Brookite, the Least Known TiO2 Photocatalyst
Abstract
:1. Introduction
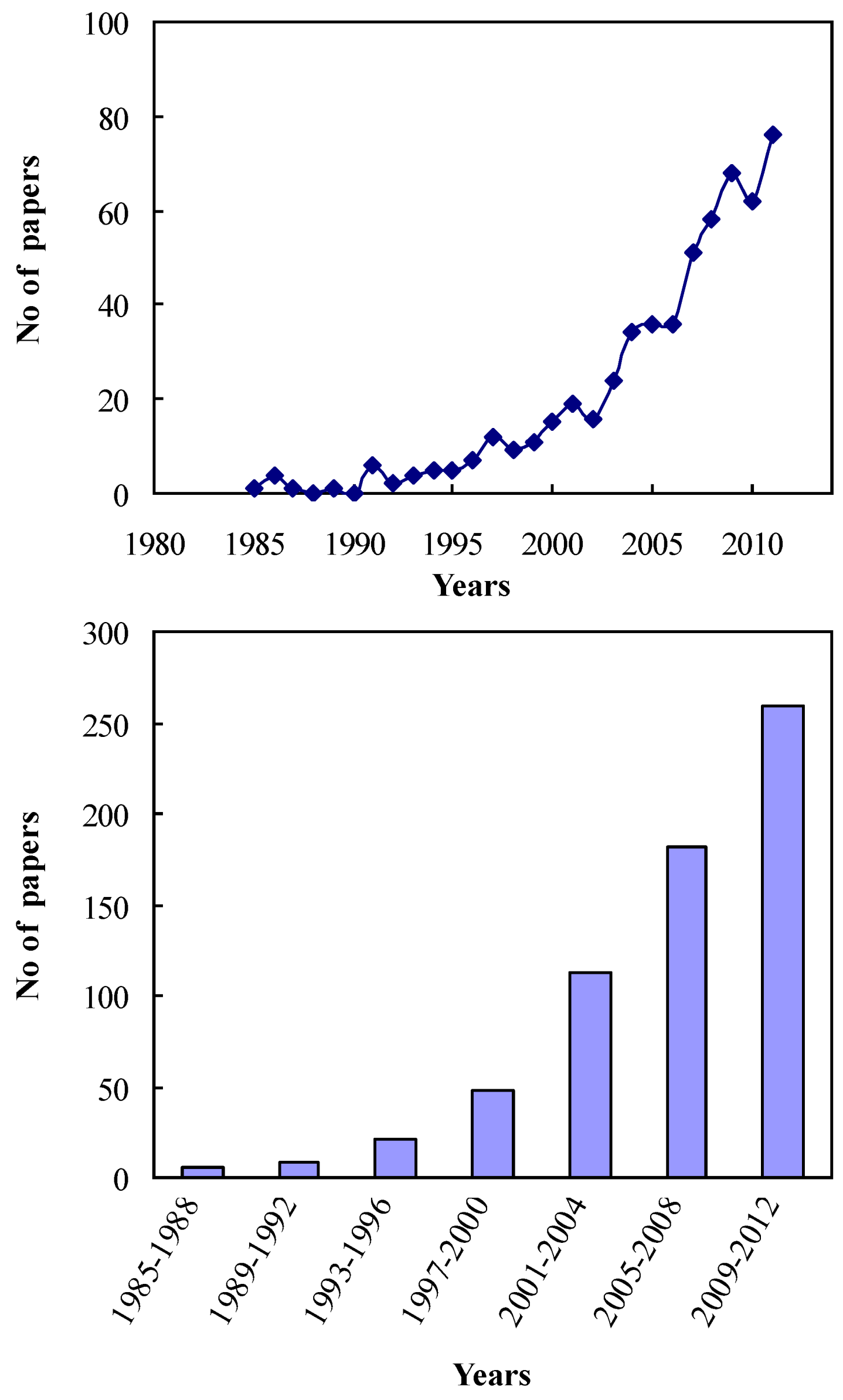
2. Brookite
2.1. Characterization
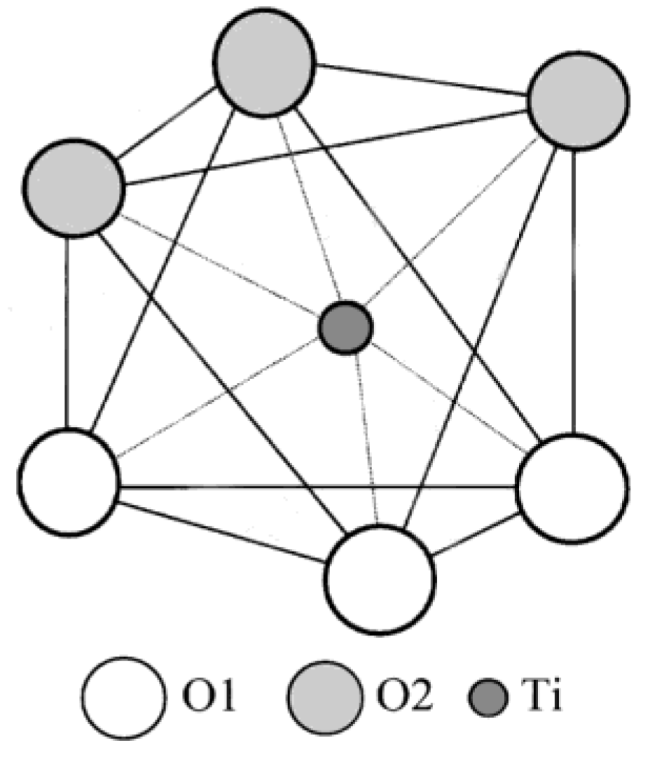
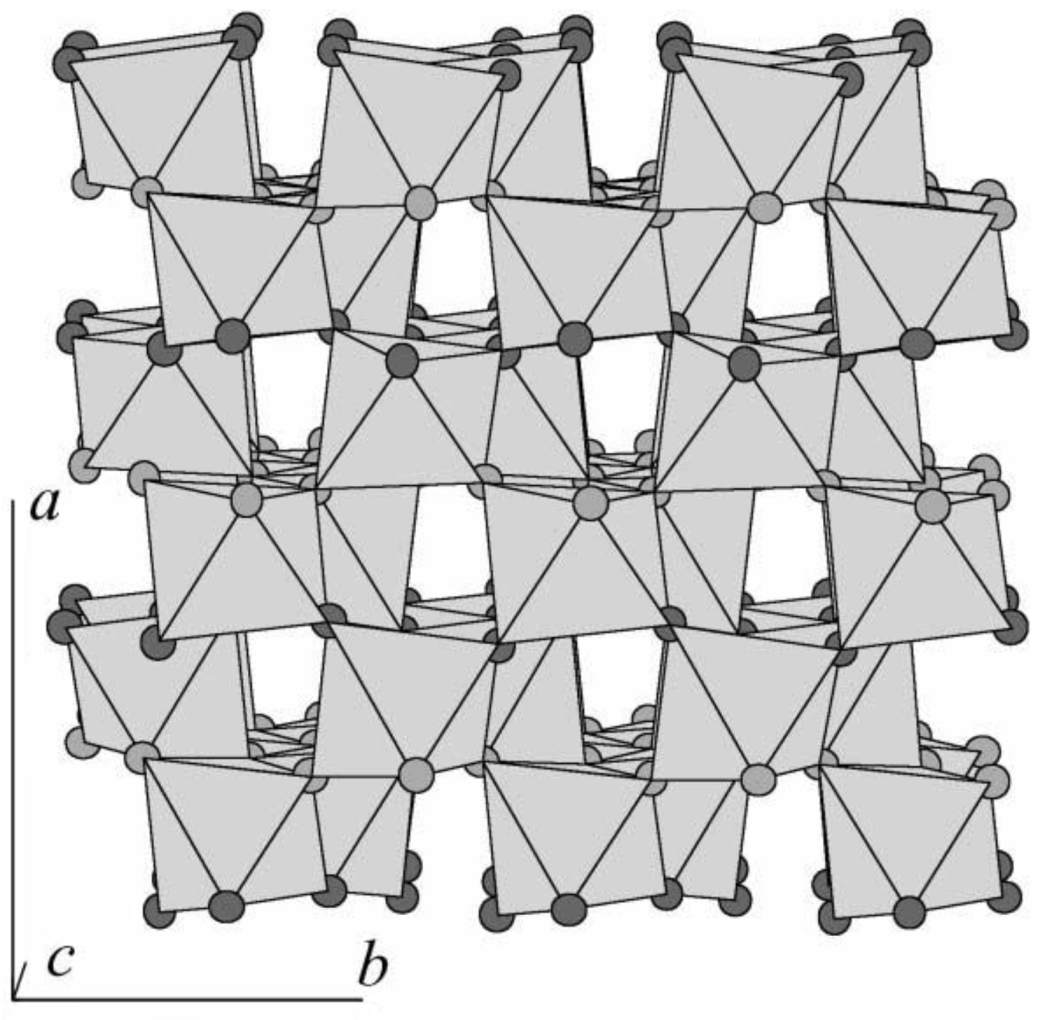
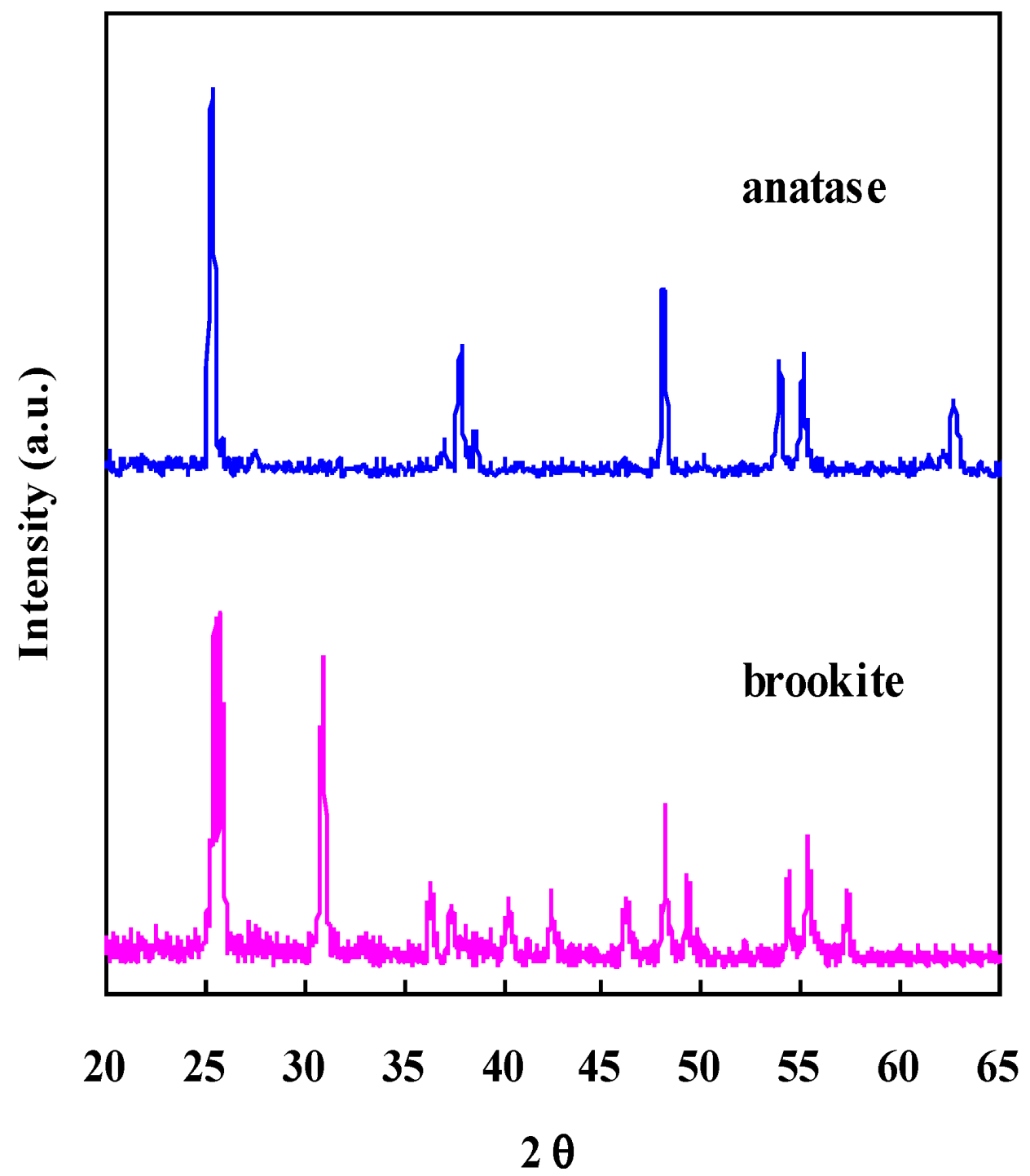
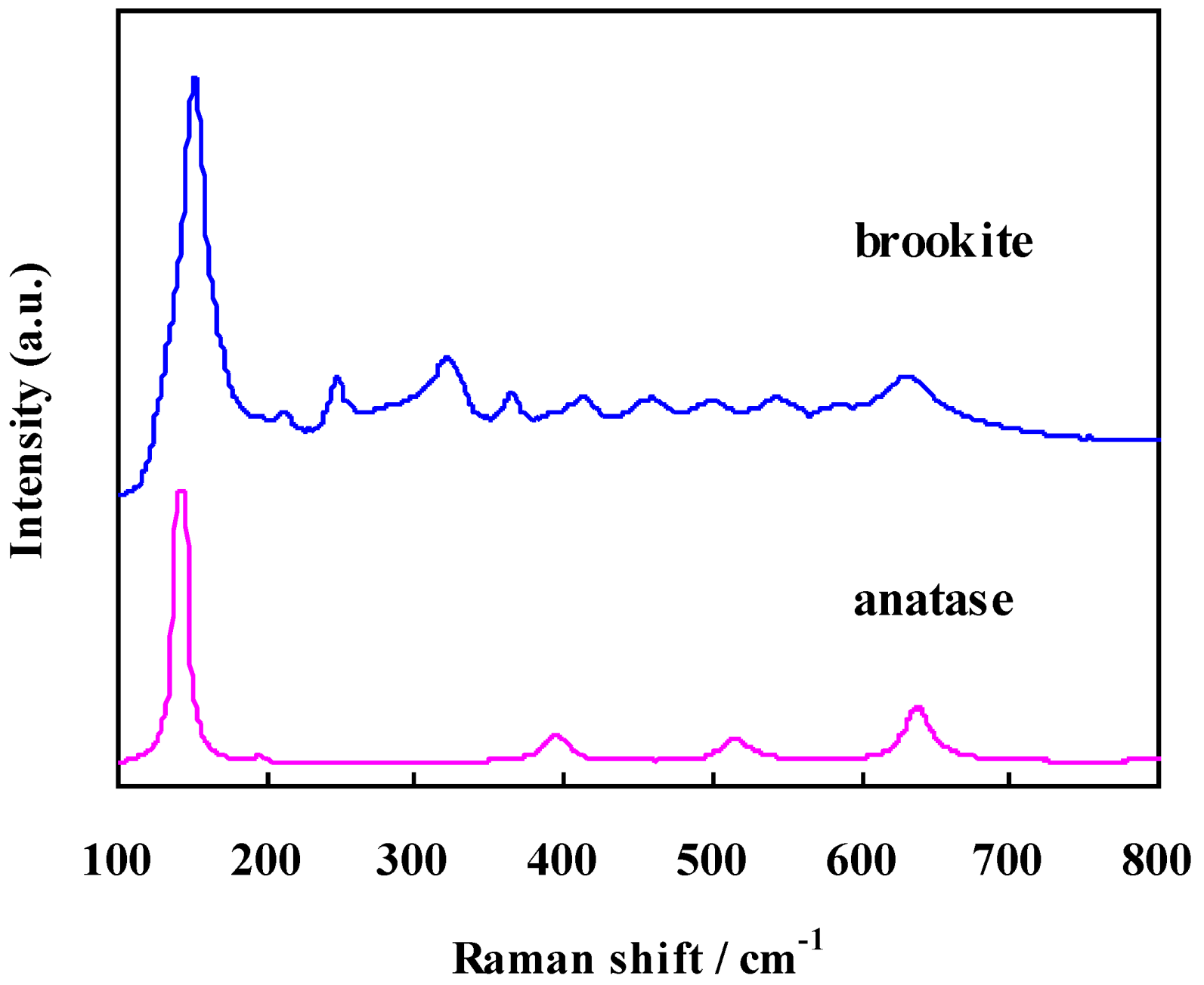
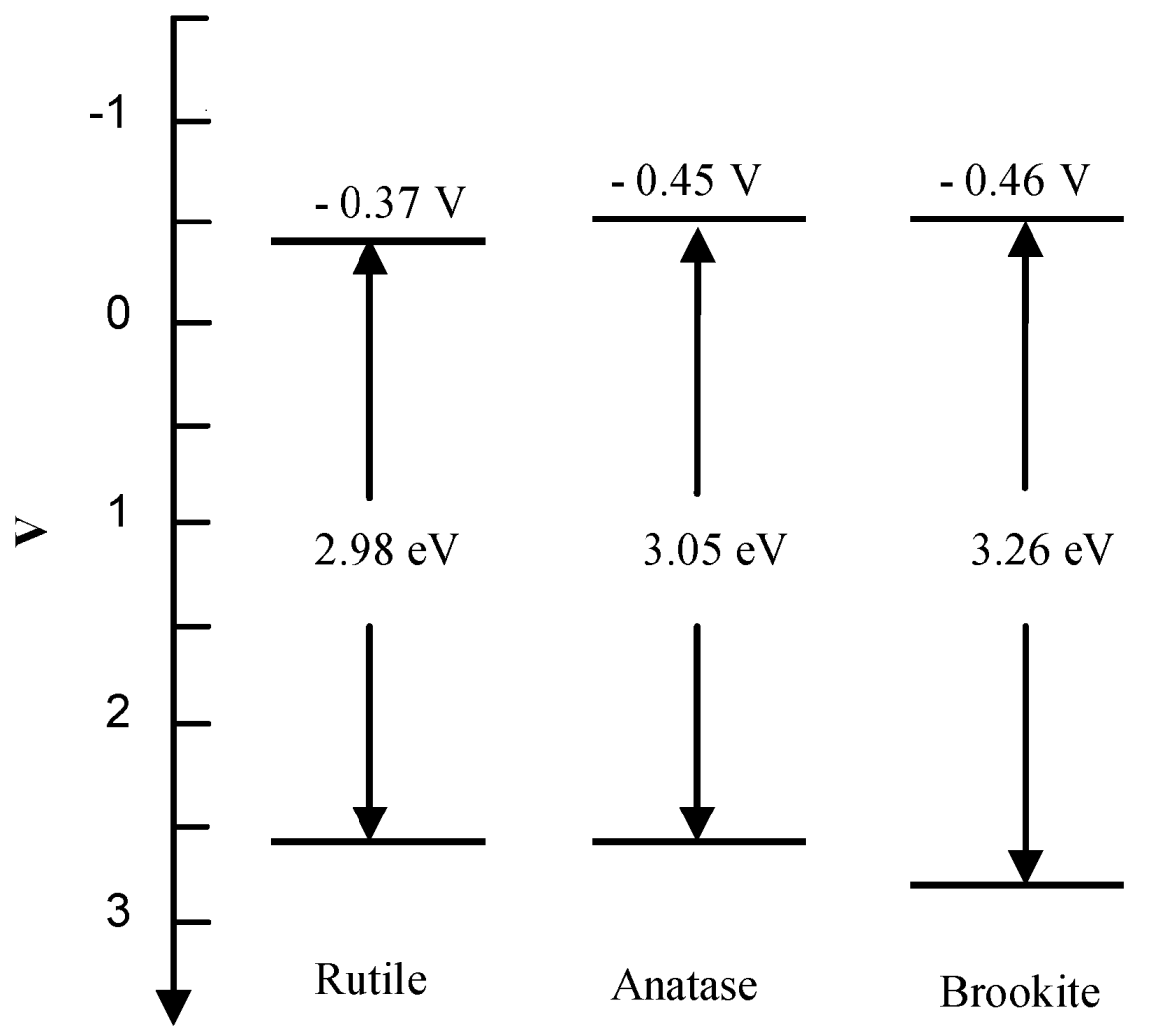
2.2. Electronic Properties
3. Brookite Preparation
3.1. Pure Brookite Powders
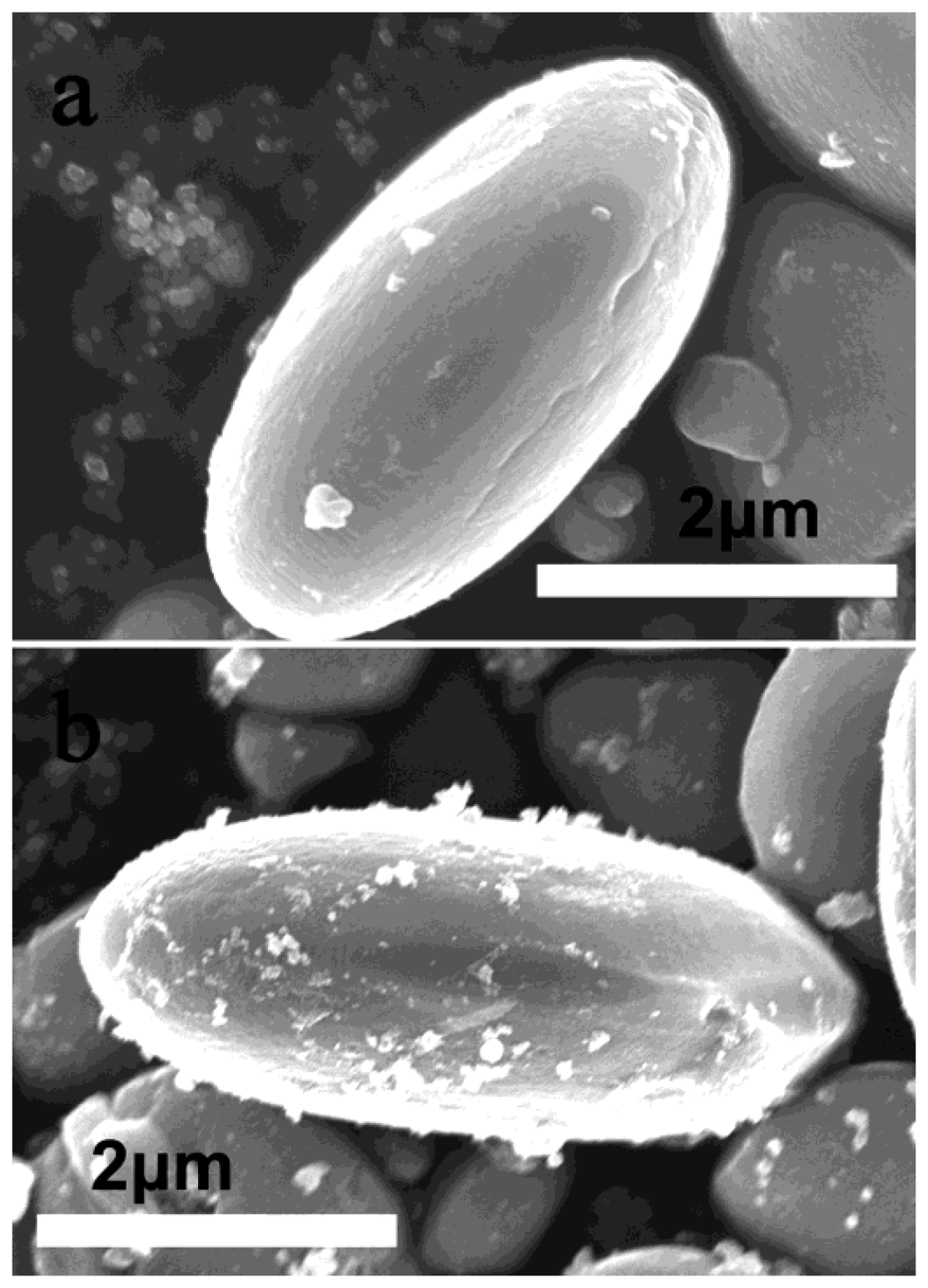
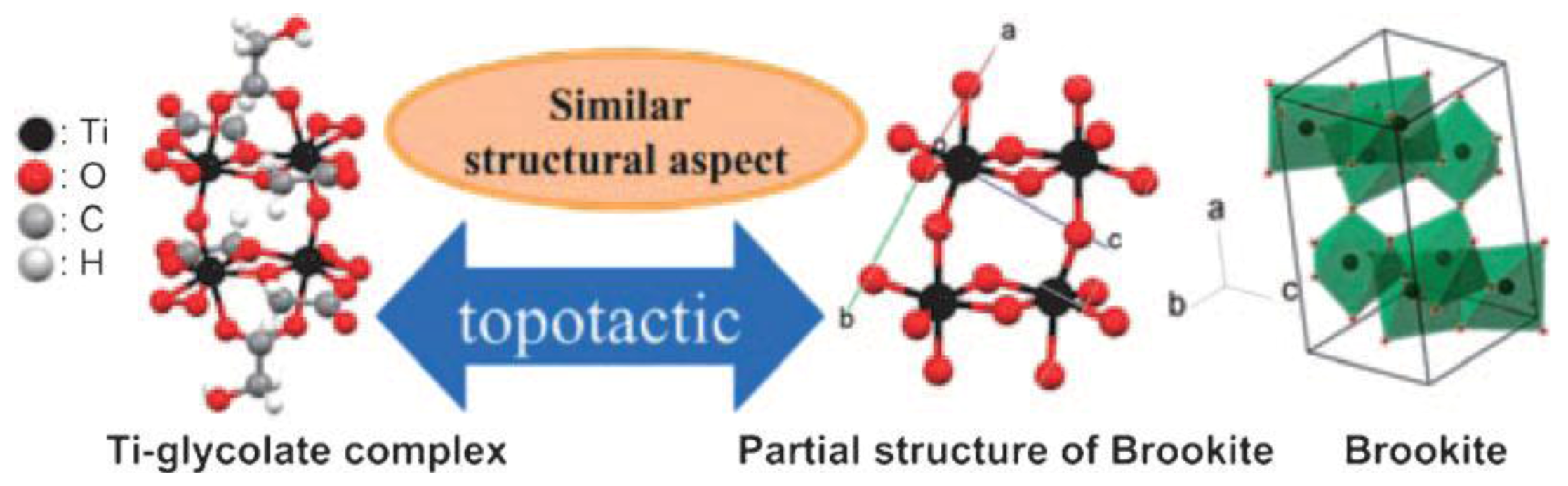
3.2. Brookite Nanomaterials
| Morphology | Precursor | Synthesis route | Reference |
|---|---|---|---|
| nanocrystals | titania powder | hydrothermal treatment | [66] |
| nanotubes | titanate nanotubes | hydrothermal treatment | [71] |
| nanorods with bipyramidal shape | titanate nanosheets | hydrothermal treatment | [72] |
| nanorods | titanium oleate complex | high-temperature aminolysis | [57] |
| nanoplatelets | titanium oxychloridehydrate | solvothermal treatment | [70] |
| nanosheets | titanium lactate | hydrothermal treatment | [27] |
| nanoflowers | titanium oxysulfate | hydrothermal treatment | [5] |
| nanoflowers | titanium butoxide | hydrothermal treatment | [79] |
| humming-top-like nanostructures | titanium isopropoxide | hydrothermal treatment | [25] |
| macroporous spherical nanoparticles | brookite nanoparticles | spray drying with colloidal templating | [96] |
| hollow nanospheres | titanium-peroxo-glycolate complex | deposition on spherical polystyrene and hydrothermal treatment | [97] |
| pseudo-cube shaped nanocrystals | titanium-peroxo complex | oleate-modified hydrothermal treatment | [95] |
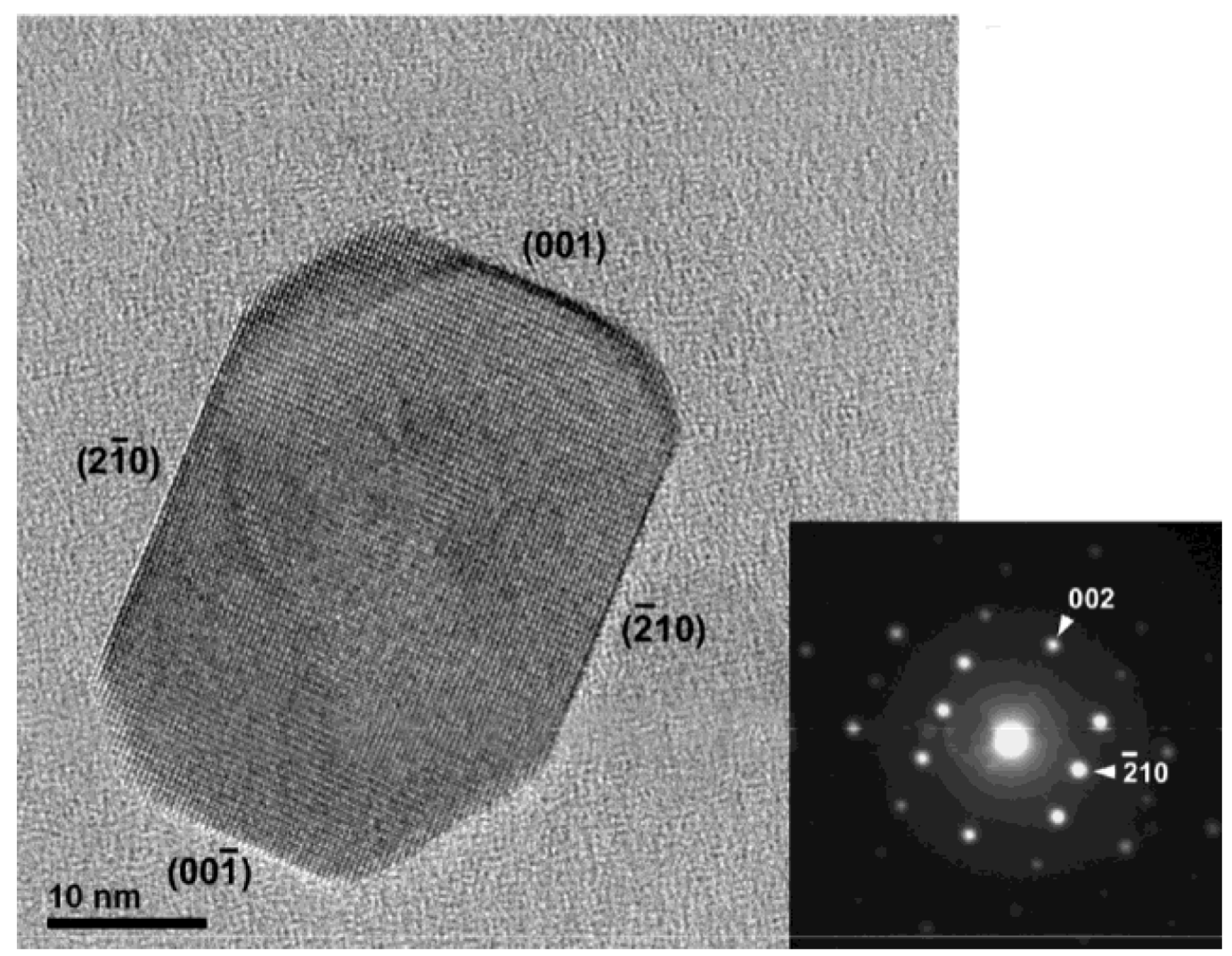
3.3. Brookite Films
4. Photoactivity
4.1. Pure Brookite
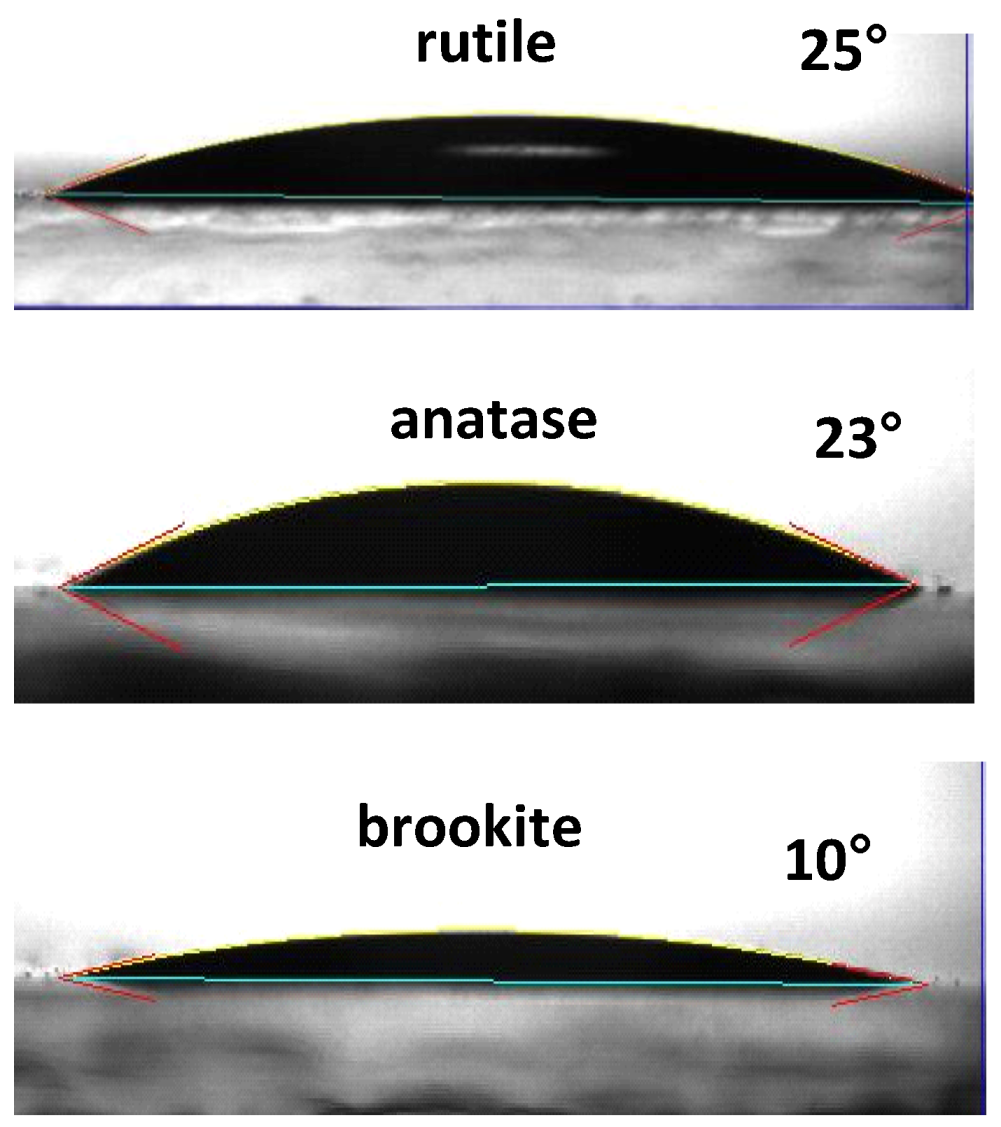
4.2. Doped and Loaded Brookite
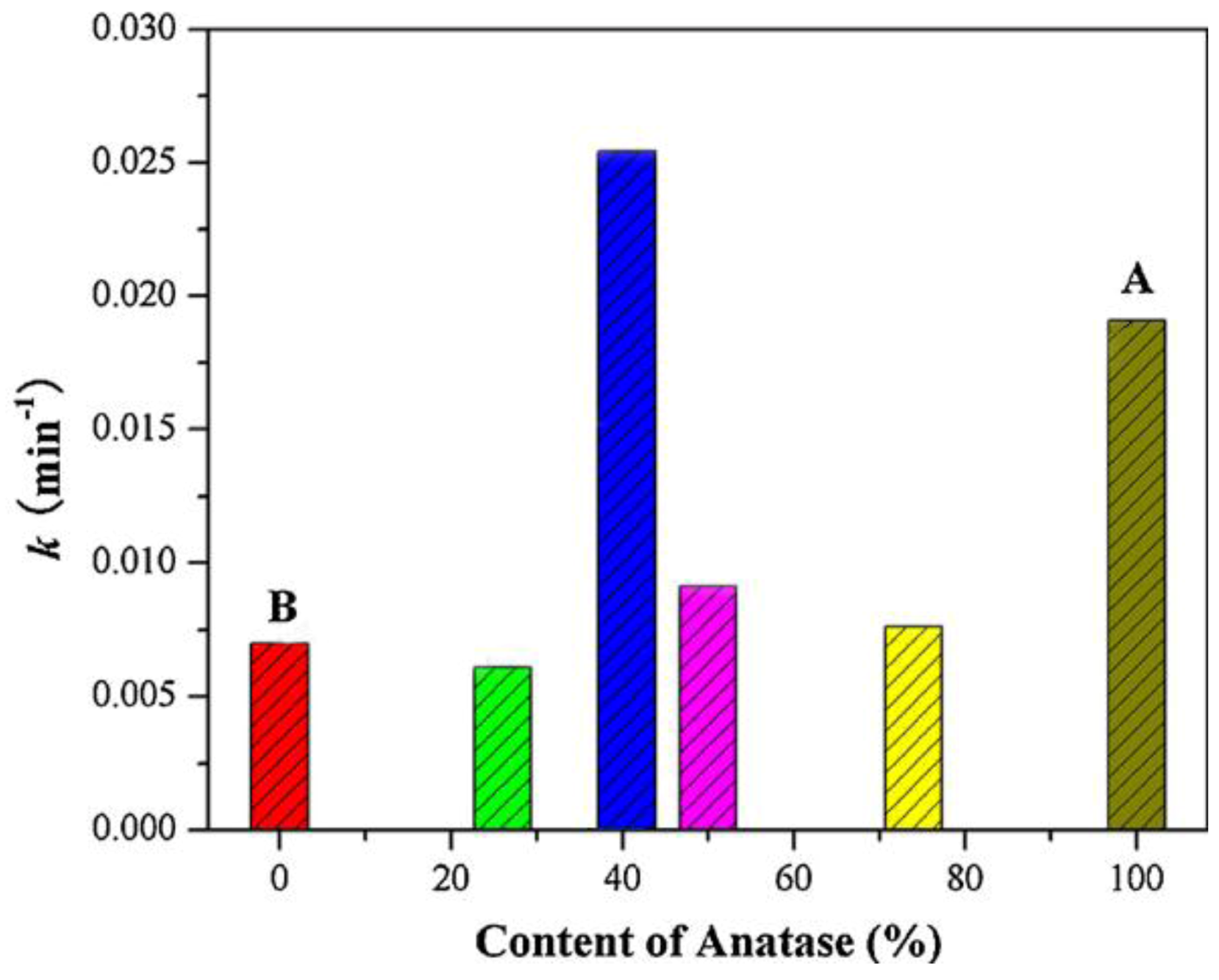
4.3. Mixtures of Brookite with Anatase and/or Rutile
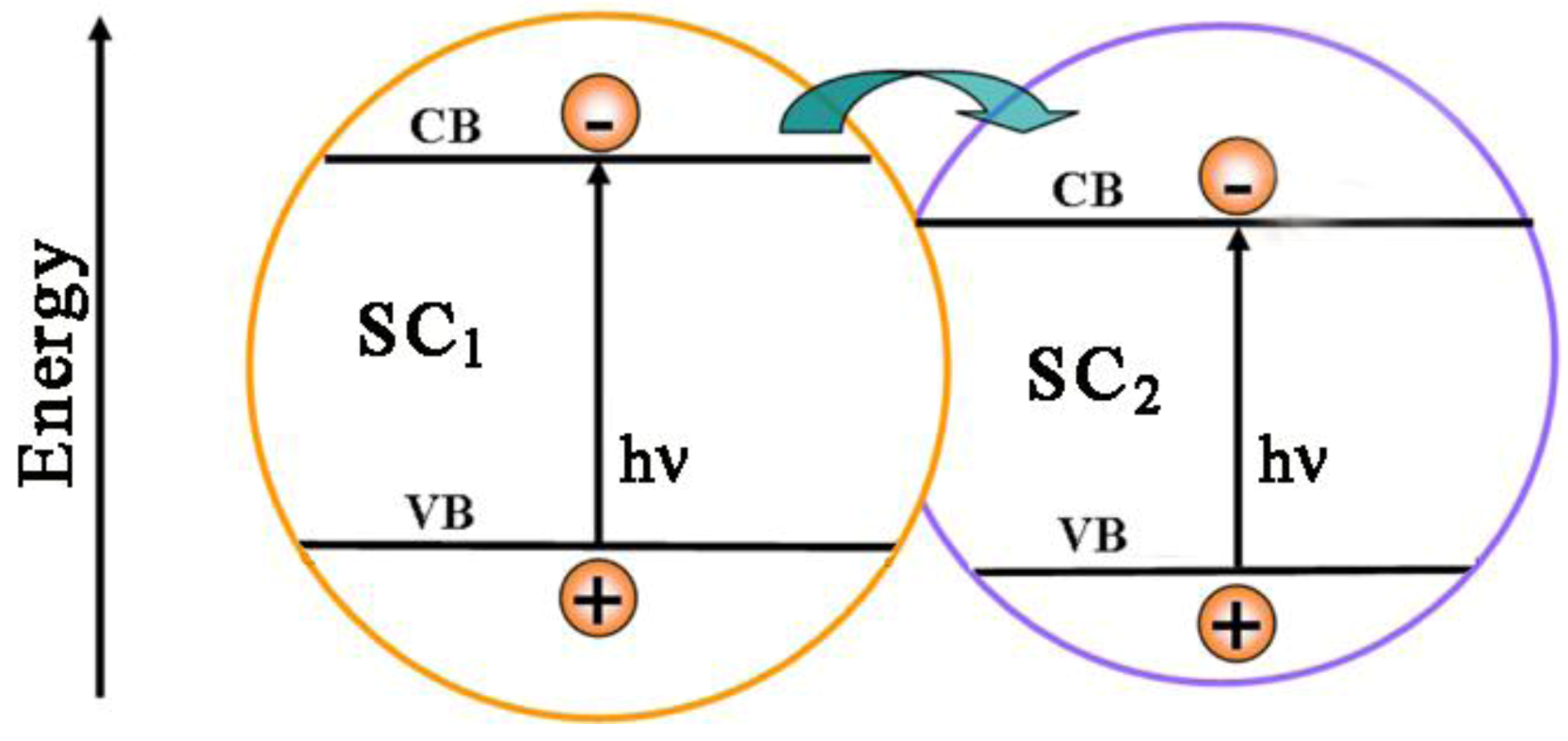
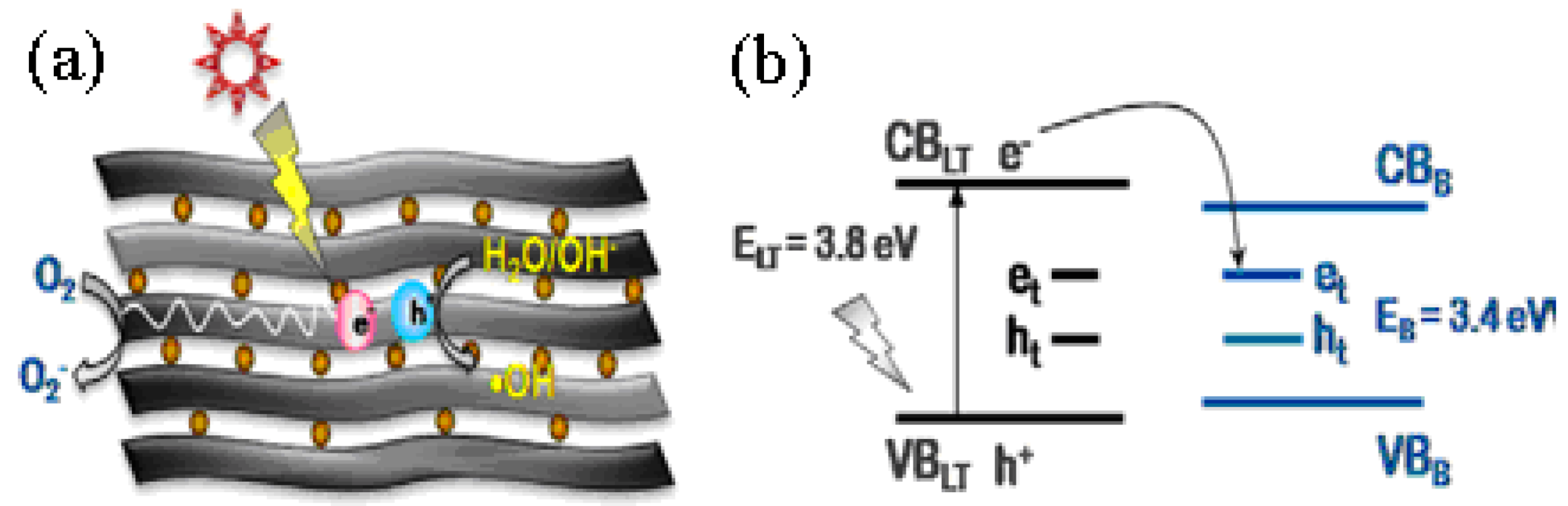
4.4. Doped and Loaded Mixtures of Brookite with Anatase and/or Rutile
5. Conclusions
References
- Pauling, L.; Sturdivant, J.H. The crystal structure of brookite. Z. Kristall. 1928, 68, 239–256. [Google Scholar]
- Bokhimi, X.; Morales, A.; Aguilar, M.; Toledo-Antonio, J.A.; Pedraza, F. Local order in titania polymorphs. Int. J. Hydrogen Energy 2001, 26, 1279–1287. [Google Scholar] [CrossRef]
- Li, J.-G.; Ishigaki, T.; Sun, X. Anatase, brookite, and rutile nanocrystals via redox reactions conditions: phase-selective synthesis and physicochemical properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar]
- Kuznetsova, I.N.; Blaskov, V.; Stambolova, I.; Znaidi, L.; Kanaev, A. TiO2 pure phase brookite with preferred orientation, synthesized as a spin-coated film. Mater. Lett. 2005, 59, 3820–3823. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Li, G.; Tang, C.; Sun, L. High-quality brookite TiO2 flowers: Synthesis, characterization, and dielectric performance. Cryst. Growth Des. 2009, 9, 3676–3682. [Google Scholar] [CrossRef]
- Bokhimi, X.; Pedraza, F. Characterization of brookite and a new corundum-like titania phase synthesized under hydrothermal conditions. J. Solid State Chem. 2004, 177, 2456–2463. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (Pbca, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates:Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar]
- Zhu, K.-R.; Zhang, M.-S.; Hong, J.-M.; Yin, Z. Size effect on phase transition sequence of TiO2 nanocrystal. Mater. Sci. Eng. A 2003, 403, 87–93. [Google Scholar]
- Grätzel, M.; Rotzinger, F.P. The influence of the crystal lattice structure on the conduction band energy of oxides of titanium(IV). Chem. Phys. Lett. 1985, 118, 474–477. [Google Scholar]
- Mo, S.D.; Ching, W.Y. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase and brookite. Phys. Rev. B 1995, 51, 13023–13032. [Google Scholar]
- Park, J.-Y.; Lee, C.; Jung, K.-W.; Jung, D. Structure related photocatalytic properties of TiO2. Bull. Korean Chem. Soc. 2009, 30, 402–404. [Google Scholar] [CrossRef]
- Landmann, M.; Rauls, E.; Schmidt, W.G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys. 2012, 24. [Google Scholar] [CrossRef]
- Kim, Y.I.; Atherton, S.J.; Brigham, E.S.; Mallouk, T.E. Sensitized layered metal oxide semiconductor particles for photochemical hydrogen evolution from nonsacrificial electron donors. J. Phys. Chem. 1993, 97, 11802–11810. [Google Scholar]
- tengl, V.; Králová, D. Photoactivity of brookite-rutile TiO2 nanocrystalline mixtures obtained by heat treatment of hydrothermally prepared brookite. Mater. Chem. Phys. 2011, 129, 794–801. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Xie, J.; Lü, X.; Liu, J.; Shu, H. Brookite titania photocatalytic nanomaterials: Synthesis, properties, and applications. Pure Appl. Chem. 2009, 81, 2407–2415. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Tian, B.; Anpo, M. Tartaric acid-assisted preparation and photocatalytic performance of titania nanoparticles with controllable phases of anatase and brookite. J. Mater. Sci. 2012, 47, 5743–5751. [Google Scholar] [CrossRef]
- Mattsson, A.; Österlund, L. Adsorption and photoinduced decomposition of acetone and acetic acid on anatase, brookite, and rutile TiO2 nanoparticles. J. Phys. Chem. C 2010, 114, 14121–14132. [Google Scholar] [CrossRef]
- Koelsch, M.; Cassaignon, S.; Guillemoles, J.F.; Jolivet, J.-P. Comparison of optical and electrochemical properties of anatase and brookite TiO2 synthesized by the sol-gel method. Thin Solid Films 2002, 403-404, 312–319. [Google Scholar] [CrossRef]
- Magne, C.; Dufour, F.; Labat, F.; Lancel, G.; Durupthy, O.; Cassaignon, S.; Pauporté, T. Effects of TiO2 nanoparticle polymorphism on dye-sensitized solar cell photovoltaic properties. J. Photochem. Photobiol. A 2012, 232, 22–31. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored titanium dioxide nanomaterials: Anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Di Paola, A.; Cufalo, G.; Addamo, M.; Bellardita, M.; Campostrini, R.; Ischia, M.; Ceccato, R.; Palmisano, L. Photocatalytic activity of nanocrystalline TiO2 (brookite, rutile and brookite-based) powders prepared by thermohydrolysis of TiCl4 in aqueous chloride solutions. Colloids Surf. A 2008, 317, 366–376. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Kim, E.J.; Hahn, S.H.; Kim, W.-J.; Shin, E.W. Synthesis of hierarchical rose bridal bouquet- and humming-top-like TiO2 nanostructures and their shape-dependent degradation efficiency of dye. J. Colloid Interface Sci. 2011, 356, 138–144. [Google Scholar] [CrossRef]
- Zallen, R.; Moret, M.P. The optical absorption edge of brookite TiO2. Solid State Commun. 2006, 137, 154–157. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Zhao, M.; Huang, X.; Chen, X.; Li, G.; Yu, R. Synthesis of high-quality brookite TiO2 single-crystalline nanosheets with specific facets exposed: Tuning catalysts from inert to highly reactive. J. Am. Chem. Soc. 2012, 134, 8328–8331. [Google Scholar]
- Shibata, T.; Irie, H.; Ohmori, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Comparison of photochemical properties of brookite and anatase TiO2 films. Phys. Chem. Chem. Phys. 2004, 6, 1359–1362. [Google Scholar]
- Dung, D.; Ramsden, J.; Grätzel, M. Dynamics of interfacial electron-transfer processes in colloidal semiconductor systems. J. Am. Chem. Soc. 1982, 104, 2977–2985. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Ceccato, R.; Palmisano, L.; Parrino, F. Highly active photocatalytic TiO2 powders obtained by thermohydrolysis of TiCl4 in water. J. Phys. Chem. C 2009, 113, 15166–15174. [Google Scholar]
- Roy, A.M.; De, G.C.; Sasmal, N.; Bhattacharyya, S.S. Determination of the flat band potential of semiconductor particles in suspension by photovoltage measurement. Int. J. Hydrogen Energy 1995, 20, 627–630. [Google Scholar] [CrossRef]
- Truong, Q.D.; Le, T.H.; Liu, J.-Y.; Chung, C.-C.; Ling, Y.-C. Synthesis of TiO2 nanoparticles using novel titanium oxalate complex towards visible light-driven photocatalytic reduction of CO2 to CH3OH. Appl. Catal. A 2012, 437-438, 28–35. [Google Scholar] [CrossRef]
- Glemser, O.; Schwarzmann, E. Zur Polymorphie des Titandioxyds. Angew. Chem. 1956, 68, 791. [Google Scholar]
- Knoll, H.; Kühnhold, U. Über die Stabilität des Anatas. Naturwiss 1957, 44, 394. [Google Scholar]
- Knoll, H. Zur Bildung von Brookit. Naturwiss 1961, 48, 601. [Google Scholar] [CrossRef]
- Yamaguchi, S. Brookite film on titanium. J. Electrochem. Soc. 1961, 108, 302. [Google Scholar] [CrossRef]
- Knoll, H. Umwandlung von Anatas in Brookit. Naturwiss 1963, 50, 546. [Google Scholar] [CrossRef]
- Knoll, H. Darstellung von Brookit. Angew. Chem. 1964, 76, 592. [Google Scholar] [CrossRef]
- Keesmann, I. Zur hydrothermalen Synthese von Brookit. Z. Anorg. Allg. Chem. 1966, 346, 30–43. [Google Scholar] [CrossRef]
- Schwarzmann, E.; Ognibeni, K.H. Hydrothermal synthesis of brookite TiO2. Z. Naturforsch. B 1974, 29, 435. [Google Scholar]
- Kiyama, M.; Akita, T.; Tsutsumi, Y.; Takada, T. Formation of titanic oxides of anatase, brookite and rutile types by aerial oxidation of titanous solutions. Chem. Lett. 1972, 21–24. [Google Scholar]
- Ohtani, B.; Handa, J.-I.; Nishimoto, S.I.; Kagiya, T. Highly active semiconductor photocatalyst: Extra-fine crystallite of brookite TiO2 for redox reaction in aqueous propan-2-ol and/or silver sulfate solution. Chem. Phys. Lett. 1985, 120, 292–294. [Google Scholar] [CrossRef]
- Oota, T.; Yamai, I.; Saito, H. Brookite formation by the oxidation of titanium metal under hydrothermal conditions. J. Ceram. Soc. Jpn. 1979, 87, 375–382. (in Japanese). [Google Scholar]
- Oota, T.; Yamai, I.; Saito, H. Hydrothermal syntesis of brookite from various titanium compounds and its formation mechanism. J. Ceram. Soc. Jpn. 1979, 87, 512–519. (in Japanese). [Google Scholar]
- Mitsuhashi, T.; Watanabe, M. Brookite formation from precipitates containing calcium ions. Mineral J. 1978, 9, 236–240. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol-gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Nagase, T.; Ebina, T.; Iwasaki, T.; Hayashi, H.; Onodera, Y.; Chatterjee, M. Hydrothermal synthesis of brookite. Chem. Lett. 1999, 28, 911–912. [Google Scholar]
- Kominami, H.; Kohno, M.; Kera, Y. Synthesis of brookite-type titanium oxide nano-crystals in organic media. J. Mater. Chem. 2000, 10, 1151–1156. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, E.; Cui, S.; Li, W.; Hu, X. Hydrothermal preparation of nanosized brookite powders. J. Am. Ceram. Soc. 2000, 83, 2634–2636. [Google Scholar]
- Zheng, Y.; Shi, E.; Cui, S.; Li, W.; Hu, X. Hydrothermal preparation and characterization of brookite-type TiO2 nanocrystallites. J. Mater. Sci. Lett. 2000, 19, 1445–1448. [Google Scholar] [CrossRef]
- Pottier, A.; Chanéac, C.; Tronc, E.; Mazerolles, L.; Jolivet, J.-P. Synthesis of brookite TiO2 nanoparticles by thermolysis of TiCl4 in strongly acidic aqueous media. J. Mater. Chem. 2001, 11, 1116–1121. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. TiO2 photocatalysts prepared by thermohydrolysis of TiCl4 in aqueous solutions. Stud. Surf. Sci. Catal. 2010, 175, 225–228. [Google Scholar]
- Lee, B.I.; Wang, X.; Bhave, R.; Hu, M. Synthesis of brookite TiO2 nanoparticles by ambient condition sol process. Mater. Lett. 2006, 60, 1179–1183. [Google Scholar] [CrossRef]
- Bhave, R.C.; Lee, B.I. Experimental variables in the synthesis of brookite phase TiO2 nanoparticles. Mater. Sci. Eng. A 2007, 467, 146–149. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, Y.S. Synthesis of TiO2 nanoparticles with pure brookite at low temperature by hydrolysis of TiCl4 using HNO3 solution. J. Mater. Sci. 2006, 41, 557–559. [Google Scholar] [CrossRef]
- Cassaignon, S.; Koelsch, M.; Jolivet, J.-P. Selective synthesis of brookite, anatase and rutile nanoparticles: thermolysis of TiCl4 in aqueous nitric acid. J. Mater. Sci. 2007, 42, 6689–6695. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Kipp, T.; Cingolani, R.; Cozzoli, P.D. Nonhydrolytic synthesis of high-quality anisotropically shaped brookite TiO2 nanocrystals. J. Am. Chem. Soc. 2008, 130, 11223–11233. [Google Scholar]
- Koelsch, M.; Cassaignon, S.; Ta Thanh Minh, C.; Guillemoles, J.-F.; Jolivet, J.-P. Electrochemical comparative study of titania (anatase, brookite and rutile) nanoparticles synthesized in aqueous medium. Thin Solid Films 2004, 451-452, 86–92. [Google Scholar] [CrossRef]
- Yang, S.-F.; Luo, W.; Zhu, Y.-C.; Liu, Y.-H.; Zhao, J.-Z.; Wang, Z.-C.; Zou, G.-T. Preparation of brookite TiO2 micro-crystals with single phase. Chem. J. Chin. Univ. 2003, 24, 1933–1936. (in Chinese). [Google Scholar]
- Zhang, J.; Yan, S.; Fu, L.; Wang, F.; Yuan, M.; Luo, G.; Xu, Q.; Wang, X.; Li, C. Photocatalytic degradation of rhodamine B on anatase, rutile, and brookite TiO2. Chin. J. Catal. 2011, 32, 983–991. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, J.; Feng, Z.; Ma, Y.; Wang, X.; Li, C. Surface structural transformation and the phase transition kinetics of brookite TiO2. Chem. Asian J. 2010, 5, 2158–2161. [Google Scholar] [CrossRef]
- Luo, W.; Yang, S.F.; Wang, Z.C.; Wang, Y.; Ahuja, R.; Johansson, B.; Liu, J.; Zou, G.T. Structural phase transitions in brookite-type TiO2 under high pressure. Solid State Commun. 2005, 133, 49–53. [Google Scholar] [CrossRef]
- Dambournet, D.; Belharouak, I.; Amine, K. Tailored preparation methods of TiO2 anatase, rutile, brookite: Mechanism of formation and electrochemical properties. Chem. Mater. 2010, 22, 1173–1179. [Google Scholar] [CrossRef]
- Dambournet, D.; Belharouak, I.; Ma, J.; Amine, K. Toward high surface area TiO2 brookite with morphology control. J. Mater. Chem. 2011, 21, 3085–3090. [Google Scholar]
- Li, J.-G.; Tang, C.; Li, D.; Haneda, H.; Ishigaki, T. Monodispersed spherical particles of brookite-type TiO2: Synthesis, characterization, and photocatalytic property. J. Am. Ceram. Soc. 2004, 87, 1358–1361. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Štengl, V.; Szatmary, L.; Subrt, J.; Lukac, J.; Murafa, N.; Niznansky, D.; Cizek, K.; Jirkovskyc, J.; Petrova, N. Transformation of brookite-type TiO2 nanocrystals to rutile: Correlation between microstructure and photoactivity. J. Mater. Chem. 2006, 16, 1709–1716. [Google Scholar]
- Štengl, V.; Bakardjieva, S.; Murafa, N.; Šubrt, J.; Měšťánková, H.; Jirkovský, J. Preparation, characterization and photocatalytic activity of optically transparent titanium dioxide particles. Mater. Chem. Phys. 2007, 105, 38–46. [Google Scholar]
- Shen, X.; Tian, B.; Zhang, J. Tailored preparation of titania with controllable phases of anatase and brookite by an alkalescent hydrothermal route. Catal. Today 2013, 201, 151–158. [Google Scholar]
- Inada, M.; Iwamoto, K.; Enomoto, N.; Hojo, J. Synthesis and photocatalytic activity of small brookite particles by self-hydrolysis of TiOCl2. J. Ceram. Soc. Jpn. 2011, 119, 451–455. [Google Scholar] [CrossRef]
- Liu, C.-E.; Rouet, A.; Sutrisno, H.; Puzenat, E.; Terrisse, H.; Brohan, L.; Richard-Plouet, M. Low temperature synthesis of nanocrystallized titanium oxides with layered or tridimensional frameworks, from [Ti8O12(H2O)24]Cl8·HCl·7H2O hydrolysis. Chem. Mater. 2008, 20, 4739–4748. [Google Scholar] [CrossRef]
- Deng, Q.; Wei, M.; Ding, X.; Jiang, L.; Ye, B.; Wei, K. Brookite-type TiO2 nanotubes. Chem. Commun. 2008. [Google Scholar] [CrossRef]
- Deng, Q.; Wei, M.; Hong, Z.; Ding, X.; Jiang, L.; Wei, K. Selective synthesis of rutile, anatase and brookite nanorods by a hydrothermal route. Curr. Nanosci. 2010, 6, 479–482. [Google Scholar] [CrossRef]
- Murakami, N.; Kamai, T.; Tsubota, T.; Ohno, T. Novel hydrothermal preparation of pure brookite-type titanium(IV) oxide nanocrystal under strong acidic conditions. Catal. Commun. 2009, 10, 963–966. [Google Scholar] [CrossRef]
- Oskam, G.; de Jesús Peet Poot, F. Synthesis of ZnO and TiO2 nanoparticles. J. Sol-Gel Sci. Techn. 2006, 37, 157–160. [Google Scholar] [CrossRef]
- Srivatsa, K.M.K.; Bera, M.; Basu, A. Pure brookite titania crystals with large surface area deposited by plasma enhanced chemical vapour deposition technique. Thin Solid Films 2008, 516, 7443–7446. [Google Scholar]
- Hall, S.R.; Swinerd, V.M.; Newby, F.N.; Collins, A.M.; Mann, S. Fabrication of porous titania (brookite) microparticles with complex morphology by sol-gel replication of pollen grains. Chem. Mater. 2006, 18, 598–600. [Google Scholar]
- Shi, J.; Shang, S.; Yang, L.; Yan, J. Morphology and crystalline phase-controllable synthesis of TiO2 and their morphology-dependent photocatalytic properties. J. Alloys Compd. 2009, 479, 436–439. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, F.; Huang, Q.; Zhang, J. Brookite TiO2 nanoflowers. Chem. Commun. 2009, 5115–5117. [Google Scholar]
- Zhao, B.; Chen, F.; Jiao, Y.; Zhang, J. Phase transition and morphological evolution of titania/titanate nanomaterials under alkalescent hydrothermal treatment. J. Mater. Chem. 2010, 20, 7990–7997. [Google Scholar]
- Jiao, Y.; Chen, F.; Zhao, B.; Yang, H.; Zhang, J. Anatase grain loaded brookite nanoflower hybrid with superior photocatalytic activity for organic degradation. Colloids Surf. A 2012, 402, 66–71. [Google Scholar] [CrossRef]
- Arıer, Ü.Ö.A.; Tepehan, F.Z. Controlling the particle size of nanobrookite TiO2 thin films. J. Alloys Compd. 2011, 509, 8262–8267. [Google Scholar] [CrossRef]
- Zhang, L.; Menendez-Flores, V.M.; Murakami, N.; Ohno, T. Improvement of photocatalytic activity of brookite titanium dioxide nanorods by surface modification using chemical etching. Appl. Surf. Sci. 2012, 238, 5803–5809. [Google Scholar]
- Li, J.-G.; Ishigaki, T. Brookite→rutile phase transformation of TiO2 studied with monodispersed particles. Acta Mater. 2004, 52, 5143–5150. [Google Scholar] [CrossRef]
- Kakihana, M.; Kobayashi, M.; Tomita, K.; Petrykin, V. Application of water-soluble titanium complexes as precursors for synthesis of titanium-containing oxides via aqueous solution processes. Bull. Chem. Soc. Jpn. 2010, 83, 1285–1308. [Google Scholar] [CrossRef]
- Tomita, K.; Petrykin, V.; Kobayashi, M.; Shiro, M.; Yoshimura, M.; Kakihana, M. A water-soluble titanium complex for the selective synthesis of nanocrystalline brookite, rutile, and anatase by a hydrothermal method. Angew. Chem. Int. Ed. 2006, 45, 2378–2381. [Google Scholar]
- Kobayashi, M.; Tomita, K.; Petrykin, V.; Yoshimura, M.; Kakihana, M. Direct synthesis of brookite-type titanium oxide by hydrothermal method using water-soluble titanium complexes. J. Mater. Sci. 2008, 43, 2158–2162. [Google Scholar] [CrossRef]
- Morishima, Y.; Kobayashi, M.; Petrykin, V.; Kakihana, M.; Tomita, K. Microwave-assisted hydrothermal synthesis of brookite nanoparticles from a water-soluble titanium complex and their photocatalytic activity. J. Ceram. Soc. Jpn. 2007, 115, 826–830. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomita, K.; Petrykin, V.; Yin, S.; Sato, S.; Yoshimura, M.; Kakihana, M. Hydrothermal synthesis of nanosized titania photocatalysts using novel water-soluble titanium complexes. Solid State Phenom. 2007, 124-126, 723–726. [Google Scholar] [CrossRef]
- Ismail, A.A.; Kandiel, T.A.; Bahnemann, D.W. Novel (and better?) titania-based photocatalysts: Brookite nanorods and mesoporous structures. J. Photochem. Photobiol. A 2010, 216, 183–193. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kobayashi, M.; Kato, H.; Kakihana, M. Hydrothermal synthesis of hierarchical TiO2 microspheres using a novel titanium complex coordinated by picolinic acid. J. Ceram. Soc. Jpn. 2011, 119, 513–516. [Google Scholar] [CrossRef]
- Morishima, Y.; Kobayashi, M.; Petrykin, V.; Yin, S.; Sato, T.; Kakihana, M.; Tomita, K. Hydrothermal synthesis of brookite type TiO2 photocatalysts using a water-soluble Ti-complex coordinated by ethilenediaminetetraacetic. J. Ceram. Soc. Jpn. 2009, 117, 320–325. [Google Scholar] [CrossRef]
- Ozawa, T.C.; Sasaki, T. An alkali-metal ion extracted layered compound as a template for a metastable phase synthesis in a low-temperature solid-state reaction: preparation of brookite from K0.8Ti1.73Li0.27O4. Inorg. Chem. 2010, 49, 3044–3050. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Fujiwara, T.; Ishihara, K.N.; Shingu, P.H. Formation of brookite-type TiO2 titania by mechanical alloying. J. Jpn. Soc. Powder Powder Metall. 2001, 48, 950–954. [Google Scholar] [CrossRef]
- Perego, C.; Wang, Y.-H.; Durupthy, O.; Cassaignon, S.; Revel, R.; Jolivet, J.-P. Nanocrystalline brookite with enhanced stability and photocatalytic activity: influence of lanthanum(III) doping. ACS Appl. Mater. Interfaces 2012, 4, 752–760. [Google Scholar]
- Ohno, Y.; Tomita, K.; Komatsubara, Y.; Taniguchi, T.; Katsumata, K.; Matsushita, N.; Kogure, T.; Okada, K. Pseudo-cube shaped brookite (TiO2) nanocrystals synthesized by an oleate-modified hydrothermal growth method. Cryst. Growth Des. 2011, 11, 4831–4836. [Google Scholar] [CrossRef]
- Iskandar, F.; Nandiyanto, A.B.D.; Yun, K.M.; Hogan, C.J., Jr.; Okuyama, K.; Biswas, P. Enhanced photocatalytic performance of brookite TiO2 macroporous particles prepared by spray drying with colloidal templating. Adv. Mater. 2007, 19, 1408–1412. [Google Scholar] [CrossRef]
- Katagiri, K.; Inami, H.; Koumoto, K.; Inumaru, K.; Tomita, K.; Kobayashi, M.; Kakihana, M. Preparation of hollow TiO2 spheres of the desired polymorphs by layer-by-layer assembly of a water-soluble titanium complex and hydrothermal treatment. Eur. J. Inorg. Chem. 2012, 2012, 3267–3272. [Google Scholar]
- Takahashi, Y.; Wakayama, S.; Ogiso, A.; Sugiyama, K. Selective deposition of anatase, rutile and brookite films by vapour phase decomposition of alkyl titanates. Kinzoko Hyomen Gijutsu 1984, 35, 584–589. (in Japanese). [Google Scholar]
- Arsov, L.D.; Kormann, C.; Plieth, W. in situ Raman spectra of anodically formed titanium dioxide layers in solutions of H2SO4, KOH, and HNO3. J. Electrochem. Soc. 1991, 138, 2964–2970. [Google Scholar] [CrossRef]
- Arsov, L.D.; Kormann, C.; Plieth, W. Electrochemical synthesis and in situ Raman spectroscopy of thin films of titanium dioxide. J. Raman Spectrosc. 1991, 22, 573–575. [Google Scholar] [CrossRef]
- Moret, M.P.; Zallen, R.; Vijay, D.P.; Desu, S.B. Brookite-rich titania films made by pulsed laser deposition. Thin Solid Films 2000, 366, 8–10. [Google Scholar] [CrossRef]
- Djaoued, Y.; Brüning, R.; Bersani, D.; Lottici, P.P.; Badilescu, S. Sol-gel nanocrystalline brookite-rich titania films. Mater. Lett. 2004, 58, 2618–2622. [Google Scholar] [CrossRef]
- Jiang, K.-J.; Kitamura, T.; Yin, H.; Ito, S.; Yanagida, S. Dye-sensitized solar cells using brookite nanoparticle TiO2 films as electrodes. Chem. Lett. 2002, 31, 872–873. [Google Scholar]
- Ohara, C.; Hongo, T.; Yamazaki, A.; Nagoya, T. Synthesis and characterization of brookite/anatase complex thin film. Appl. Surf. Sci. 2008, 254, 6619–6622. [Google Scholar] [CrossRef]
- Pan, H.; Qiu, X.; Ivanov, I.N.; Meyer, H.M.; Wang, W.; Zhu, W.; Paranthaman, M.P.; Zhang, Z.; Eres, G.; Gu, B. Fabrication and characterization of brookite-rich, visible light-active TiO2 films for water splitting. Appl. Catal. B 2009, 93, 90–95. [Google Scholar] [CrossRef]
- Bach, H.; Schroeder, H. Kristallstruktur und optische eigenschaften von dünnen organogenen titanoxyd-schichten auf glasunterlagen. Thin Solid Films 1968, 1, 255–276. [Google Scholar] [CrossRef]
- López, A.; Acosta, D.; Martínez, A.I.; Santiago, J. Nanostructured low crystallized titanium dioxide thin films with good photocatalytic activity. Powder Technol. 2010, 202, 111–117. [Google Scholar] [CrossRef]
- Novotna, P.; Krysa, J.; Maixner, J.; Kluson, P.; Novak, P. Photocatalytic activity of sol-gel TiO2 thin films deposited on soda lime glass and soda lime glass precoated with a SiO2 layer. Surf. Coat. Technol. 2010, 204, 2570–2575. [Google Scholar] [CrossRef]
- Krýsa, J.; Novotná, P.; Kment, Š.; Mills, A. Effect of glass substrate and deposition technique on the properties of sol gel TiO2 thin films. J. Photochem. Photobiol. A 2011, 222, 81–86. [Google Scholar] [CrossRef]
- Addamo, M.; Bellardita, M.; Di Paola, A.; Palmisano, L. Preparation and photoactivity of nanostructured anatase, rutile and brookite TiO2 thin films. Chem. Commun. 2006. [Google Scholar] [CrossRef]
- Di Paola, A.; Addamo, M.; Bellardita, M.; Cazzanelli, E.; Palmisano, L. Preparation of photocatalytic brookite thin films. Thin Solid Films 2007, 515, 3527–3529. [Google Scholar]
- Di Paola, A.; Addamo, M.; Bellardita, M.; García-López, E.; Marcì, G.; Palmisano, L. Preparation of photocatalytic nanostructured TiO2 thin films. Mater. Sci. Forum 2008, 587-588, 795–799. [Google Scholar] [CrossRef]
- Magne, C.; Cassaignon, S.; Lancel, G.; Pauporté, T. Brookite TiO2 nanoparticle films for dye-sensitized solar cells. ChemPhysChem 2011, 12, 2461–2467. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, K.; Kim, J.H.; Lee, N.-H.; Kim, S.-J. Preparation of brookite phase TiO2 colloidal sol for thin film coating. Mater. Lett. 2006, 60, 364–367. [Google Scholar] [CrossRef]
- Caricato, A.P.; Buonsanti, R.; Catalano, M.; Cesaria, M.; Cozzoli, P.D.; Luches, A.; Manera, M.G.; Martino, M.; Taurino, A.; Rella, R. Films of brookite TiO2 nanorods/nanoparticles deposited by matrix-assisted pulsed laser evaporation as NO2 gas-sensing layers. Appl. Phys. A 2011, 104, 963–968. [Google Scholar] [CrossRef]
- Manera, M.G.; Taurino, A.; Catalano, M.; Rella, R.; Caricato, A.P.; Buonsanti, R.; Cozzoli, P.D.; Martino, M. Enhancement of the optically activated NO2 gas sensing response of brookite TiO2 nanorods/nanoparticles thin films deposited by matrix-assisted pulsed-laser evaporation. Sens. Actuators B 2012, 161, 869–879. [Google Scholar]
- Li, W.-K.; Gong, X.-Q.; Lu, G.; Selloni, A. Different reactivities of TiO2 polymorphs: comparative DFT calculations of water and formic acid adsorption at anatase and brookite TiO2 surfaces. J. Phys. Chem. C 2008, 112, 6594–6596. [Google Scholar]
- Kominami, H.; Ishii, Y.; Kohno, M.; Konishi, S.; Kera, Y.; Ohtani, B. Nanocrystalline brookite-type titanium(IV) oxide photocatalysts prepared by a solvothermal method: correlation between their physical properties and photocatalytic activities. Catal. Lett. 2003, 91, 41–47. [Google Scholar] [CrossRef]
- Kominami, H.; Kato, J.; Murakami, S.; Ishii, Y.; Kohno, M.; Yabutani, K.; Yamamoto, T.; Kera, Y.; Inoue, M.; Inui, T.; Ohtani, B. Solvothermal syntheses of semiconductor photocatalysts of ultra-high activities. Catal. Today 2003, 84, 181–189. [Google Scholar]
- Zhou, L.-J.; Yan, S.-S.; Tian, B.-Z.; Chen, F.; Zhang, J.-L.; Huang, J.-Z.; Zhang, L.-Z. Preparation and characterization of anatase-brookite TiO2 film on the PET surface. Acta Phys. Chim. Sin. 2006, 22, 569–573. (in Chinese). [Google Scholar]
- Bellardita, M.; Di Paola, A.; Palmisano, L.; Parrino, F.; Buscarino, G.; Amadelli, R. Preparation and photoactivity of samarium loaded anatase, brookite and rutile catalysts. Appl. Catal. B 2011, 104, 291–299. [Google Scholar] [CrossRef]
- Yin, S.; Ihara, K.; Liu, B.; Wang, Y.; Li, R.; Sato, T. Preparation of anatase, rutile and brookite type anion doped titania photocatalyst nanoparticles and thin films. Phys. Scr. 2007, T129, 268–273. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Di Paola, A.; Palmisano, L.; Selli, E. Effect of titanium dioxide crystalline structure on the photocatalytic production of hydrogen. Photochem. Photobiol. Sci. 2011, 10, 355–360. [Google Scholar] [CrossRef]
- Lee, B.I.; Kaewgun, S.; Kim, W.; Choi, W.; Lee, J.S.; Kim, E. Visible light photocatalytic properties of polymorphic brookite titania. J. Renew. Sustain. Energy 2009, 1, 023101. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Satya Kishore, M.; Pralong, V.; Varadaradju, U.V.; Raveau, B. Lithium intercalation into nanocrystalline brookite TiO2. Electrochem. Solid-State Lett. 2007, 10, A29–A31. [Google Scholar]
- Anji Reddy, M.; Pralong, V.; Varadaradju, U.V.; Raveau, B. Crystallite size constraints on lithium insertion into brookite TiO2. Electrochem. Solid State Lett. 2008, 11, A132–A134. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, J.-G.; Choi, K.J.; Choi, H.-J.; Kim, D.-W. Preparation of brookite-type TiO2/carbon nanocomposite electrodes for application to Li ion batteries. Eur. J. Inorg. Chem. 2008, 2008, 878–882. [Google Scholar]
- Lee, D.-H.; Kim, D.-W.; Park, J.-G. Enhanced rate capabilities of nanobrookite with electronically conducting MWCNT networks. Cryst. Growth Des. 2008, 8, 4506–4510. [Google Scholar] [CrossRef]
- Ohtani, B.; Kawaguchi, J.; Kozawa, M.; Nakaoka, Y.; Nosaka, Y.; Nishimoto, S. Effect of platinum loading on the photocatalytic activity of cadmium(II) sulfide particles suspended in aqueous amino acid solutions. J. Photochem. Photobiol. A 1995, 90, 75–80. [Google Scholar] [CrossRef]
- Maldotti, A.; Amadelli, R.; Samiolo, L.; Molinari, A.; Penoni, A.; Tollari, S.; Cenini, S. Photocatalytic formation of a carbamate through ethanol-assisted carbonylation of p-nitrotoluene. Chem. Commun. 2005, 1749–1751. [Google Scholar]
- Addamo, M.; Augugliaro, V.; Bellardita, M.; Di Paola, A.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Environmentally friendly photocatalytic oxidation of aromatic alcohol to aldehyde in aqueous suspension of brookite TiO2. Catal. Lett. 2008, 126, 58–62. [Google Scholar] [CrossRef]
- Augugliaro, V.; Loddo, V.; López-Muñoz, M.J.; Márquez-Álvarez, C.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Home-prepared anatase, rutile, and brookite TiO2 for selective photocatalytic oxidation of 4-methoxybenzyl alcohol in water: reactivity and ATR-FTIR study. Photochem. Photobiol. Sci. 2009, 8, 663–669. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Bellardita, M.; Di Paola, A.; García López, E.; Loddo, V.; Marcì, G.; Palmisano, G.; Yurdakal, S. Titania photocatalysts for selective oxidations in water. ChemSusChem 2011, 4, 1431–1438. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Aita, Y.; Komatsu, M.; Yin, S.; Sato, T. Phase-compositional control and visible light photocatalytic activity of nitrogen-doped titania via solvothermal process. J. Solid State Chem. 2004, 177, 3235–3238. [Google Scholar]
- Yin, S.; Aita, Y.; Komatsu, M.; Wang, J.; Tang, Q.; Sato, T. Synthesis of excellent visible-light responsive TiO2−xNy photocatalyst by a homogeneous precipitation-solvothermal process. J. Mater. Chem. 2005, 15, 674–682. [Google Scholar]
- Sato, T.; Aita, Y.; Komatsu, M.; Yin, S. Solvothermal synthesis of visible light responsive nitrogen-doped titania nanocrystals. J. Mater. Sci. 2006, 41, 1433–1438. [Google Scholar]
- Bonsu, P.O.; Lü, X.; Xie, J.; Jiang, D.; Chen, M.; Wei, X. Photoenhanced degradation of rhodamine blue on monometallic gold (Au) loaded brookite titania photocatalysts activated by visible light. React. Kinet. Mech. Catal. 2012, 107, 487–502. [Google Scholar] [CrossRef]
- Yan, W.; Chen, B.; Mahurin, S.M.; Dai, S.; Overbury, S.H. Brookite-supported highly stable gold catalytic system for CO oxidation. Chem. Commun. 2004. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Cohen, H.; Willer, I.; Bossmann, S.; Braun, A.M. Lanthanide oxide-doped titanium dioxide: effective photocatalysts for the degradation of organic pollutants. J. Mater. Sci. 1999, 34, 5273–5280. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Willer, I.; Bossmann, S.; Braun, A.M. Lanthanide oxide-doped titanium dioxide photocatalysts: Novel photocatalysts for the enhanced degradation of p-chlorophenoxyacetic acid. Environ. Sci. Technol. 2001, 35, 1544–1549. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Palmisano, L.; Parrino, F.; Buscarino, G.; Amadelli, R. Preparation of Sm-loaded brookite TiO2 photocatalysts. Catal. Today 2011, 161, 35–40. [Google Scholar]
- Luca, D.; Mardare, D.; Iacomi, F.; Teodorescu, C.M. Increasing surface hydrophilicity of titania films by doping. Appl. Surf. Sci. 2006, 252, 6122–6126. [Google Scholar] [CrossRef]
- Eshaghi, A.; Eshaghi, A. Optical and hydrophilic properties of nanostructure Cu loaded brookite TiO2 thin film. Thin Solid Films 2011, 520, 1053–1056. [Google Scholar] [CrossRef]
- Lü, X.; Liu, J.; Zhang, H.; Ding, J.; Xie, J. Structure and property of mesoporous molybdenum/carbon co-doped brookite titania. Trans. Nonferrous Met. Soc. China 2009, 19, 669–673. [Google Scholar] [CrossRef]
- Hotchandani, S.; Kamat, P.V. Charge-transfer processes in coupled semiconductor systems. Photochemistry and photoelectrochemistry of the colloidal CdS-ZnO system. J. Phys. Chem. 1992, 96, 6834–6839. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Di Paola, A.; Palmisano, L.; Derrigo, M.; Augugliaro, V. Preparation and characterization of tungsten chalcogenide photocatalysts. J. Phys. Chem. B 1997, 101, 876–883. [Google Scholar] [CrossRef]
- Ovenstone, J. Preparation of novel titania photocatalysts with high activity. J. Mater. Sci. 2001, 36, 1325–1329. [Google Scholar] [CrossRef]
- Li, Y.; Lee, N.-H.; Hwang, D.-S.; Song, J.S.; Lee, E.G.; Kim, S.-J. Synthesis and characterization of nano titania powder with high photoactivity for gas-phase photo-oxidation of benzene from TiOCl2 aqueous solution at low temperatures. Langmuir 2004, 20, 10838–10844. [Google Scholar]
- Li, Y.; Lee, N.-H.; Song, J.S.; Lee, E.G.; Kim, S.-J. Synthesis and photocatalytic properties of nano bi-crystalline titania of anatase and brookite by hydrolyzing TiOCl2 aqueous solution at low temperatures. Res. Chem. Intermed. 2005, 31, 309–318. [Google Scholar]
- Yu, J.C.; Yu, J.; Ho, W.; Zhang, L. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem. Commun. 2001. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.C.; Leung, M.K.-P.; Ho, W.; Cheng, B.; Zhao, X.; Zhao, J. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J. Catal. 2003, 217, 69–78. [Google Scholar]
- Yu, J.C.; Yu, J.; Zhang, L.; Ho, W. Enhancing effects of water content and ultrasonic irradiation on the photocatalytic activity of nano-sized TiO2 powders. J. Photochem. Photobiol. A 2002, 148, 263–271. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Yu, J. Direct sonochemical preparation and characterization of highly active mesoporous TiO2 with a bicrystalline framework. Chem. Mater. 2002, 14, 4647–4653. [Google Scholar] [CrossRef]
- Ozawa, T.; Iwasaki, M.; Tada, H.; Akita, T.; Tanaka, K.; Ito, S. Low-temperature synthesis of anatase-brookite composite nanocrystals: The junction effect on photocatalytic activity. J. Colloid Interface Sci. 2005, 281, 510–513. [Google Scholar]
- Perera, S.; Gillan, E.G. A facile solvothermal route to photocatalytically active nanocrystalline anatase TiO2 from peroxide precursors. Solid State Sci. 2008, 10, 864–872. [Google Scholar] [CrossRef]
- Tian, G.; Fu, H.; Jing, L.; Xin, B.; Pan, K. Preparation and characterization of stable biphase TiO2 photocatalyst with high crystallinity, large surface area, and enhanced photoactivity. J. Phys. Chem. C 2008, 112, 3083–3089. [Google Scholar] [CrossRef]
- Dufour, F.; Cassaignon, S.; Durupthy, O.; Colbeau-Justin, C.; Chanéac, C. Do TiO2 nanoparticles really taste better when cooked in a microwave oven? Eur. J. Inorg. Chem. 2012, 2012, 2707–2715. [Google Scholar]
- Lee, S.C.; Lee, H.U.; Lee, S.M.; Lee, G.; Hong, W.G.; Lee, J.; Kim, H.J. Preparation and characterization of bicrystalline TiO2 photocatalysts with high crystallinity and large surface area. Mater. Lett. 2012, 79, 191–194. [Google Scholar]
- Boppella, R.; Basak, P.; Manorama, S.V. Viable method for the synthesis of biphasic TiO2 nanocrystals with tunable phase composition and enabled visible-light photocatalytic performance. ACS Appl. Mater. Interfaces 2012, 4, 1239–1246. [Google Scholar] [CrossRef]
- Carrera, R.; Vázquez, A.L.; Castillo, S.; Arce, E. Photocatalytic degradation of acetaldehyde by sol-gel TiO2 nanoparticles: effect of the physicochemical properties on the photocatalytic activity. Mater. Sci. Forum 2011, 691, 92–98. [Google Scholar] [CrossRef]
- Yu, J.; Su, Y.; Cheng, B.; Zhou, M. Effects of pH on the microstructures and photocatalytic activity of mesoporous nanocrystalline titania powders prepared via hydrothermal method. J. Mol. Catal. A 2006, 258, 104–112. [Google Scholar] [CrossRef]
- Liu, A.R.; Wang, S.M.; Zhao, Y.R.; Zheng, Z. Low-temperature preparation of nanocrystalline TiO2 photocatalyst with a very large specific surface area. Mater. Chem. Phys. 2006, 99, 131–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Zeng, Q.; Zhi, J. An approach for controllable synthesis of different-phase titanium dioxide nanocomposites with peroxotitanium complex as precursor. J. Phys. Chem. C 2008, 112, 16457–16462. [Google Scholar] [CrossRef]
- Qin, W.; Liu, J.-J.; Zuo, S.-L.; Yu, Y.-C.; Hao, Z.-P. Solvothermal synthesis of nanosized TiO2 particles with different crystal structures and their photocatalytic activities. J. Inorg. Mater. 2007, 22, 931–936. (In Chinese) [Google Scholar]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Gialanella, S.; Pirola, C.; Ragaini, V. Tailored anatase/brookite nanocrystalline TiO2. The optimal particle features for liquid and gas-phase photocatalytic reactions. J. Phys. Chem. C 2007, 111, 13222–13231. [Google Scholar]
- Cappelletti, G.; Bianchi, C.L.; Ardizzone, S. Nano-titania assisted photoreduction of Cr(VI). The role of the different TiO2 polymorphs. Appl. Catal. B 2008, 78, 193–201. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Naldoni, A.; Pirola, C. Photocatalytic degradation of toluene in the gas phase: Relationship between surface species and catalyst features. Environ. Sci. Technol. 2008, 42, 6671–6676. [Google Scholar]
- Hao, H.; Zhang, J. Low temperature synthesis of crystalline mesoporous titania with high photocatalytic activity by post-treatment in nitric acid ethanol solution. Mater. Lett. 2009, 63, 106–108. [Google Scholar] [CrossRef]
- Zheng, R.; Meng, X.; Tang, F. Synthesis, characterization and photodegradation study of mixed-phase titania hollow submicrospheres with rough surface. Appl. Surf. Sci. 2009, 255, 5989–5994. [Google Scholar] [CrossRef]
- Mahdjoub, N.; Allen, N.; Kelly, P.; Vishnyakov, V. Thermally induced phase and photocatalytic activity evolution of polymorphous titania. J. Photochem. Photobiol. A 2010, 210, 125–129. [Google Scholar] [CrossRef]
- Nolph, C.A.; Sievers, D.E.; Kaewgun, S.; Kucera, C.J.; McKinney, D.H.; Rientjes, J.P.; White, J.L.; Bhave, R.; Lee, B.I. Photocatalytic study of polymorphic titania synthesized by ambient condition sol process. Catal. Lett. 2007, 117, 102–106. [Google Scholar]
- Kaewgun, S.; Nolph, C.A.; Lee, B.I. Enhancing photocatalytic activity of polymorphic titania nanoparticles by NMP solvent-based ambient condition process. Catal. Lett. 2008, 123, 173–180. [Google Scholar] [CrossRef]
- Kaewgun, S.; Mckinney, D.; White, J.; Smith, A.; Tinker, M.; Ziska, J.; Lee, B.I. Study of visible light photocatalytic activity achieved by NMP solvent treatment of polymorphic titania. J. Photochem. Photobiol. A 2009, 202, 154–158. [Google Scholar] [CrossRef]
- Kaewgun, S.; Nolph, C.A.; Lee, B.I.; Wang, L.-Q. Influence of hydroxyl contents on photocatalytic activities of polymorphic titania nanoparticles. Mater. Chem. Phys. 2009, 114, 439–445. [Google Scholar] [CrossRef]
- Tzikalos, N.; Belessi, V.; Lambropoulou, D. Photocatalytic degradation of Reactive Red 195 using anatase/brookite TiO2 mesoporous nanoparticles: optimization using response surface methodology (RSM) and kinetics studies. Environ. Sci. Pollut. Res. 2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, H.; Zhang, G. A novel mixed-phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar] [CrossRef]
- Ohtani, B. Preparing articles on photocatalysis-beyond the illusions, misconceptions, and speculation. Chem. Lett. 2008, 37, 217–229. [Google Scholar]
- Prieto-Mahaney, O.O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between photocatalytic activities and structural and physical properties of titanium(IV) oxide powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, D. Single-crystalline TiO2 nanorods: Highly active and easily recycled photocatalysts. Appl. Catal. B 2007, 73, 166–171. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L. Controllable one-pot synthesis and enhanced photocatalytic activity of mixed-phase TiO2 nanocrystals with tunable brookite/rutile ratios. J. Phys. Chem. C 2009, 113, 1785–1790. [Google Scholar]
- Lopez, T.; Gomez, R.; Sanchez, E.; Tzompantzi, F.; Vera, L. Photocatalytic activity in the 2,4-dinitroaniline decomposition over TiO2 sol-gel derived catalysts. J. Sol-Gel Sci. Technol. 2001, 22, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Nan, J. Hydrothermal-hydrolysis synthesis and photocatalytic properties of nano-TiO2 with an adjustable crystalline structure. J. Hazard. Mater. 2010, 176, 617–622. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kuo, C.-S.; Huang, C.-H.; Li, Y.-Y.; Chou, P.-W.; Cheng, C.-L.; Wong, M.-S. Visible-light-responsive nano-TiO2 with mixed crystal lattice and its photocatalytic activity. Nanotechnology 2006, 17, 2490–2497. [Google Scholar]
- Luís, A.M.; Neves, M.C.; Mendonça, M.H.; Monteiro, O.C. Influence of calcination parameters on the TiO2 photocatalytic properties. Mater. Chem. Phys. 2011, 125, 20–25. [Google Scholar] [CrossRef]
- Liao, Y.; Que, W.; Jia, Q.; He, Y.; Zhang, J.; Zhong, P. Controllable synthesis of brookite/anatase/rutile TiO2 nanocomposites and single-crystalline rutile nanorods array. J. Mater. Chem. 2012, 22, 7937–7944. [Google Scholar]
- Liu, J.; Qin, W.; Zuo, S.; Yu, Y.; Hao, Z. Solvothermal-induced phase transition and visible photocatalytic activity of nitrogen-doped titania. J. Hazard. Mater. 2009, 163, 273–278. [Google Scholar] [CrossRef]
- Shao, G.-S.; Zhang, X.-J.; Yuan, Z.-Y. Preparation and photocatalytic activity of hierarchically mesoporous-macroporous TiO2−xNx. Appl. Catal. B 2008, 82, 208–218. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.-Y. Facile synthesis of anatase-brookite mixed-phase N-doped TiO2 nanoparticles with high visible-light photocatalytic activity. Eur. J. Inorg. Chem. 2009, 2009, 3727–3733. [Google Scholar] [CrossRef]
- Pap, Z.; Baia, L.; Mogyorósi, K.; Dombi, A.; Oszkó, A.; Danciu, V. Correlating the visible light photoactivity of N-doped TiO2 with brookite particle size and bridged-nitro surface species. Catal. Commun. 2012, 17, 1–7. [Google Scholar] [CrossRef]
- Popa, M.; Diamandescu, L.; Vasiliu, F.; Teodorescu, C.M.; Cosoveanu, V.; Baia, M.; Feder, M.; Baia, L.; Danciu, V. Synthesis, structural characterization, and photocatalytic properties of iron-doped TiO2 aerogels. J. Mater. Sci. 2009, 44, 358–364. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, J. The study of iron (III) and nitrogen co-doped mesoporous TiO2 photocatalysts: Synthesis, characterization and activity. Microporous Mesoporous Mater. 2009, 121, 52–57. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Ackerman, E.A.; Gajdardziska-Josifovska, M.; Li, H. Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels. Appl. Catal. A 2011, 400, 195–202. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, F.; Jiao, Y.; Yang, H.; Zhang, J. Ag0-loaded brookite/anatase composite with enhanced photocatalytic performance towards the degradation of methyl orange. J. Mol. Catal. A 2011, 348, 114–119. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag-TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Lu, X. Preparation of silver-modified TiO2 via microwave-assisted method and its photocatalytic activity for toluene degradation. J. Hazard. Mater. 2010, 177, 639–647. [Google Scholar] [CrossRef]
- Nassoko, D.; Li, Y.-F.; Li, J.-L.; Li, X.; Yu, Y. Neodymium-doped TiO2 with anatase and brookite two phases: Mechanism for photocatalytic activity enhancement under visible light and the role of electron. Int. J. Photoenergy 2012, Article ID 716087. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36-73. https://doi.org/10.3390/catal3010036
Di Paola A, Bellardita M, Palmisano L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts. 2013; 3(1):36-73. https://doi.org/10.3390/catal3010036
Chicago/Turabian StyleDi Paola, Agatino, Marianna Bellardita, and Leonardo Palmisano. 2013. "Brookite, the Least Known TiO2 Photocatalyst" Catalysts 3, no. 1: 36-73. https://doi.org/10.3390/catal3010036
APA StyleDi Paola, A., Bellardita, M., & Palmisano, L. (2013). Brookite, the Least Known TiO2 Photocatalyst. Catalysts, 3(1), 36-73. https://doi.org/10.3390/catal3010036





