A Novel Polymer-Derived Ni/SiOC Catalyst for the Dry Reforming of Methane
Abstract
1. Introduction
2. Results
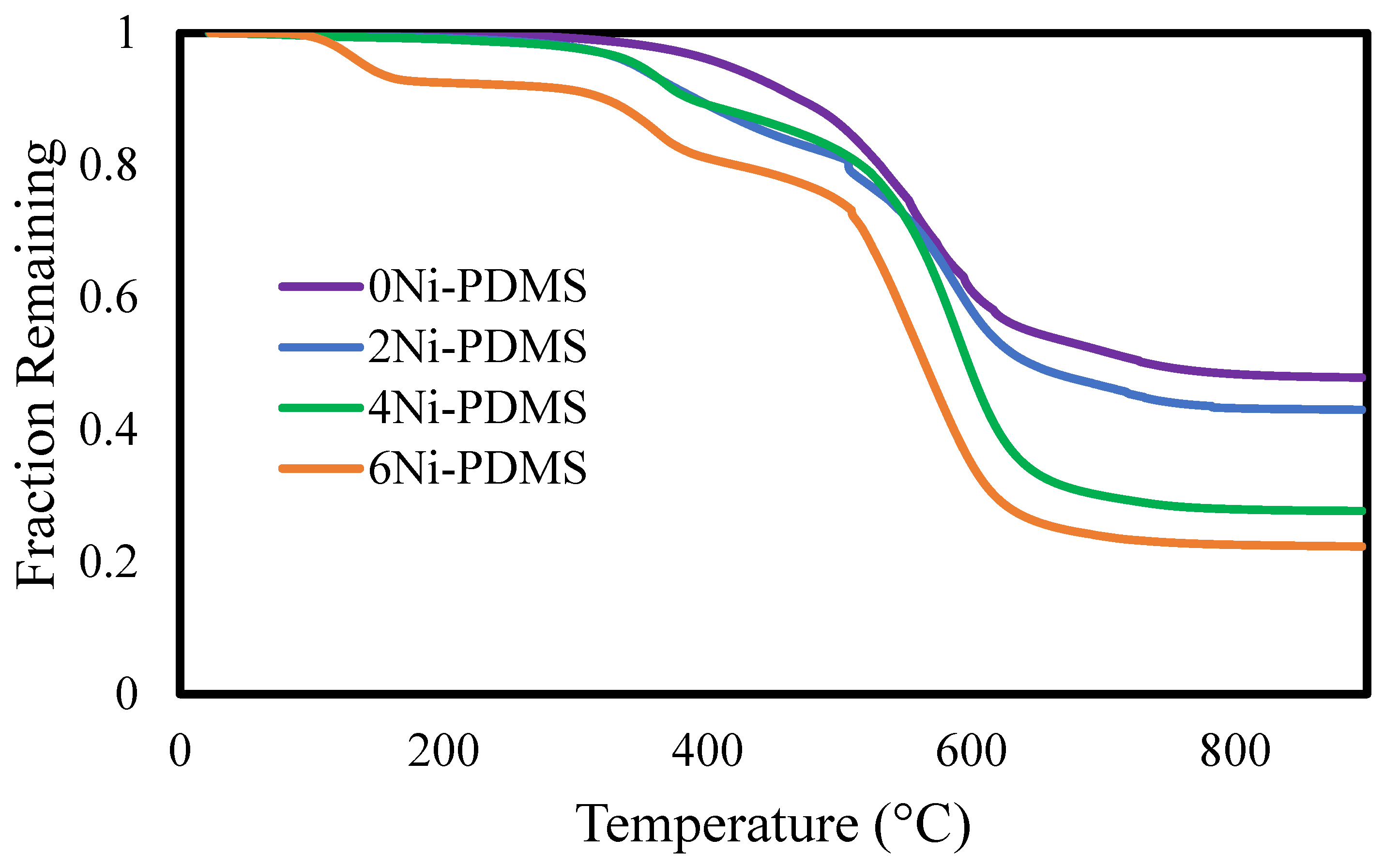
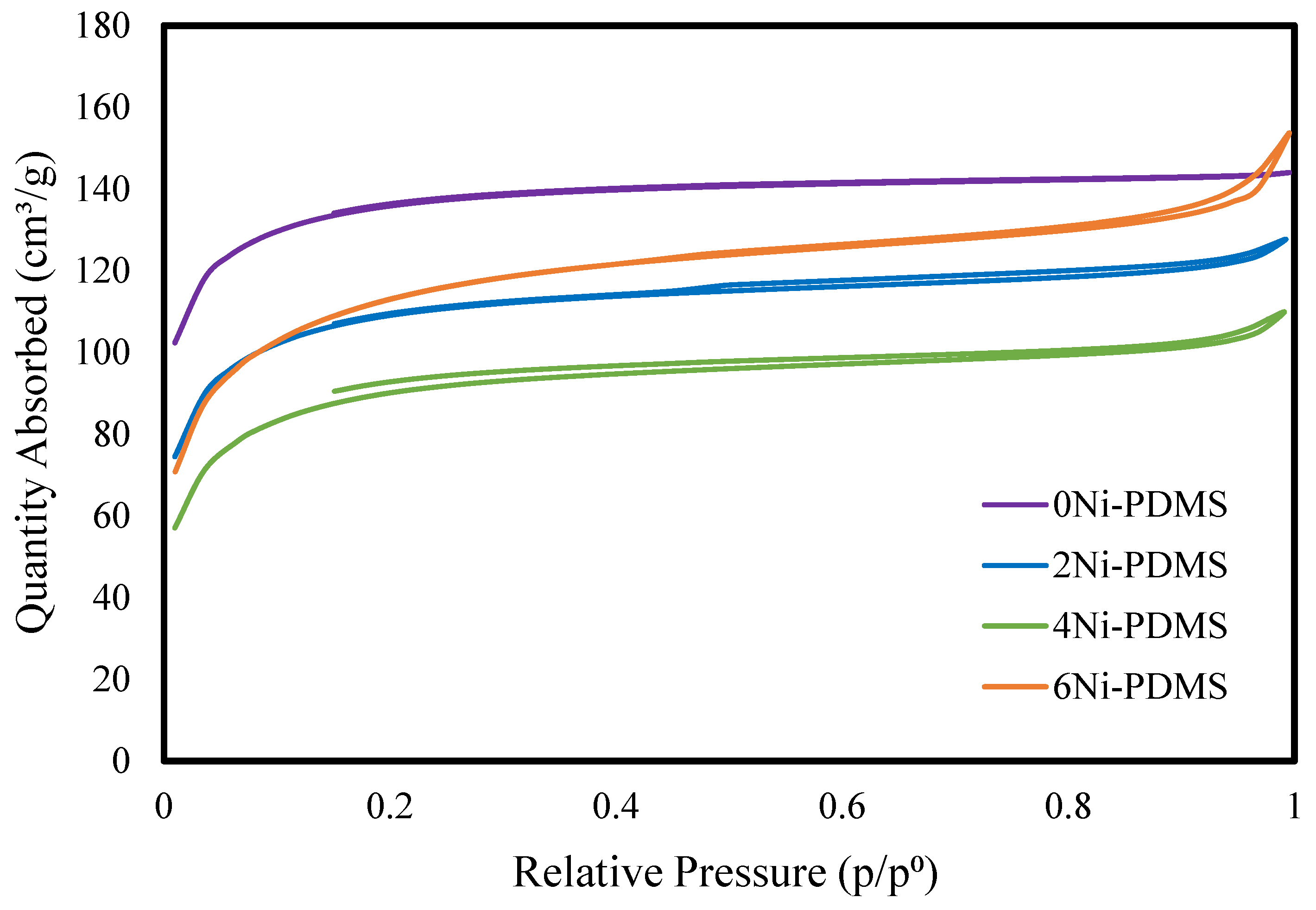
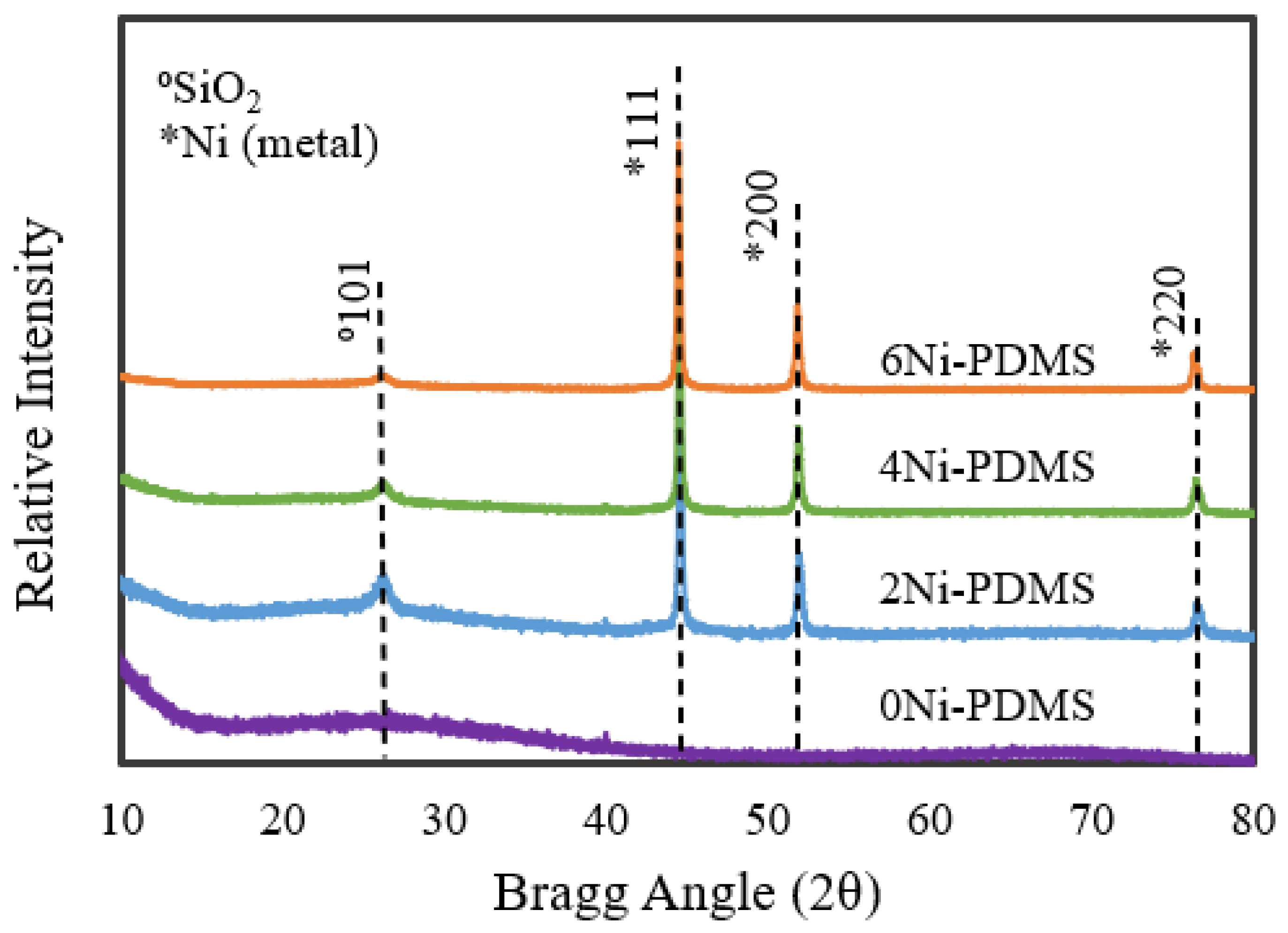
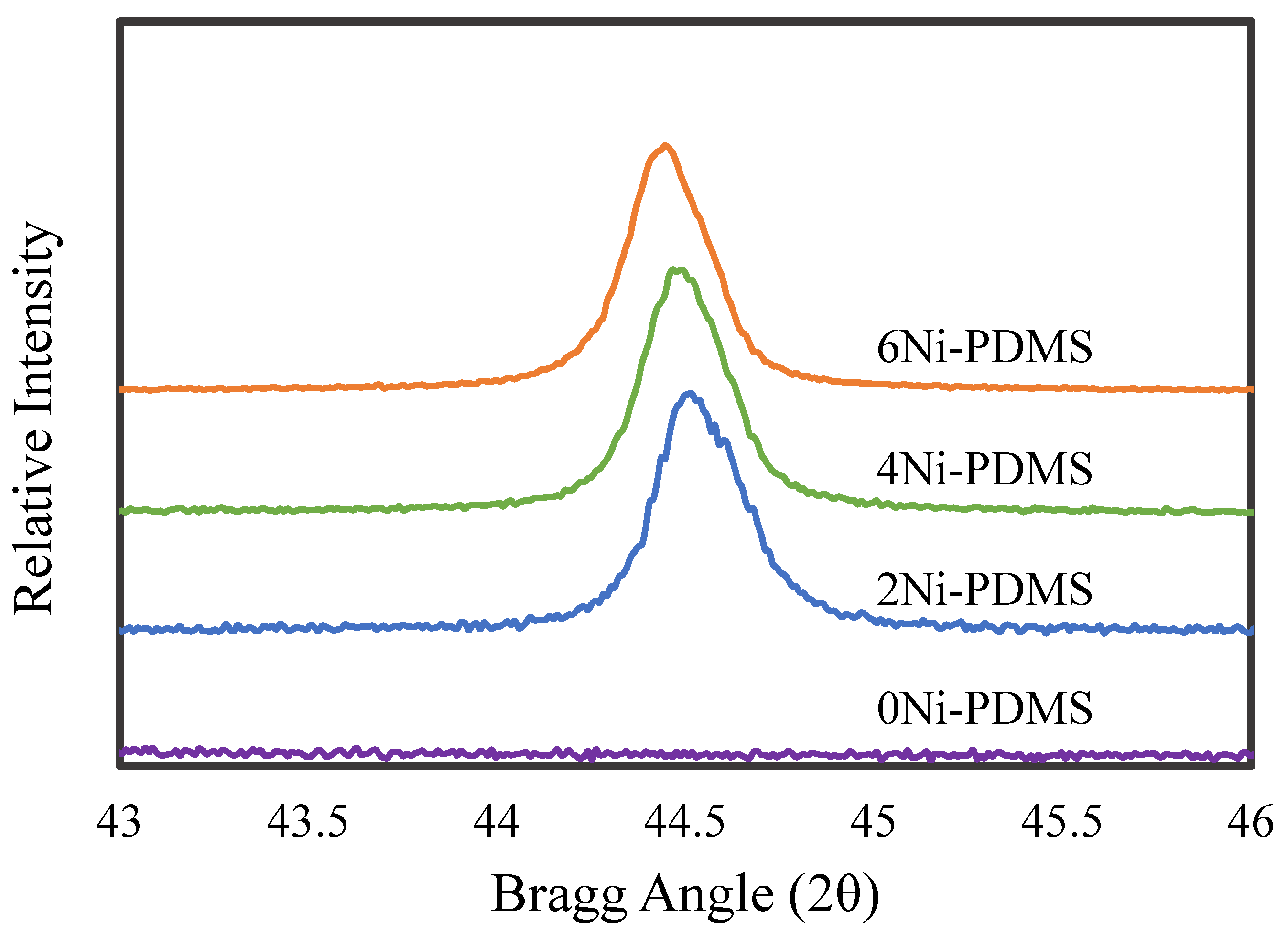
2.1. Catalyst Synthesis and Characterization
2.2. Catalytic Activity
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization Techniques
- 🡺
- The intercept of the BET plot must be positive;
- 🡺
- increases with .
4.3. Catalytic Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavoie, J.M. Review on dry reforming of methane, a potential more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Hussain, I.; Hassan, N.S.; Hambali, H.U.; Siang, T.J.; Vo, D.V.N. Catalytic systems for enhanced carbon dioxide reforming of methane: A review. Environ. Chem. Lett. 2021, 19, 2157–2183. [Google Scholar] [CrossRef]
- Daly, M. EPA Proposes ‘Methane Fee’ for Waste Generated by Oil and Natural Gas Companies. PBS News Hour, 12 January 2024. Available online: https://www.pbs.org/newshour/politics/epa-proposes-methane-fee-for-waste-generated-by-oil-and-natural-gas-companies (accessed on 29 June 2025).
- Obi, I. New Study Quantifies Natural Gas Wasted on U.S. Public and Tribal Lands. Environmental Defense Fund, 26 January 2023. Available online: https://www.edf.org/media/new-study-quantifies-natural-gas-wasted-us-public-and-tribal-lands#:~:text=This%20analysis%20also%20highlights%20that,gas%20lost%20through%20flaring%20alone (accessed on 29 May 2025).
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type. J. Chem. Eng. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Hussien, A.G.S.; Polychronopoulou, K. A review on the different aspects and challenges of the dry reforming of methane (DRM) reaction. Nanomaterials 2022, 12, 3400. [Google Scholar] [CrossRef] [PubMed]
- Wender, I. Reactions of synthesis gas. Fuel Process. Technol. 1996, 48, 189–297. [Google Scholar] [CrossRef]
- Araújo, P.M.; da Costa, K.M.; Passos, F.B. Hydrogen production from methane autothermal reforming over CaTiO3, BaTiO3 and SrTiO3 supported nickel catalysts. Int. J. Hydrogen Energy 2021, 46, 24107–24116. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Roldan Cuenya, B.; Fierro, J.L.G. Partial oxidation of methane to syngas over nickel-based catalysts: Influence of support type, addition of rhodium, and preparation method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef]
- Ganguli, A.; Bhatt, V. Hydrogen production using advanced reactors by steam methane reforming: A review. Front. Therm. Eng. 2023, 3, 1143987. [Google Scholar] [CrossRef]
- Manna, J. Chapter 1.1—Hydrogen economy and international hydrogen strategies. In Towards Hydrogen Infrastructure; Jaiswal-Nagar, D., Dixit, V., Devasahayam, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–38. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Zeng, C.; Sun, W.; Fan, H.; Ma, Q.; Zhao, T.-S. Recent research progress of methane dry reforming to syngas. Fuel 2025, 398, 135535. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Tran, A.V.; Vo, D.-V.N.; Nguyen, H.T.; Rajamohan, N.; Trinh, T.H.; Nguyen, T.L.; Le, Q.V.; Nguyen, T.M. Methane dry reforming: A catalyst challenge awaits. J. Ind. Eng. Chem. 2024, 140, 169–189. [Google Scholar] [CrossRef]
- Baharudin, L.; Rahmat, N.; Othman, N.H.; Shah, N.; Syed-Hassan, S.S.A. Formation, control, and elimination of carbon on Ni-based catalyst during CO2 and CH4 conversion via dry reforming process: A review. J. CO2 Util. 2022, 61, 102050. [Google Scholar] [CrossRef]
- Agún, B.; Abánades, A. Comprehensive review on dry reforming of methane: Challenges and potential for greenhouse gas mitigation. Int. J. Hydrogen Energy 2025, 103, 395–414. [Google Scholar] [CrossRef]
- Essmeister, J.; Schachtner, L.; Szoldatits, E.; Schwarz, S.; Lichtenegger, A.; Baumann, B.; Föttinger, K.; Konegger, T. Polymer-derived Ni/SiOC materials structured by vat-based photopolymerization with catalytic activity in CO2 methanation. Open Ceram. 2023, 14, 100350. [Google Scholar] [CrossRef]
- Xu, Z.; Park, E.D. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2024, 14, 176. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Hussain, I.; Taialla, O.A.; Awad, M.M.; Tanimu, A.; Alhooshani, K.; Ganiyu, S.A. Advances in catalytic dry reforming of methane (DRM): Emerging trends, current challenges, and future perspectives. J. Clean. Prod. 2023, 423, 138638. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F. A review on catalyst advances for photothermal dry reforming of methane reaction. Sep. Purif. Technol. 2025, 354, 128799. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.-Y.; Zheng, H.-Y.; Xu, K.-D.; Hu, Z.; Lei, Y. Enhanced photothermal catalytic performance of dry reforming of methane over Ni/mesoporous TiO2 composite catalyst. Chem. Eng. J. 2022, 429, 132507. [Google Scholar] [CrossRef]
- Lv, H.; Dong, X.; Li, R.; Zeng, C.; Zhang, X.; Song, Y.; Liu, H.; Shao, J.; Ta, N.; Zhao, Q.; et al. Super-dry reforming of methane using a tandem electro-thermocatalytic system. Nat. Chem. 2025, 17, 695–702. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z. Tandem electro-thermocatalytic system: Redefining CO2 utilization efficiency in methane dry reforming. Natl. Sci. Rev. 2025, 12, nwaf212. [Google Scholar] [CrossRef]
- Alawi, N.M.; Al-Mohammedawi, H.H.; Nguyen, H.M.; Azeez, R.A.; Shams, O.A.; Sukkar, K.A. Catalysts for Reforming of Methane (A Review). Pet. Chem. 2024, 64, 964–971. [Google Scholar] [CrossRef]
- Bhaskaran, A.; Roy, S. Exploring dry reforming of CH4 to syngas using high-entropy materials: A novel emerging approach. ChemCatChem 2024, 17, e202401297. [Google Scholar] [CrossRef]
- Chai, Y.; Fu, Y.; Feng, H.; Kong, W.; Yuan, C.; Pan, B.; Zhang, J.; Sun, Y. A nickel-based perovskite catalyst with a bimodal size distribution of nickel particles for dry reforming of methane. ChemCatChem 2018, 10, 2078–2086. [Google Scholar] [CrossRef]
- Baudouina, D.; Rodemerck, U.; Krumeich, F.; de Mallmann, A.; Szeto, K.C.; Ménard, H.; Veyre, L.; Candy, J.P.; Webb, P.B.; Thieuleux, C.; et al. Particle size effect in the low temperature reforming of methane by carbon dioxide on silica-supported Ni nanoparticles. J. Catal. 2013, 297, 27–34. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [PubMed]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Lale, A.; Schmidt, M.; Mallmann, M.D.; Bezerra, A.V.A.; Acosta, E.D.; Machado, R.A.F.; Demirci, U.B.; Bernard, S. Polymer-derived ceramics with engineered mesoporosity: From design to application in catalysis. Surf. Coat. Technol. 2018, 350, 569–586. [Google Scholar] [CrossRef]
- Erb, D.; Lu, K. Additive and pyrolysis atmosphere effects on polysiloxane-derived porous SiOC ceramics. J. Eur. Ceram. Soc. 2017, 37, 4547–4557. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Hourlier, D. Heat treatment of commercial Polydimethylsiloxane PDMS precursors: Part I. Towards conversion of patternable soft gels into hard ceramics. Ceram. Int. 2019, 45, 6255–6262. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, A.; Xu, J. Tailored synthesis of monodispersed nano/submicron porous silicon oxycarbide (SiOC) spheres with improved Li-storage performance as an anode material for Li-ion batteries. J. Power Sources 2017, 364, 288–298. [Google Scholar] [CrossRef]
- Wang, J.; Gili, A.; Grünbacher, M.; Praetz, S.; Epping, J.D.; Görke, O.; Schuck, G.; Penner, S.; Schlesiger, C.; Schomäcker, R.; et al. Silicon oxycarbonitride ceramic containing nickel nanoparticles: From design to catalytic application. Mater. Adv. 2021, 2, 1715–1730. [Google Scholar] [CrossRef]
- De Jesus, J.C.; González, I.; Quevedo, A.; Puerta, T. Thermal decomposition of nickel acetate tetrahydrate: An integrated study by TGA, QMS and XPS techniques. J. Mol. Catal. A Chem. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer 2002, 43, 2011–2015. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, L.; Ma, Q. Influence of Ni on the structural evolution of polymer-derived SiOC ceramics. J. Mater. Res. Technol. 2022, 20, 4515–4524. [Google Scholar] [CrossRef]
- Rupasinghe, B. Transformations of Siloxane-Based Materials Toward a Reuse and Recycling Loop: Catalytic Methods and Photochemistry. Ph.D. Thesis, College of Bowling Green, Bowling Green State University, Bowling Green, OH, USA, 2022. Available online: https://etd.ohiolink.edu/acprod/odb_etd/ws/send_file/send?accession=bgsu164795589014941&disposition=inline (accessed on 30 May 2025).
- Zhang, C.; Xu, Z.; Hu, Y.; He, J.; Tian, M.; Zhou, J.; Zhou, Q.; Chen, S.; Chen, D.; Chen, P.; et al. Novel insights into the hydroxylation behaviors of α-quartz (101) surface and its effects on the adsorption of sodium oleate. Minerals 2019, 9, 450. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Zhang, J.; Li, J. Large scale synthesis and characterization of Ni nanoparticles by solution reaction method. Bull. Mater. Sci. 2008, 31, 97–100. [Google Scholar] [CrossRef]
- Kumar, R.; Kar, M. Correlation between lattice strain and magnetic behavior in non-magnetic Ca substituted nano-crystalline cobalt ferrite. Ceram. Int. 2016, 42, 6640–6647. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, S.; Yang, B. Promoted coke resistance of Ni by surface carbon for the dry reforming of methane. Science 2023, 26, 106237. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.M.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Su, D.; Li, Y.L.; An, H.J.; Liu, X.; Hou, F.; Li, J.Y.; Fu, X. Pyrolytic transformation of liquid precursors to shaped bulk ceramics. J. Eur. Ceram. Soc. 2010, 30, 1503–1511. [Google Scholar] [CrossRef]
- Pan, J.; Yan, X.; Cheng, X.; Shen, W.; Li, S.; Cai, X. In situ synthesis and electrical properties of porous SiOC ceramics decorated with SiC nanowires. Ceram. Int. 2016, 42, 12345–12351. [Google Scholar] [CrossRef]
- Johnson, T.J.; Myers, T.L.; Su, Y.F.; Tonkyn, R.G.; Kelly-Gorham, M.R.K.; Danby, T.O. Quartz (SiO2) Infrared Spectrum, NIST Chem. WebBook. 2017. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C14808607&Contrib=IARPA-IR-S&Type=IR-SPEC&Index=0#Notes (accessed on 30 May 2025).
- Wang, F.; Yu, Z.; Shi, K.; Li, X.; Lu, K.; Huang, W.; Yu, C.; Yang, K. One-Pot Synthesis of N-Doped NiO for Enhanced Photocatalytic CO2 Reduction with Efficient Charge Transfer. Molecules 2023, 28, 2435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cannizzaro, F.; Liu, L.; Zhang, H.; Kosinov, N.; Filot, I.A.W.; Rabeah, J.; Brückner, A.; Hensen, E.J.M. Ni–in synergy in CO2 hydrogenation to methanol. ACS Catal. 2021, 11, 11371–11384. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, M.K.; Laorenza, D.W.; Schaller, R.D.; Freedman, D.E. Nickel(II) metal complexes as optically addressable qubit candidates. J. Am. Chem. Soc. 2020, 142, 14826–14830. [Google Scholar] [CrossRef]
- Szoldatits, E.; Essmeister, J.; Schachtner, L.; Konegger, T.; Föttinger, K. Polymer-derived SiOC as support material for Ni-based catalysts: CO2 methanation performance and effect of support modification with La2O3. Front. Chem. 2023, 11, 1163503. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B. 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Yue, L.; Li, J.; Chen, C.; Fu, X.; Gong, Y.; Xia, X.; Hou, J.; Xiao, C.; Chen, X.; Zhao, L.; et al. Thermal-stable Pd@mesoporous silica core-shell nanocatalysts for dry reforming of methane with good coke-resistant performance. Fuel 2018, 218, 335–341. [Google Scholar] [CrossRef]
- Chein, R.; Yang, Z. Experimental study on dry reforming of biogas for syngas production over Ni-based catalysts. ACS Omega 2019, 4, 20911–20922. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis gas production via the biogas reforming reaction over Ni/MgO–Al2O3 and Ni/CaO–Al2O3 catalysts. Waste Biomass Valori. 2016, 7, 725–736. [Google Scholar] [CrossRef]
- Donphai, W.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J. Effect of Ni-CNTs/mesocellular silica composite catalysts on carbon dioxide reforming of methane. Appl. Catal. A Gen. 2014, 475, 16–26. [Google Scholar] [CrossRef]
- Wang, J.; Grünbacher, M.; Penner, S.; Bekheet, M.F.; Gurlo, A. Porous silicon oxycarbonitride ceramics with palladium and Pd2Si nanoparticles for dry reforming of methane. Polymers 2022, 14, 3470. [Google Scholar] [CrossRef] [PubMed]
- Fitting and Optimizing BET Surface Area. Available online: https://www.azom.com/article.aspx?ArticleID=10480 (accessed on 4 April 2025).
- Choma, J.; Jaroniec, M.; Kloske, M. Improved pore-size analysis of carbonaceous adsorbents. Adsorpt. Sci. Technol. 2002, 20, 307–315. [Google Scholar] [CrossRef]
- Alabi, W.O.; Wang, H.; Adesanmi, B.M.; Shakouri, M.; Hu, Y. Support composition effect on the structures, metallic sites formation, and performance of Ni-Co-Mg-Al-O composite for CO2 reforming of CH4. J. CO2 Util. 2021, 43, 101355. [Google Scholar] [CrossRef]

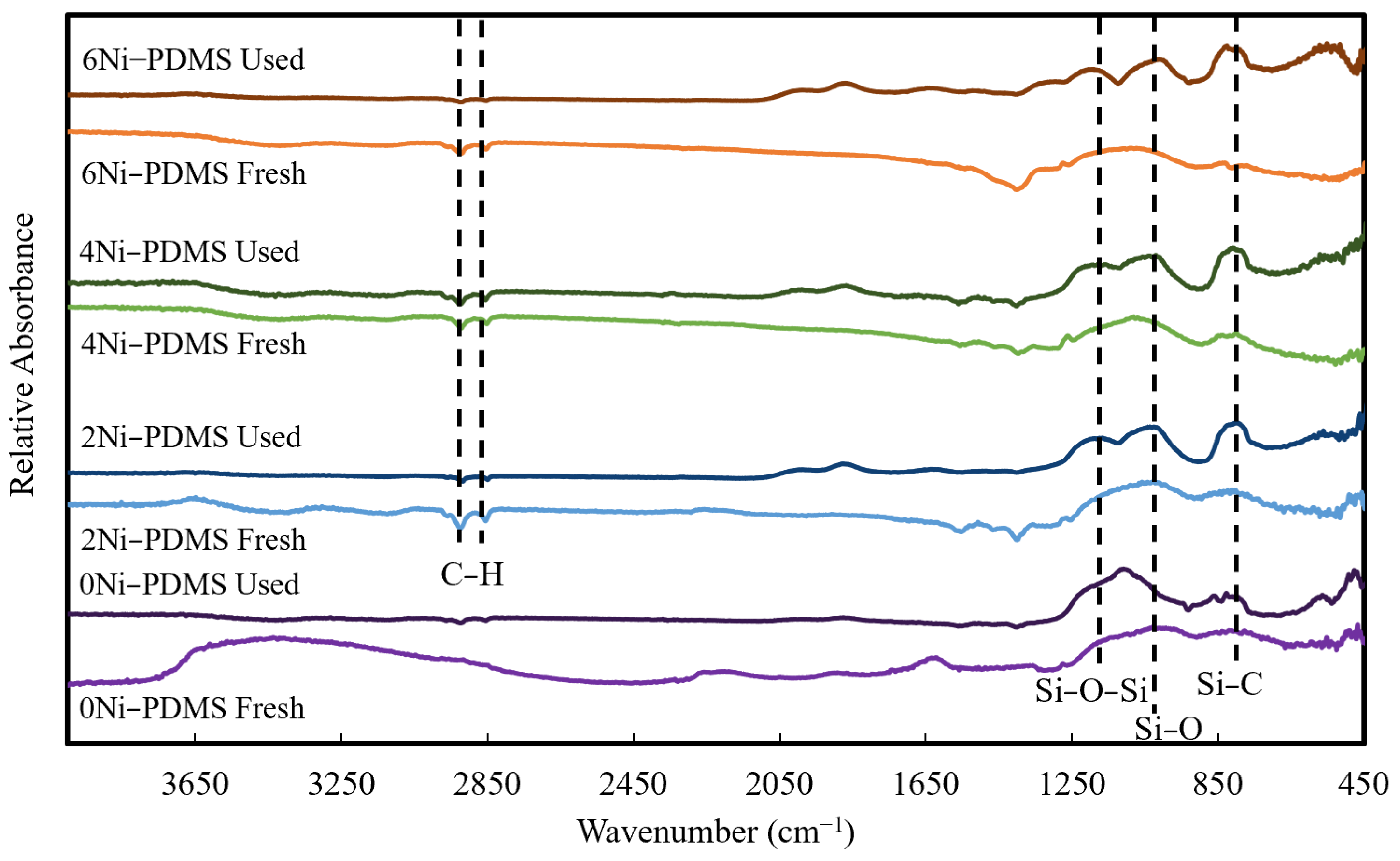
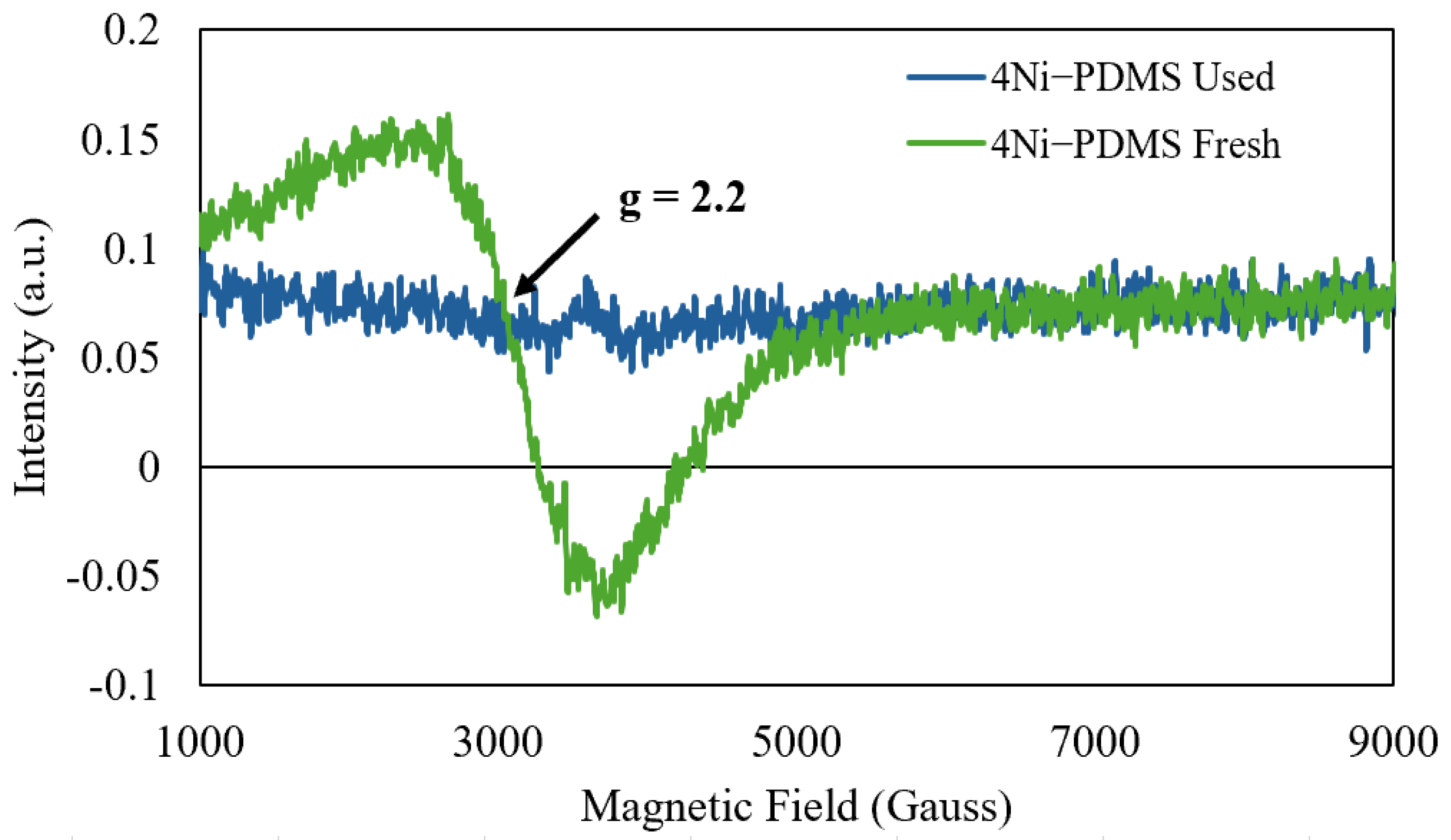

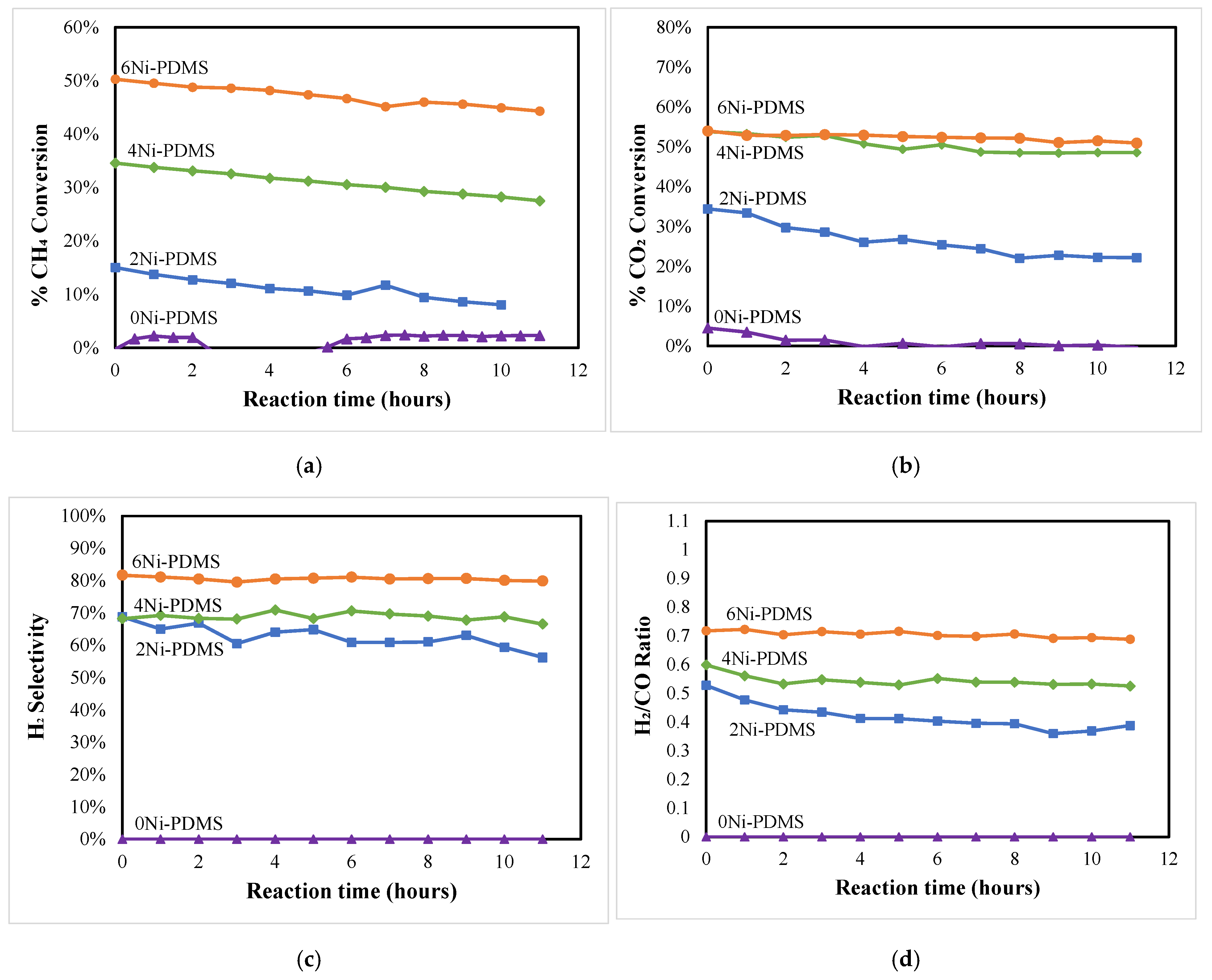

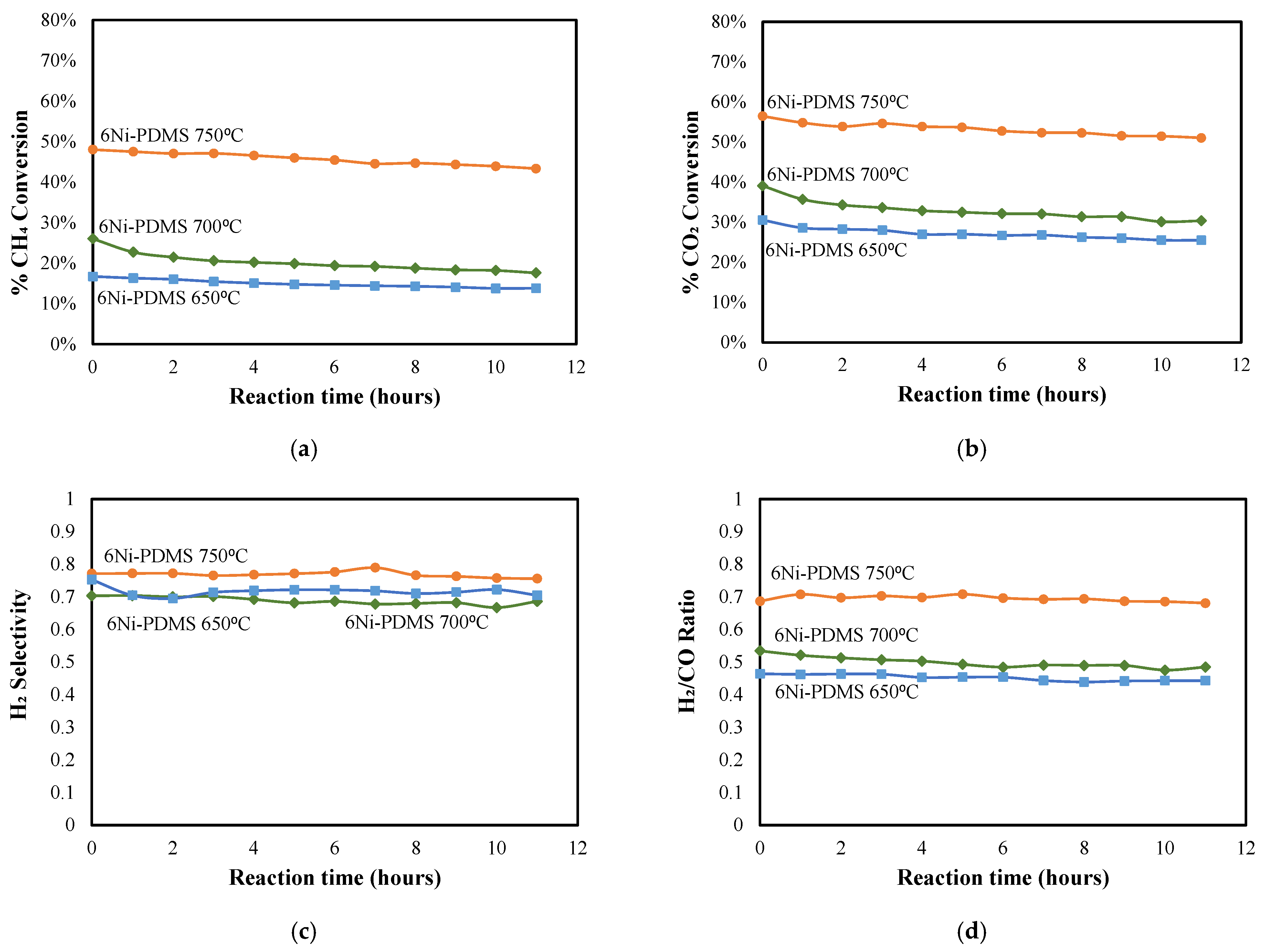
| Catalyst ID | Ni-PDMS Before Pyrolysis | Ni-PDMS After Pyrolysis | ||
|---|---|---|---|---|
| Nominal wt% Ni | Estimated Molar Ratio Ni/Si | Estimated wt% Ni 1 | EDS Molar Ratio Ni/Si | |
| 0Ni-PDMS | 0% | 0 | 0% | 0 |
| 2Ni-PDMS | 1.85% | 0.053 | 4.3% | 0.147 |
| 4Ni-PDMS | 3.44% | 0.106 | 12.4% | 0.202 |
| 6Ni-PDMS | 5.94% | 0.210 | 21.8% | 0.190+/−0.105 |
| Catalyst ID | <300 °C | 300–500 °C | >500 °C | Total |
|---|---|---|---|---|
| 0Ni-PDMS | 0.8% | 13.1% | 44.5% | 52.1% |
| 2Ni-PDMS | 2.2% | 16.9% | 47.1% | 57.0% |
| 4Ni-PDMS | 2.3% | 15.9% | 66.3% | 72.3% |
| 6Ni-PDMS | 8.7% | 18.3% | 70.1% | 77.7% |
| Catalyst | BET Surface Area | Pore Volume | Micropore Volume 1 | Average Pore Width 2 | Average Micropore Width 1 | Ni Crystal Size 3 |
|---|---|---|---|---|---|---|
| ID | (m2/g) | (cm3/g) | (cm3/g) | (Å) | (Å) | (Å) |
| 0Ni-PDMS | 520 | 0.222 | 0.198 | 17.2 | 16.2 | N/A |
| 2Ni-PDMS | 409 | 0.195 | 0.153 | 19.3 | 16.56 | 334 |
| 4Ni-PDMS | 337 | 0.168 | 0.123 | 20.2 | 16.63 | 362 |
| 6Ni-PDMS | 417 | 0.231 | 0.172 | 22.8 | 17.82 | 306 |
| Catalyst | Catalyst Synthesis Method | Operating Temperature | CH4 Conversion | CO2 Conversion | H2/CO Ratio | TOF 1 | Activity Loss | Reference |
|---|---|---|---|---|---|---|---|---|
| ID | (°C) | (%) | (%) | (min−1) | (%) | |||
| 6Ni-PDMS | Pyrolyzed Ni-PDMS | 750 | 47 | 54 | 0.70 | 2.07 ± 0.079 | 9.8 | This work |
| 6Ni-PDMS | Pyrolyzed Ni-PDMS | 700 | 22 | 34 | 0.51 | 0.70 ± 0.36 | 30 | This work |
| 6Ni-PDMS | Pyrolyzed Ni-PDMS | 650 | 16 | 29 | 0.46 | 0.52 ± 0.05 | 19 | This work |
| 4Ni-PDMS | Pyrolyzed Ni-PDMS | 750 | 37 | 51 | 0.53 | 3.11 ± 0.53 | 17 | This work |
| 2Ni-PDMS | Pyrolyzed Ni-PDMS | 750 | 15 | 25 | 0.41 | 3.90 ± 1.06 | 49 | This work |
| Ni@SiO2 | SiO2 core–shell Ni nanoparticle catalyst | 750 | 58 | 72 | 0.75 | 0.998 | 5.0 | [51] |
| Pd/SiO2 | Impregnation | 750 | 65 | 70 | - | 75 | 66 | [52] |
| Pt/Al2O3 | Impregnation | 750 | 60 | 76 | 0.73 | 26.1 | 6.7 | [53] |
| Ni/Al2O3 | Impregnation | 750 | 75 | 76 | 0.75 | 2.95 | 14 | [53] |
| Ni/Al2O3 | Impregnation | 750 | 50 | 65 | 0.80 | 11.0 | - | [54] |
| Ni-CNTs, mesocellular silica | Chemical vapor deposition technique | 650 | 50 | 58 | 0.62 | 2.62 | 7.5 | [55] |
| Trials | Reaction Temperature | Feed Gas Molar Ratio | Catalysts |
|---|---|---|---|
| 1–4 | 750 °C | 1:1:1 CH4:CO2:N2 | all Ni-PDMS catalysts |
| 5 | 650 °C | 1:1:1 CH4:CO2:N2 | 6Ni-PDMS |
| 6 | 700 °C | 1:1:1 CH4:CO2:N2 | 6Ni-PDMS |
| 7 | 750 °C | 1:2:1 CH4:CO2:N2 | 6Ni-PDMS |
| 8 | 750 °C | 2:1:1 CH4:CO2:N2 | 6Ni-PDMS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olp, R.; Hohn, K.L.; Almquist, C.B. A Novel Polymer-Derived Ni/SiOC Catalyst for the Dry Reforming of Methane. Catalysts 2025, 15, 645. https://doi.org/10.3390/catal15070645
Olp R, Hohn KL, Almquist CB. A Novel Polymer-Derived Ni/SiOC Catalyst for the Dry Reforming of Methane. Catalysts. 2025; 15(7):645. https://doi.org/10.3390/catal15070645
Chicago/Turabian StyleOlp, Rachel, Keith L. Hohn, and Catherine B. Almquist. 2025. "A Novel Polymer-Derived Ni/SiOC Catalyst for the Dry Reforming of Methane" Catalysts 15, no. 7: 645. https://doi.org/10.3390/catal15070645
APA StyleOlp, R., Hohn, K. L., & Almquist, C. B. (2025). A Novel Polymer-Derived Ni/SiOC Catalyst for the Dry Reforming of Methane. Catalysts, 15(7), 645. https://doi.org/10.3390/catal15070645









