Efficient Method for the Synthesis of 5-Methylfurfural from l-Rhamnose Using a Biphasic System
Abstract
1. Introduction
2. Results and Discussion
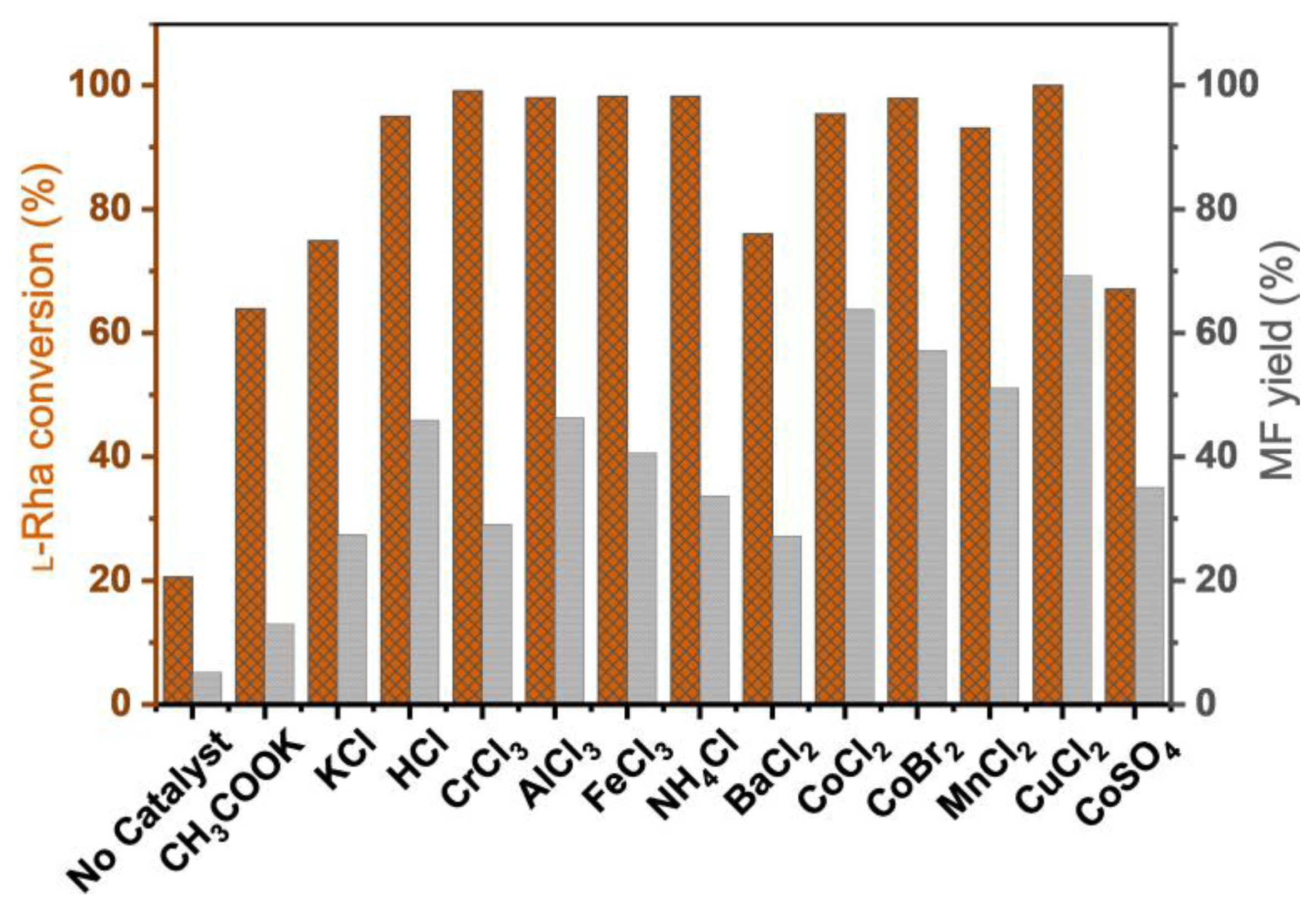
2.1. Influence of Catalyst Species on l-Rhamnose Conversion to MF
2.2. Effect of Extraction Solvent on l-Rhamnose Conversion to MF
2.3. Effect of the Reaction Temperature and Time
2.4. Effect of the Catalyst and Substrate Concentration
2.5. Recyclability of the Concentrated CuCl2 Solution
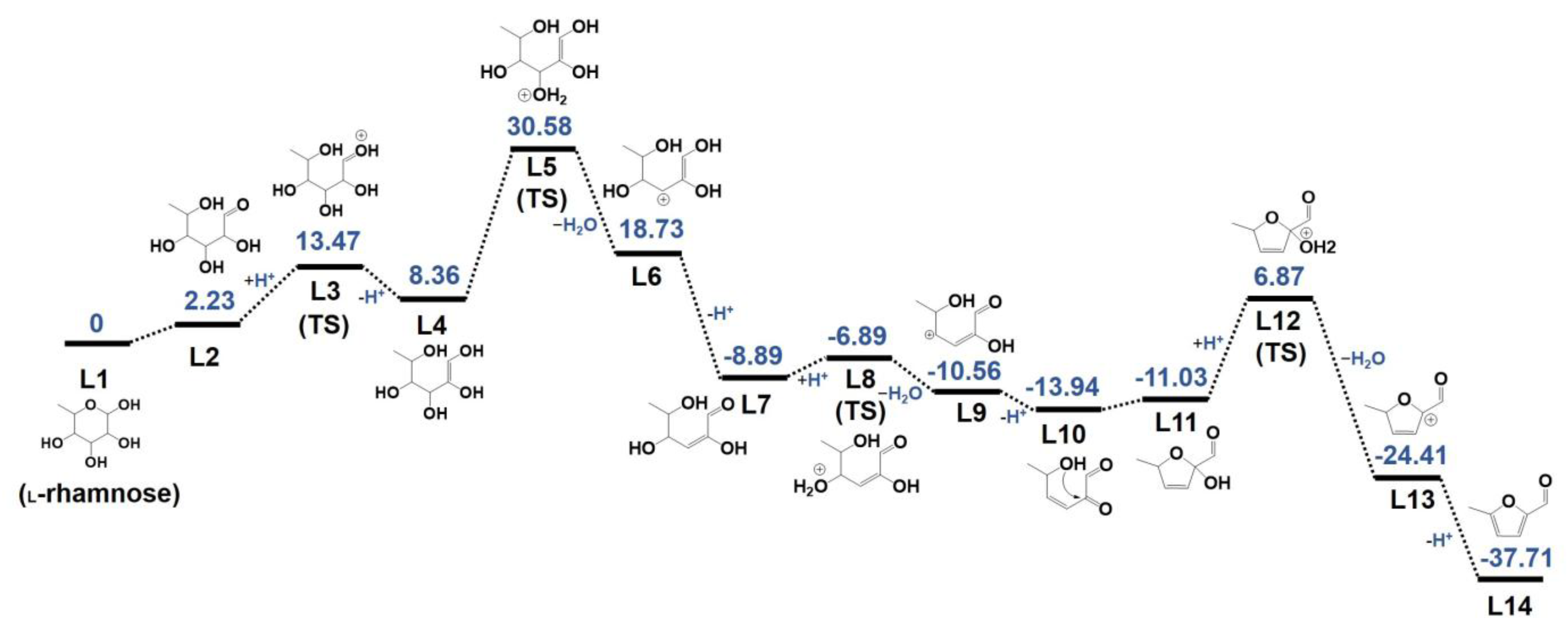
2.6. Mechanism of l-Rhamnose Dehydration to MF
3. Experimental Section
3.1. Materials
3.2. Experimental Procedure
3.3. Analytical Method
3.4. Computational Methods
3.5. Determination of Yields for Products and Partition Coefficient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Niu, H.; Zheng, N.; Liu, S.; Li, Y. Poly (ethylene-co-propylene)/poly (ethylene glycol) elastomeric hydrogels with thermoreversibly cross-linked networks. Polym. Chem. 2019, 10, 4789–4800. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Ordomsky, V.; van der Schaaf, J.; Schouten, J.; Nijhuis, T. The effect of solvent addition on fructose dehydration to 5-hydroxymethylfurfural in biphasic system over zeolites. J. Catal. 2012, 287, 68–75. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.; Dumesic, J. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono-and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Dunlop, A.P.; Peters, F.N. The Furans; Reinhold: New York, NY, USA, 1953. [Google Scholar]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Galkin, K.I.; Ananikov, V.P. Towards Improved Biorefinery Technologies: 5-Methylfurfural as a Versatile C6 Platform for Biofuels Development. ChemSusChem 2019, 12, 185–189. [Google Scholar] [CrossRef]
- Yang, W.; Sen, A. Direct Catalytic Synthesis of 5-Methylfurfural from Biomass-Derived Carbohydrates. Chemsuschem 2011, 4, 349–352. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Nagata, Y.; Masumoto, K.; Inomata, Y.; Hatakeyama, K.; Quitain, A.T.; Shotipruk, A.; Kida, T. Manganese doped graphene oxide: Selective hydrogenation catalyst for converting 5-hydroxymethyl furfural to 5-methyl furfural. Mol. Catal. 2024, 553, 113787. [Google Scholar] [CrossRef]
- Peng, Y.; Li, X.; Gao, T.; Li, T.; Yang, W. Preparation of 5-Methylfurfural from Starch in One Step by Iodide Mediated Metal-Free Hydrogenolysis. Green Chem. 2019, 21, 4169–4177. [Google Scholar] [CrossRef]
- Qin, S.; Li, T.; Zhang, M.; Liu, H.; Yang, X.; Rong, N.; Jiang, J.; Wang, Y.; Zhang, H.; Yang, W. Catalyst-free synthesis of biodiesel precursors from biomass-based furfuryl alcohols in the presence of H2O and air. Green Chem. 2019, 21, 6326–6334. [Google Scholar] [CrossRef]
- Ban, H.; Zhang, Y.; Chen, S.; Cheng, Y.; Pan, T.; Wang, L.; Li, X. Production of 2, 5-furandicarboxylic acid by optimization of oxidation of 5-methyl furfural over homogeneous Co/Mn/Br catalysts. ACS Sustain. Chem. Eng. 2020, 8, 8011–8023. [Google Scholar] [CrossRef]
- Sun, D.; Mo, J.; Liu, W.; Yan, N.; Qiu, X. Ultra-Strong and Tough Bio-Based Polyester Elastomer with Excellent Photothermal Shape Memory Effect and Degradation Performance. Adv. Funct. Mater. 2024, 34, 2403333. [Google Scholar] [CrossRef]
- Hirota, M. Process for Preparing 5-Methyl-2-furfural. Patent US 20070078273, 5 April 2007. [Google Scholar]
- Aggarwal, S.; Jilling, T.; Doran, S.; Ahmad, I.; Eagen, J.E.; Gu, S.; Gillespie, M.; Albert, C.J.; Ford, D.; Matalon, S.; et al. Phosgene inhalation causes hemolysis and acute lung injury. Toxicol. Lett. 2019, 312, 204–213. [Google Scholar] [CrossRef]
- Yang, W.; Grochowski, M.R.; Sen, A. Selective reduction of biomass by hydriodic acid and its in situ regeneration from iodine by metal/hydrogen. ChemSusChem 2012, 5, 1218–1222. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Dramatic advancements in the saccharide to 5-(chloromethyl) furfural conversion reaction. ChemSusChem 2009, 2, 859–861. [Google Scholar] [CrossRef]
- Hamada, K.; Suzukamo, G.; Fujisawa, K. Process for Producing 5-Methylfurfural. Patent US 4335049, 15 June 1982. Available online: https://patentimages.storage.googleapis.com/pdfs/US4335049.pdf (accessed on 6 May 2024).
- Sun, G.; An, J.; Hu, H.; Li, C.; Zuo, S.; Xia, H. Green catalytic synthesis of 5-methylfurfural by selective hydrogenolysis of 5-hydroxymethylfurfural over size-controlled Pd nanoparticle catalysts. Catal. Sci. Technol. 2019, 9, 1238–1244. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Long, S.; Sun, Y.; Tang, X.; Zeng, X.; Lin, L. Direct conversion of biomass derived l-rhamnose to 5-methylfurfural in water in high yield. Green Chem. 2020, 22, 5984–5988. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, P.; Chen, P.; Wu, D. Highly efficient enzymatic conversion of rutin to isoquercitrin and l-rhamnose using deep eutectic solvents. ACS Sustain. Chem. Eng. 2020, 8, 14905–14913. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Zhou, Y.; Tong, D.; Hu, C. Selective conversion of hemicellulose in macroalgae Enteromorpha prolifera to rhamnose. ACS Omega 2019, 4, 7023–7028. [Google Scholar] [CrossRef]
- Marcotullio, G.; De Jong, W. Chloride ions enhance furfural formation from D-xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739–1746. [Google Scholar] [CrossRef]
- Ribeiro, A.; Esteso, M.; Lobo, V.; Valente, A.; Simões, S.; Sobral, A.; Burrows, H. Interactions of copper (II) chloride with sucrose, glucose, and fructose in aqueous solutions. J. Mol. Struct. 2007, 826, 113–119. [Google Scholar] [CrossRef]
- Mensah, J.B.; Delidovich, I.; Hausoul, P.J.C.; Weisgerber, L.; Schrader, W.; Palkovits, R. Mechanistic studies of the Cu(OH)+-catalyzed isomerization of glucose into gructose in water. ChemSusChem 2018, 11, 2579–2586. [Google Scholar] [CrossRef]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z.-G. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar] [CrossRef] [PubMed]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Rakić, V.; Auroux, A. CeO2-Nb2O5 mixed oxide catalysts: Preparation, characterization and catalytic activity in fructose dehydration reaction. Catal. Today 2012, 192, 160–168. [Google Scholar] [CrossRef]
- Tucker, M.H.; Crisci, A.J.; Wigington, B.N.; Phadke, N.; Alamillo, R.; Zhang, J.; Scott, S.L.; Dumesic, J.A. Acid-functionalized SBA-15-type periodic mesoporous organosilicas and their use in the continuous production of 5-hydroxymethylfurfural. ACS Catal. 2012, 2, 1865–1876. [Google Scholar] [CrossRef]
- Nimlos, M.R.; Blanksby, S.J.; Qian, X.; Himmel, M.E.; Johnson, D.K. Mechanisms of glycerol dehydration. J. Phys. Chem. A 2006, 110, 6145–6156. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Assessment of Gaussian-3 and density-functional theories on the G3/05 test set of experimental energies. J. Chem. Phys. 2005, 123, 124107. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Chabalowski, C.; Devlin, F.; Jalkanen, K. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Mayes, H.B.; Nolte, M.W.; Beckham, G.T.; Shanks, B.H.; Broadbelt, L.J. The Alpha-Bet(a) of glucose pyrolysis: Computational and experimental investigations of 5-hydroxymethylfurfural and levoglucosan formation reveal implications for cellulose pyrolysis. ACS Sustain. Chem. Eng. 2014, 2, 1461–1473. [Google Scholar] [CrossRef]
- Bernales, V.S.; Marenich, A.V.; Contreras, R.; Cramer, C.J.; Truhlar, D.G. Quantum mechanical continuum solvation models for ionic liquids. J. Phys. Chem. B 2012, 116, 9122–9129. [Google Scholar] [CrossRef]





| Routes | Raw Materials | Catalysts | Reaction Conditions | MF Yield (%) | Drawbacks | References |
|---|---|---|---|---|---|---|
| 1 | 2-Methylfuran | COCl2 | 60 °C, 12 h | 96 | Highly toxic reactant | [18,19] |
| 2 | Glucose, Fructose | H2, HI, RuCl3, or Pd/C | 90 °C, 0.5–1 h | 68 | Highly deleterious and corrosive | [20] |
| 3 | Glucose, Sucrose, Cellulose | (1) aq. HCl/(ClCH2)2; (2) H2, Pd/C | (1) 100 °C, 3 h; (2) 40 °C, 5 h | (1) CMF yield 90%; (2) MF yield 95% | Two steps, long time, large amount of organic solvent | [21,22] |
| 4 | HMF | HCOOH, PVP/Pd | THF, 200–220 °C, 7.5 h | 80 | High temperature, corrosive solvent, long time | [23] |
| 5 | l-Rhamnose | [BMIM]Cl/CrCl2 | 110 °C, 2 h | 61 | Expensive ionic liquid, low yield | [11] |
| 6 | l-Rhamnose | NaCl-H2O/AlCl3 | 155 °C, 0.5 h | 97 | Large amount of organic solvent | [24] |
| Entry | Catalyst | Concentration | Solvent | Volume Ratio | MF Yield |
|---|---|---|---|---|---|
| Species | mol/L | (%) | |||
| 1 | / | 0.1 | Toluene | 1 | 5 |
| 2 | CuCl2 | 0.1 | / | 1 | 27 |
| 3 | CuCl2 | 0.1 | CCl4 | 1 | 54 |
| 4 | CuCl2 | 0.1 | DIPE | 1 | 85 |
| 5 | CuCl2 | 0.1 | MIBK | 1 | 82 |
| 6 | CuCl2 | 0.1 | 1-butanol | 1 | 47 |
| 7 | CuCl2 | 0.1 | DIPE | 0.25 | 65 |
| 8 | CuCl2 | 0.1 | DIPE | 2 | 94 |
| 9 | CuCl2 | 0.05 | DIPE | 2 | 63 |
| 10 | CuCl2 | 0.15 | DIPE | 2 | 91 |
| 11 | CuCl2 | 0.2 | DIPE | 2 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Jiang, P.; Cui, Q.; Wang, Z.; Wei, Y.; Luo, C.; Guo, J.; Liu, C.; Zhang, W. Efficient Method for the Synthesis of 5-Methylfurfural from l-Rhamnose Using a Biphasic System. Catalysts 2025, 15, 465. https://doi.org/10.3390/catal15050465
He Z, Jiang P, Cui Q, Wang Z, Wei Y, Luo C, Guo J, Liu C, Zhang W. Efficient Method for the Synthesis of 5-Methylfurfural from l-Rhamnose Using a Biphasic System. Catalysts. 2025; 15(5):465. https://doi.org/10.3390/catal15050465
Chicago/Turabian StyleHe, Zongke, Pengfei Jiang, Qianqian Cui, Ziyue Wang, Yaozhong Wei, Chao Luo, Jichang Guo, Chang Liu, and Wei Zhang. 2025. "Efficient Method for the Synthesis of 5-Methylfurfural from l-Rhamnose Using a Biphasic System" Catalysts 15, no. 5: 465. https://doi.org/10.3390/catal15050465
APA StyleHe, Z., Jiang, P., Cui, Q., Wang, Z., Wei, Y., Luo, C., Guo, J., Liu, C., & Zhang, W. (2025). Efficient Method for the Synthesis of 5-Methylfurfural from l-Rhamnose Using a Biphasic System. Catalysts, 15(5), 465. https://doi.org/10.3390/catal15050465







