Abstract
Photocatalytic H2 evolution was examined using Pt-loaded TiO2-photocatalyst in the presence of amines as sacrificial agents. In the case of amines with all of the carbon attached to the hetero-atom such as 2-aminoethanol, 1,2-diamonoethane, 2-amino-1,3-propanediol, and 3-amino-1,2-propanediol, they were completely decomposed into CO2and water to quantitatively evolve H2. On the other hand, the amines with both hetero-atoms and one methyl group at the β-positions (neighboring carbons) of amino group such as 2-amino-1-propanol and 1,2-diaminopropane were partially decomposed. Also, the photocatalytic H2 evolution using amines without the hetero-atoms at the β-positions such as ethylamine, propylamine, 1-butylamine, 1,3-diaminopropane, 2-propylamine, and 2-butylamine was inefficient. Thus, it was found that the neighboring hetero-atom strongly assisted the degradation of sacrificial amines. Moreover, rate constants for H2 evolution were compared among amines. In conclusion, the neighboring hetero-atom did not affect the rate constants but enhanced the yield of hydrogen evolution.
1. Introduction
Hydrogen production from water has received a great amount of interest in development of a renewable and clean energy source [1]. Titanium oxide (TiO2)-photocatalytic reaction has been one promising approach to hydrogen production since the discovery of photoelectrochemical hydrogen evolution with TiO2 by Honda and Fujishima [2]. The TiO2-photocatalytic reaction is initiated by charge-separation into electrons and holes (h+) under irradiation [3]. The electron reduces water to generate H2 while h+ oxidizes the HO− to give a hydroxyl (HO·) radical [4]. It is well known that the use of electron-donating sacrificial agents remarkably accelerates the TiO2-photocatalyzed hydrogen evolution since the HO• radical is consumed by the sacrificial agents [5]. Therefore, the selection of sacrificial agents will strongly affect the efficiency of the hydrogen evolution. Recently, we have found that sacrificial agents with all of the carbon attached the hydroxy groups such as saccharides (e.g., glucose and xylose) and polyalcohols (l,2-ethandiol, glycerol, arabitol) and continued to serve as an electron source until their sacrificial ability was exhausted in the photocatalytic hydrogen evolution by Pt-loaded TiO2 (Pt/TiO2) [6,7,8]. Thus, it was found that a neighboring oxygen-atom substituted at β-position strongly assisted the decarboxylation from alcoholic sacrificial agents [8]. On the other hand, it is well known that amines are good electron-donating sacrificial agents [9]. However, the degradation process of the amine is still insufficient to be elucidated. Here, the degradation process of the amines in the photocatalyic H2 evolution using the Pt/TiO2 was examined by a quantitative and a kinetic analysis in order to examine whether or not neighboring oxygen-atom affects the yield and the rate of H2 evolution.
2. Results
2.1. Yields of H2 and CO2 Evolved from the Photocatalytic Reaction
Table 1 lists the sacrificial agents such as amine and carboxylic acid used in the photocatalytic H2 evolution. Recently we have elucidated that the alcohols, the carbons of which all have hydroxyl groups, were completely decomposed into CO2 and water by the TiO2 photocatalytic reaction [8]. Therefore, the amines used in the present study were classified into three groups (Groups A, B and C) from the substituent positions of the hetero-atoms such as O and N. Group A involved the amines with all of the carbon-substituted hetero-atoms. Group B involved the amines with both continuous hetero-atom-substituted carbon and one non-hetero-atom-substituted carbon (methyl group). Group C involved the amines without the hetero-atoms at the β-position of amino group.
An aqueous solution (150 mL) containing varying amounts of sacrificial agents (0.25–1.25 mmol) and Pt/TiO2 (100 mg) was irradiated under vigorous stirring with a magnetic stirrer for 14–160 h until the gas evolution ceased. The evolved gas was analyzed by GLC and the gas volumes of H2 and CO2evolved from given amounts of sacrificial agents are summarized in Supporting Information (Table S1). A typical example can be observed in the results of 1,2-diaminoethane (1a) where the evolved H2 increased as increase of the amounts of 1a used. However, the molar ratio of the evolved H2 to 1a (H2/1a) was dependent on the amount of 1a used. Therefore, the H2/1a was plotted against the molar ratio of 1a to the catalyst (1a/catalyst) which was adjusted to 0.2, 0.4, 0.6, 0.8, and 1.0, as shown in Figure 1. As the 1a/catalyst decreased, the H2/1a values increased. The intercept of the plots represented a limiting amount of H2 (H2max) obtained from one mole of sacrificial agent at an infinite amount of the catalyst.

Table 1.
Yields and rate constants for Pt/TiO2-photocatalytic hydrogen evolution using sacrificial amines and carboxylic acids.
| Sacrificial agents | H2max | CO2max | CH4max | Yield/% a | k/h−1 b | ||
|---|---|---|---|---|---|---|---|
| Run | Formula | PE | /mol mol−1 | /mol mol−1 | /mol mol−1 | ||
| Amines with all of carbons attached hetero-atom (Group A) | |||||||
| 1 | H2NCH2CH2NH2 (1a) | 10 | 5.2 | 2.1 | 0 | 100 | 3.84 |
| 2 | MeNH2 (1b) | 6 | 2.9 | 1.0 | 0 | 97 | 3.83 |
| 3 | HOCH2CH2NH2 (1c) | 10 | 5.0 | 1.8 | 0 | 100 | 7.64 |
| 4 | (HOCH2)2CHNH2 (1d) | 14 | 7.0 | 2.5 | 0 | 100 | 6.70 |
| 5 | HOCH2CH(OH)CH2NH2 (1e) | 14 | 6.4 | 1.8 | 0 | 91 | 7.64 |
| Amines with both hetero-atoms and methyl group at β-positions (Group B) | |||||||
| 6 | MeCH(NH2)CH2OH (1f) | 16 | 3.8 | 0.7 | 0 | 48 | 9.43 |
| 7 | MeCH(NH2)CH2NH2 (1g) | 16 | 4.0 | 0 | 0 | 50 | 3.93 |
| Amines without hetero-atom at β-positions (Group C) | |||||||
| 8 | MeCH2NH2 (1h) | 12 | 3.3 | 0.5 | 0 | 55 | 5.21 |
| 9 | MeCH2CH2NH2 (1i) | 18 | 4.0 | 0.0 | 0 | 44 | 4.09 |
| 10 | MeCH2CH2CH2NH2 (1j) | 24 | 4.1 | 0.1 | 0 | 34 | 2.53 |
| 11 | H2NCH2CH2CH2NH2 (1k) | 16 | 4.6 | 0.0 | 0 | 58 | 6.23 |
| 12 | MeCH(NH2)Me (1l) | 18 | 2.4 | 0.0 | 0 | 27 | 6.70 |
| 13 | MeCH2CH(NH2)Me (1m) | 24 | 3.3 | 0.0 | 0 | 25 | 2.40 |
| Carboxylic acids and carbonyl compounds | |||||||
| 14 | HCO2H (2a) | 2 | 1.0 | 1.0 | 0 | 100 | 4.88 |
| 15 | HO2CCO2H (2b) | 2 | 1.0 | 2.0 | 0 | 100 | 0.76 |
| 16 | HO2CCH2OH (2c) | 6 | 2.8 | 1.8 | 0 | 93 | 6.38 |
| 17 | MeCO2H (2d) | 8 | 2.9 | 1.7 | 0.27 | 100 | 0.07 |
| 18 | MeCOCH2OH (2e) | 14 | 4.9 | 2.5 | 0.30 | 89 | 0.34 |
| 19 | MeCH(OH)CO2H (2f) | 12 | 4.1 | 2.3 | 0.30 | 88 | 12.48 |
| 20 | MeCOCO2H (2g) | 10 | 3.9 | 2.7 | 0.30 | 102 | 0.37 |
| 21 | CH2(CO2H)2 (2h) | 8 | 2.6 | 2.7 | 0.31 | 96 | 0.10 |
| 22 | MeCH2CO2H (2i) | 14 | 2.3 | 1.0 | 0 | 33 | 0.18 |
a Yield = 100(2H2max + 8CH4max)/PE; b Rate constants for H2 evolution.
Also, the amounts of the evolved CO2 were dependent on the amount of sacrificial agents used. The plots of the molar ratio of CO2 to 1a (CO2/1a) against 1a/catalyst are shown in Figure 1. From the intercept of the plots, the limiting amount of CO2 (CO2max) from one mole of 1a was obtained. Thus, the 1a was decomposed by HO• radical to five equivalents of H2 and two equivalents of CO2.
Similar treatments were applied to other sacrificial agents. The H2max and CO2max are summarized in Table 1. In many cases, the CO2 evolved from the amines was smaller than the case of carboxylic acids. The resulting CO2 might be partially trapped with NH3 or amines in aqueous solution to form RNH3+HCO3− [10]. If sacrificial agents which have formula CnHmNpOq, is entirely decomposed into CO2, NH3, and H2O by HO• radical (Equation (1)), the sacrificial agents will be capable of serving as 4n + m − 3p − 2q electron sources. We defined these 4n + m − 3p − 2q values as the potentially electron-donating ability (PE) of sacrificial agents. In other words, sacrificial agent theoretically has the ability to evolve (4n + m − 3p − 2q)/2 equivalents of the H2 in the TiO2-photocatalytic reaction. The PE values are listed in Table 1. The H2 evolution yield was defined to be 100 × 2H2max/PE.
CnHmNpOq + (4n + m − 3p − 2q)HO·→ nCO2 + pNH3 + (2n + m − 3p − q)H2O
PE = 4n + m − 3p − 2q
PE = 4n + m − 3p − 2q
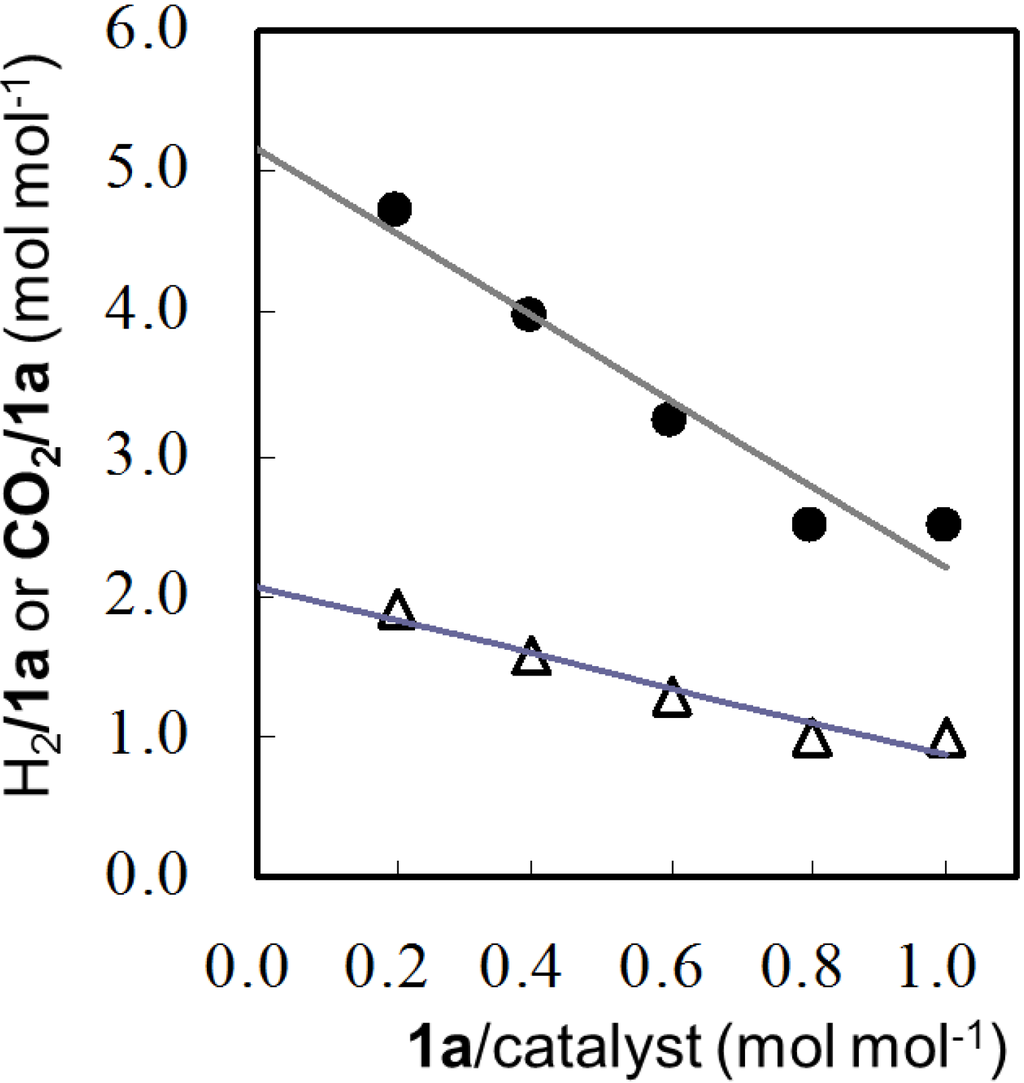
Figure 1.
Plots of H2/1a (●) and CO2/1a (Δ) against 1a/catalyst in TiO2-photocatalytic reaction of 1,2-aminoethane (1a).
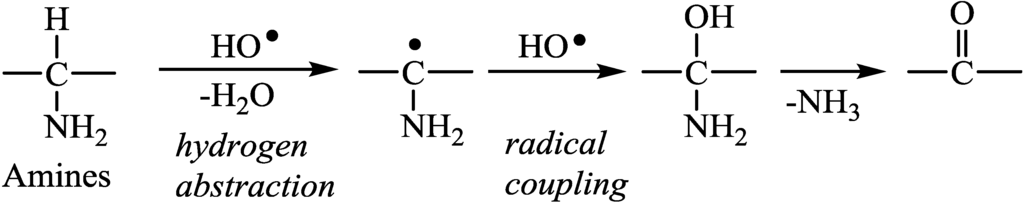
Figure 2.
Degradation scheme of amine (1) by HO• radical.
2.2. Kinetic Analysis of Hydrogen Evolution
Figure 2 shows the degradation scheme of the amines by HO• radical. The HO• radical abstracted a hydrogen atom from the α-carbon of the amines, and the resulting radicals underwent to radical coupling with HO• radical [11]. Elimination of ammonia occurred to form the carbonyl groups. The evolution of one equivalent of H2 corresponded to the consumption of two equivalent of HO• radicals and one equivalent of sacrificial agent. Therefore, the rate of H2 evolution can be presented by Equation (2). It was suggested that the concentration of HO• radical ([HO•]) was constant under same irradiation conditions. Therefore, pseudo-first order rate constant (k) for H2 evolution can be determined by the plots of—ln(1 − V/22.4) against the irradiation time (t) where V was the gas volume in mL evolved from 1 mmol of sacrificial agent at each irradiation time (Equation (3)). In the case of carboxylic acids, two equivalent HO• radicals induced to evolution of one equivalent of CO2 along with one equivalent of H2. The k values were determined according to the plots of—ln(1 − V/44.8) against the irradiation time.
rate = d[H2]/dt = k' [HO•]2[amine] = k[amine], where [amine] = 1 − [H2]
– ln(1 − V/22.4) = k t
3. Discussion
3.1. Degradation Mechanism of Carboxylic Acids (2a–i)
Several kinds of carboxylic acids and carbonyl compounds (2a–i) which were thought to be intermediates in the degradation process of the amines were subjected to the photocatalytic H2 evolution. Formic acid (2a), oxalic acid (2b), and glycolic acid (2c) were completely decomposed to CO2 and water along with the formation of H2 in almost quantitative yields (runs 14–16). On the other hand, acetic acid (2d) was decomposed into CO2 and water along with the formation of methane (run 17), as has been reported by Zheng et al. [12]. Therefore, the limiting amount of CH4 (CH4max) from one mole of 1c was obtained from the intercept of the plots of the molar ratio of CH4 to 1c (CH4/1c) against 1c/catalyst. 2d was thought to consume 5.8 equivalents of HO· radicals to produce 1.7CO2 and 0.3CH4, since 2.9 equivalents of H2 were evolved. Therefore, decomposition scheme of 2d is written as Figure 3. 1-Hydroxy-2-propanone (2e) consumed 9.8 equivalents of HO• radicals to produce 2.5CO2 and 0.30CH4 (run 18). Also lactic acid (2f) consumed 8.2 equivalents of HO• radicals to produce 2.3CO2 and 0.30CH4 (run 19). Pyruvic acid (2g) consumed 7.8 equivalents of HO• radicals to produce 2.7CO2 and 0.30CH4 (run 20). Malonic acid (2h) consumed 5.2 equivalents of HO• radicals to produce 2.7CO2 and 0.31CH4 (run 21). Since the decomposition of 2d–h by HO• radical occurred along with the formation of methane, the degradation of 2f–h proceeded through 2e (Figure 3). The yields from 2d–h were calculated to be near 100% according to the equation: Yield = 100 × (2H2max + 8CH4max)/PE.
3.2. Degradation Mechanism of Amines (1a–i)
At first, we checked whether initial hydrogen abstraction occurs at α-hydrogen of amino group or amino group itself, since amino group is good sacrificial agent. The photocatalytic H2 evolution was performed using t-BuNH2 which had no α-hydrogen as sacrificial agent. H2 was evolved from t-BuNH2(H2max = 1.0) whereas H2 was not evolved from t-BuOH (H2max = 0). Also the photocatalytic reaction using NH3 as sacrificial agents evolved H2. Therefore, hydrogen abstraction from amino group occurred resulting in the oxidation of amino group. However, H-abstraction from amino group was slower than the H-abstraction from the α-carbons of amine and alcohols [13]. Moreover, the photocatalytic H2 evolution using methylamine (1b) produced in 3.0 of H2max values (Table 1, run 1). Nitoromethane was not formed from 1b. Therefore, we thought that the degradation of 1b proceeded through the formation of formaldehyde and formic acid (2a) in a similar manner to the case of methanol [14,15] (Figure 4), although the oxidation of amino group of methylamine has been reported [16,17]. This suggested that the amino group was not oxidized in the case of usual 1 having hydrogen at the α-carbons.
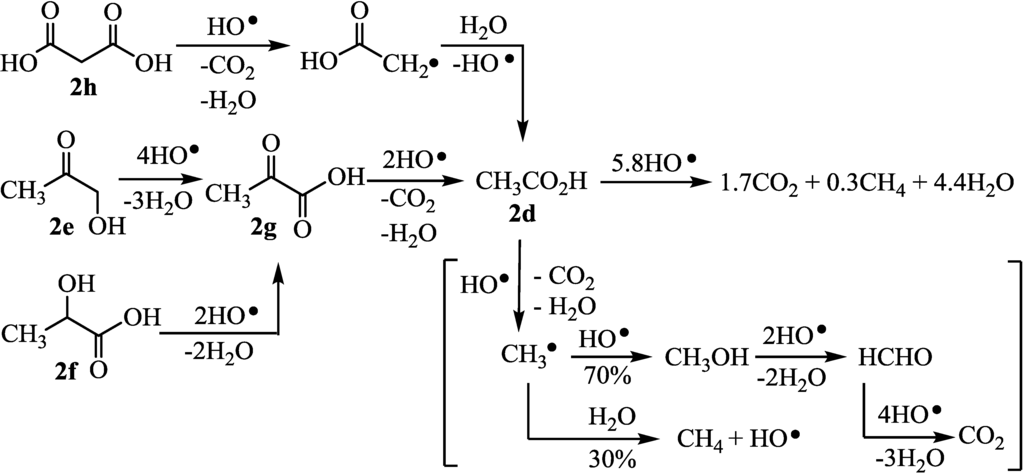
Figure 3.
Decomposition of carboxylic acid (2d–h) by HO• radical.
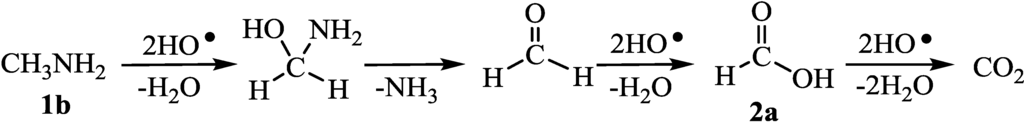
Figure 4.
Decomposition of methylamine (1b) and formic acid (2a) by HO• radical.
In Group A, 1a and 2-aminoethanol (1c) whose H2max were 5.0–5.2 were completely decomposed by HO· radicals (runs 1 and 3). These results suggested that the decomposition proceeded through the formation of 2b (Figure 5). Also, 2-amino-1,3-propanediol (1d) and 3-amino-1,2-propanediol (1e) were completely decomposed via 2b (runs 4–5) in a similar manner to the case of glycerol [18]. Thus, in the cases of 1b–e with all of the carbon attached the hetero atoms (Group A), the decomposition was able to proceed quantitatively. On the other hand, the yields of H2 evolution using Group B such as 2-amino-1-propanol (1f) and 1,2-diaminopropane (1g) did not reach 100% (runs 6–7), as has been reported for 1,2-propanediol [19]. These were decomposed through the formations of 2g to give 2d which were detected by LC-MS analysis (Figure 6). It was found that 1f–g with one methyl group (Group B) did not completely decompose, resulting in H2 in 48-88% yields. In the case of Group C, ethylamine (1h) and propylamine (1i) were partially decomposed (Figure 7) and the H2 yields did not reach 100% (run 8) in a similar manner to the case of ethanol [20]. The decomposition occurred through the formation of 2d which could be detected by LC-MS analysis. Butylamine (1j) consumed four equivalents of HO• radicals to give butanoic acid which was slowly decomposed to propanal [21] since butanoic acid was detected by LC-MS (run 10). Also, the 1,3-diaminopropane (1k) consumed eight equivalents of HO• radicals to give 2h (run 11). However, it was thought that 2h smoothly underwent further oxidation to 2d, since 2h could not be detected by the LC-MS analysis of the photolysates but 2d was detected. Thus, terminal amines (1h–1k) underwent the partial CO2 evolution (runs 8–11). Carboxylic acid tended to react with amines and/or ammonia to form salts (RNH3+HCO3−) which were inactive toward the HO• radical. Therefore, the degradation of amines with HO• radical was retarded compared with those of carboxylic acids. The amines, such as 2-propylamine (1l) and 2-butylamine (1m), whose amino group attached on secondary carbons, were inefficient (runs 12–13), as has been reported for the oxidation of secondary alcohols such as 2-propanol [22] and 2-butanol [22]. Acetone from 1l [23] and 2-butanone from 1m were detected by GLC analysis of photolysates.
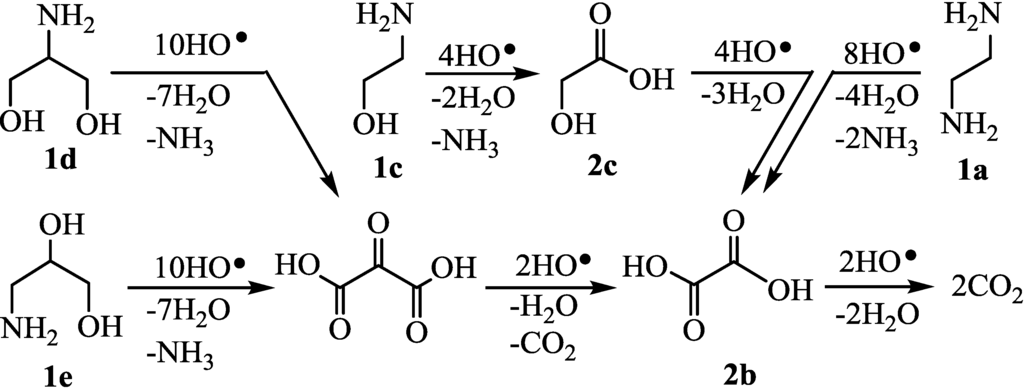
Figure 5.
Degradation of 1a, 1c–e (Group A) by HO• radical.
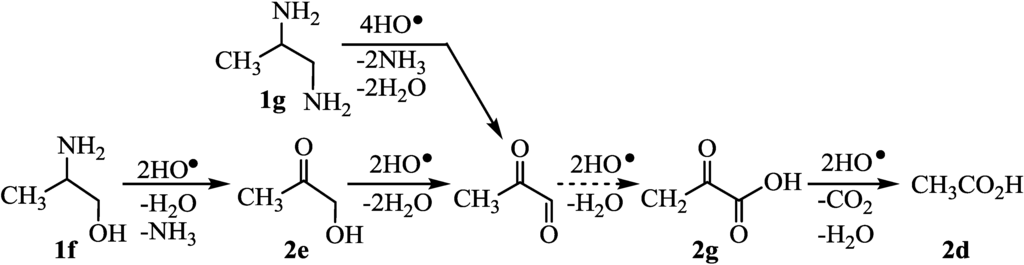
Figure 6.
Degradation of 1f–g (Group B) by HO• radical.
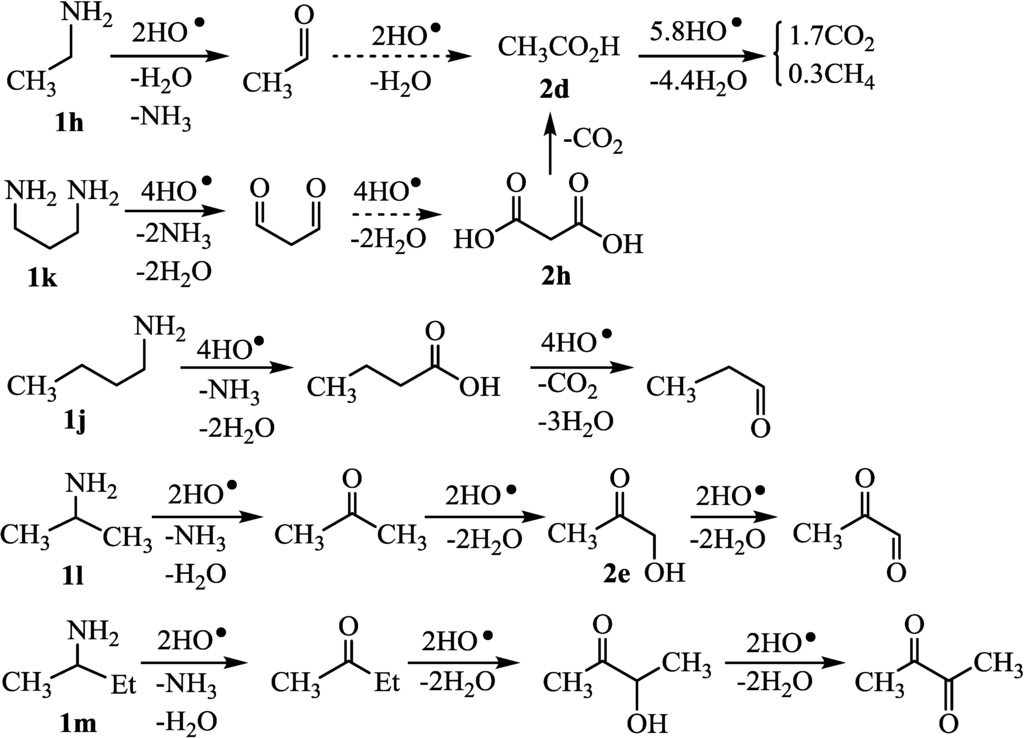
Figure 7.
Degradation of 1h–m (Group C) by HO• radical.
3.3. Rate Constants for the Hydrogen Abstraction with HO• Radical from 1 and 2
As a preliminary experiment, the time-conversion of H2 evolution was examined using three carbons-containing sacrificial agents such as 1-propylamine (1i), propanal, and propanoic acid (2i). According to Figure 8, 1i was oxidized to propanal which was further oxidized to 2i, which was subjected to the decarboxylation to give ethanol (run 22). The time courses of gas evolution are shown in Figure 9. Apparently, gas evolutions from 1i were faster than that from 2i (runs 9 and 22). Therefore, the analysis of the H2-evolutions in the amines was safely performed without the effects of the further oxidation of aldehydes and carboxylic acids. The results of rate constants for H2 evolution using a variety of amines are summarized in Table 1. Moreover, the k values (3.83–9.43 h−1) of 1a–g which belong to groups A and B were almost same as those (2.40–6.70 h−1) of 1h–m (Group C). Therefore, neighboring hetero-atoms which are substituted at the β-positions did not affect the rate constants for hydrogen abstraction from α carbons of the amine.
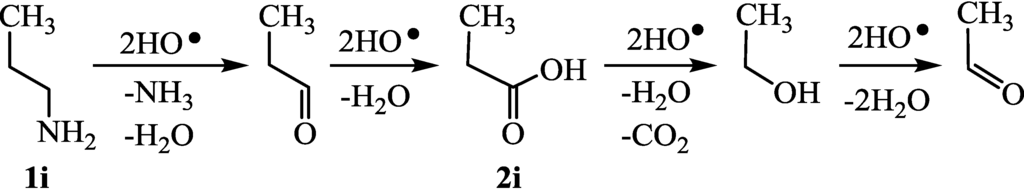
Figure 8.
Degradation of 1-propylamine (1i) and propanoic acid (2i) by HO• radical.
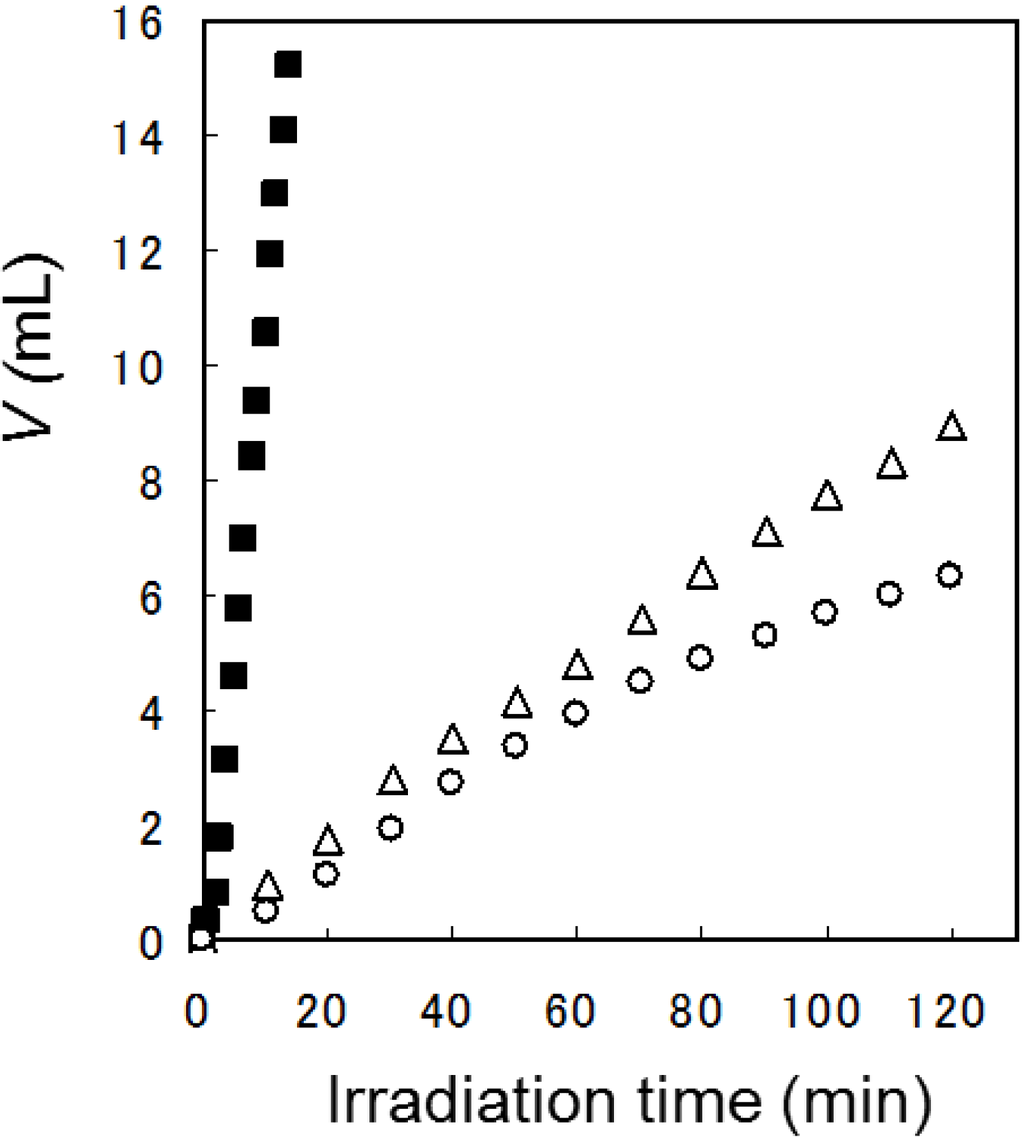
Figure 9.
Time-conversion plots of the evolved gas volume (V) in the Pt/TiO2 photocatalytic reaction using 1-propylamine (1i, ■), propanal (Δ), and propanoic acid (2i, ○).
3.4. Relationship between the Actual Electron-Donating Ability and PE of Sacrificial Agents
Figure 10 shows plots of the actual electron-donating ability (2H2max) against the PE values in the Pt/TiO2-photocatalytic H2 evolution using the amines. The amines with all of the carbon attached the hetero-atom (Group A) were completely decomposed into CO2 and water in almost 100% yields. Group B with both one methyl group and hetero-atoms at the β-positions of amino and hydroxyl groups (neighboring hetero-atom) and the Group C, without neighboring hetero-atom, were partially decomposed. As has been reported previously for alcoholic sacrificial agents [8], it was found that the neighboring hetero-atom remarkably assisted the decarboxylation from carboxylic acids (Figure 11). The lone pair electrons of the hetero-atom stabilized the radical (3). Therefore, the degradation of sacrificial agents having neighboring hetero-atom proceeded to evolve H2 in high yields.
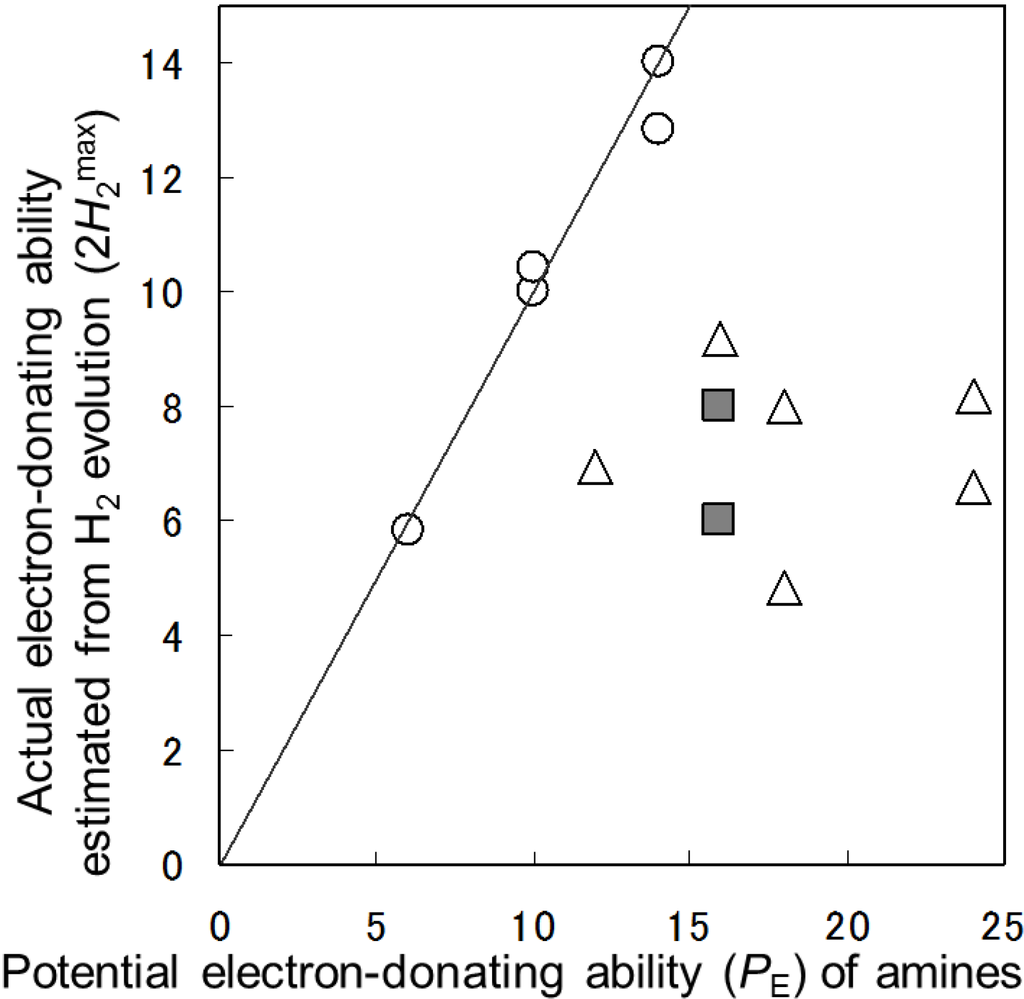
Figure 10.
Plots of the actual electron-donating ability (2H2max) against PE in the Pt/TiO2-photocatalytic H2 evolution using 1a–m: Group A (○), Group B (■), and Group C (Δ). A line was 2H2max = PE.
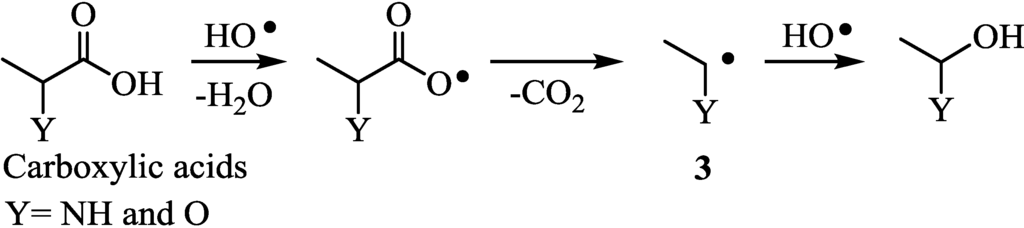
Figure 11.
Neighboring hetero-atom assistance to the decarboxylation with HO• radical.
4. Experimental Section
4.1. Preparation of the Photocatalyst
Anatase-type of TiO2 (Ishihara Sangyo Kasei Ltd. ST-01) was purchased from Ishihara Sangyo, Japan. According to previous research [24], the Pt/TiO2 was prepared by irradiation of a deaerated aqueous solution (400 mL) containing TiO2 (4.0 g), K2PtCl6 (20–200 mg), and 2-propanol (2.4 g, 3.0 mL) by high-pressure mercury lamp (100 W, UVL-100HA, Riko, Japan) for 24 h under stirring. Water was entirely removed from the photolysate by evaporator. The resulting precipitate was washed with water on filter and then dried under reduced pressure to give Pt/TiO2. The structure of Pt/TiO2 was analyzed by a Shimadzu (Kyoto, Japan) XRD 7000 diffractometer. Figure 12 shows that an anatase structure of TiO2 was kept after the loading of Pt. The Pt-content of TiO2 was optimized to be 2.0 wt % by the comparison of H2 amounts evolved from the photocatalytic reaction using the Pt/TiO2 with different amounts of Pt (1.0–3.0 wt %), which was performed in the presence of glycerol (115 mg, 1.25 mmol) under irradiation for 6 h [8].
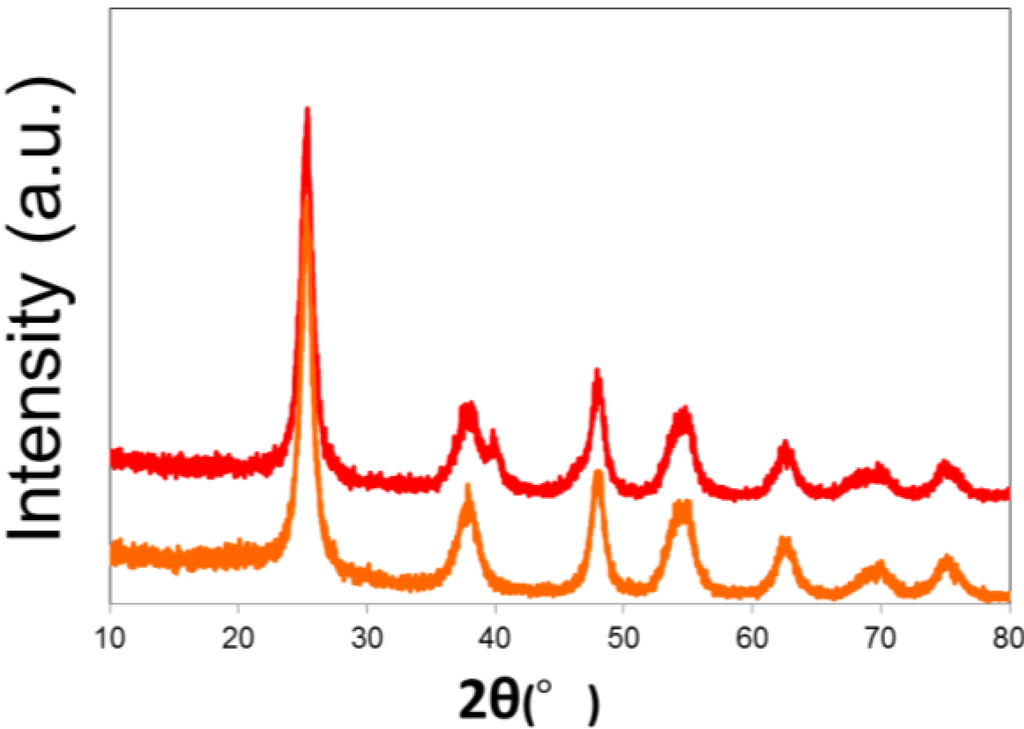
Figure 12.
X-ray diffraction of Pt-loaded TiO2 (red line) and TiO2 (ST-01, orange line).
4.2. Photocatalytic Reaction
The amines and carboxylic acids were purchased from Wako Chemicals (Osaka, Japan) and used without further purification. The volume of the aqueous solution containing sacrificial agents (0.25–1.25 mmol) was adjusted to 150 mL by adding water and the solution was introduced into a reaction vessel. The optimized amount of Pt/TiO2 (100 mg, 1.25 mmol) was added into a reaction vessel and suspended by vigorous stirring by a magnetic stirrer. A high-pressure mercury lamp (100 W, UVL-100HA, Riko, Chiba, Japan) was inserted into the reaction vessel, which was attached to a measuring cylinder with a gas-impermeable tube to collect the evolved gas. The reaction vessel was set in a water bath to keep it at a constant temperature (usually 20 °C). After the O2 was purged from the reaction vessel by N2 gas, irradiation was performed. The evolved gas was collected by the measuring cylinder to measure the volume of the evolved gas. The evolved gas was analyzed on a Shimadzu (Kyoto, Japan) GC-8A equipped with a TCD detector at a temperature raised from 40 °C to 180 °C using a stainless column (3 mmΦ × 6 m) packed with a SHINCARBON ST (Shimadzu, Kyoto, Japan). H2, CO2, and methane were detected in addition to N2 which was used as the purging gas.
4.3. Analysis of Photolysate Solutions
LC-MS analysis of the photolysate solutions were performed on a Waters Alliance (Tokyo, Japan) 2695 under conditions (ESI ionization, capillary voltage 3.5 kV, source temperature 120 °C and desolvation temperature 350 °C) using column (Waters Tokyo, Japan) SunFire (Tokyo, Japan) C18, 2.1 mmΦ × 150 mm) and eluent solution (water). Under these conditions, acetic acid (2d) appeared at 2.10-min retention time where acetic acid had a mass peak at m/z 60 (M+). Also, propanoic acid (2i, m/z 74 (M+)) and pyruvic acid (2e, m/z 88 (M+)) were detected at 3.16 min and 2.68 min, respectively. Butanoic acid (m/z 71 (M+-17)) and malonic acid (2f, m/z 104 (M+), 87 (M+–OH)) appeared at 6.20 min and 2.44 min, respectively. 2-Butaonone and acetone were measured by GLC analysis at a temperature raised from 50 °C to 250 °C on a Shimadzu (Kyoto, Japan) 14A with FID detector using a capillary column (J&W CP-Sil 5CB, Folsom, CA, USA). 32 mmΦ × 50 m). 2-Butaonone and acetone appeared at 10.5 min and 6.9 min retention time, respectively.
5. Conclusions
The Pt/TiO2-photocatalytic H2 evolution was examined using amines as sacrificial agents. The degradation pathways of amines by HO• radical were elucidated. The yields of the evolved H2 depended on their structure of amines. The presence of neighboring hetero-atom strongly accelerated the decarboxylation, resulting in high yields of H2 evolution. However, the neighboring hetero-atoms did not affect the hydrogen abstraction from α carbons of amines.
Acknowledgments
This study was partially supported by a Grant-in-Aid for Scientific Research (C) No 24610055 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author Contributions
Masahide Yasuda conceived experimental idea and analyzed experimental data. Also Masahide Yasuda prepared the manuscript and revised it. Takayuki Tomo carried out the kinetic analysis. Shoichi Hirata carried out the quantitative analysis of hydrogen evolution under supervision of Tsutomu Shiragami. Tomoko Matsumoto carried out the product analysis and X-ray diffraction.
References
- Navarro, R.M.; Peña, M.A.; Fierro, J.L.G. Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar]
- Galinska, A.; Walendziewski, J. Photocatalytic water splitting over Pt-TiO2 in the presence of sacrificial reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Shiragami, T.; Tomo, T.; Tsumagari, H.; Yuki, R.; Yamashita, T.; Yasuda, M. Pentose acting as a sacrificial multi-electron source in photocatalytic hydrogen evolution from water by Pt-doped TiO2. Chem. Lett. 2012, 41, 29–30. [Google Scholar] [CrossRef]
- Shiragami, T.; Tomo, T.; Tsumagari, H.; Ishii, Y.; Yasuda, M. Hydrogen evolution from napiergrass by the combination of biological treatment and a Pt-loaded TiO2-photocatalytic reaction. Catalysis 2012, 2, 56–67. [Google Scholar]
- Shiragami, T.; Tomo, T.; Matsumoto, T.; Yasuda, M. Structural dependence of alcoholic sacrificial agents on TiO2-photocatalytic hydrogen evolution. Bull. Chem. Soc. Jpn. 2013, 86, 382–389. [Google Scholar] [CrossRef]
- Nishimoto, S.-I.; Ohtani, B.; Yoshikawa, T.; Kagiya, T. Photocatalytic conversion of primary amines to secondary amines and cyclization of polymethylene-α,ω-diamines by an aqueous suspension of TiO2/Pt. J. Am. Chem. Soc. 1983, 105, 7180–7182. [Google Scholar] [CrossRef]
- Kominami, H.; Nishimune, H.; Ohita, Y.; Arakawa, Y.; Inaba, T. Photocatalytic hydrogen formation from ammonia and methyl amine in an aqueous suspension of metal-loaded titanium(IV) oxide particles. Appl. Catal. B 2012, 111–112, 297–302. [Google Scholar] [CrossRef]
- Klare, M.; Scheen, J.; Vogelsang, K.; Jacobs, H.; Broekaert, J.A.C. Degradation of short-chain alkyl- and alkanolamines by TiO2- and Pt/TiO2-assisted photocatalysis. Chemosphere 2000, 41, 353–362. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Wei, L.-F.; Zhang, Z.-H.; Jiang, Q.-J.; Wei, Y.-J.; Xie, B.; Wei, M.-B. Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation. Int. J. Hydrog. Energy 2009, 34, 9033–9041. [Google Scholar] [CrossRef]
- Helali, S.; Puzenat, E.; Perol, N.; Safi, M.-J.; Guillard, C. Methylamine and dimethylamine photocatalytic degradation—Adsorption isotherms and kinetics. Appl. Catal. A 2011, 402, 201–207. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar]
- Al-Mazroai, L.S.; Bowker, M.; Davies, P.; Dickinson, A.; Greaves, J.; James, D.; Millard, L. The photocatalytic reforming of methanol. Catal. Today 2007, 122, 46–50. [Google Scholar] [CrossRef]
- Kim, S.; Choi, W. Kinetics and Mechanisms of Photocatalytic Degradation of (CH3)nNH4−n+ (0 ≤ n ≤ 4) in TiO2 Suspension: The Role of OH Radicals. Environ. Sci. Technol. 2002, 36, 2019–2025. [Google Scholar] [CrossRef]
- Helali, S.; Dappozze, F.; Horikoshi, S.; Bui, T.H.; Perol, N.; Guillard, C. Kinetics of the photocatalytic degradation of methylamine: Influence of pH and UV-A/UV-B radiant fluxes. J. Photochem. Photobiol. A 2013, 255, 50–57. [Google Scholar]
- Daskalaki, V.D.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Chang, C.-H.; Idriss, H. Photo-catalytic production of hydrogen from ethanol over M/TiO2 catalyst (M = Pd, Pt, and Rh). Appl. Catal. B 2006, 67, 217–222. [Google Scholar] [CrossRef]
- Guillard, C. Photocatalytic degradation of butanoic acid: Influence of its ionisation state on the degradation pathway: Comparison with O3/UV process. J. Photochem. Photobiol. A 2000, 135, 65–75. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, Y.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrog. Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Ohtani, B.; Kakimoto, M.; Nishimoto, S.; Kagiya, T. Photocatalytic reaction of neat alcohols by metal-loaded titanium(IV) oxide particles. J. Photochem. Photobiol. A 1993, 70, 265–272. [Google Scholar] [CrossRef]
- Kennedy, J.C., III; Datye, A.K. Photothermal heterogeneous oxidation of ethanol over Pt/TiO2. J. Catal. 1998, 179, 375–389. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).