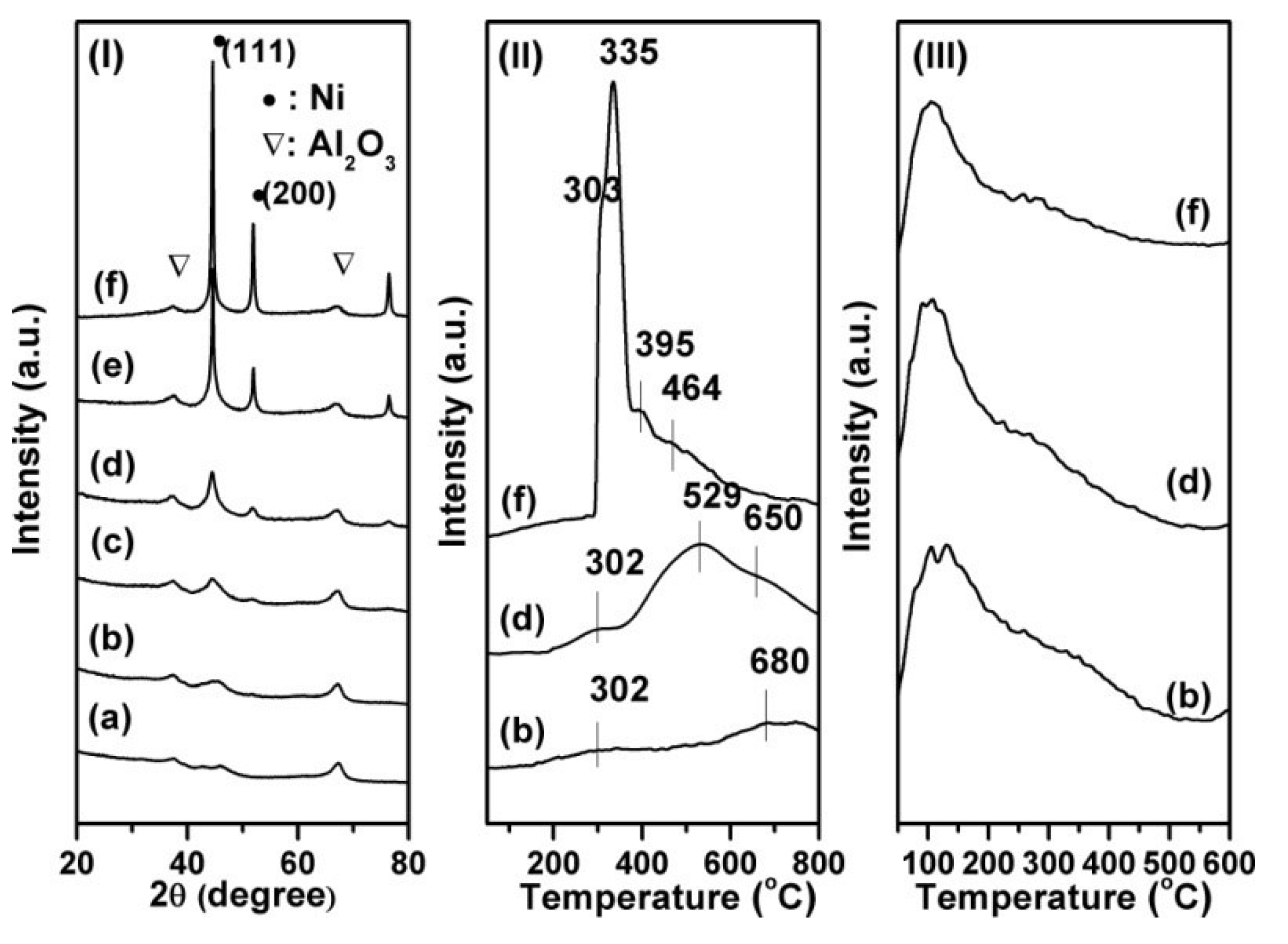
| Ni loading (%) | Time (h) | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
| 1,2-PDO | EG | Others | |||
| 5 | 12 | 50.6 | 84.2 | 4.8 | 11.0 |
| 9.5 | 7.3 | 51.5 | 75.8 | 14.3 | 9.9 |
| 17.5 | 5 | 52.8 | 61.4 | 25.1 | 13.5 |
| 29.6 | 4 | 47.2 | 60.5 | 26.2 | 13.3 |
| 40 | 7 | 45.9 | 59.1 | 27.0 | 13.9 |
| Entry | Calcination temperature (°C) | Time (h) | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|
| 1,2-PDO | EG | Others | ||||
| 1 | 450 | 6 | 59.8 | 61.4 | 25.1 | 13.5 |
| 2 a | 450 | 6 | 23.4 | 78.0 | 9.3 | 12.7 |
| 3 | 500 | 6 | 42.4 | 66.5 | 21.6 | 11.9 |
| 4 | 600 | 6 | 27.1 | 78.6 | 9.4 | 12.0 |

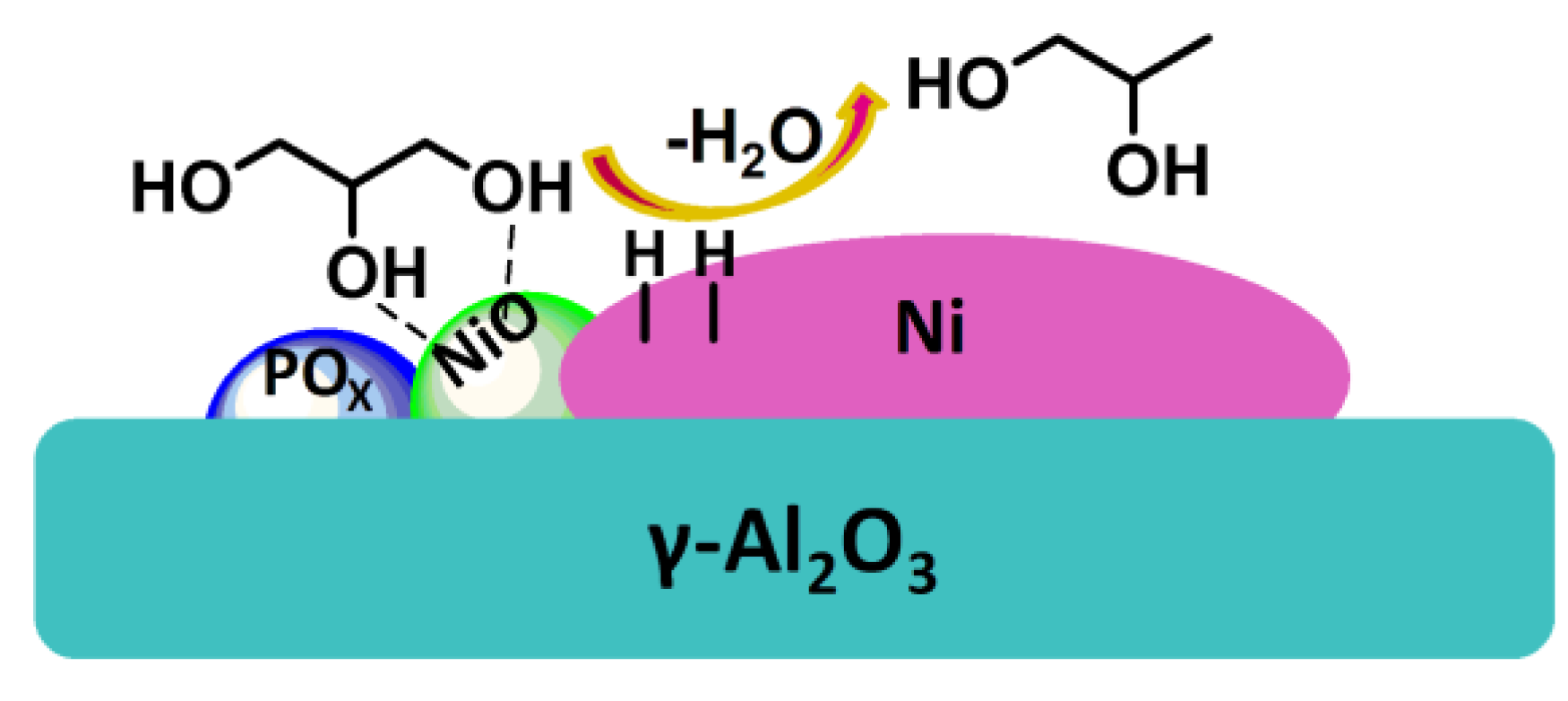
| Entry | Catalysts | Time (h) | Conv. (%) | Selectivity (%) | Rate (h−1) a | ||
|---|---|---|---|---|---|---|---|
| 1,2-PDO | EG | Others | |||||
| 1 b | 17.5% Ni/ Al2O3-500 | 6 | 42.4 | 66.5 | 21.6 | 11.9 | 0.99 |
| 2 c | 17.5% Ni/ Al2O3-500 | 18 | 33.9 | 66.1 | 20.8 | 13.1 | 0.79 |
| 3 b | 17.5% Ni-2.9% P/Al2O3-500 | 6 | 42.7 | 85.7 | 6.3 | 8.0 | 1.00 |
| 4 c | 17.5% Ni-2.9% P/Al2O3-500 | 18 | 31.2 | 89.7 | 5.7 | 4.6 | 0.73 |
| Catalyst | Reaction condition | Conversion (%) | S1,2-PDO (%) | Ref. |
|---|---|---|---|---|
| Raney Ni | 190 °C, 1 MPa, 44 h | 97 | 71 | [10] |
| Ni/AC-CB | 200 °C, 5 MPa, 24 h | 63.2 | 77.4 | [12] |
| 7.5%Ce-Ni/SBA | 200 °C, 2.4 MPa, 8 h | 51 | 29 | [14] |
| Ni2P/SiO2 | 220 °C, 3 MPa, 1.13 h | 95.1 | 85.9 | [15] |
| 17.5% Ni-2.9% P/Al2O3 | 230 °C, 3 MPa, 15 h | 95.7 | 84.7 | This work |
3. Experimental Section
3.1. Materials
3.2. Catalysts Preparation
3.3. Characterization
3.4. Hydrogenolysis of Glycerol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Cwielag, I.; Jerzykiewicz, W. The antioxidant and anticorrosive properties of crude glycerol fraction from biodiesel production. J. Chem. Technol. Biotechnol. 2009, 84, 1196–1201. [Google Scholar] [CrossRef]
- Zhou, C.H.C.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol conversion in the aqueous solution under hydrogen over Ru/C + an ion-exchange resin and its reaction mechanism. J. Catal. 2006, 240, 213–221. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Wang, L.N.; Wang, J.H.; Xia, S.X.; Chen, P.; Hou, Z.Y.; Zheng, X.M. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Hao, S.L.; Peng, W.C.; Zhao, N.; Xiao, F.K.; Wei, W.; Sun, Y.H. Hydrogenolysis of glycerol to 1,2-propanediol catalyzed by Cu-H4SiW12O40/Al2O3 in liquid phase. J. Chem. Technol. Biotechnol. 2010, 85, 1499–1503. [Google Scholar]
- Akiyama, M.; Sato, S.; Takahashi, R.; Inui, K.; Yokota, M. Dehydration-hydrogenation of glycerol into 1,2-propanediol at ambient hydrogen pressure. Appl. Catal. A 2009, 371, 60–66. [Google Scholar] [CrossRef]
- Mizugaki, T.; Arundhathi, R.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Selective Hydrogenolysis of Glycerol to 1,2-Propanediol Using Heterogeneous Copper Nanoparticle Catalyst Derived from Cu–Al Hydrotalcite. Chem. Lett. 2013, 42, 729–731. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P. Selective hydrogenolysis of glycerol with Raney nickel. Ind. Eng. Chem. Res. 2005, 44, 8535–8537. [Google Scholar] [CrossRef]
- Van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Green, M.J.; Friedrich, H.B. A catalytic route to lower alcohols from glycerol using Ni-supported catalysts. Green Chem. 2011, 13, 1819–1827. [Google Scholar] [CrossRef]
- Yu, W.Q.; Xu, J.; Ma, H.; Chen, C.; Zhao, J.; Miao, H.Q. Song, A remarkable enhancement of catalytic activity for KBH4 treating the carbothermal reduced Ni/AC catalyst in glycerol hydrogenolysis. Catal. Commun. 2010, 11, 493–497. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.L.; Zheng, H.Y.; Li, Y.W.; Zeng, Z.Y. Continuous production of 1,2-propanediol by the selective hydrogenolysis of solvent-free glycerol under mild conditions. J. Chem. Technol. Biotechnol. 2008, 83, 1670–1675. [Google Scholar] [CrossRef]
- Jimenez-Morales, I.; Vila, F.; Mariscal, R.; Jimenez-Lopez, A. Hydrogenolysis of glycerol to obtain 1,2-propanediol on Ce-promoted Ni/SBA-15 catalysts. Appl. Catal. B 2012, 117, 253–259. [Google Scholar] [CrossRef]
- Huang, J.H.; Chen, J.X. Comparison of Ni2P/SiO2 and Ni/SiO2 for hydrogenolysis of glycerol: A consideration of factors influencing catalyst activity and product selectivity. Chin. J. Catal. 2012, 33, 790–796. [Google Scholar] [CrossRef]
- Montassier, C.; Ménézo, J.C.; Hoang, L.C.; Renaud, C.; Barbier, J. Aqueous polyol conversions on ruthenium and on sulfur-modified ruthenium. J. Mol. Catal. 1991, 70, 99–110. [Google Scholar] [CrossRef]
- Rode, C.V.; Mane, R.B.; Ghalwadkar, A.A.; Hengne, A.M.; Suryawanshi, Y.R. Role of promoters in copper chromite catalysts for hydrogenolysis of glycerol. Catal. Today 2011, 164, 447–450. [Google Scholar]
- Oyama, S.T.; Wang, X.; Lee, Y.K.; Chun, W.J. Active phase of Ni2P/SiO2 in hydroprocessing reactions. J. Catal. 2004, 221, 263–273. [Google Scholar] [CrossRef]
- Sawhill, S.; Layman, K.; Vanwyk, D.; Engelhard, M.; Wang, C.; Bussell, M. Thiophene hydrodesulfurization over nickel phosphide catalysts: Effect of the precursor composition and support. J. Catal. 2005, 231, 300–313. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Shi, R.; Yang, M. Effect of metal–support interaction on the catalytic performance of Ni/Al2O3 for selective hydrogenation of isoprene. J. Mol. Catal. A 2011, 344, 122–127. [Google Scholar] [CrossRef]
- Hoffer, B.W.; van Langeveld, A.D.; Janssens, J.P.; Bonne, R.L.C.; Lok, C.M.; Moulijn, J.A. Stability of highly dispersed Ni/Al2O3 catalysts: Effects of pretreatment. J. Catal. 2000, 192, 432–440. [Google Scholar] [CrossRef]
- Mahata, N.; Cunha, A.F.; M. Órfão, J.J.; Figueiredo, J.L. Hydrogenation of nitrobenzene over nickel nanoparticles stabilized by filamentous carbon. Appl. Catal. A 2008, 351, 204–209. [Google Scholar] [CrossRef]
- Chen, J.; Ci, D.; Wang, R.; Zhang, J. Hydrodechlorination of chlorobenzene over NiB/SiO2 and NiP/SiO2 amorphous catalysts after being partially crystallized: A consideration of electronic and geometrical factors. Appl. Surf. Sci. 2008, 255, 3300–3309. [Google Scholar] [CrossRef]
- Abu, I.; Smith, K. The effect of cobalt addition to bulk MoP and Ni2P catalysts for the hydrodesulfurization of 4,6-dimethyldibenzothiophene. J. Catal. 2006, 241, 356–366. [Google Scholar] [CrossRef]
- Diskin, A.M.; Cunningham, R.H.; Ormerod, R.M. The oxidative chemistry of methane over supported nickel catalysts. Catal. Today 1998, 46, 147–154. [Google Scholar] [CrossRef]
- Nagaoka, K.; Sato, K.; Nishiguchi, H.; Takita, Y. Influence of support on catalytic behavior of nickel catalysts in oxidative steam prereforming of n-butane for fuel cell applications. Appl. Catal. A 2007, 327, 139–146. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, Y.W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Lu, H.B.; Yin, H.B.; Liu, Y.M.; Jiang, T.S.; Yu, L.B. Influence of support on catalytic activity of Ni catalysts in p-nitrophenol hydrogenation to p-aminophenol. Catal. Commun. 2008, 10, 313–316. [Google Scholar] [CrossRef]
- Gavalas, G.R.; Phichitkul, C.; Voecks, G.E. Structure and activity of NiO-α-Al2O3 and NiO-ZrO2 calcined at high temperatures: I. Structure. J. Catal. 1984, 88, 54–64. [Google Scholar] [CrossRef]
- Chen, Y.G.; Ren, J. Conversion of methane and carbon dioxide into synthesis gas over alumina-supported nickel catalysts: Effect of Ni-Al2O3 interactions. Catal. Lett. 1994, 29, 39–48. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; Guemez, M.B.; Fierro, J.L.G. Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst: The role of acid and metal sites on product selectivity and the reaction mechanism. Appl. Catal. B 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Sharma, R.V.; Kumar, P.; Dalai, A.K. Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr:Zr mixed metal oxides catalyst. Appl. Catal. A 2014, 477, 147–156. [Google Scholar] [CrossRef]
- Miranda, B.C.; Chimentao, R.J.; Santos, J.B.O.; Gispert-Guirado, F.; Llorca, J.; Medina, F.; Bonillo, F.L.; Sueiras, J.E. Conversion of glycerol over 10% Ni/gamma-Al2O3 catalyst. Appl. Catal. B 2014, 147, 464–480. [Google Scholar] [CrossRef]
- Medlin, J.W. Understanding and Controlling Reactivity of Unsaturated Oxygenates and Polyols on Metal Catalysts. ACS Catal. 2011, 1, 1284–1297. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Cheng, H.; Liang, G.; He, L.; Lin, W.; Yu, Y.; Zhao, F. Effect of Phosphine Doping and the Surface Metal State of Ni on the Catalytic Performance of Ni/Al2O3 Catalyst. Catalysts 2015, 5, 759-773. https://doi.org/10.3390/catal5020759
Li X, Cheng H, Liang G, He L, Lin W, Yu Y, Zhao F. Effect of Phosphine Doping and the Surface Metal State of Ni on the Catalytic Performance of Ni/Al2O3 Catalyst. Catalysts. 2015; 5(2):759-773. https://doi.org/10.3390/catal5020759
Chicago/Turabian StyleLi, Xiaoru, Haiyang Cheng, Guanfeng Liang, Limin He, Weiwei Lin, Yancun Yu, and Fengyu Zhao. 2015. "Effect of Phosphine Doping and the Surface Metal State of Ni on the Catalytic Performance of Ni/Al2O3 Catalyst" Catalysts 5, no. 2: 759-773. https://doi.org/10.3390/catal5020759
APA StyleLi, X., Cheng, H., Liang, G., He, L., Lin, W., Yu, Y., & Zhao, F. (2015). Effect of Phosphine Doping and the Surface Metal State of Ni on the Catalytic Performance of Ni/Al2O3 Catalyst. Catalysts, 5(2), 759-773. https://doi.org/10.3390/catal5020759






