Regulation of Ag1Cux/SBA-15 Catalyst for Efficient CO Catalytic Degradation at Room Temperature
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Ag/Cu Molar Ratio and Pretreated Condition on Catalytic Activity for CO Oxidation
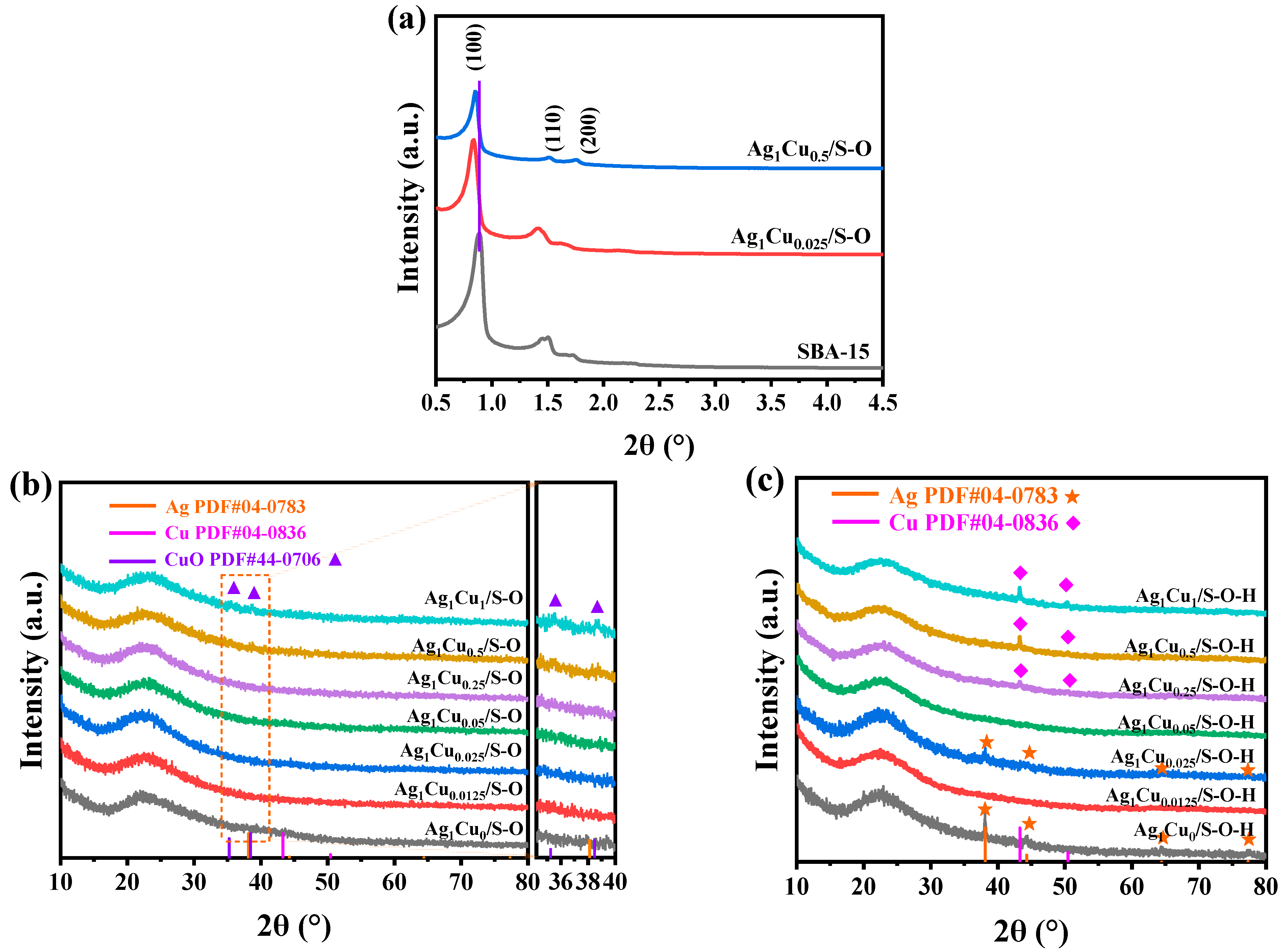
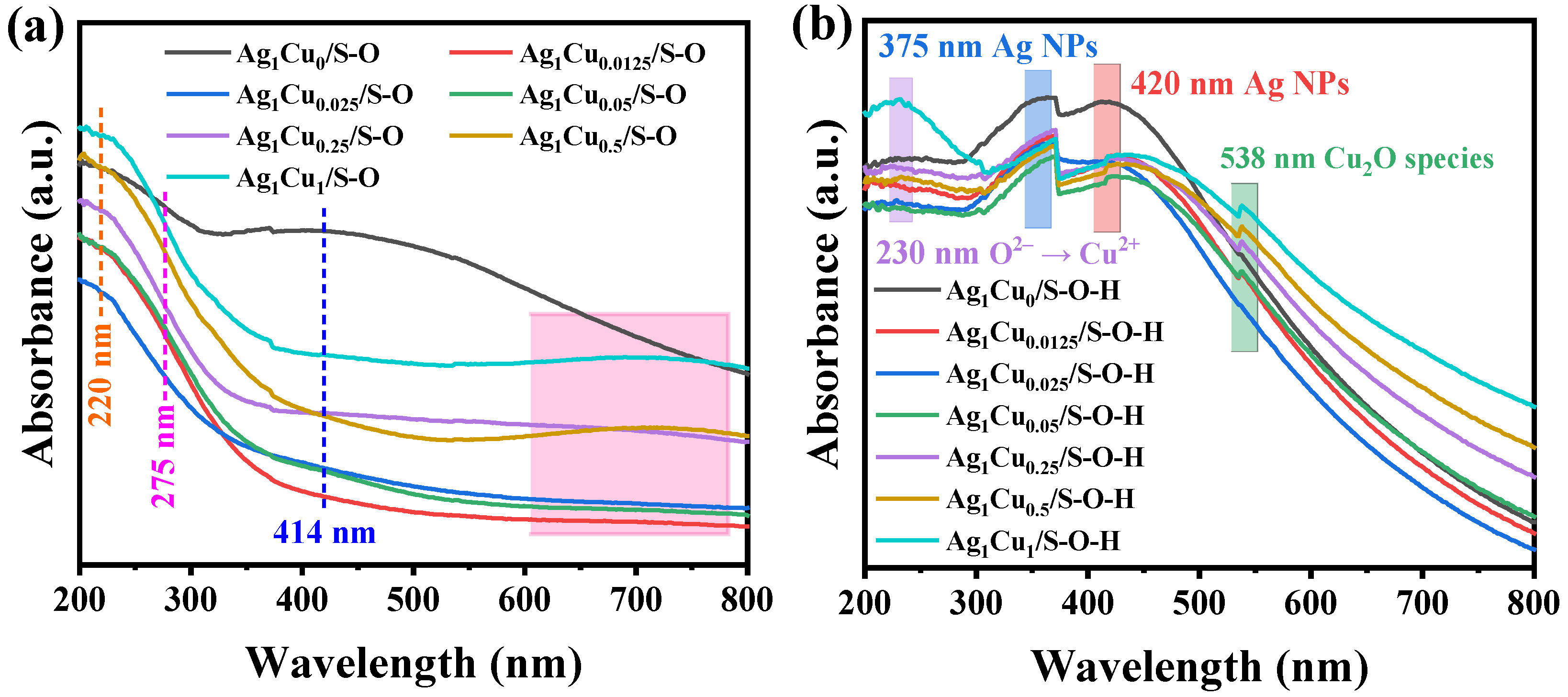
2.1.1. Characterizations of Ag1Cux/S-O and Ag1Cux/S-O-H Catalysts
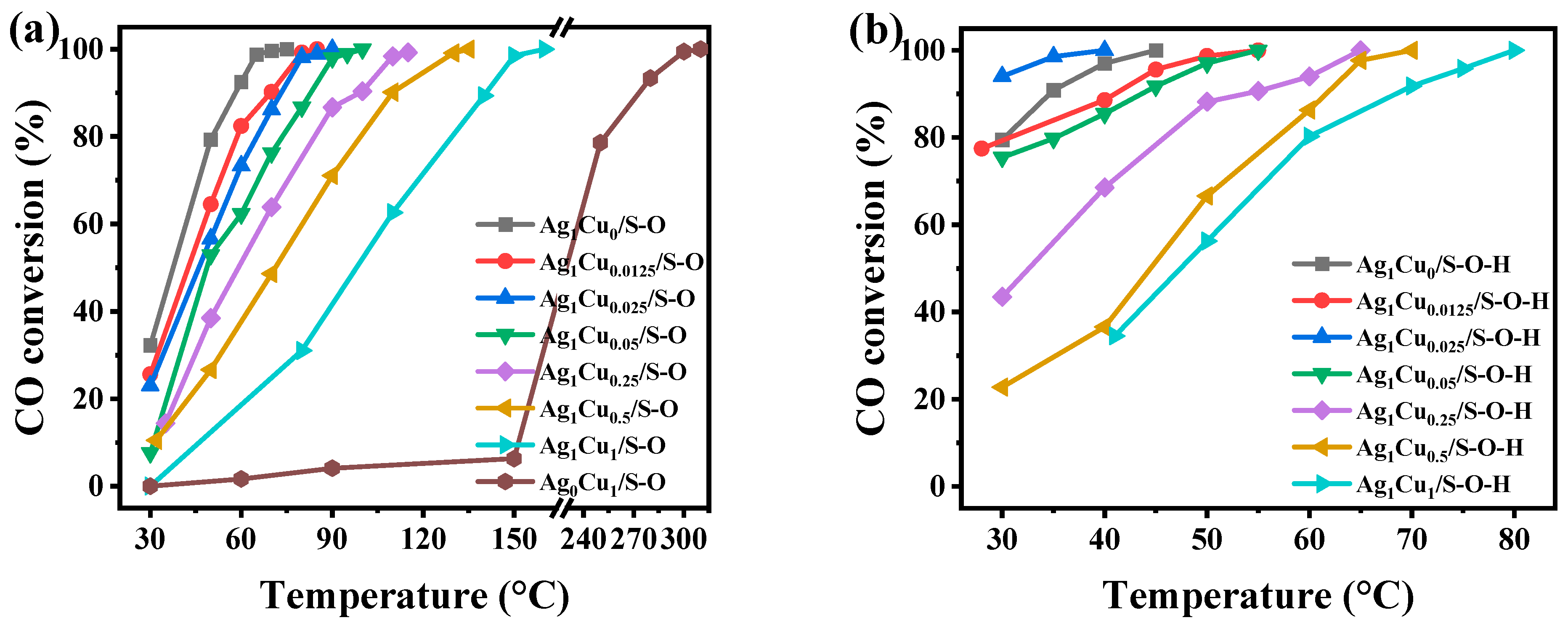
2.1.2. Catalytic Performance of Ag1Cux/S-O and Ag1Cux/S-O-H for CO Oxidation
2.2. Influence of H2 Treatment Temperature on the Catalytic Activity of CO Oxidation
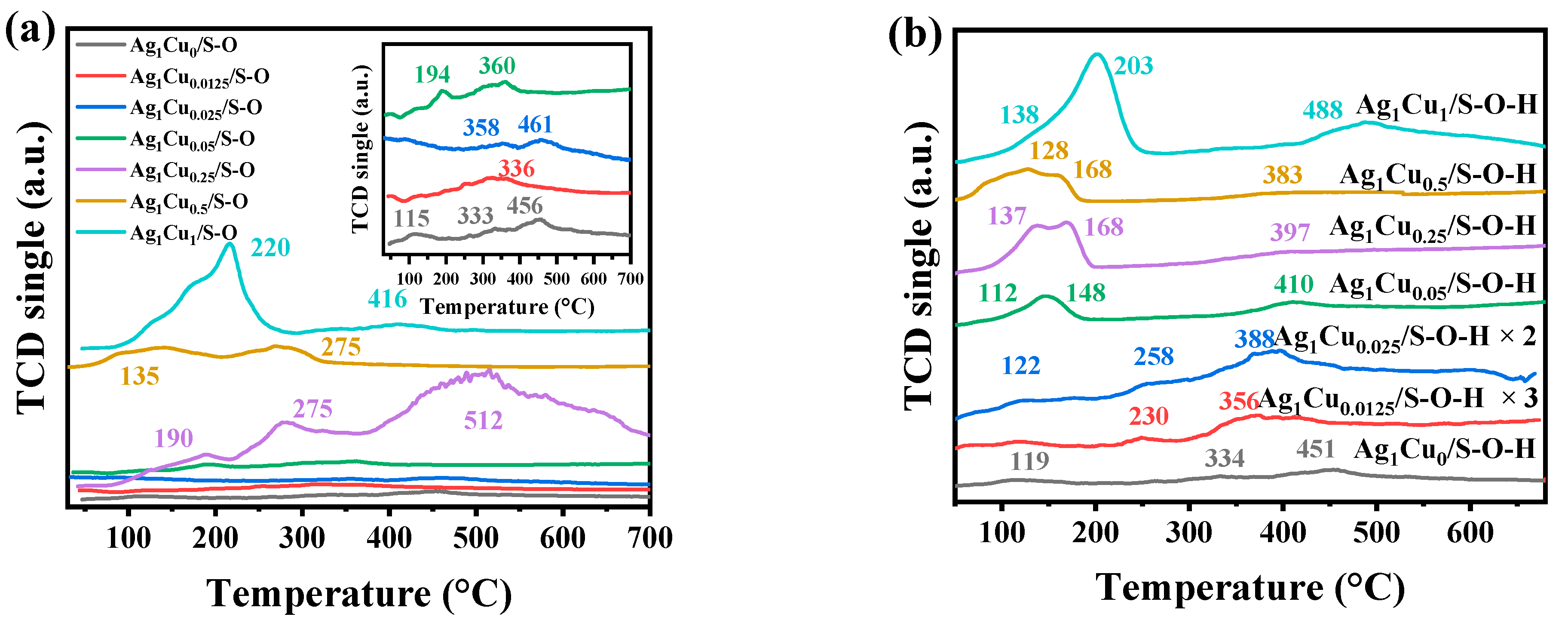
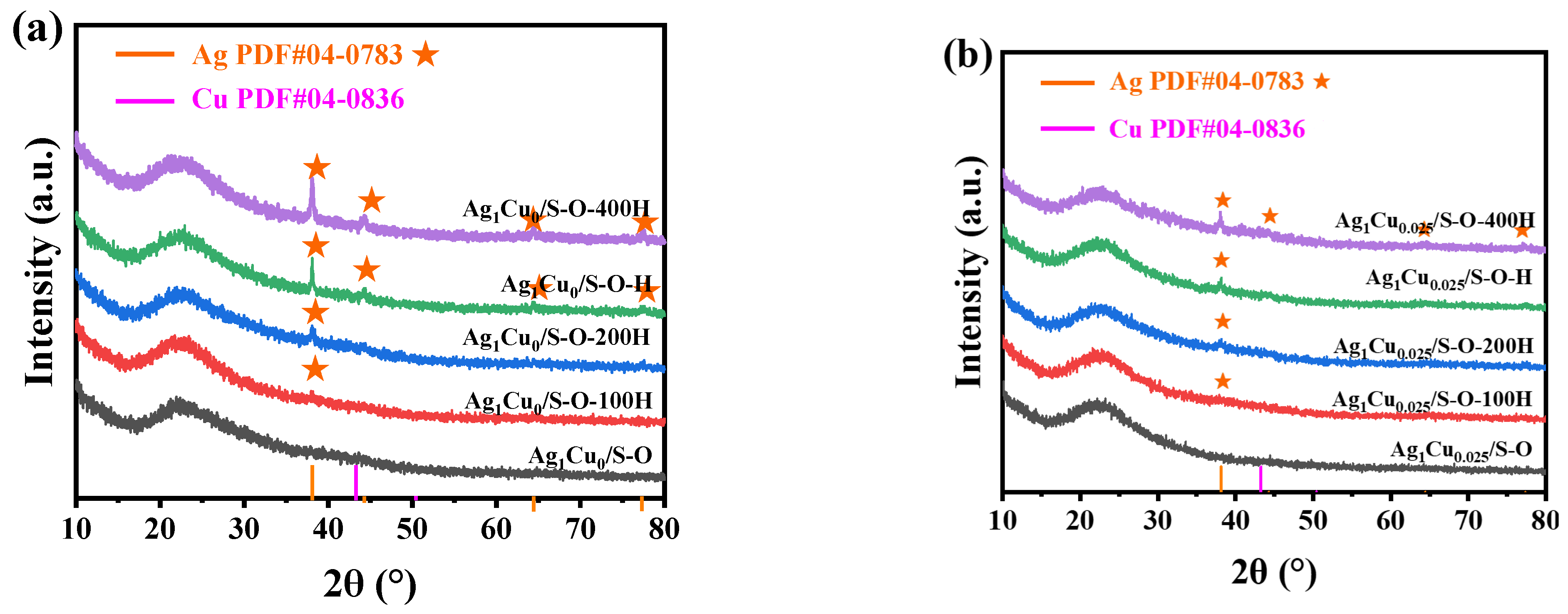
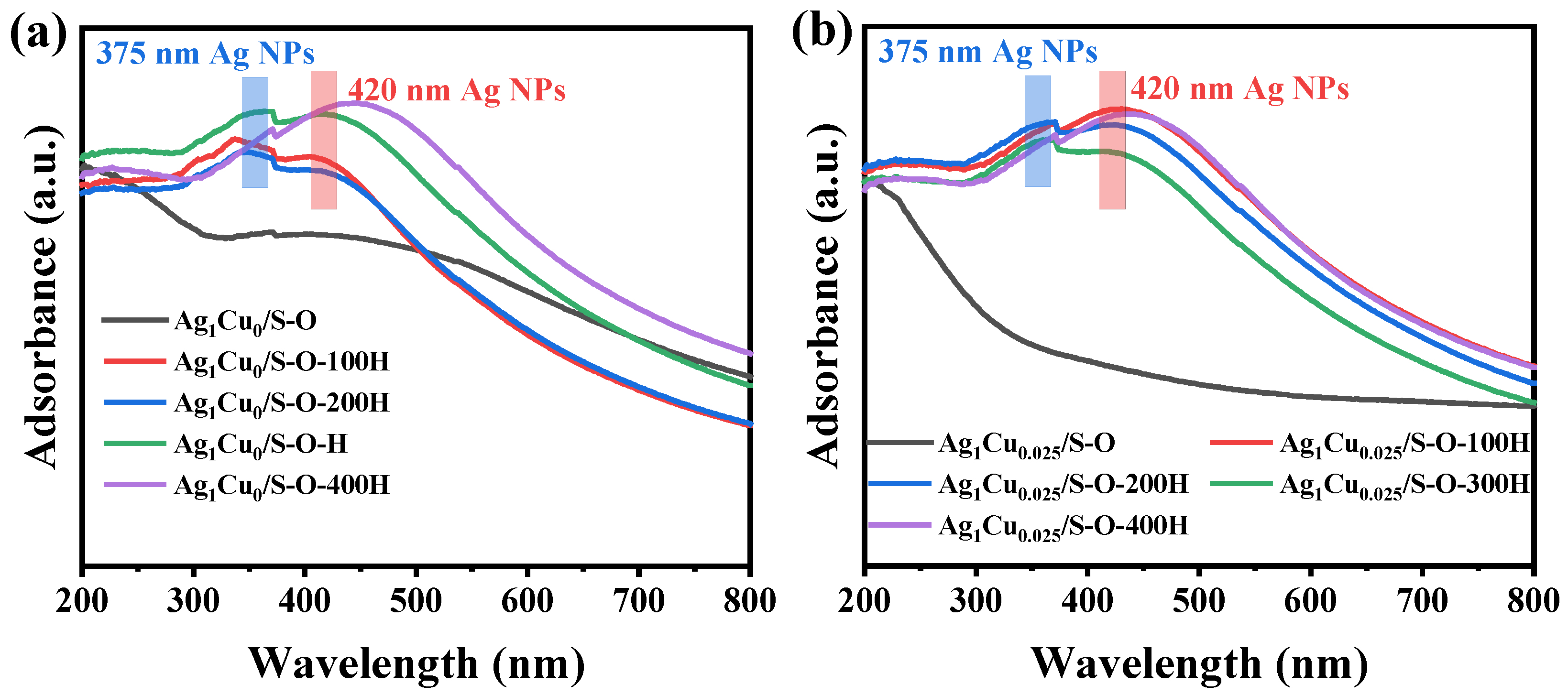
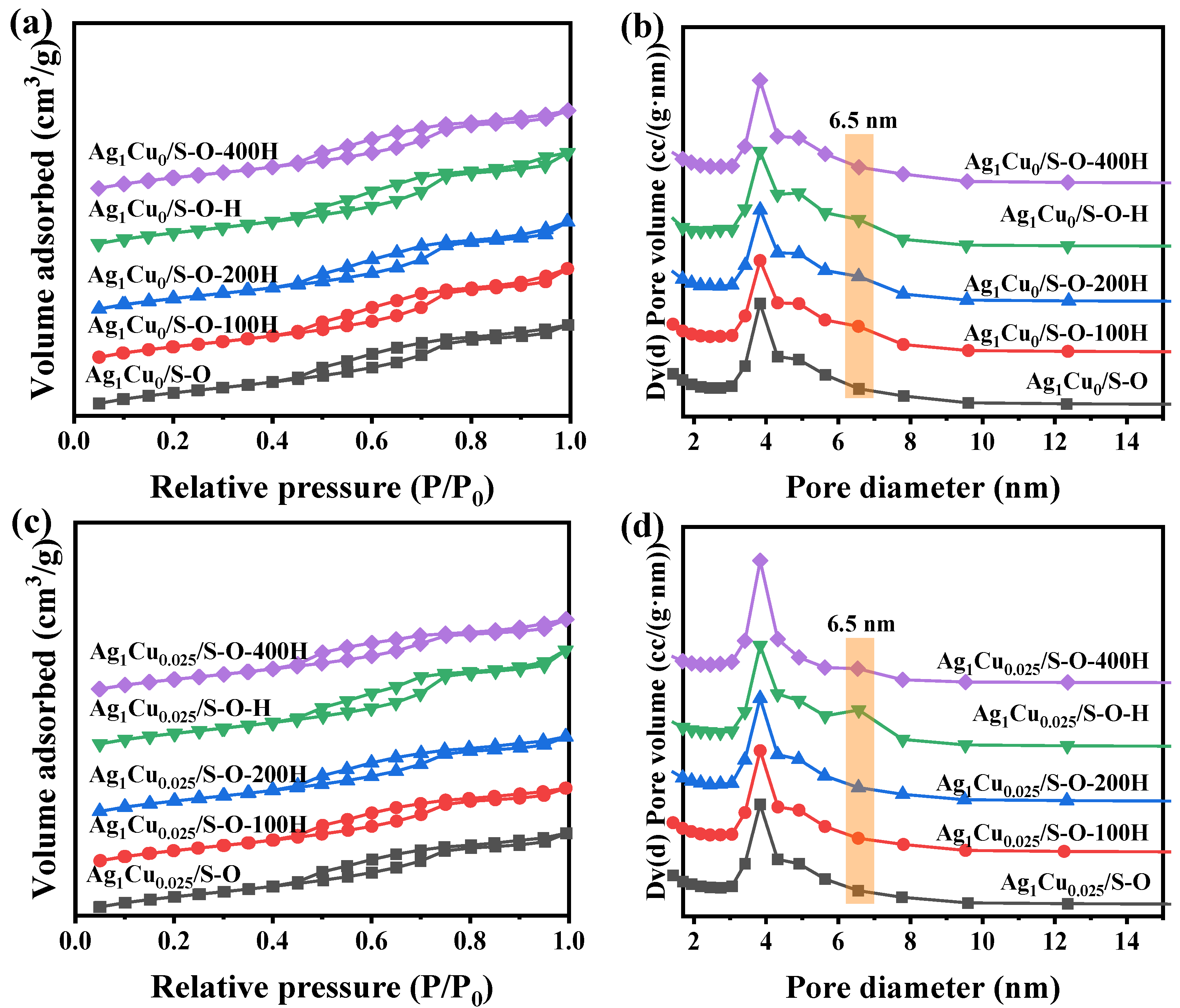
2.2.1. Characterization of the Ag1Cu0/S-O-yH and Ag1Cu0.025/S-O-yH Catalysts
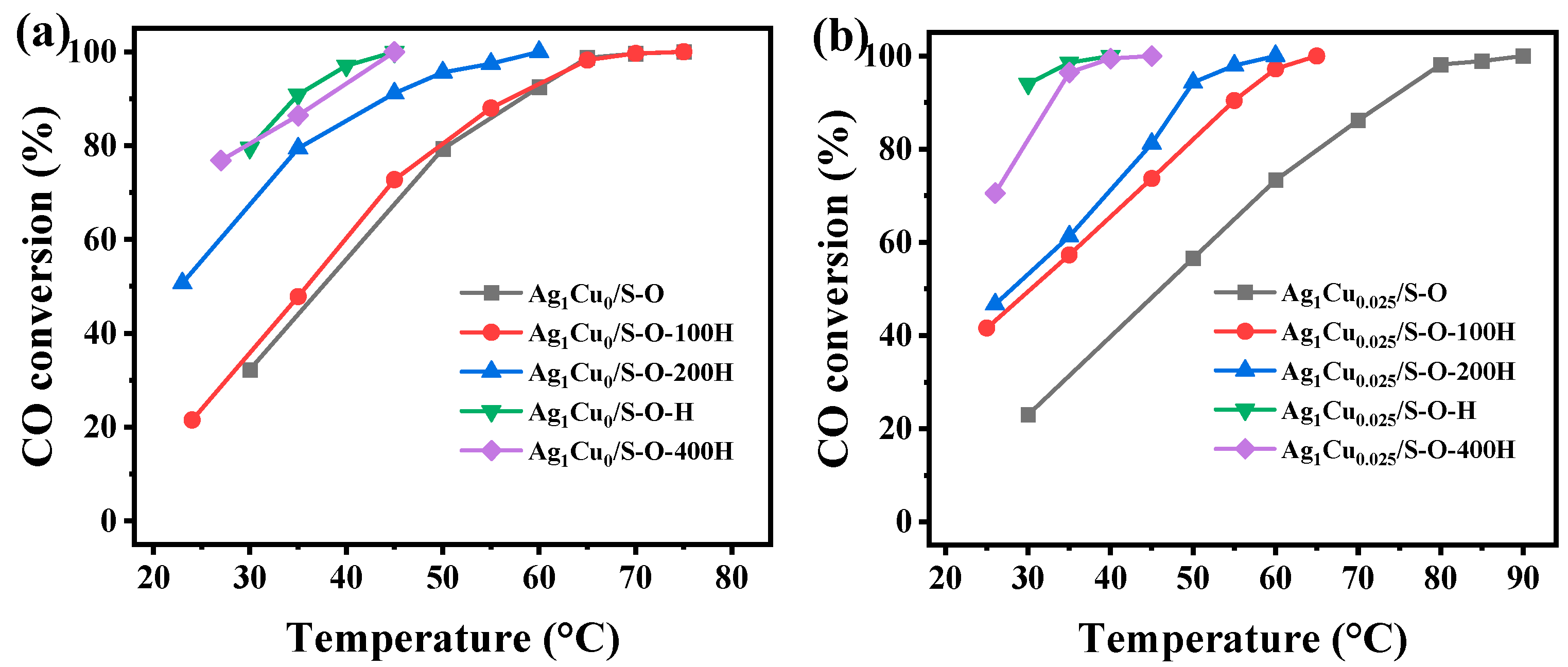
2.2.2. Catalytic Performance of the Ag1Cu0/S-O-yH and Ag1Cu0.025/S-O-yH Catalysts
2.3. Discussion
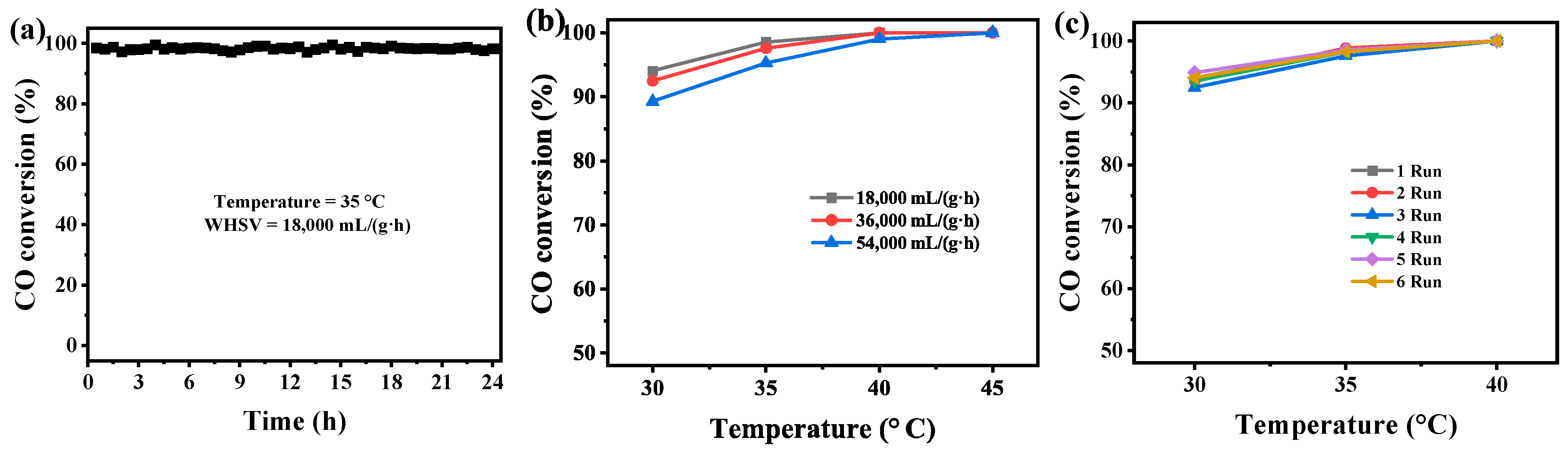
2.4. Catalytic Stability, Effect of Reaction Velocity, and Reusability
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Synthesis
3.2.1. Preparation of the Support
3.2.2. Synthesis of Ag1Cux/SBA-15 Catalysts
3.3. Characterizations
3.4. Catalytic Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daniels, S.T. Products of incomplete combustion (Ox, COx, HOx, NOx, SOx ROx, MOx and POx). J. Hazard. Mater. 1989, 22, 161–173. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, M.; Niu, Y.; Yi, L.; Yi, H.; Zhou, Y.; Zhao, S.; Tang, X.; Gao, F. Advances in CO catalytic oxidation on typical noble metal catalysts: Mechanism, performance and optimization. Chem. Eng. J. 2024, 495, 153523. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Landaw, S.A. Carbon Monoxide and Human Health. Science 1968, 162, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Shu, Q.; Zhang, R.; Zhang, Q. Structured Mesh-Type Pt/Mn/γ-Al2O3/Al Catalyst Enhanced the CO Oxidation at Room Temperature by In Situ Generation of Hydroxyl: Behavior and Mechanism. Catalysts 2025, 15, 430. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Xue, D.; Liu, X.; Jin, M.; Sun, L. Incorporation of Cu(II) and its selective reduction to Cu(I) within confined spaces: Efficient active sites for CO adsorption. J. Mater. Chem. A 2018, 6, 8930–8939. [Google Scholar] [CrossRef]
- Ko, K.J.; Kim, H.; Cho, Y.H.; Lee, H.; Kim, K.M.; Lee, C.H. Overview of Carbon Monoxide Adsorption Performance of Pristine and Modified Adsorbents. J. Chem. Eng. Data 2022, 67, 1599–1616. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Hardenbergh, J.H. The catalytic reduction of carbon monoxide over iron surfaces: A surface science investigation. J. Catal. 1984, 87, 66–76. [Google Scholar] [CrossRef]
- Huang, B.; Shi, Z.; Yang, Z.; Dai, M.; Wen, Z.; Li, W.; Zi, G.; Luo, L. Mechanism of CO selective catalytic reduction denitration on Fe-Mn/AC catalysts at medium and low temperatures under oxygen atmosphere. Chem. Eng. J. 2022, 446, 137412. [Google Scholar] [CrossRef]
- Liang, C.; Sun, Y.; Li, P.; Jiang, Y.; Sun, X.; Yang, Z. Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts. Catalysts 2025, 15, 353. [Google Scholar] [CrossRef]
- Lyu, Y.; Xu, J.; Chen, S.; Wang, S.; Liu, X. Simultaneous catalytic oxidation of toluene and CO over Cu-V/Al-Ce catalysts: Physicochemical properties-activity relationship and simultaneous oxidation mechanism. J. Hazard. Mater. 2024, 466, 133507. [Google Scholar] [CrossRef]
- Eid, K.; Gamal, A.; Abdullah, A.M. Graphitic carbon nitride-based nanostructures as emergent catalysts for carbon monoxide (CO) oxidation. Green Chem. 2023, 25, 1276–1310. [Google Scholar] [CrossRef]
- Kovalskii, A.M.; Volkov, I.N.; Evdolimenko, N.D.; Tkachenko, O.P.; Leybo, D.V.; Chepkasov, I.V.; Popov, Z.I.; Matveev, A.T.; Manakhov, A.; Permyakova, E.S.; et al. Hexagonal BN- and BNO-supported Au and Pt nanocatalysts in carbon monoxide oxidation and carbon dioxide hydrogenation reactions. Appl. Catal. B 2022, 303, 120891. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, Y.; Hu, H.; Wei, J.; Qiao, R.; Bi, F.; Zhang, X. Recent progress of cerium-based catalysts for the catalytic oxidation of volatile organic compounds: A review. Fuel 2025, 399, 135603. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, F.; Wei, J.; Han, X.; Gao, B.; Qiao, R.; Xu, J.; Liu, N.; Zhang, X. Boosting the Photothermal Oxidation of Multicomponent VOCs in Humid Conditions: Synergistic Mechanism of Mn and K in Different Oxygen Activation Pathways. Environ. Sci. Technol. 2025, 59, 11341–11352. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Bi, F.; Liu, N.; Zhang, X. Degradation of volatile organic pollutants over manganese-based catalysts by defect engineering: A review. Sep. Purif. Technol. 2025, 362, 131934. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N. Atomic dispersion of bulk/nano metals to atomic-sites catalysts and their application in thermal catalysis. Nano Res. 2023, 16, 6380–6401. [Google Scholar] [CrossRef]
- Xiao, M.; Han, D.; Yang, X.; Yu, J.; Shi, B.; Guo, Y.; Yu, X.; Ge, M. Active Interfacial Perimeter in Pt/CeO2 Catalysts with Embedding Structure for Water-Tolerant Toluene Combustion. Environ. Sci. Technol. 2024, 58, 22808–22817. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Huang, J.; Ma, S.; Zhang, Y.; Bi, F.; Zhang, X. Electron Beam Irradiation Modified UiO-66 Supported Pt Catalysts for Low-Temperature Ethyl Acetate Catalytic Degradation. Catalysts 2025, 15, 220. [Google Scholar] [CrossRef]
- Zhang, N.; He, C.; Jing, Y.; Qian, Y.; Obuchi, M.; Toyoshima, R.; Kondoh, H.; Oka, K.; Wu, B.; Li, L.; et al. Enhanced Nitrous Oxide Decomposition on Zirconium-Supported Rhodium Catalysts by Iridium Augmentation. Environ. Sci. Technol. 2025, 59, 1598–1607. [Google Scholar] [CrossRef]
- Zhang, N.; He, C.; Jing, Y.; Qian, Y.; Toyao, T.; Shimizu, K. Enhanced N2O decomposition on Rh/ZrO2 catalysts through the promotional effect of palladium. Surf. Interf. 2024, 46, 104120. [Google Scholar] [CrossRef]
- Huang, J.; Tao, H.; Chen, Z.; Zhou, M.; Na, J.; Chen, X.; Zhang, X. Advances in photocatalytic removal of NOx and VOCs from flue gas. Energy Environ. Prot. 2024, 38, 144–154. [Google Scholar]
- Ma, M.; Zhang, R.; Shen, Y.; Zhou, X.; Zhai, Y.; Han, Y.; Wang, D.; Zhang, L.; Song, X.; Fang, D.; et al. Mesoporous Ce-Ti Catalysts Modified by Phosphotungstic Acid and Chitosan for the Synergistic Catalysis of CVOCs and NOx. Catalysts 2025, 15, 119. [Google Scholar] [CrossRef]
- Cao, X.; Liu, Y.; Huang, Q.; Chen, Z.; Sun, J.; Sun, J.; Pang, S.; Liu, P.; Wang, W.; Zhang, Y.; et al. Single Droplet Tweezer Revealing the Reaction Mechanism of Mn(II)-Catalyzed SO2 Oxidation. Environ. Sci. Technol. 2024, 58, 5068–5078. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Su, L.; Chen, G.; Deng, Y.; Liu, Y.; Cen, W. Nondissociative activation of O2 for SO2 oxidation on metal-free N-doped carbocatalyst. Surf. Sci. 2022, 723, 122116. [Google Scholar] [CrossRef]
- Le, P.H.; Kitamoto, Y.; Yamashita, S.; Cao, K.L.A.; Hirano, T.; Amen, T.W.M.; Tsunoji, N.; Ogi, T. Macropore-Size Engineering toward Enhancing the Catalytic Performance of CO Oxidation over Three-Way Catalyst Particles. ACS Appl. Mater. Interfaces 2023, 15, 54073–54084. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, S.; Liu, H.; Shi, J.; Mi, J.; Liu, H.; Gong, Z.; Oliviero, L.; Mauge, F.; Li, J. Reverse oxygen spillover triggered by CO adsorption on Sn-doped Pt/TiO2 for low-temperature CO oxidation. Nat. Commun. 2023, 14, 3477. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, F.; Wang, Y.; Jia, M.; Tao, X.; Jin, Y.; Zhang, X. MOF-derived CeO2 supported Ag catalysts for toluene oxidation: The effect of synthesis method. Mol. Catal. 2021, 515, 111922. [Google Scholar] [CrossRef]
- Biabani-Ravandi, A.; Rezaei, M.; Fattah, Z. Catalytic performance of Ag/Fe2O3 for the low temperature oxidation of carbon monoxide. Chem. Eng. J. 2013, 219, 124–130. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.; Qin, L.; Huang, D.; Yi, H.; Liu, X.; Liu, S.; Zhang, M.; Li, B.; Li, L.; et al. Recent progress of noble metals with tailored features in catalytic oxidation for organic pollutants degradation. J. Hazard. Mater. 2022, 422, 126950. [Google Scholar] [CrossRef]
- Xu, W.W.; Dong, M.Y.; Di, L.B.; Zhang, X.L. A Facile Method for Preparing UiO-66 Encapsulated Ru Catalyst and its Application in Plasma-Assisted CO2 Methanation. Nanomaterials 2019, 9, 1432. [Google Scholar] [CrossRef]
- Shesterkina, A.; Zhdanova, C.; Davshan, N.; Medvedev, A.; Kalmykov, K.; Dunaev, S.; Kustov, L.; Kustov, A. CO2 hydro-genation to methanol on the incapsulated Cu-Zn catalyst: Effect of the MCM-41 and SBA-15 supports and the method of preparation on catalytic activity. Green Synth. Catal. 2025. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, Z.; Dong, C.; Huang, N. Effect of pretreatment conditions on catalytic activity of Ag/SBA-15 catalyst for toluene oxidation. Chin. J. Catal. 2017, 38, 1603–1612. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Z.; Yu, F.; Wang, Y. High-temperature diffusion induced high activity of SBA-15 supported Ag particles for low temperature CO oxidation at room temperature. J. Catal. 2013, 297, 264–271. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.I.; Jun, K.D.; Koh, H.L. Effect of Cu promoter and alumina phases on Pt/Al2O3 for propane dehydrogenation. Korean J. Chem. Eng. 2017, 345, 1337–1345. [Google Scholar] [CrossRef]
- Wang, J.; He, G.; Wang, C.; Chen, X.; Liu, X.; Li, Y.; Shan, W.; He, H. HCHO oxidation on Pt-Na/SiO2 catalyst with ultralow Pt loading: New insight into the effect of Si support and Na promoter. Appl. Catal. B Environ. Energy 2024, 347, 123787. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Hussien, A.G.S.; Dabbawala, A.A.; Anjum, D.H.; Charisiou, N.D.; Almaksoud, W.; AbouHamad, E.; Kobayashi, Y.; Goula, M.A.; Polychronopoulou, K. Merits of the CeLaCuO ternary oxide as promoter of the Ni/SBA-15 catalyst for the dry reforming of methane reaction. Chem. Eng. J. 2024, 496, 153948. [Google Scholar] [CrossRef]
- Sareen, S.; Mutreja, V.; Singh, S.; Pal, B. Highly dispersed Au, Ag and Cu nanoparticles in mesoporous SBA-15 for highly selective catalytic reduction of nitroaromatics. RSC Adv. 2015, 5, 184–190. [Google Scholar] [CrossRef]
- Gu, J.; Huang, Y.; Elangovan, S.P.; Li, Y.; Zhao, W.; Toshio, I.; Yamazaki, Y.; Shi, J. Highly Dispersed Copper Species within SBA-15 Introduced by the Hydrophobic Core of a Surfactant Micelle as a Carrier and Their Enhanced Catalytic Activity for Cyclohexane Oxidation. J. Phys. Chem. C 2011, 115, 21211–21217. [Google Scholar] [CrossRef]
- Bi, F.; Wei, J.; Gao, B.; Ma, S.; Liu, N.; Xu, J.; Liu, B.; Huang, Y.; Zhang, X. How the Most Neglected Residual Species in MOF-Based Catalysts Involved in Catalytic Reactions to Form Toxic Byproducts. Environ. Sci. Technol. 2024, 58, 19797–19806. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Meunier, F.C.; Avalos-Borja, M.; Breen, J.P.; Pestryakov, A. On the nature of the silver phases of Ag/Al2O3 catalysts for reactions involving nitric oxide. Appl. Catal. B 2002, 36, 287–297. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; He, G.; Chen, M.; Zhang, C.; He, H. Nanosize effect of Al2O3 in Ag/Al2O3 catalyst for the selective catalytic oxidation of ammonia. ACS Catal. 2018, 8, 2670–2682. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Wang, D.; Hong, Z.; Qi, Z.; Li, X. Hollow ZSM-5 zeolite encapsulated Ag nanoparticles for SO2-resistant selective catalytic oxidation of ammonia to nitrogen. Sep. Purif. Technol. 2019, 209, 1016–1026. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Czaplinsks, J.; Sobczak, I.; Ziolek, M. Bimetallic AgCu/SBA-15 System: The Effect of Metal Loading and Treatment of Catalyst on Surface Properties. J. Phys. Chem. C 2014, 118, 12796–12810. [Google Scholar] [CrossRef]
- Bi, F.; Wei, J.; Zhou, Z.; Zhang, Y.; Gao, B.; Liu, N.; Xu, J.; Liu, B.; Huang, Y.; Zhang, X. Insight into the Synergistic Effect of Binary Nonmetallic Codoped Co3O4 Catalysts for Efficient Ethyl Acetate Degradation under Humid Conditions. JACS Au 2025, 5, 363–380. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Wang, Y.; Liu, N.; Zuo, Y.; Cui, L. Study of catalytic activity at the Ag/Al-SBA-15 catalysts for CO oxidation and selective CO oxidation. Chem. Eng. J. 2016, 283, 1097–1107. [Google Scholar] [CrossRef]
- Wen, Z.; Duan, X.; Hu, M.; Cao, Y.; Ye, L.; Jiang, L.; Yuan, Y. Efficient low-temperature soot combustion by bimetallic Ag-Cu/SBA-15 catalysts. J. Environ. Sci. 2018, 64, 122–129. [Google Scholar] [CrossRef]
- Xue, J.; Wu, M.; Song, Y.; Zhao, J.; Wu, J.; Quan, Y.; Run, J. Study on performance of Ag-modified layered copper silicate catalyst for hydrogenation of dimethyl oxalate to methyl glycolate. J. Fuel Chem. Technol. 2022, 50, 1014–1022. [Google Scholar] [CrossRef]
- Mahara, Y.; Ishikawa, H.; Ohyama, J.; Sawabe, K.; Satsuma, A. Ag-M (M: Ni, Co, Cu, Fe) bimetal catalysts prepared by galvanic deposition method for CO oxidation. Catal. Today 2016, 265, 2–6. [Google Scholar] [CrossRef]
- Jabłońska, M.; Beala, A.M.; Nocuń, M.; Palkovits, R. Ag-Cu based catalysts for the selective ammonia oxidation into nitrogen and water vapour. Appl. Catal. B 2018, 232, 275–287. [Google Scholar] [CrossRef]
- Zhang, N.; Tong, J.; Miyazaki, S.; Zhao, S.; Kubota, H.; Jing, Y.; Mine, S.; Toyao, T.; Shimizu, K. Mechanism of NH3-SCR over P/CeO2 Catalysts Investigated by Operando Spectroscopies. Environ. Sci. Technol. 2023, 57, 16289–16295. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, Y.; Zhou, Z.; Guo, L.; Zhu, Z.; Liu, B.; Zhang, X. Electron Beam Irradiation-Induced Defects Enhance Pt-TiO2 Photothermal Catalytic Degradation in PAEs: A Performance and Mechanism Study. Molecules 2025, 30, 697. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Guo, L.; Bi, F.; Ma, S.; Shen, Q.; Qiao, R.; Zhang, X. Insight into the degradation mechanism of mixed VOCs oxidation over Pd/UiO-66(Ce) catalysts: Combination of operando spectroscopy and theoretical calculation. Sep. Technol. Technol. 2025, 354, 129443. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Wang, Z.; Feng, Y.; Liu, Y.; Dai, H.; Wang, Z.; Deng, J. Photothermal synergistic catalytic oxidation of ethyl acetate over MOFs-derived mesoporous N-TiO2 supported Pd catalysts. Appl. Catal. B 2023, 322, 122075. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H.; Zhang, X.; Mao, Y.; Wu, Y.; Liu, W.; Gao, H.; Zhang, M.; Song, Z. Catalytic oxidation of toluene by manganese oxides: Effect of K+ doping on oxygen vacancy. J. Environ. Sci. 2024, 142, 43–56. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Wang, L.; Tang, M.; Lu, S. Removal of toluene and SO2 by hierarchical porous carbons: A study on adsorption selectivity and mechanism. Environ. Sci. Pollut. Res. 2022, 29, 29117–29129. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Kardash, T.Y.; Slavinskaya, E.M.; Stonkus, O.A.; Koscheev, S.V.; Boronin, A.I. The decomposition of mixed oxide Ag2Cu2O3: Structural features and the catalytic properties in CO and C2H4 oxidation. Appl. Surf. Sci. 2018, 427, 363–374. [Google Scholar] [CrossRef]
- Yin, C.; Lei, Y.; Wu, H.; Xu, Y.; Luo, H.; Wang, H. Synergistic effect of Cu and Ag on porous (Cu, Ag) co-doped CeO2 nanosheets for low-temperature CO oxidation. Inorg. Chem. Commun. 2025, 172, 113729. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Zaikovskii, V.I.; Liotta, L.F.; Vodyankina, O.V. Low-temperature CO oxidation over Ag/SiO2 catalysts: Effect of OH/Ag ratio. Appl. Catal. B 2018, 221, 598–609. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Peng, H.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Ag supported on meso-structured SiO2 with different morphologies for CO oxidation: On the inherent factors influencing the activity of Ag catalysts. Microporous Mesoporous Mater. 2017, 242, 90–98. [Google Scholar] [CrossRef]
- Soni, Y.; Pradhan, S.; Bamnia, M.K.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Khan, T.S.; Haider, M.A.; Vinod, C.P. Spectroscopic Evidences for the Size Dependent Generation of Pd Species Responsible for the Low Temperature CO Oxidation Activity on Pd-SBA-15 Nanocatalyst. Appl. Catal. B 2020, 272, 118934. [Google Scholar] [CrossRef]
- Zhang, S.; An, K.; Wang, X.; Wang, H.; Li, S.; Sun, H.; Li, N.; Wu, Y.; Liu, Y. PtPd nanoparticles on LaCoO3 with ruthenium in the interfaces for CO oxidation. Appl. Surf. Sci. 2023, 609, 155285. [Google Scholar] [CrossRef]
- Samadi, P.; Binczarski, M.J.; Maniukiewicz, W.; Pawlaczyk, A.; Rogowski, J.; Szubiakiewicz, E.; Szynkowska-Jozwik, M.I.; Witonska, I.A. Zn Modification of Pd/TiO2/Ti Catalyst for CO Oxidation. Materials 2023, 16, 1216. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, X.; Yu, F.; Liu, X.; Fu, Q. Role of the Al chemical environment in the formation of silver species and its CO oxidation activity. J. Catal. 2015, 321, 113–122. [Google Scholar] [CrossRef]
| Ag1Cux/SBA-15 Catalysts | Metal Loading (wt%) | |||
|---|---|---|---|---|
| Pretreated Under 30.0 vol.% O2/Ar Atmosphere at 500 °C for 2 h | Pretreated Under 30.0 vol.% O2/Ar Atmosphere at 500 °C for 2 h and H2 Atmosphere at 300 °C for 2 h | |||
| Ag | Cu | Ag | Cu | |
| Ag1Cu0/SBA-15 | 3.95 | / | 3.93 | / |
| Ag1Cu0.0125/SBA-15 | 3.94 | 0.026 | 3.94 | 0.024 |
| Ag1Cu0.025/SBA-15 | 3.90 | 0.054 | 3.92 | 0.052 |
| Ag1Cu0.05/SBA-15 | 3.88 | 0.112 | 3.87 | 0.115 |
| Ag1Cu0.25/SBA-15 | 3.85 | 0.536 | 3.85 | 0.532 |
| Ag1Cu0.5/SBA-15 | 3.82 | 1.108 | 3.83 | 1.110 |
| Ag1Cu1/SBA-15 | 3.80 | 2.321 | 3.81 | 2.324 |
| Ag1Cux/SBA-15 Catalysts | Catalytic Performance CO Oxidation (°C) | |||
|---|---|---|---|---|
| Pretreated Under 30.0 vol.% O2/Ar Atmosphere at 500 °C for 2 h | Pretreated Under 30.0 vol.% O2/Ar Atmosphere at 500 °C for 2 h and H2 Atmosphere at 300 °C for 2 h | |||
| T50 | T98 | T50 | T98 | |
| Ag1Cu0/SBA-15 | 37 | 65 | <30 | 41 |
| Ag1Cu0.0125/SBA-15 | 42 | 79 | <30 | 48 |
| Ag1Cu0.025/SBA-15 | 45 | 80 | <30 | 34 |
| Ag1Cu0.05/SBA-15 | 48 | 90 | <30 | 51 |
| Ag1Cu0.25/SBA-15 | 59 | 110 | 32 | 63 |
| Ag1Cu0.5/SBA-15 | 71 | 128 | 44 | 66 |
| Ag1Cu1/SBA-15 | 98 | 149 | 47 | 77 |
| Ag0Cu1/SBA-15 | 210 | 295 | - | - |
| Pretreated Conditions | Ag1Cu0/SBA-15 | Ag1Cu0.025/SBA-15 | ||||
|---|---|---|---|---|---|---|
| SBET (m2/g) | V (cm3/g) | D (nm) | SBET (m2/g) | V (cm3/g) | D (nm) | |
| 500 °C O2 | 494 | 0.592 | 3.0–7.7 | 477 | 0.560 | 3.0–7.7 |
| 500 °C O2-100 °C H2 | 490 | 0.650 | 3.0–7.7 | 481 | 0.560 | 3.0–7.7 |
| 500 °C O2-200 °C H2 | 492 | 0.640 | 3.0–7.7 | 485 | 0.570 | 3.0–7.7 |
| 500 °C O2-300 °C H2 | 503 | 0.663 | 3.0–7.7 | 492 | 0.680 | 3.0–7.7 |
| 500 °C O2-400 °C H2 | 491 | 0.590 | 3.0–7.7 | 467 | 0.540 | 3.0–7.7 |
| Pretreated Conditions | Catalytic Performance CO Oxidation (°C) | |||
|---|---|---|---|---|
| Ag1Cu0/SBA-15 | Ag1Cu0.025/SBA-15 | |||
| T50 | T98 | T50 | T98 | |
| 500 °C O2 | 37 | 65 | 46 | 81 |
| 500 °C O2-100 °C H2 | 35 | 65 | 30 | 61 |
| 500 °C O2-200 °C H2 | <30 | 56 | <30 | 55 |
| 500 °C O2-300 °C H2 | <30 | 41 | <30 | 34 |
| 500 °C O2-400 °C H2 | <30 | 43 | <30 | 37 |
| Catalysts | Preparation Method | Noble Metal Loadings (wt%) | Flow Rate (mL/min) | Preparation Conditions | Catalytic Activity (°C) | Ref. |
|---|---|---|---|---|---|---|
| Ag2Cu2O3 | Co-precipitation | / | 1000 | / | 160 (T100) | [58] |
| CuAg/CeO2 | Urea-assisted | 5.71 | 30 | 550 °C-air-2 h | 100 (T100) | [59] |
| 8Ag/SiO2-500 | Wetness impregnation | 8 | 20 | 500 °C-air-0.5 h 200 °C-10%H2/Ar-0.5 h | 66 (T98) | [60] |
| Ag/SBA-15 | Impregnation | 7 | 30 | 550 °C-N2-2 h 550 °C-air-6 h | 150 (T100) | [61] |
| Pd/SBA-15 | Precipitation | 2.8 | 25 | 300 °C-air-4 h | 115 (T100) | [62] |
| Pt/Sn0.2Ti0.8O2 | Impregnation | 0.5 | 100 | 300 °C-5%H2-1 h | 120 (T100) | [26] |
| PtPdRu/LCO | Sol–gel | / | 40 | 300 °C-H2 | 165 (T100) | [63] |
| Pd-Zn/TiO2/Ti | Plasma electrolytic oxidation | 5 | 50 | 400 °C-H2-2 h | 180 (T100) | [64] |
| Ag1Cu0.025/S-O-H | Impregnation | 4 | 30 | 500 °C-30%O2/Ar-2 h 300 °C-H2-2 h | 35 (T98) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, F.; Hu, H.; Zheng, Y.; Wang, Y.; Wang, Y.; Liu, B.; Dong, H.; Zhang, X. Regulation of Ag1Cux/SBA-15 Catalyst for Efficient CO Catalytic Degradation at Room Temperature. Catalysts 2025, 15, 676. https://doi.org/10.3390/catal15070676
Bi F, Hu H, Zheng Y, Wang Y, Wang Y, Liu B, Dong H, Zhang X. Regulation of Ag1Cux/SBA-15 Catalyst for Efficient CO Catalytic Degradation at Room Temperature. Catalysts. 2025; 15(7):676. https://doi.org/10.3390/catal15070676
Chicago/Turabian StyleBi, Fukun, Haotian Hu, Ye Zheng, Yanxuan Wang, Yuxin Wang, Baolin Liu, Han Dong, and Xiaodong Zhang. 2025. "Regulation of Ag1Cux/SBA-15 Catalyst for Efficient CO Catalytic Degradation at Room Temperature" Catalysts 15, no. 7: 676. https://doi.org/10.3390/catal15070676
APA StyleBi, F., Hu, H., Zheng, Y., Wang, Y., Wang, Y., Liu, B., Dong, H., & Zhang, X. (2025). Regulation of Ag1Cux/SBA-15 Catalyst for Efficient CO Catalytic Degradation at Room Temperature. Catalysts, 15(7), 676. https://doi.org/10.3390/catal15070676









