Efficient Production of (R)-3-Aminobutyric Acid by Biotransformation of Recombinant E. coli
Abstract
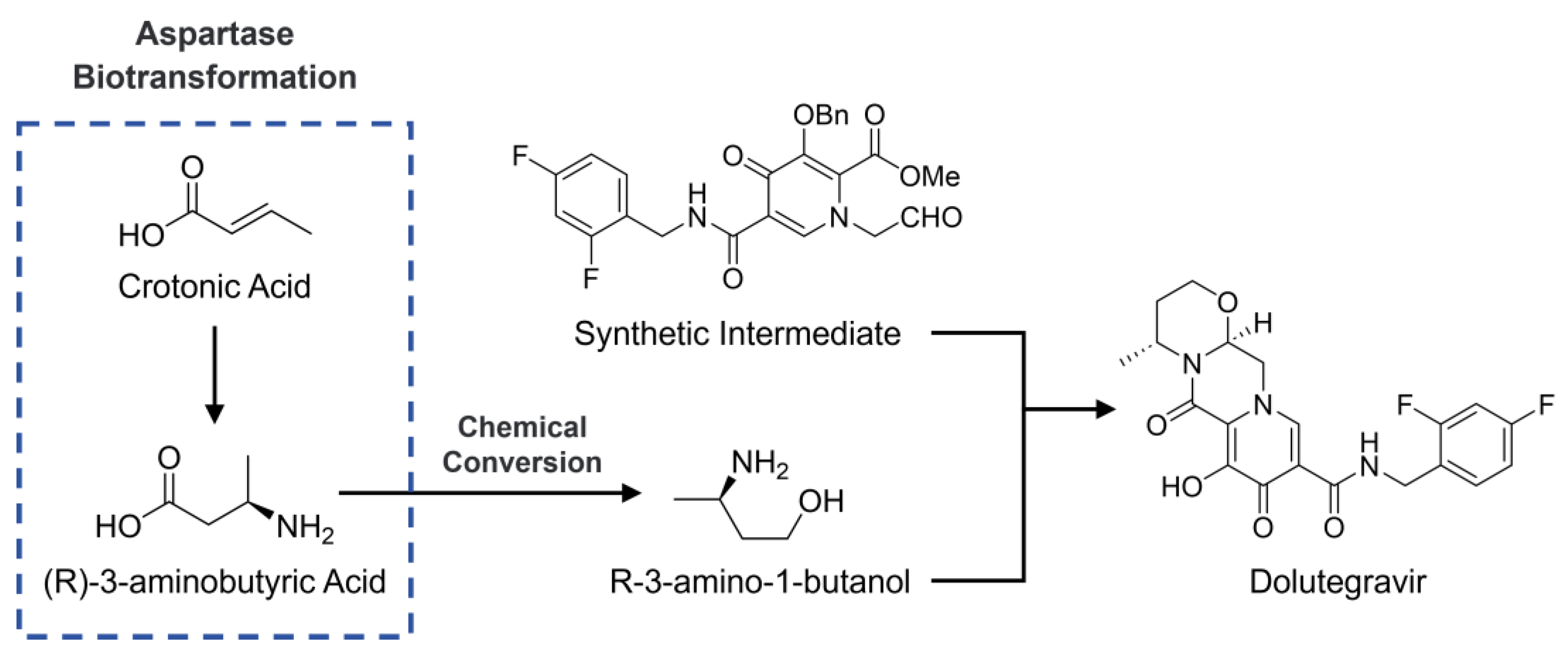
1. Introduction
2. Results and Discussion
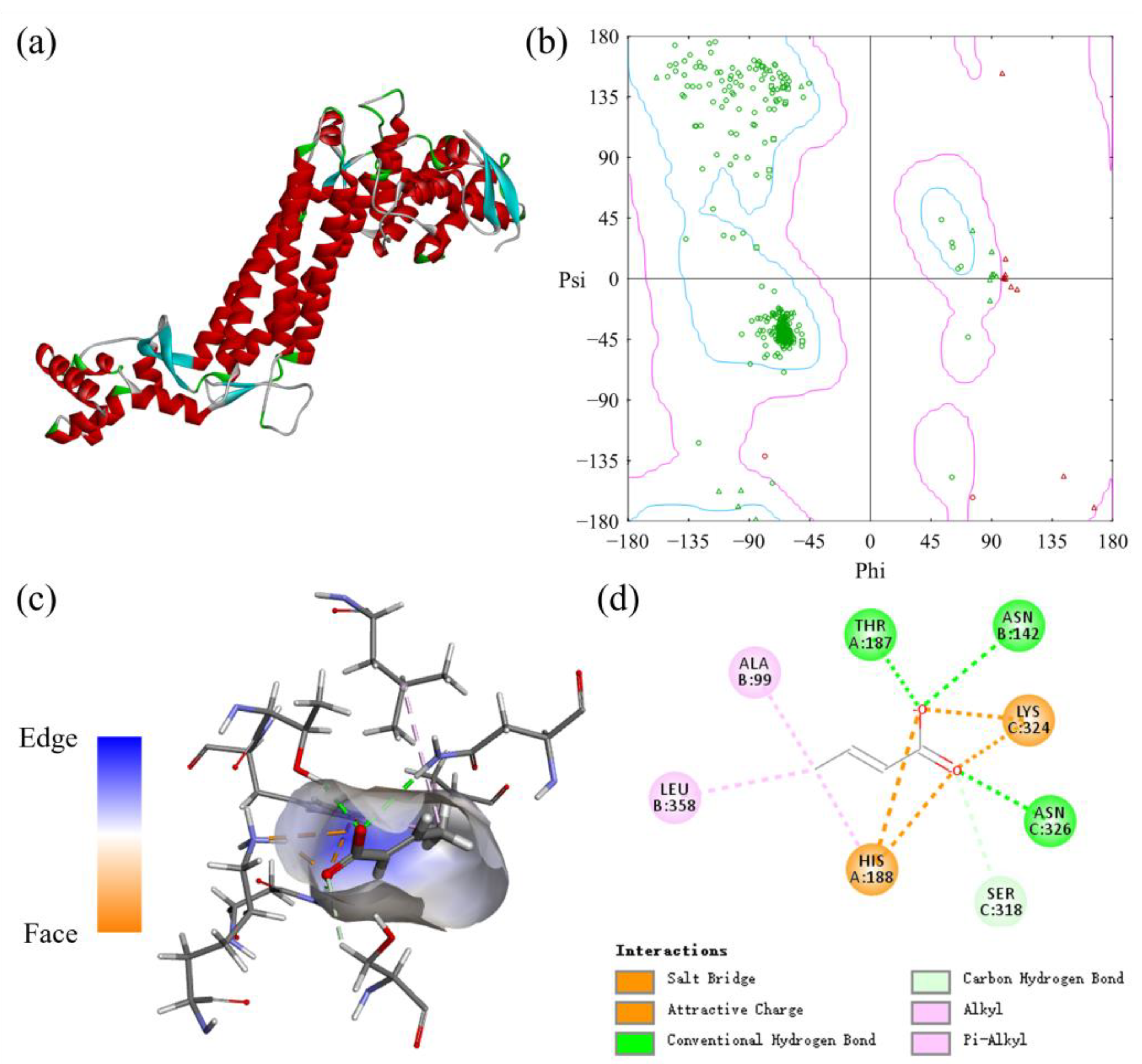
2.1. Structure and Catalytic Mechanism Analysis of Aspartase
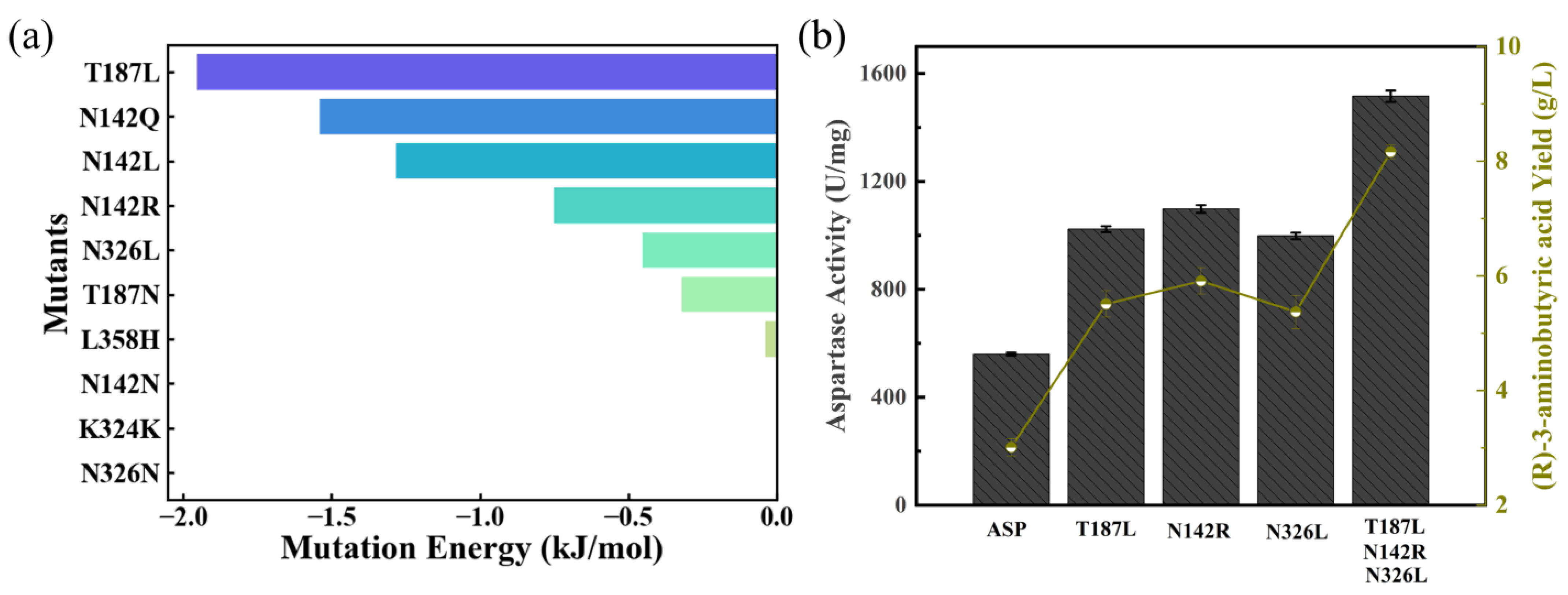
2.2. Rational Design of Aspartase to Enhance Catalytic Activity
2.3. Catalysis of (R)-3-Aminobutyric Acid Production by Engineered Aspartase Mutant
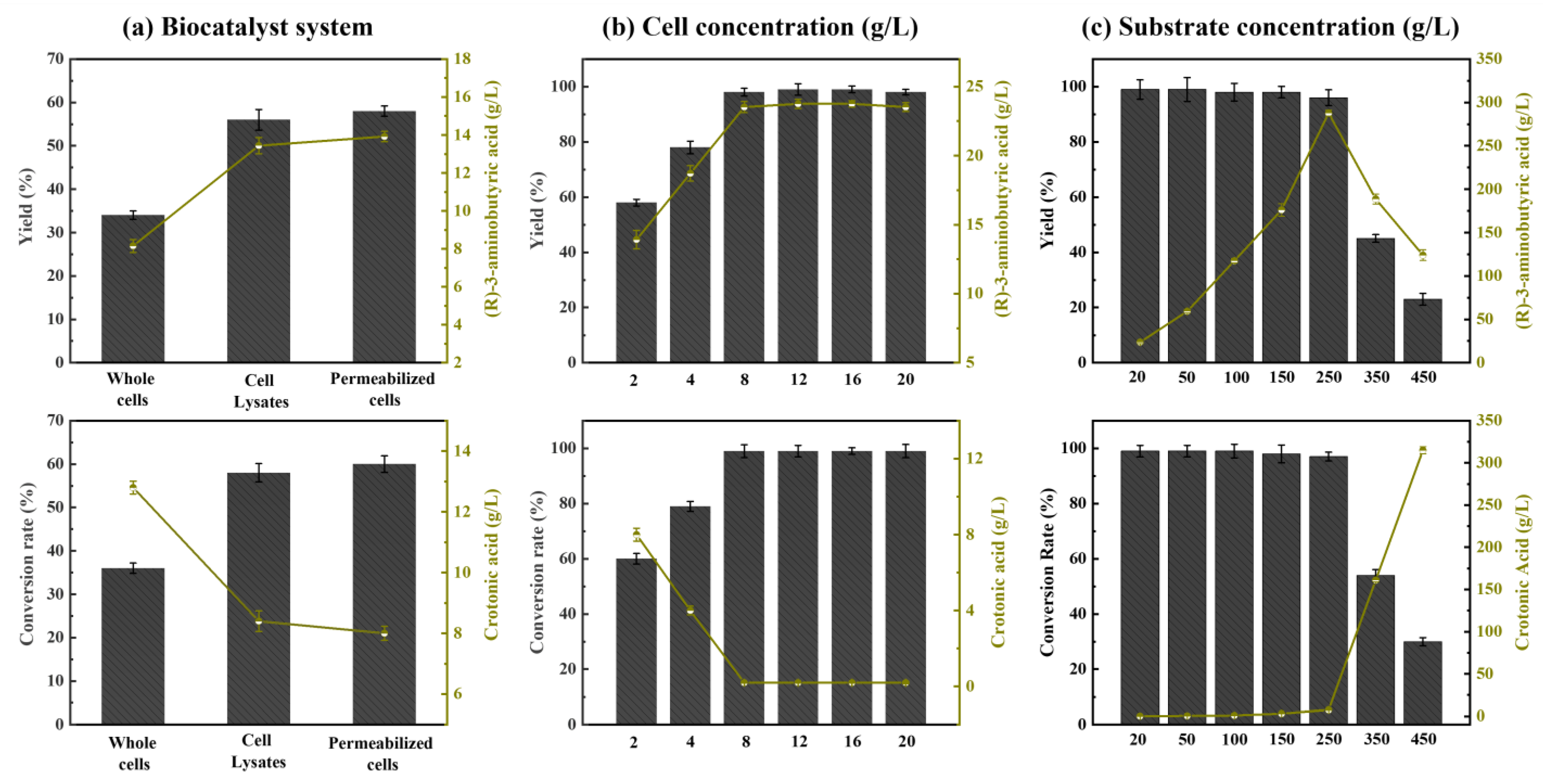
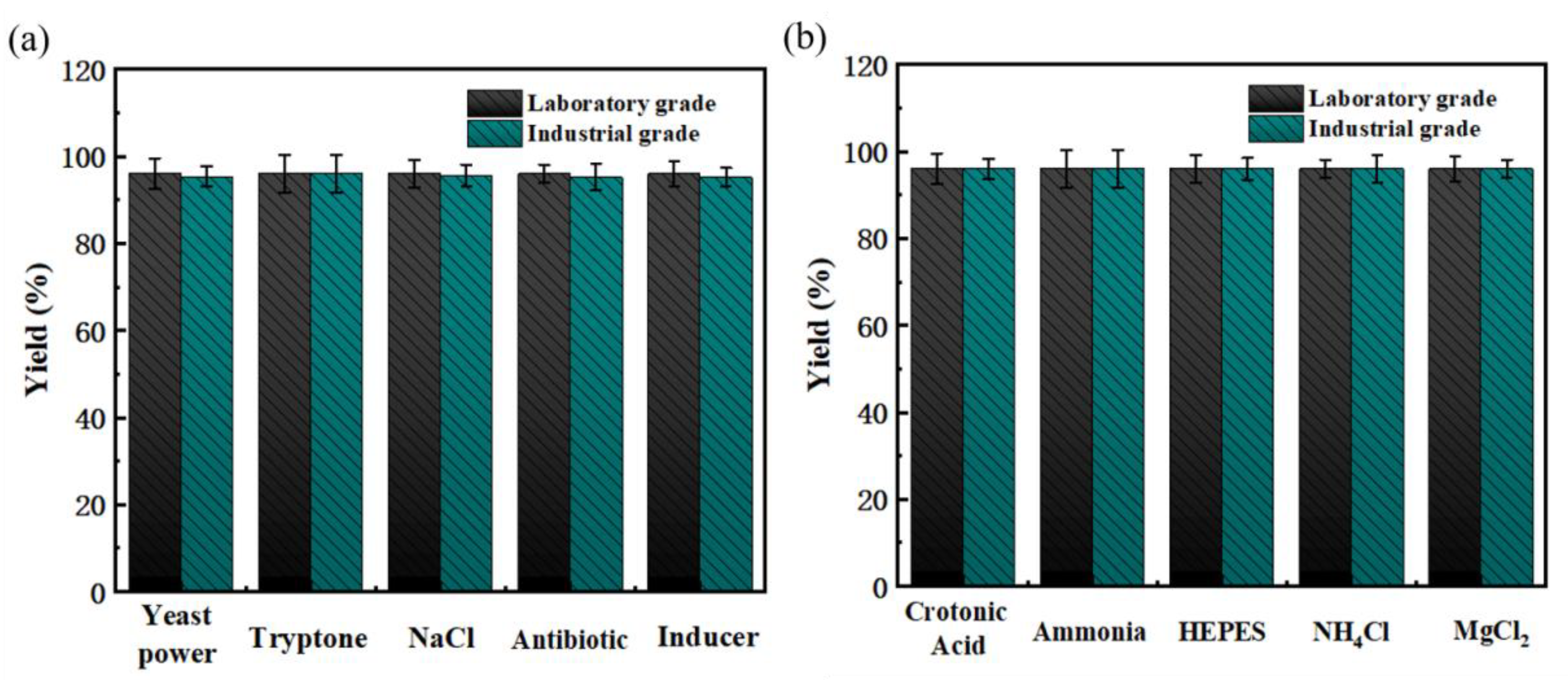
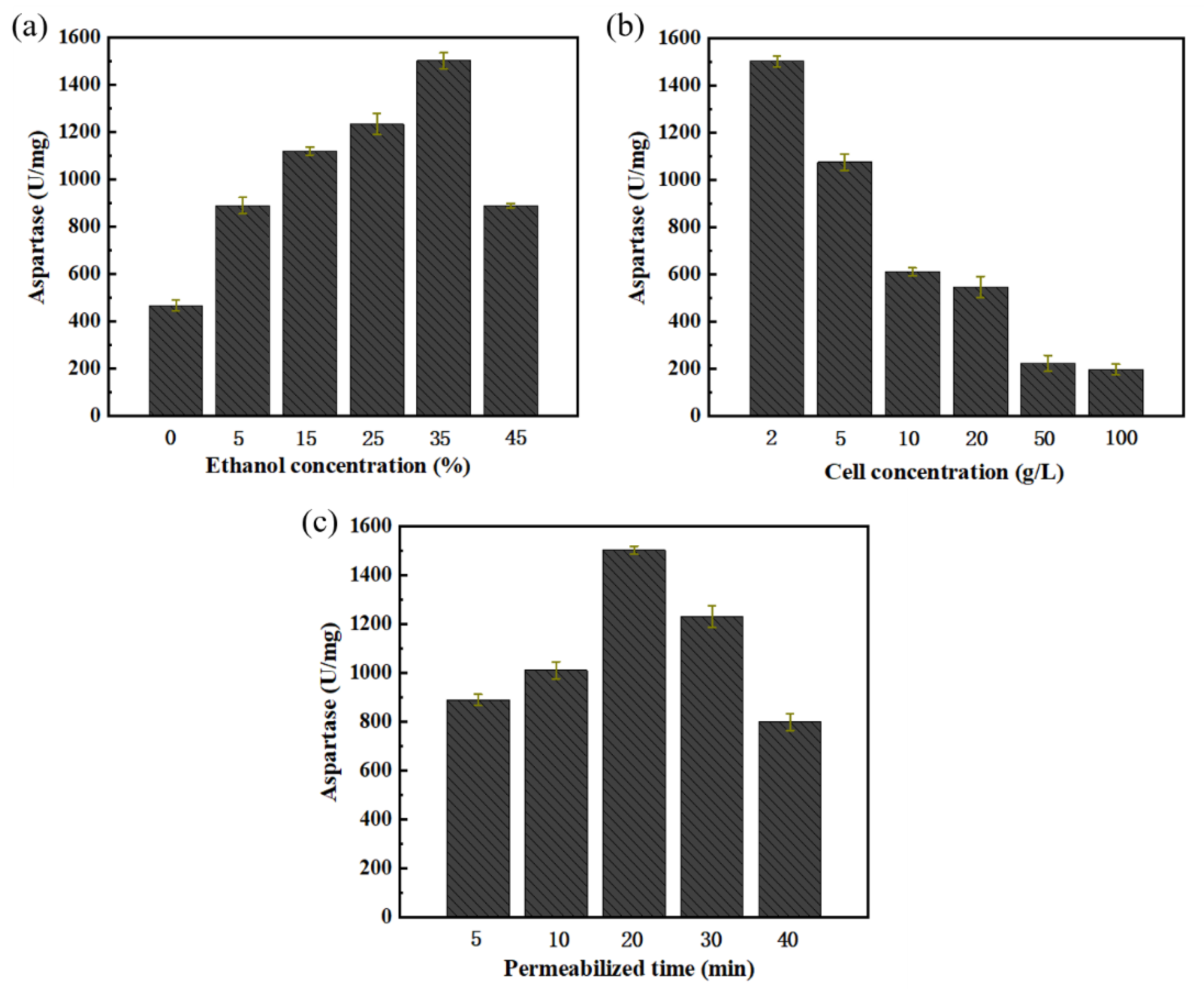
2.4. Optimization of Biocatalytic Process and Industrial Raw Material Substitution
2.5. High-Efficiency and Low-Cost (R)-3-Aminobutyric Acid Production in the Liter-Scale Integrated Strategy
3. Materials and Methods
3.1. Strains, Media, and Reagents
3.2. Rational Design of Aspartase
3.3. Construction of Engineered E. coli Strain for (R)-3-Aminobutyric Acid Production
3.4. Cultivation in Shaken Flasks
3.5. Preparation of Biocatalyst
3.6. Biocatalytic Production of (R)-3-Aminobutyric in Shaken Flasks
3.7. Optimization of Catalytic Reaction Conditions
3.8. Replacement of Industrial Materials
3.9. Product Preparation in Small-Scale Process
3.10. Activity Assay
3.11. Product Detection Method
3.12. Data Processing and Statistical Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LB | Luria–Bertani |
| IPTG | Isopropyl-beta-D-thiogalactopyranoside |
| AIDS | Acquired immune deficiency syndrome |
References
- Mbhele, N.; Chimukangara, B.; Gordon, M. HIV-1 integrase strand transfer inhibitors: A review of current drugs, recent advances and drug resistance. Int. J. Antimicrob. Agents 2021, 57, 106343. [Google Scholar] [CrossRef]
- Dow, D.E.; Bartlett, J.A. Dolutegravir, the Second-Generation of Integrase Strand Transfer Inhibitors (INSTIs) for the Treatment of HIV. Infect. Dis. Ther. 2014, 3, 83–102. [Google Scholar] [CrossRef]
- Cook, N.J.; Li, W.; Berta, D.; Badaoui, M.; Ballandras-Colas, A.; Nans, A.; Kotecha, A.; Rosta, E.; Engelman, A.N.; Cherepanov, P. Structural basis of second-generation HIV integrase inhibitor action and viral resistance. Science 2020, 367, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Landovitz, R.J.; Sax, P.E.; Smith, D.M.; Springer, S.A.; Günthard, H.F.; Thompson, M.A.; Bedimo, R.J.; Benson, C.A.; Buchbinder, S.P.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV in Adults: 2024 Recommendations of the International Antiviral Society–USA Panel. JAMA 2025, 333, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ran, X.; Qiang, F.; Xiaojing, S.; Yang, H.; Xiaoli, D.; Li, T. A preliminary study on plasma concentration, short-term efficacy, and safety profile of dolutegravir in Chinese people with HIV. Expert Rev. Clin. Pharmacol. 2024, 17, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-M.; Alharbi, N.S.; Sun, B.; Shantharam, C.S.; Rakesh, K.P.; Qin, H.-L. Synthetic routes and structure-activity relationships (SAR) of anti-HIV agents: A key review. Eur. J. Med. Chem. 2019, 181, 111566. [Google Scholar] [CrossRef]
- Slagman, S.; Fessner, W.-D. Biocatalytic routes to anti-viral agents and their synthetic intermediates. Chem. Soc. Rev. 2021, 50, 1968–2009. [Google Scholar] [CrossRef]
- Hughes, D.L. Review of Synthetic Routes and Final Forms of Integrase Inhibitors Dolutegravir, Cabotegravir, and Bictegravir. Org. Process Res. Dev. 2019, 23, 716–729. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.; Yang, T.; Zhang, X.; Rao, Z. Surface charge-based rational design of aspartase modifies the optimal pH for efficient β-aminobutyric acid production. Int. J. Biol. Macromol. 2020, 164, 4165–4172. [Google Scholar] [CrossRef]
- Jefford, C.W.; Wang, J. An enantiospecific synthesis of β-amino acids. Tetrahedron Lett. 1993, 34, 1111–1114. [Google Scholar] [CrossRef]
- Weiß, M.; Brinkmann, T.; Gröger, H. Towards a greener synthesis of (S)-3-aminobutanoic acid: Process development and environmental assessment. Green Chem. 2010, 12, 1580–1588. [Google Scholar] [CrossRef]
- Eissen, M.; Weiß, M.; Brinkmann, T.; Steinigeweg, S. Comparison of Two Alternative Routes to an Enantiomerically Pure β-Amino Acid. Chem. Eng. Technol. 2010, 33, 629–637. [Google Scholar] [CrossRef]
- Santaniello, E.; Ferraboschi, P.; Grisenti, P.; Manzocchi, A. The biocatalytic approach to the preparation of enantiomerically pure chiral building blocks. Chem. Rev. 1992, 92, 1071–1140. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Asymmetric biocatalysis with microbial enzymes and cells. Curr. Opin. Microbiol. 2010, 13, 283–292. [Google Scholar] [CrossRef]
- Bhardwaj, K.K.; Gupta, R. Synthesis of Chirally Pure Enantiomers by Lipase. J. Oleo Sci. 2017, 66, 1073–1084. [Google Scholar] [CrossRef]
- Klotz, S.; Kaufmann, N.; Kuenz, A.; Prüße, U. Biotechnological production of enantiomerically pure d-lactic acid. Appl. Microbiol. Biotechnol. 2016, 100, 9423–9437. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ruth, K.; Thöny-Meyer, L.; Zinn, M. Enatiomerically pure hydroxycarboxylic acids: Current approaches and future perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-P.; Cao, C.-H.; Zheng, Y.-G. Enzymatic asymmetric synthesis of chiral amino acids. Chem. Soc. Rev. 2018, 47, 1516–1561. [Google Scholar] [CrossRef]
- Li, R.; Wijma, H.J.; Song, L.; Cui, Y.; Otzen, M.; Tian, Y.e.; Du, J.; Li, T.; Niu, D.; Chen, Y.; et al. Computational redesign of enzymes for regio- and enantioselective hydroamination. Nat. Chem. Biol. 2018, 14, 664–670. [Google Scholar] [CrossRef]
- Liu, M.; Sibi, M.P. Recent advances in the stereoselective synthesis of β-amino acids. Tetrahedron 2002, 58, 7991–8035. [Google Scholar] [CrossRef]
- Kim, J.; Kyung, D.; Yun, H.; Cho, B.-K.; Seo, J.-H.; Cha, M.; Kim, B.-G. Cloning and Characterization of a Novel β-Transaminase from Mesorhizobium sp. Strain LUK: A New Biocatalyst for the Synthesis of Enantiomerically Pure β-Amino Acids. Appl. Environ. Microbiol. 2007, 73, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y. A QM/MM study of the catalytic mechanism of aspartate ammonia lyase. J. Mol. Graph. Model. 2014, 51, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Puthan Veetil, V.; Fibriansah, G.; Raj, H.; Thunnissen, A.-M.W.H.; Poelarends, G.J. Aspartase/Fumarase Superfamily: A Common Catalytic Strategy Involving General Base-Catalyzed Formation of a Highly Stabilized aci-Carboxylate Intermediate. Biochemistry 2012, 51, 4237–4243. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Sakai, H.; Kawata, Y.; Hata, Y. Crystal Structure of Thermostable Aspartase from Bacillus sp. YM55-1: Structure-based Exploration of Functional Sites in the Aspartase Family. J. Mol. Biol. 2003, 328, 635–654. [Google Scholar] [CrossRef]
- Kawata, Y.; Tamura, K.; Kawamura, M.; Ikei, K.; Mizobata, T.; Nagai, J.; Fujita, M.; Yano, S.; Tokushige, M.; Yumoto, N. Cloning and over-expression of thermostable Bacillus sp. YM55-1 aspartase and site-directed mutagenesis for probing a catalytic residue. Eur. J. Biochem. 2000, 267, 1847–1857. [Google Scholar] [CrossRef]
- Fibriansah, G.; Veetil, V.P.; Poelarends, G.J.; Thunnissen, A.-M.W.H. Structural Basis for the Catalytic Mechanism of Aspartate Ammonia Lyase. Biochemistry 2011, 50, 6053–6062. [Google Scholar] [CrossRef]
- Puthan Veetil, V.; Raj, H.; Quax, W.J.; Janssen, D.B.; Poelarends, G.J. Site-directed mutagenesis, kinetic and inhibition studies of aspartate ammonia lyase from Bacillus sp. YM55-1. FEBS J. 2009, 276, 2994–3007. [Google Scholar] [CrossRef]
- de Villiers, M.; Puthan Veetil, V.; Raj, H.; de Villiers, J.; Poelarends, G.J. Catalytic Mechanisms and Biocatalytic Applications of Aspartate and Methylaspartate Ammonia Lyases. ACS Chem. Biol. 2012, 7, 1618–1628. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, C.; Shen, X.; Sun, X.; Wang, J.; Yuan, Q. Unlocking the potential of enzyme engineering via rational computational design strategies. Biotechnol. Adv. 2024, 73, 108376. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Q.; Wu, W.; Pu, Z.; Yu, H. Rational design of enzyme activity and enantioselectivity. Front. Bioeng. Biotechnol. 2023, 11, 1129149. [Google Scholar] [CrossRef]
- Ding, Y.; Perez-Ortiz, G.; Peate, J.; Barry, S.M. Redesigning Enzymes for Biocatalysis: Exploiting Structural Understanding for Improved Selectivity. Front. Mol. Biosci. 2022, 9, 908285. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Fernandes, P.A.; Ramos, M.J. Modern computational methods for rational enzyme engineering. Chem Catal. 2022, 2, 2481–2498. [Google Scholar] [CrossRef]
- Lutz, S. Beyond directed evolution—Semi-rational protein engineering and design. Curr. Opin. Biotechnol. 2010, 21, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; You, S.; Zhang, J.; Zhang, H.; Wang, H.; Zhang, W.; Qi, W.; Su, R.; He, Z. Rational design of 17β-hydroxysteroid dehydrogenase type3 for improving testosterone production with an engineered Pichia pastoris. Bioresour. Technol. 2021, 341, 125833. [Google Scholar] [CrossRef] [PubMed]
- Gran-Scheuch, A.; Wijma, H.J.; Capra, N.; van Beek, H.L.; Trajkovic, M.; Baldenius, K.; Breuer, M.; Thunnissen, A.-M.W.H.; Janssen, D.B. Bioinformatics and Computationally Supported Redesign of Aspartase for β-Alanine Synthesis by Acrylic Acid Hydroamination. ACS Catal. 2025, 15, 928–938. [Google Scholar] [CrossRef]
- Asano, Y.; Kira, I.; Yokozeki, K. Alteration of substrate specificity of aspartase by directed evolution. Biomol. Eng. 2005, 22, 95–101. [Google Scholar] [CrossRef]
- Sun, C.; Cheng, Z.; Jiao, J.; Ding, S.; Tian, Z. Method for Enzymatic Preparation of R-3-Aminobutyric. Acid. Patent WO2019062222A1, 4 April 2019. [Google Scholar]
- Vogel, A.; Schmiedel, R.; Hofmann, U.; Gruber, K.; Zangger, K. Converting Aspartase into a β-Amino Acid Lyase by Cluster Screening. ChemCatChem 2014, 6, 965–968. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, Y.; Zhang, J.; Zhou, Y.; Wang, M.; You, S.; Su, R.; Qi, W. Enhanced catalytic activity of polyethylene terephthalate hydrolase by structure-guided loop-focused iterative mutagenesis strategy. J. Hazard. Mater. 2025, 490, 137837. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; You, S.; Lin, W.; Su, R.; Qi, W. Efficient thermophilic polyethylene terephthalate hydrolase enhanced by cross correlation-based accumulated mutagenesis strategy. Bioresour. Technol. 2024, 406, 130929. [Google Scholar] [CrossRef]
- Falzone, C.J.; Karsten, W.E.; Conley, J.D.; Viola, R.E. L-Aspartase from Escherichia coli: Substrate specificity and role of divalent metal ions. Biochemistry 1988, 27, 9089–9093. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Min, P.S.; Jae, C.W. Integration of cell permeabilization and medium engineering for enhanced enantioselective synthesis of ethyl-S-3-hydroxy-3-phenylpropanoate (S-EHPP). Biochem. Eng. J. 2019, 148, 24–28. [Google Scholar] [CrossRef]
- Catania, C.; Ajo-Franklin, C.M.; Bazan, G.C. Membrane permeabilization by conjugated oligoelectrolytes accelerates whole-cell catalysis. RSC Adv. 2016, 6, 100300–100306. [Google Scholar] [CrossRef]

| Fermentation | Bioproduction by Permeabilized Cells | ||||
|---|---|---|---|---|---|
| Type | OD600 | Aspartase (U/mg) | Reaction Time (h) | (R)-3-Aminobutyric Acid (g/L) | Yield (%) |
| Lab scale (Reagents) | 4.43 ± 0.37 | 1516 ± 19 | 24 | 287 ± 1.25 | 95.9 ± 0.55 |
| Lab scale (Industrial materials) | 4.35 ± 0.21 | 1503 ± 12 | 24 | 281 ± 0.65 | 93.9 ± 0.25 |
| Bioreactor scale (Industrial materials) | 4.29 ± 0.21 | 1496 ± 16 | 48 | 215 ± 0.94 | 71.8 ± 0.42 |
| Bioreactor scale after the process optimization (Industrial materials) | 4.32 ± 0.28 | 1502 ± 23 | 24 | 284 ± 1.07 | 94.9 ± 0.87 |
| Reagents | Industrial Materials | ||
|---|---|---|---|
| Component | Cost (¥) | Component | Cost (¥) |
| Tryptone (35 g) | 17.50 | Tryptone (35 g) | 0.35 |
| Yeast extract (17.5 g) | 4.55 | Yeast extract (17.5 g) | 0.16 |
| Sodium chloride (35 g) | 0.70 | Sodium chloride (35 g) | 0.02 |
| Seed fermentation broth (100 mL) | 0.65 | Seed fermentation broth (100 mL) | 0.02 |
| Resistance (0.35 mL) | 3.96 | Resistance (0.35 mL) | 0.14 |
| IPTG (0.35 mL) | 9.8 | IPTG (0.35 mL) | 2.4 |
| 35% ethanol (3.5 L) | 36.75 | 35% ethanol (3.5 L) | 12.01 |
| Butenoic acid (225 g) | 42.3 | Butenoic acid (225 g) | 13.95 |
| Ammonia (230 mL) | 2.34 | Ammonia (230 mL) | 0.525 |
| HEPES buffer (0.9 L) | 1.21 | HEPES buffer (0.9 L) | 0.05 |
| MgCl2 (2 mL) | 0.05 | MgCl2 (2 mL) | 0.01 |
| NH4Cl (2 mL) | 2.9 | NH4Cl (2 mL) | 0.12 |
| Total | 122.71 | Total | 29.72 |
| (R)-3-aminobutyric acid (1 kg) | 479.76 | (R)-3-aminobutyric acid (1 kg) | 116.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, Q.; Lv, J.; Zhang, J.; Dou, T.; You, S.; Su, R.; Qi, W. Efficient Production of (R)-3-Aminobutyric Acid by Biotransformation of Recombinant E. coli. Catalysts 2025, 15, 466. https://doi.org/10.3390/catal15050466
Zhang H, Xu Q, Lv J, Zhang J, Dou T, You S, Su R, Qi W. Efficient Production of (R)-3-Aminobutyric Acid by Biotransformation of Recombinant E. coli. Catalysts. 2025; 15(5):466. https://doi.org/10.3390/catal15050466
Chicago/Turabian StyleZhang, Hongtao, Qing Xu, Jiajia Lv, Jiaxing Zhang, Tongyi Dou, Shengping You, Rongxin Su, and Wei Qi. 2025. "Efficient Production of (R)-3-Aminobutyric Acid by Biotransformation of Recombinant E. coli" Catalysts 15, no. 5: 466. https://doi.org/10.3390/catal15050466
APA StyleZhang, H., Xu, Q., Lv, J., Zhang, J., Dou, T., You, S., Su, R., & Qi, W. (2025). Efficient Production of (R)-3-Aminobutyric Acid by Biotransformation of Recombinant E. coli. Catalysts, 15(5), 466. https://doi.org/10.3390/catal15050466








