Rationally Designed 2D CZIS/2D Ti3CNTx Heterojunctions for Photocatalytic Hydrogen Evolution Reaction
Abstract
1. Introduction
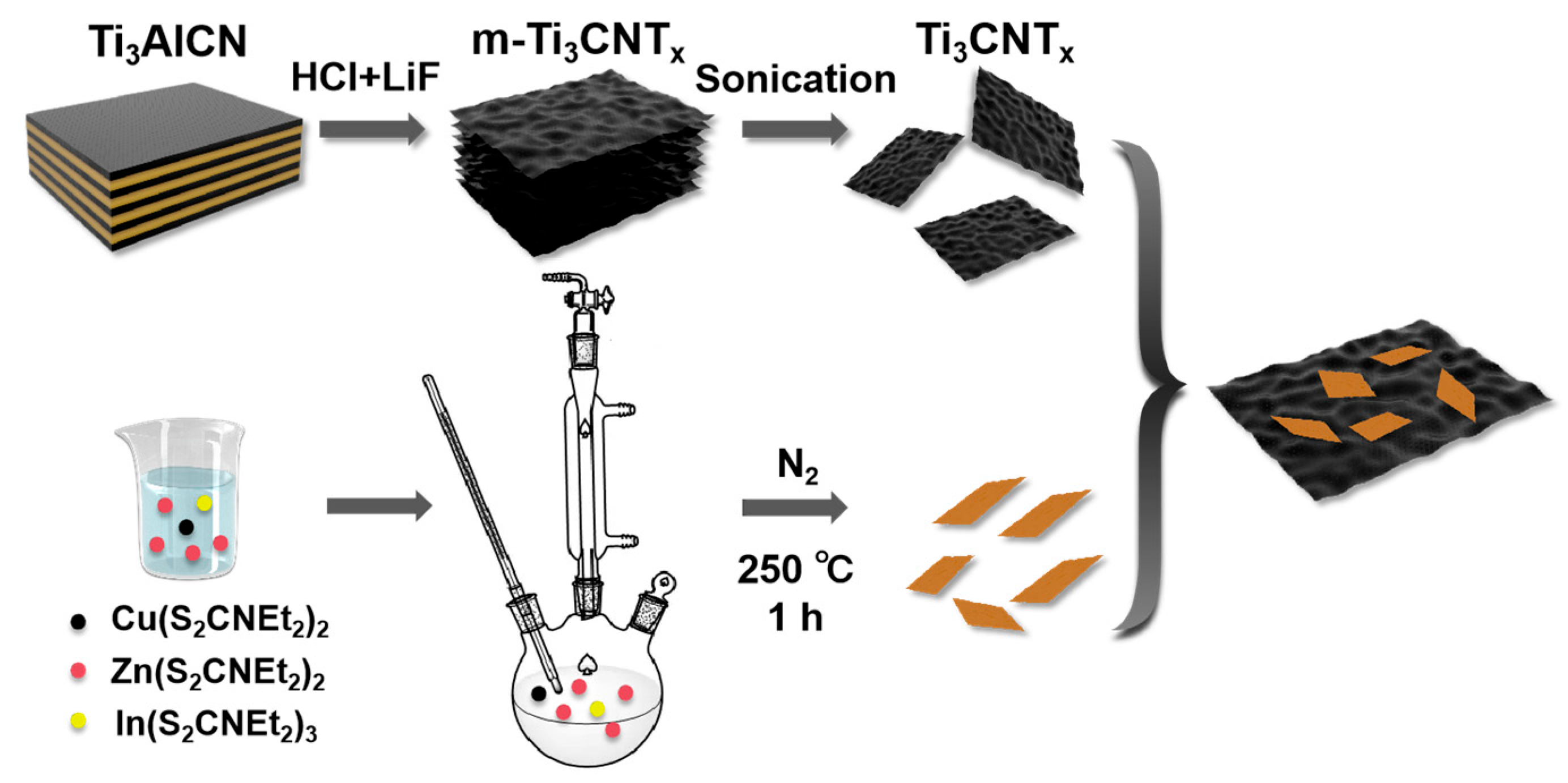
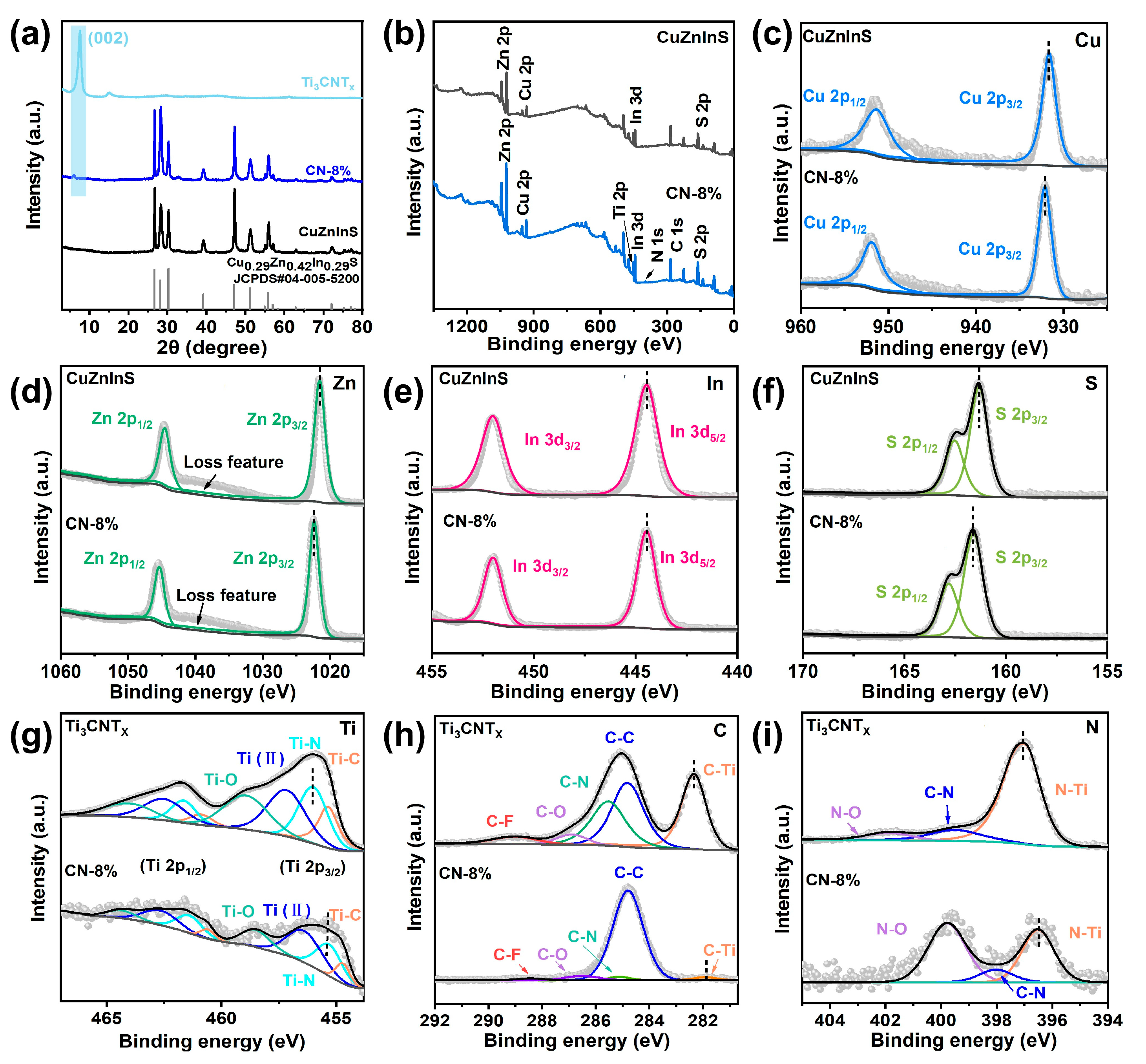
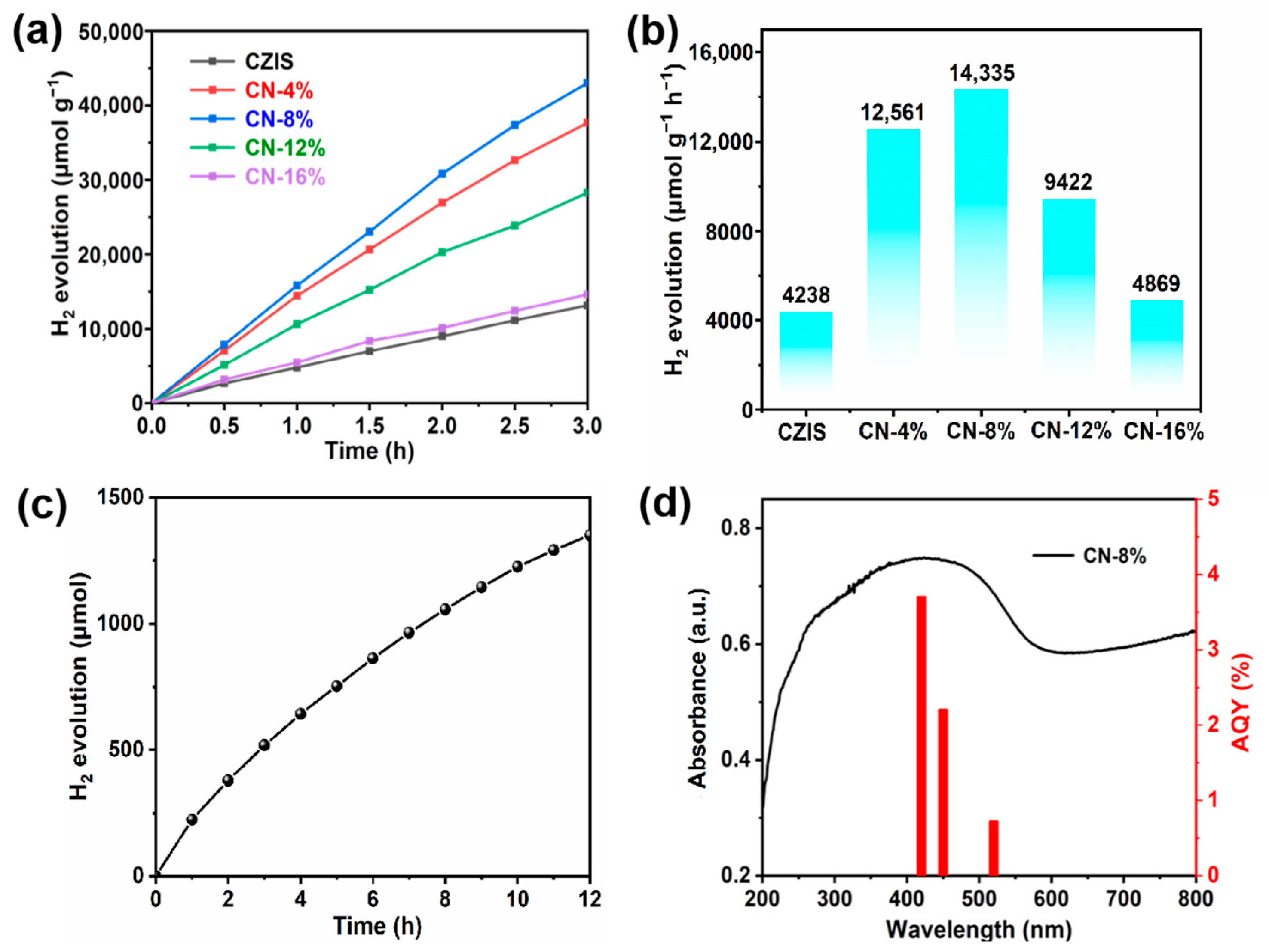
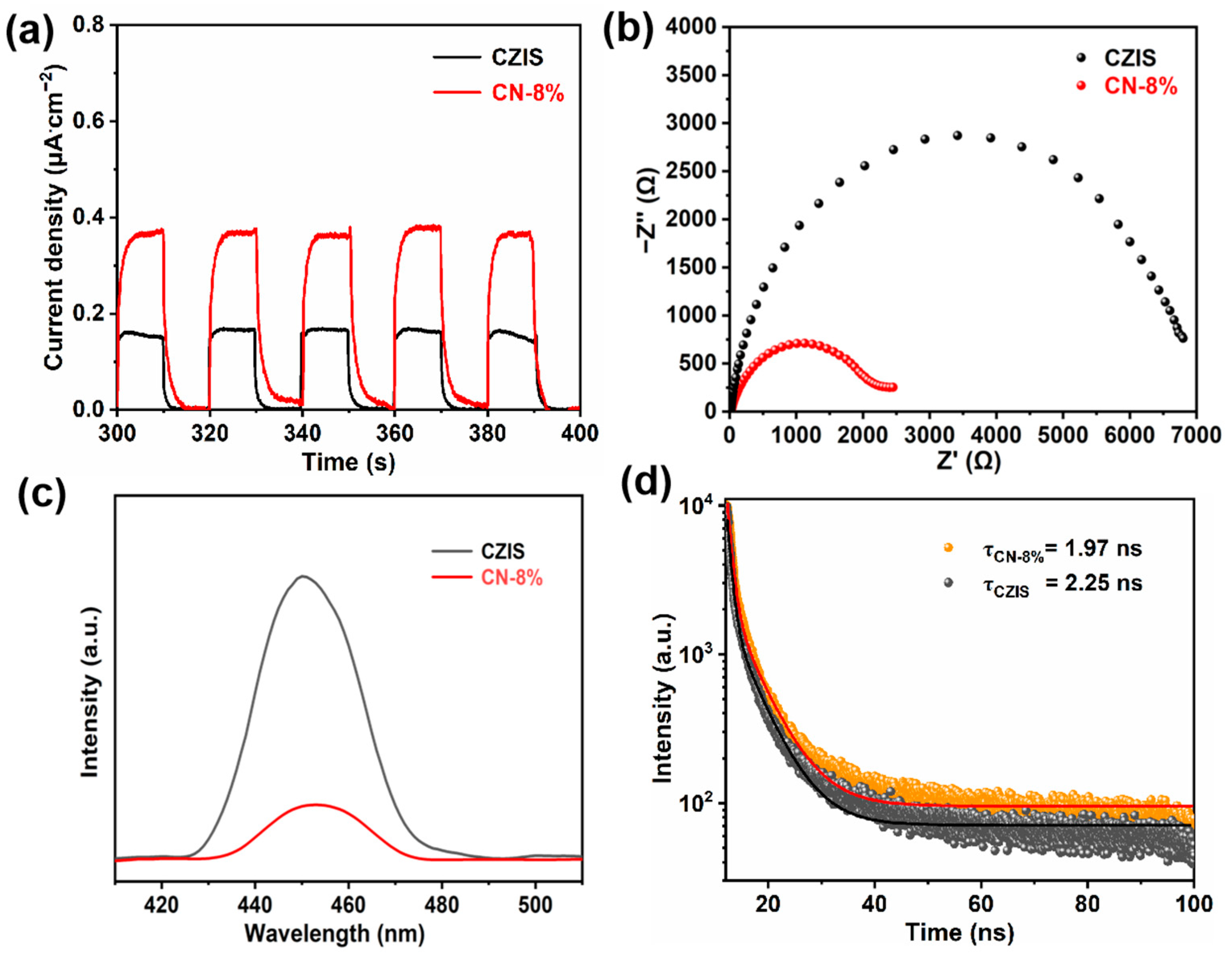
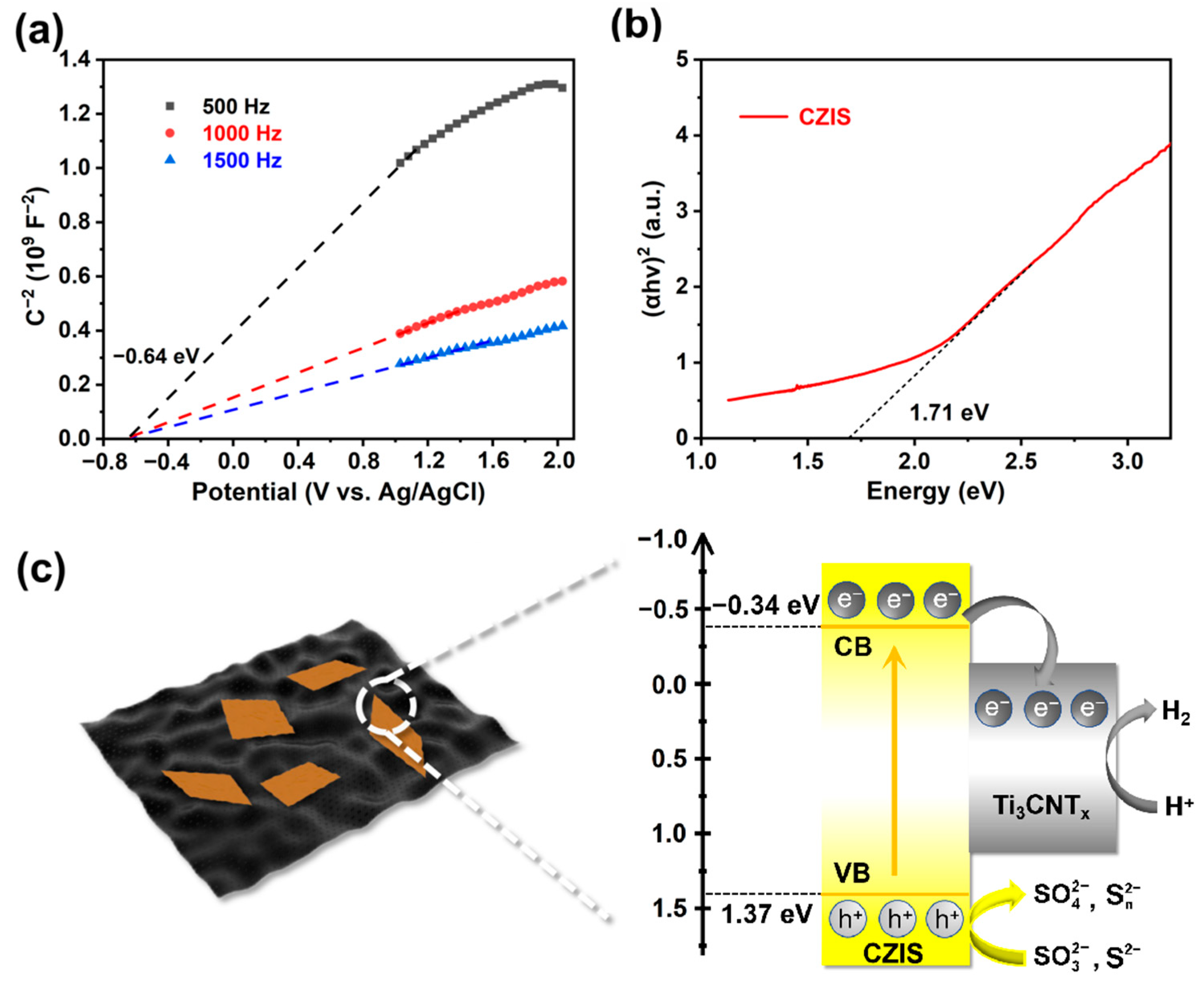
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A Review on Heterogeneous Photocatalysis for Environmental Remediation: From Semiconductors to Modification Strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Liu, B.-J.; Chen, Q.; Mo, Q.-L.; Xiao, F.-X. Robust, Versatile, Green and Emerging Layer-by-Layer Self-Assembly Platform for Solar Energy Conversion. Coord. Chem. Rev. 2023, 493, 215285. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-Hydrogen Efficiency of More than 9% in Photocatalytic Water Splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current Understanding and Challenges of Solar-Driven Hydrogen Generation Using Polymeric Photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting Polymers for Oxygen Evolution Reaction under Light Illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Wrede, S.; Liu, A.; Brnovic, A.; Wang, S.; Axelsson, M.; Tian, H. Preparation, Characterization, Evaluation and Mechanistic Study of Organic Polymer Nano-Photocatalysts for Solar Fuel Production. Chem. Soc. Rev. 2022, 51, 6909–6935. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, M.; Cao, X.; Wang, H.; Xia, L.; Jiang, W.; Huang, M.; He, L.; Zhao, X.; Zhou, Y. Progress in Preparation, Identification and Photocatalytic Application of Defective g-C3N4. Coord. Chem. Rev. 2024, 510, 215849. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kibria, M.G.; Mullins, C.B. Metal-Free Photocatalysts for Hydrogen Evolution. Chem. Soc. Rev. 2020, 49, 1887–1931. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q.; Du, M.; Zeng, X.; Zhong, G.; Qiu, B.; Zhang, J. Recent Progress of Metal Sulfide Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, 2202929. [Google Scholar] [CrossRef]
- Zheng, X.; Song, Y.; Gao, Q.; Lin, J.; Zhai, J.; Shao, Z.; Li, J.; Wu, D.; Shi, X.; Liu, W.; et al. Controllable-Photocorrosion Balance Endows ZnCdS Stable Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2025, 35, 2506159. [Google Scholar] [CrossRef]
- Murtaza, G.; Alderhami, S.; Alharbi, Y.T.; Zulfiqar, U.; Hossin, M.; Alanazi, A.M.; Almanqur, L.; Onche, E.U.; Venkateswaran, S.P.; Lewis, D.J. Scalable and Universal Route for the Deposition of Binary, Ternary, and Quaternary Metal Sulfide Materials from Molecular Precursors. ACS Appl. Energy Mater. 2020, 3, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wu, Z.; Huang, K.; Ye, J.; Zhang, H. Surface Modification of 2D Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, 2200180. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Q.; Zhuang, T.-T.; Li, Y.; Zhang, G.; Liu, G.-Q.; Fan, F.-J.; Shi, L.; Yu, S.-H. Single Crystalline Quaternary Sulfide Nanobelts for Efficient Solar-to-Hydrogen Conversion. Nat. Commun. 2020, 11, 5194. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Su, F.; Liu, T.; Liu, G.-Q.; Li, Y.; Ma, T.; Wang, Y.; Zhang, C.; Yang, Y.; Yu, S.-H. Phosphorus-Doped Single-Crystalline Quaternary Sulfide Nanobelts Enable Efficient Visible-Light Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2022, 144, 20620–20629. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Yu, L.; Nguyen, T.H.; Ikeda, S.; Jiang, F. Over 1% Efficient Unbiased Stable Solar Water Splitting Based on a Sprayed Cu2 ZnSnS4 Photocathode Protected by a HfO2 Photocorrosion-Resistant Film. ACS Energy Lett. 2018, 3, 1875–1881. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, A.; Liu, J.; Zhu, D.; Shi, X.; Kong, Q.; Wang, Z.; Qu, S.; Teng, F.; Wang, Z. Non-Injection Synthesis of L-Shaped Wurtzite Cu–Ga–Zn–S Alloyed Nanorods and Their Advantageous Application in Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2018, 6, 18649–18659. [Google Scholar] [CrossRef]
- Ding, M.; Cui, S.; Lin, Z.; Yang, X. Crystal Facet Engineering of Hollow Cadmium Sulfide for Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2024, 357, 124333. [Google Scholar] [CrossRef]
- Tong, R.; Ng, K.W.; Wang, X.; Wang, S.; Wang, X.; Pan, H. Two-Dimensional Materials as Novel Co-Catalysts for Efficient Solar-Driven Hydrogen Production. J. Mater. Chem. A 2020, 8, 23202–23230. [Google Scholar] [CrossRef]
- Guan, Y.; Zhao, R.; Cong, Y.; Chen, K.; Wu, J.; Zhu, H.; Dong, Z.; Zhang, Q.; Yuan, G.; Li, Y.; et al. Flexible Ti2C MXene Film: Synthesis, Electrochemical Performance and Capacitance Behavior. Chem. Eng. J. 2022, 433, 133582. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Motta Meira, D.; Colón, G.; Kubacka, A.; Fernández-García, M. Phase-Contact Engineering in Mono- and Bimetallic Cu-Ni Co-catalysts for Hydrogen Photocatalytic Materials. Angew. Chem. Int. Ed. 2018, 57, 1199–1203. [Google Scholar] [CrossRef]
- Indra, A.; Acharjya, A.; Menezes, P.W.; Merschjann, C.; Hollmann, D.; Schwarze, M.; Aktas, M.; Friedrich, A.; Lochbrunner, S.; Thomas, A.; et al. Boosting Visible-Light-Driven Photocatalytic Hydrogen Evolution with an Integrated Nickel Phosphide–Carbon Nitride System. Angew. Chem. Int. Ed. 2017, 56, 1653–1657. [Google Scholar] [CrossRef]
- Feng, W.; Yuan, J.; Gao, F.; Weng, B.; Hu, W.; Lei, Y.; Huang, X.; Yang, L.; Shen, J.; Xu, D.; et al. Piezopotential-Driven Simulated Electrocatalytic Nanosystem of Ultrasmall MoC Quantum Dots Encapsulated in Ultrathin N-Doped Graphene Vesicles for Superhigh H2 Production from Pure Water. Nano Energy 2020, 75, 104990. [Google Scholar] [CrossRef]
- Tang, R.; Xiong, S.; Gong, D.; Deng, Y.; Wang, Y.; Su, L.; Ding, C.; Yang, L.; Liao, C. Ti3C2 2D MXene: Recent Progress and Perspectives in Photocatalysis. ACS Appl. Mater. Inter. 2020, 12, 56663–56680. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Z.; Sa, B.; Miao, N.; Zhou, J.; Sun, Z. Computational Design of Double Transition Metal MXenes with Intrinsic Magnetic Properties. Nanoscale Horiz. 2022, 7, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Li, C.; Liu, S.-Y.; Fang, B.; Yang, H. Z-Scheme Systems: From Fundamental Principles to Characterization, Synthesis, and Photocatalytic Fuel-Conversion Applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Mai, T.; Guo, W.-Y.; Wang, P.-L.; Chen, L.; Qi, M.-Y.; Liu, Q.; Ding, Y.; Ma, M.-G. Bilayer Metal-Organic Frameworks/MXene/Nanocellulose Paper with Electromagnetic Double Loss for Absorption-Dominated Electromagnetic Interference Shielding. Chem. Eng. J. 2023, 464, 142517. [Google Scholar] [CrossRef]
- Ying, Y.; Lin, Z.; Huang, H. “Edge/Basal Plane Half-Reaction Separation” Mechanism of Two-Dimensional Materials for Photocatalytic Water Splitting. ACS Energy Lett. 2023, 8, 1416–1423. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Z.; Wang, R.; Guo, Y.; Wang, L.; Hu, Q.; Liu, K.; Li, N.; Zhou, A. 2D MoB MBene: An Efficient Co-Catalyst for Photocatalytic Hydrogen Production under Visible Light. ACS Nano 2024, 18, 12524–12536. [Google Scholar] [CrossRef]
- Kwon, N.H.; Yun, S.Y.; Lim, J.; Hwang, S.-J. Recent Advances in High-Performance Catalysts Hybridized with Two-Dimensional Conductive Inorganic Nanosheets. Nano Energy 2024, 122, 109315. [Google Scholar] [CrossRef]
- Liu, G.; Zhen, C.; Kang, Y.; Wang, L.; Cheng, H.-M. Unique Physicochemical Properties of Two-Dimensional Light Absorbers Facilitating Photocatalysis. Chem. Soc. Rev. 2018, 47, 6410–6444. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, J.; Hao, G.; Jiang, W.; Di, J. 2D Atomic Layers for CO2 Photoreduction. Small 2024, 20, 2306742. [Google Scholar] [CrossRef]
- Yu, H.; Dai, M.; Zhang, J.; Chen, W.; Jin, Q.; Wang, S.; He, Z. Interface Engineering in 2D/2D Heterogeneous Photocatalysts. Small 2023, 19, 2205767. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, X.; Zhang, K.; Ma, Y.; Wang, H.; Li, H.; Huang, H.; Sun, X.; Ma, T. Construction of a 2D/2D Crystalline Porous Materials Based S-Scheme Heterojunction for Efficient Photocatalytic H2 Production. Adv. Energy Mater. 2024, 14, 2303638. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Liu, Y.; Wang, J.; Xu, X.; Guan, R.; Zhang, Y.; Shi, W.; Liu, Y.; Zhao, Z. Bisphenol A Assisted Ti3C2Tx/CuZnInS Schottky Heterojunction for Highly Efficient Photocatalytic Nitrogen Fixation. Colloid. Surf. A 2022, 648, 129430. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Zhao, C.; Liu, Y.; Liang, Q.; Zhou, M.; Li, Z.; Zhou, Y. Construction ZnIn2S4/Ti3C2 of 2D/2D Heterostructures with Enhanced Visible Light Photocatalytic Activity: A Combined Experimental and First-Principles DFT Study. Appl. Surf. Sci. 2021, 570, 151183. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, X.; Wang, S.; Ma, F.; Znad, H.; Shen, Z.; Liu, L.; Liu, S. MXene as a Non-Metal Charge Mediator in 2D Layered CdS@Ti3C2@TiO2 Composites with Superior Z-Scheme Visible Light-Driven Photocatalytic Activity. Environ. Sci. Nano 2019, 6, 3158–3169. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, N.; Tang, Z.-R.; Anpo, M.; Xu, Y.-J. Ti3C2Tx MXene as a Janus Cocatalyst for Concurrent Promoted Photoactivity and Inhibited Photocorrosion. App. Catal. B Environ. 2018, 237, 43–49. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhang, F.; Chen, Y.; Li, J.-Y.; Xu, Y.-J. Photoredox-Catalyzed Biomass Intermediate Conversion Integrated with H2 Production over Ti3C2Tx/CdS Composites. Green. Chem. 2020, 22, 163–169. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Meng, X. In-Situ Construction of Ternary Ti3C2 MXene@TiO2/ZnIn2S4 Composites for Highly Efficient Photocatalytic Hydrogen Evolution. J. Colloid. Interface Sci. 2020, 580, 669–680. [Google Scholar] [CrossRef]
- Li, S.; Shao, L.; Yang, Z.; Cheng, S.; Yang, C.; Liu, Y.; Xia, X. Constructing Ti3C2 MXene/ZnIn2S4 Heterostructure as a Schottky Catalyst for Photocatalytic Environmental Remediation. Green Energy Environ. 2022, 7, 246–256. [Google Scholar] [CrossRef]
- Peng, J.; Shen, J.; Yu, X.; Tang, H.; Zulfiqar; Liu, Q. Construction of LSPR-Enhanced 0D/2D CdS/MoO3− S-Scheme Heterojunctions for Visible-Light-Driven Photocatalytic H2 Evolution. Chin. J. Catal. 2021, 42, 87–96. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.-F. 2D MXenes as Co-Catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiong, Y.; Wang, X.; Xue, Y.; Liu, W.; Tian, J. Dual Cocatalysts and Vacancy Strategies for Enhancing Photocatalytic Hydrogen Production Activity of Zn3In2S6 Nanosheets with an Apparent Quantum Efficiency of 66.20%. J. Colloid. Interface Sci. 2023, 640, 31–40. [Google Scholar] [CrossRef]
- Biswal, L.; Acharya, L.; Mishra, B.P.; Das, S.; Swain, G.; Parida, K. Interfacial Solid-State Mediator-Based Z-Scheme Heterojunction TiO2@Ti3C2/MgIn2S4 Microflower for Efficient Photocatalytic Pharmaceutical Micropollutant Degradation and Hydrogen Generation: Stability, Kinetics, and Mechanistic Insights. ACS Appl. Energy Mater. 2023, 6, 2081–2096. [Google Scholar] [CrossRef]
- Yang, B.; Han, J.; Zhang, Q.; Liao, G.; Cheng, W.; Ge, G.; Liu, J.; Yang, X.; Wang, R.; Jia, X. Carbon Defective G-C3N4 Thin-Wall Tubes for Drastic Improvement of Photocatalytic H2 Production. Carbon 2023, 202, 348–357. [Google Scholar] [CrossRef]
- Sun, Y.; Hao, Y.; Lin, X.; Liu, Z.; Sun, H.; Jia, S.; Chen, Y.; Yan, Y.; Li, X. Efficient Electron Transport by 1D CuZnInS Modified 2D Ti3C2 MXene for Enhanced Photocatalytic Hydrogen Production. J. Colloid. Interface Sci. 2024, 653, 396–404. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of Sacrificial Reagents on Photocatalytic Hydrogen Evolution over Different Photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Younsi, M.; Saadi, S.; Bouguelia, A.; Aider, A.; Trari, M. Synthesis and Characterization of Oxygen-Rich Delafossite CuYO2+x—Application to H2-Photo Production. Sol. Energy Mater. Sol. Cells 2007, 91, 1102–1109. [Google Scholar] [CrossRef]
- Xu, F.; Li, Z.; Fan, D.; Zhao, C.; Yang, X. Construction of G-C3N4/Ti3CN MXene Schottky Heterojunctions for Boosting Photocatalytic Hydrogen Production. Surf. Interf. 2024, 51, 104658. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Wu, Y.; Tu, W.; Wu, S.; Yuan, X.; Zeng, G.; Xu, Z.J.; Li, S.; Chew, J.W. Electrical Promotion of Spatially Photoinduced Charge Separation via Interfacial-Built-in Quasi-Alloying Effect in Hierarchical Zn2In2S5/Ti3C2(O, OH)x Hybrids toward Efficient Photocatalytic Hydrogen Evolution and Environmental Remediation. App. Catal. B Environ. 2019, 245, 290–301. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Wu, C.; Zhang, H.; Sun, J.; Li, Q. Delaminating Ti3C2 MXene by Blossom of ZnIn2S4 Microflowers for Noble-Metal-Free Photocatalytic Hydrogen Production. J. Mater. Sci. Technol. 2022, 120, 89–98. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X. Rational Design of 1D/2D Heterostructured Photocatalyst for Energy and Environmental Applications. Chem. Eng. J. 2020, 395, 125030. [Google Scholar] [CrossRef]
- Li, D.; Yang, C.; Rajendran, S.; Qin, J.; Zhang, X. Nanoflower-like Ti3CN@TiO2/CdS Heterojunction Photocatalyst for Efficient Photocatalytic Water Splitting. Int. J. Hydrog. Energy 2022, 47, 19580–19589. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In Situ Construction of Protonated G-C3N4/Ti3C2 MXene Schottky Heterojunctions for Efficient Photocatalytic Hydrogen Production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Liu, S.; Fang, H.; Chen, R.; Ma, Y.; Zhu, Y.; Ye, M.; Zhang, H.; Zhang, K. Bridging CdS with Ti3C2Tx Nanosheets via Ligands for Enhanced Charge Transfer in Photocatalytic H2 Production. Int. J. Hydrog. Energy 2024, 54, 1154–1159. [Google Scholar] [CrossRef]
- Wei, P.; Chen, Y.; Zhou, T.; Wang, Z.; Zhang, Y.; Wang, H.; Yu, H.; Jia, J.; Zhang, K.; Peng, C. Manipulation of Charge-Transfer Kinetics via Ti3C2Tx(T = −O) Quantum Dot and N-Doped Carbon Dot Coloading on CdS for Photocatalytic Hydrogen Production. ACS Catal. 2023, 13, 587–600. [Google Scholar] [CrossRef]
- Huang, K.; Lv, C.; Li, C.; Bai, H.; Meng, X. Ti3C2 MXene Supporting Platinum Nanoparticles as Rapid Electrons Transfer Channel and Active Sites for Boosted Photocatalytic Water Splitting over G-C3N4. J. Colloid Interface Sci. 2023, 636, 21–32. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, H.; Wang, X.; Li, Q.; Chang, S.; Huang, Y.; Gao, L.; Cui, Y.; Liu, R.; Dai, W.-L. Robust Construction of CdSe nanorods@Ti3C2 MXene Nanosheet for Superior Photocatalytic H2 Evolution. Appl. Catal. B Environ. 2023, 328, 122537. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Ultrathin ZnIn2S4 Nanosheets Anchored on Ti3C2TX MXene for Photocatalytic H2 Evolution. Angew. Chem. 2020, 132, 11383–11388. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, Z.; Yang, X. Rationally Designed 2D CZIS/2D Ti3CNTx Heterojunctions for Photocatalytic Hydrogen Evolution Reaction. Catalysts 2025, 15, 632. https://doi.org/10.3390/catal15070632
Li P, Wang Z, Yang X. Rationally Designed 2D CZIS/2D Ti3CNTx Heterojunctions for Photocatalytic Hydrogen Evolution Reaction. Catalysts. 2025; 15(7):632. https://doi.org/10.3390/catal15070632
Chicago/Turabian StyleLi, Peize, Zhiying Wang, and Xiaofei Yang. 2025. "Rationally Designed 2D CZIS/2D Ti3CNTx Heterojunctions for Photocatalytic Hydrogen Evolution Reaction" Catalysts 15, no. 7: 632. https://doi.org/10.3390/catal15070632
APA StyleLi, P., Wang, Z., & Yang, X. (2025). Rationally Designed 2D CZIS/2D Ti3CNTx Heterojunctions for Photocatalytic Hydrogen Evolution Reaction. Catalysts, 15(7), 632. https://doi.org/10.3390/catal15070632








