Iron-Modified Functional Biochar Activates Peroxydisulfate for Efficient Degradation of Organic Pollutants
Abstract
1. Introduction
2. Results and Discussion
2.1. Biomass Characterization Results
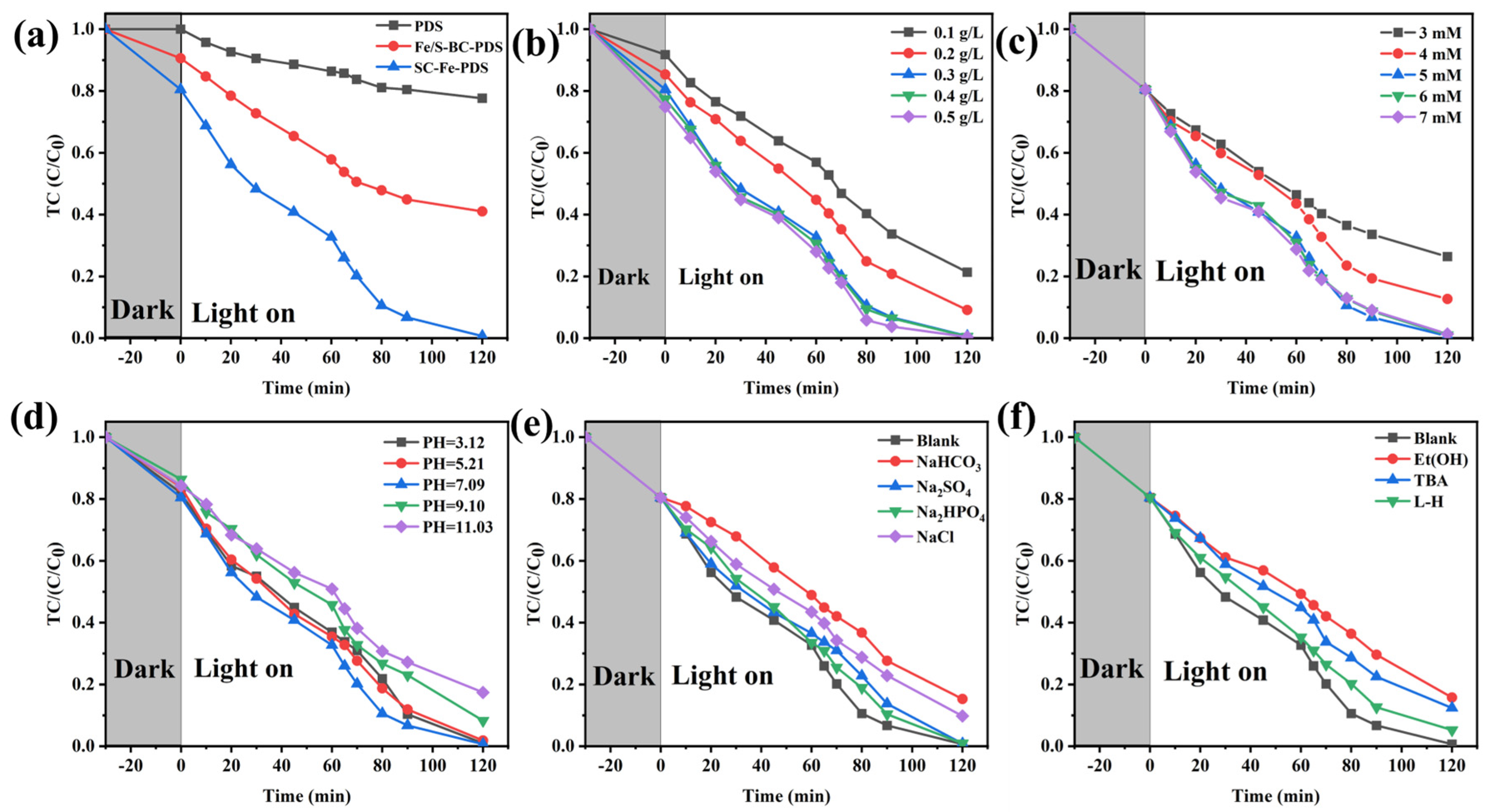
2.2. Optimization of System Parameters for TC Removal Experiment
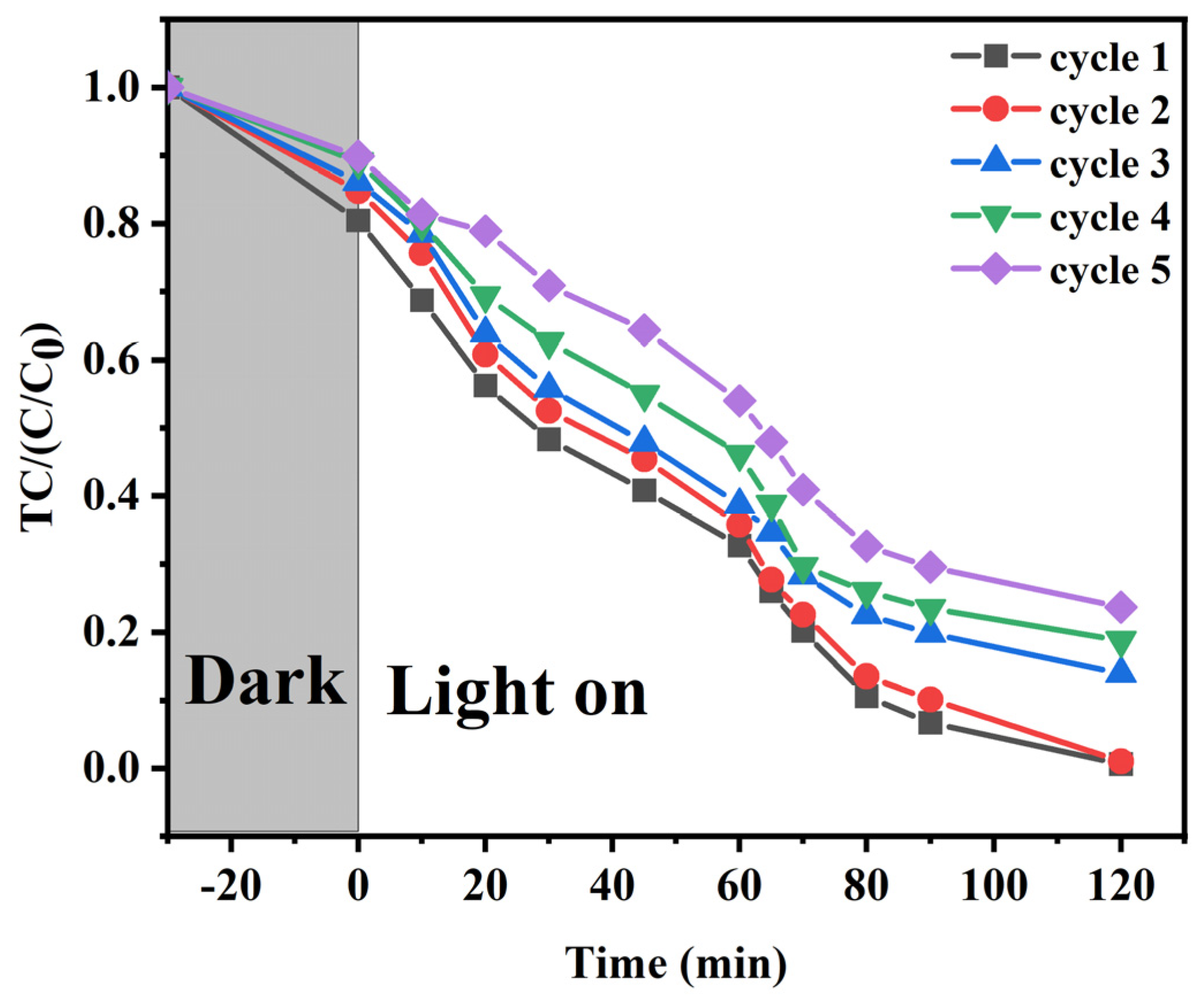
2.3. Reusability of SC-Fe
3. Materials and Methods
3.1. Raw Materials
3.2. Instruments and Equipment
3.3. Preparation of Modified Biochar
3.4. Experiment on Modified Biochar Activating PDS for TC Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Kong, J.; Yuan, J.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem. Eng. J. 2018, 331, 242–254. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Li, W.; Zhou, Y.; Wu, L.; Cheng, Z. Adsorption of tetracycline by alkali-modified biochar. J. Yichun Coll. 2022, 44, 6–12+47. [Google Scholar]
- Yuan, H.; Li, C.; Wei, Y.; Zhu, Y.; Zuo, W.; Tong, H. FeOCl Composite peanut shell-derived biochar Activated by persulfate for degradation of tetracycline. Chem. Technol. Dev. 2023, 52, 7–10. [Google Scholar]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Li, F.; Duan, F.; Ji, W.; Gui, X. Biochar-activated persulfate for organic contaminants removal: Efficiency, mechanisms and influencing factors. Ecotoxicol. Environ. Saf. 2020, 198, 110653. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X.; et al. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef]
- Xu, R.; Li, M.; Zhang, Q. Collaborative optimization for the performance of ZnO/biochar composites on persulfate activation through plant enrichment-pyrolysis method. Chem. Eng. J. 2022, 429, 132294. [Google Scholar] [CrossRef]
- Hou, X.; Dong, H.; Li, Y.; Xiao, J.; Dong, Q.; Xiang, S.; Chu, D. Activation of persulfate by graphene/biochar composites for phenol degradation: Performance and nonradical dominated reaction mechanism. J. Environ. Chem. Eng. 2023, 11, 109348. [Google Scholar] [CrossRef]
- Zhong, S.; Pan, J.; Tian, K.; Qin, J.; Qing, T.; Zhang, J. Efficient degradation of p-chlorophenol by N,S-co-doped biochar activated perxymonosulfate. Process Saf. Environ. Prot. 2023, 169, 437–446. [Google Scholar] [CrossRef]
- Sierra, I.; Iriarte-Velasco, U.; Ayastuy, J.L.; Aguayo, A.T. Production of magnetic sewage sludge biochar: Investigation of the activation mechanism and effect of the activating agent and temperature. Biomass Convers. Biorefinery 2022, 13, 17101–17118. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman spectroscopic investigations of activated carbon materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Li, C.; Xu, B.; Jin, M.; Chen, L.; Yi, G.; Chen, L.; Wu, Y.; Zhang, Y.; Xing, B. Sulfur and nitrogen co-doped biochar activated persulfate to degrade phenolic wastewater: Changes in impedance. J. Mol. Struct. 2023, 1294, 136344. [Google Scholar] [CrossRef]
- Huang, R.; Feng, T.; Wu, S.; Zhang, X.; Fan, Z.; Yu, Q.; Chen, Y.; Chen, T. In-situ synthesis of magnetic iron-chitosan-derived biochar as an efficient persulfate activator for phenol degradation. Environ. Res. 2023, 234, 116604. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yu, H.; Jin, R.; Zhou, J. Acceleration of goethite-catalyzed Fenton-like oxidation of ofloxacin by biochar. J. Hazard. Mater. 2020, 397, 122783. [Google Scholar] [CrossRef] [PubMed]
- Introduction. In Encyclopedia of Condensed Matter Physics, 2nd ed.; Chakraborty, T., Ed.; Academic Press: Oxford, UK, 2024; pp. ix–xvii. [Google Scholar]
- Hussain, I.; Li, M.; Zhang, Y.; Li, Y.; Huang, S.; Du, X.; Liu, G.; Hayat, W.; Anwar, N. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem. Eng. J. 2017, 311, 163–172. [Google Scholar] [CrossRef]
- Xi, M.; Cui, K.; Cui, M.; Ding, Y.; Guo, Z.; Chen, Y.; Li, C.; Li, X. Enhanced norfloxacin degradation by iron and nitrogen co-doped biochar: Revealing the radical and nonradical co-dominant mechanism of persulfate activation. Chem. Eng. J. 2021, 420, 129902. [Google Scholar] [CrossRef]
- Stuglik, Z.; Pawełzagórski, Z. Pulse radiolysis of neutral iron (II) solutions: Oxidation of ferrous ions by OH radicals. Radiat. Phys. Chem. 1981, 17, 229–233. [Google Scholar] [CrossRef]
- Chen, K.F.; Kao, C.M.; Wu, L.C.; Surampalli, R.Y.; Liang, S.H. Methyl tert-butyl ether (MTBE) degradation by ferrous ion-activated persulfate oxidation: Feasibility and kinetics studies. Water Environ. Res. 2009, 81, 687–694. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Xu, Z.; Chen, Y.; Song, D. Degradation of tetracycline in a schorl/H2O2 system: Proposed mechanism and intermediates. Chemosphere 2018, 202, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, C.; Zhao, X.; Bu, X.; Liao, X.; Fan, H.; Gao, W.; Hu, H.; Zhang, Y.; Huang, Z. Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: Efficiency, stability and mechanism. J. Environ. Chem. Eng. 2021, 9, 105800. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Song, H.; Li, Q.; Ye, Y.; Pan, F.; Zhang, D.; Xia, D. Degradation of cephalexin by persulfate activated with magnetic loofah biochar: Performance and mechanism. Sep. Purif. Technol. 2021, 272, 118971. [Google Scholar] [CrossRef]

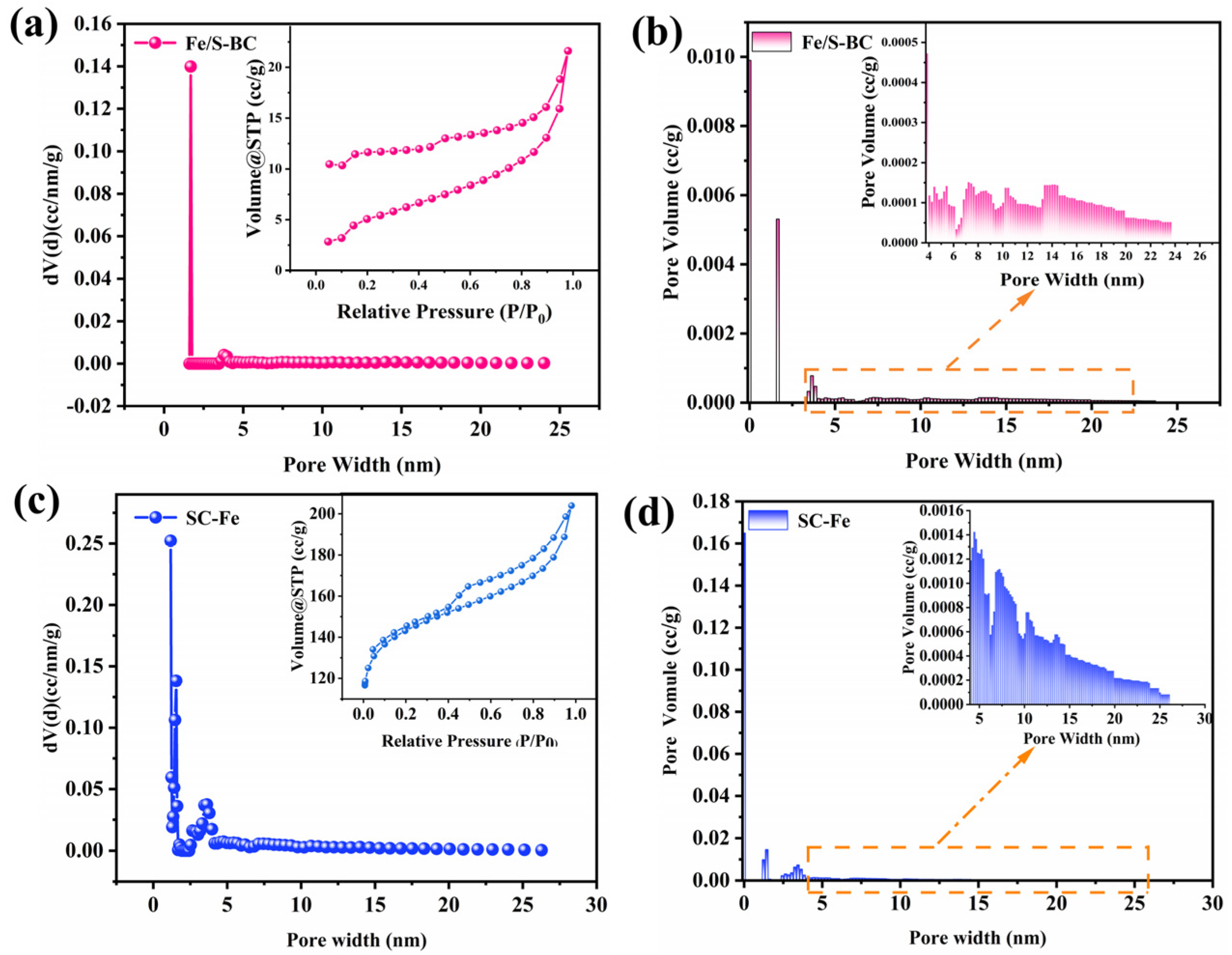
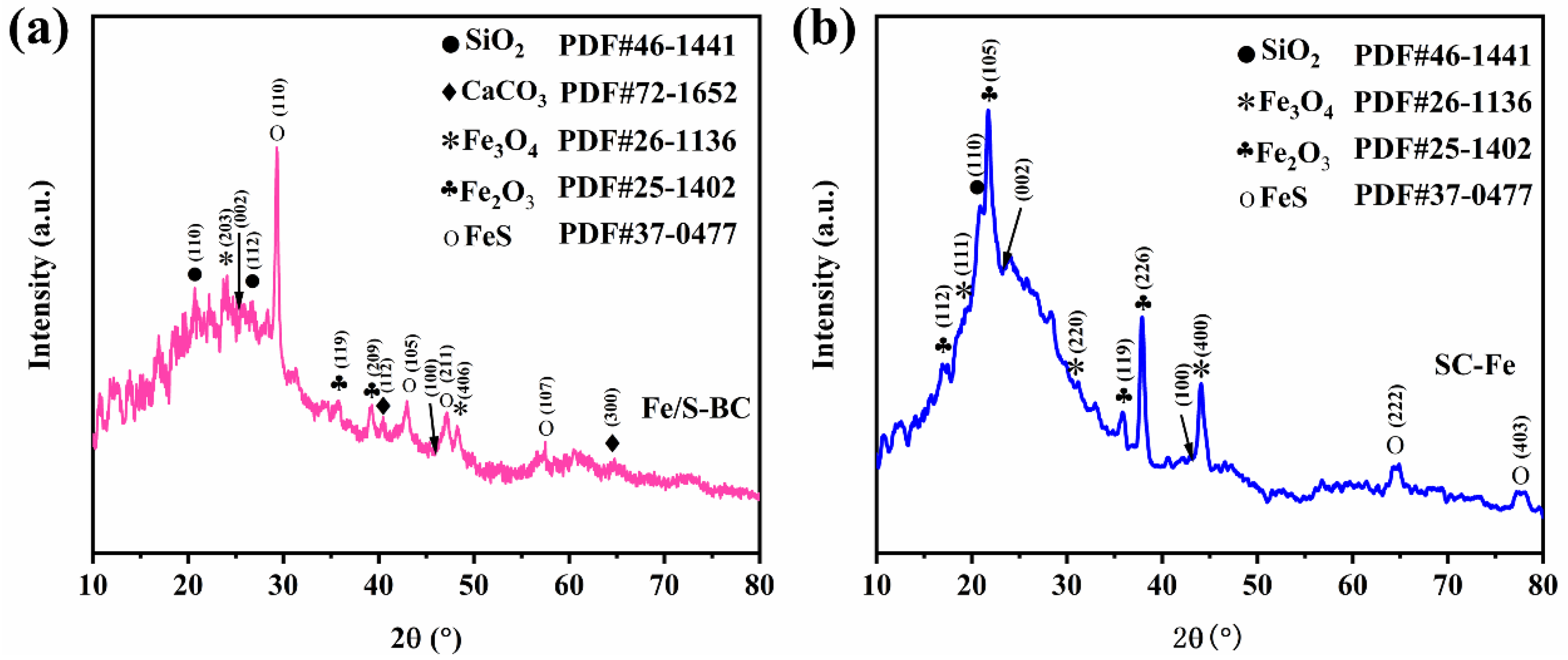
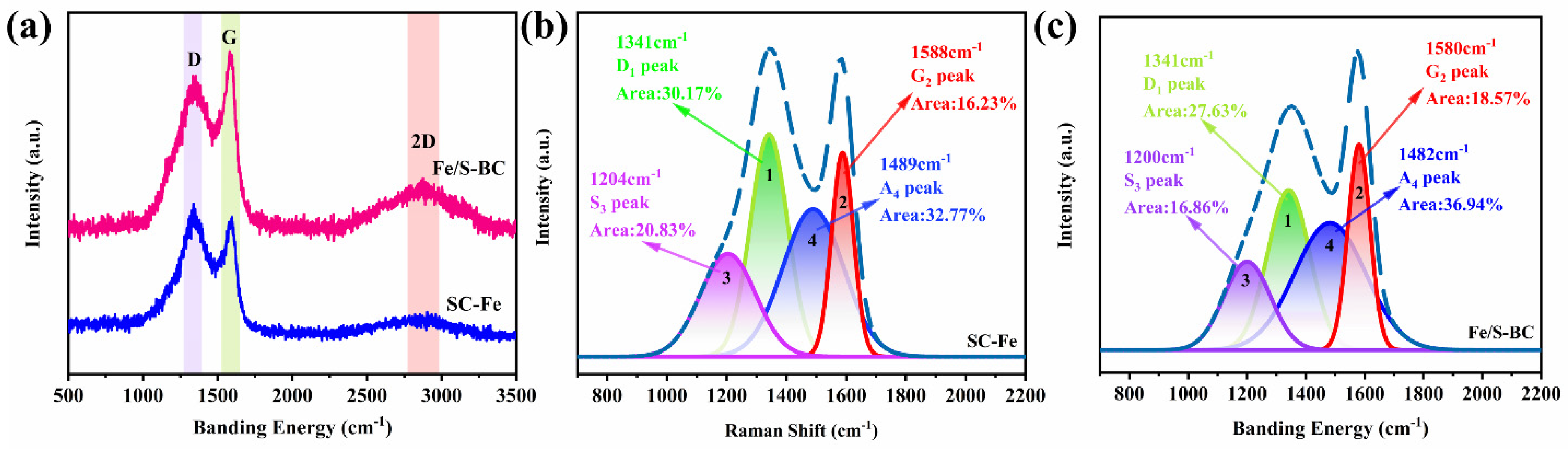

| Sample | SBET (m2 g−1) | Pore Size (nm) | Pore Volume (cc/g) |
|---|---|---|---|
| Fe/S-BC | 14 | 1.766 | 0.028 |
| SC-Fe | 480 | 1.178 | 0.280 |
| Catalysts | D1 (%) | S3 (%) | A4 (%) | G2 (%) | ID1/IG2 |
|---|---|---|---|---|---|
| Fe/S-BC | 27.63% | 16.86% | 36.94% | 18.57% | 1.49 |
| SC-Fe | 30.17% | 20.83% | 32.77% | 16.23% | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, B.; Pu, H.; Yang, Z.; Qin, Y.; An, M.; Gao, C.; Mao, K.; Wang, S.; Xue, B.; et al. Iron-Modified Functional Biochar Activates Peroxydisulfate for Efficient Degradation of Organic Pollutants. Catalysts 2025, 15, 462. https://doi.org/10.3390/catal15050462
Chen W, Zhang B, Pu H, Yang Z, Qin Y, An M, Gao C, Mao K, Wang S, Xue B, et al. Iron-Modified Functional Biochar Activates Peroxydisulfate for Efficient Degradation of Organic Pollutants. Catalysts. 2025; 15(5):462. https://doi.org/10.3390/catal15050462
Chicago/Turabian StyleChen, Weijie, Bingbing Zhang, Hao Pu, Zhao Yang, Yixue Qin, Mingze An, Chengtao Gao, Kang Mao, Sheng Wang, Bing Xue, and et al. 2025. "Iron-Modified Functional Biochar Activates Peroxydisulfate for Efficient Degradation of Organic Pollutants" Catalysts 15, no. 5: 462. https://doi.org/10.3390/catal15050462
APA StyleChen, W., Zhang, B., Pu, H., Yang, Z., Qin, Y., An, M., Gao, C., Mao, K., Wang, S., Xue, B., & Sun, C. (2025). Iron-Modified Functional Biochar Activates Peroxydisulfate for Efficient Degradation of Organic Pollutants. Catalysts, 15(5), 462. https://doi.org/10.3390/catal15050462







