Valorization of Residual Brewery Biomass for the Production of Counter Electrodes for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Results
2.1. Physical and Chemical Characteristics of the Iron Oxide/Biochar Composite
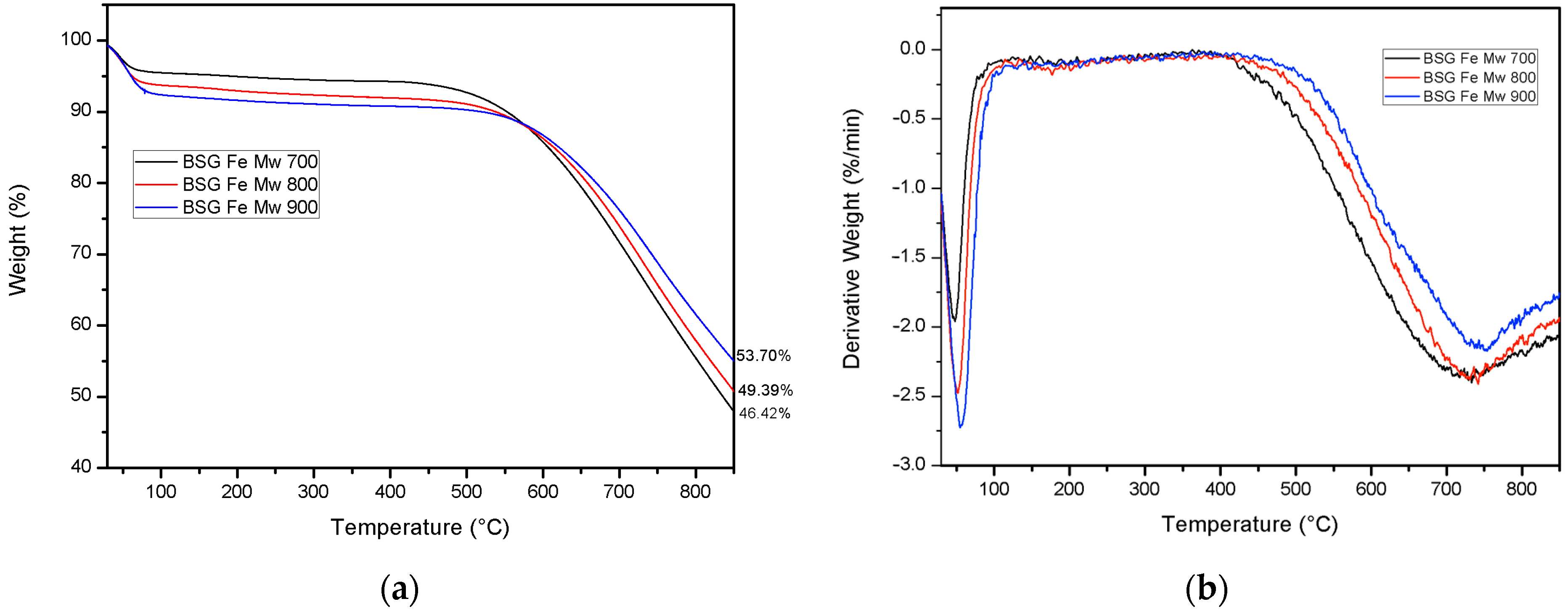
2.1.1. Thermogravimetric Analysis (TGA)
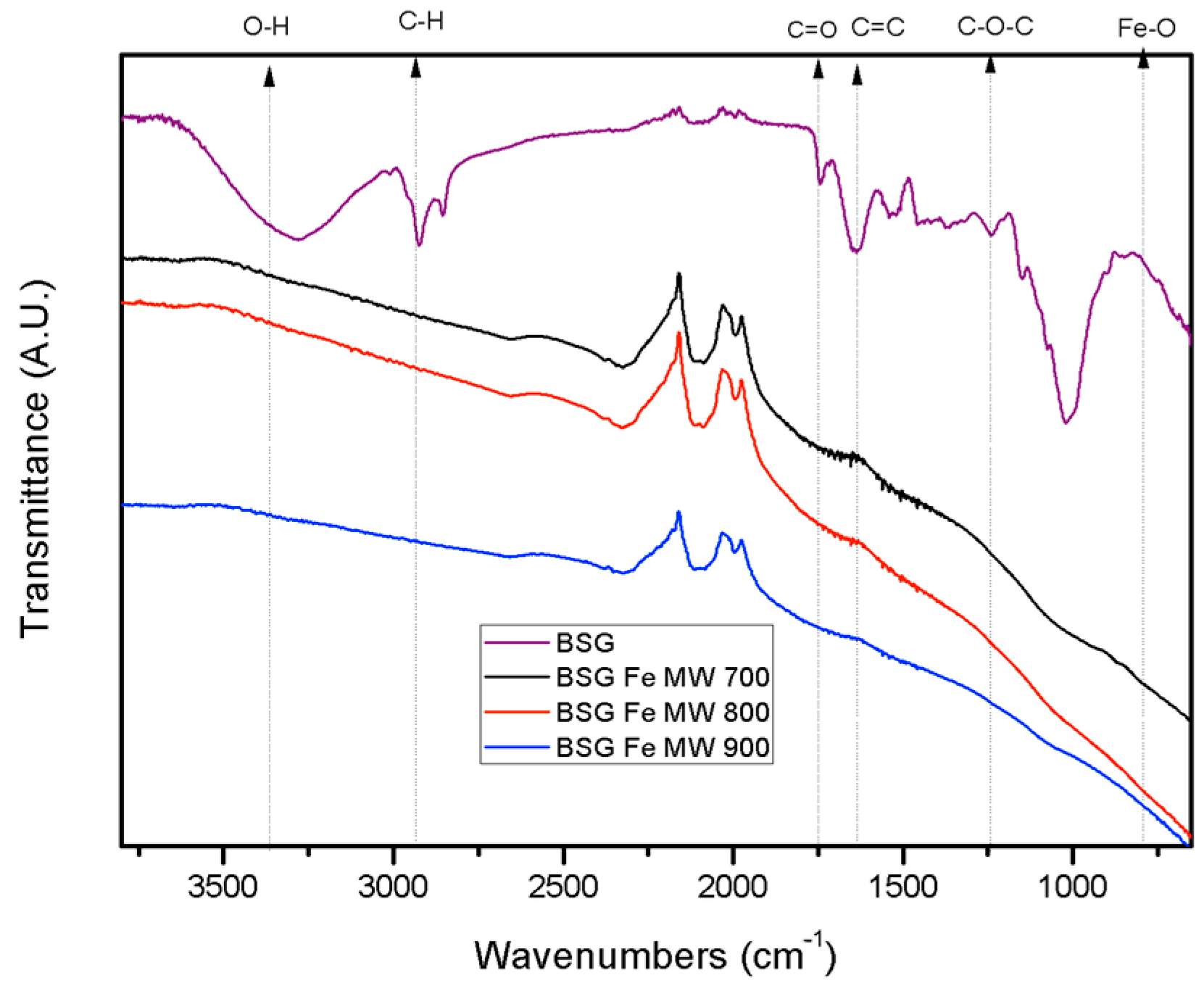
2.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.3. X-Ray Diffraction (XRD)
2.1.4. Raman Spectroscopy
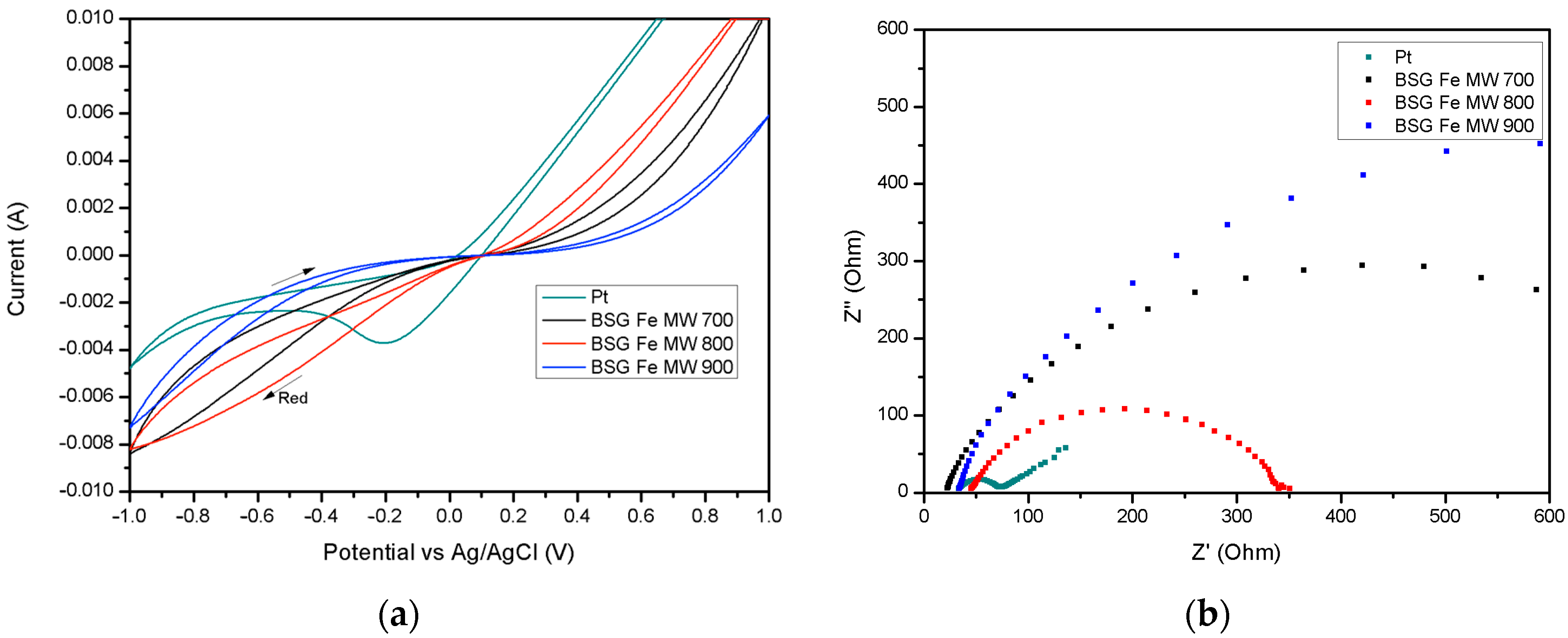
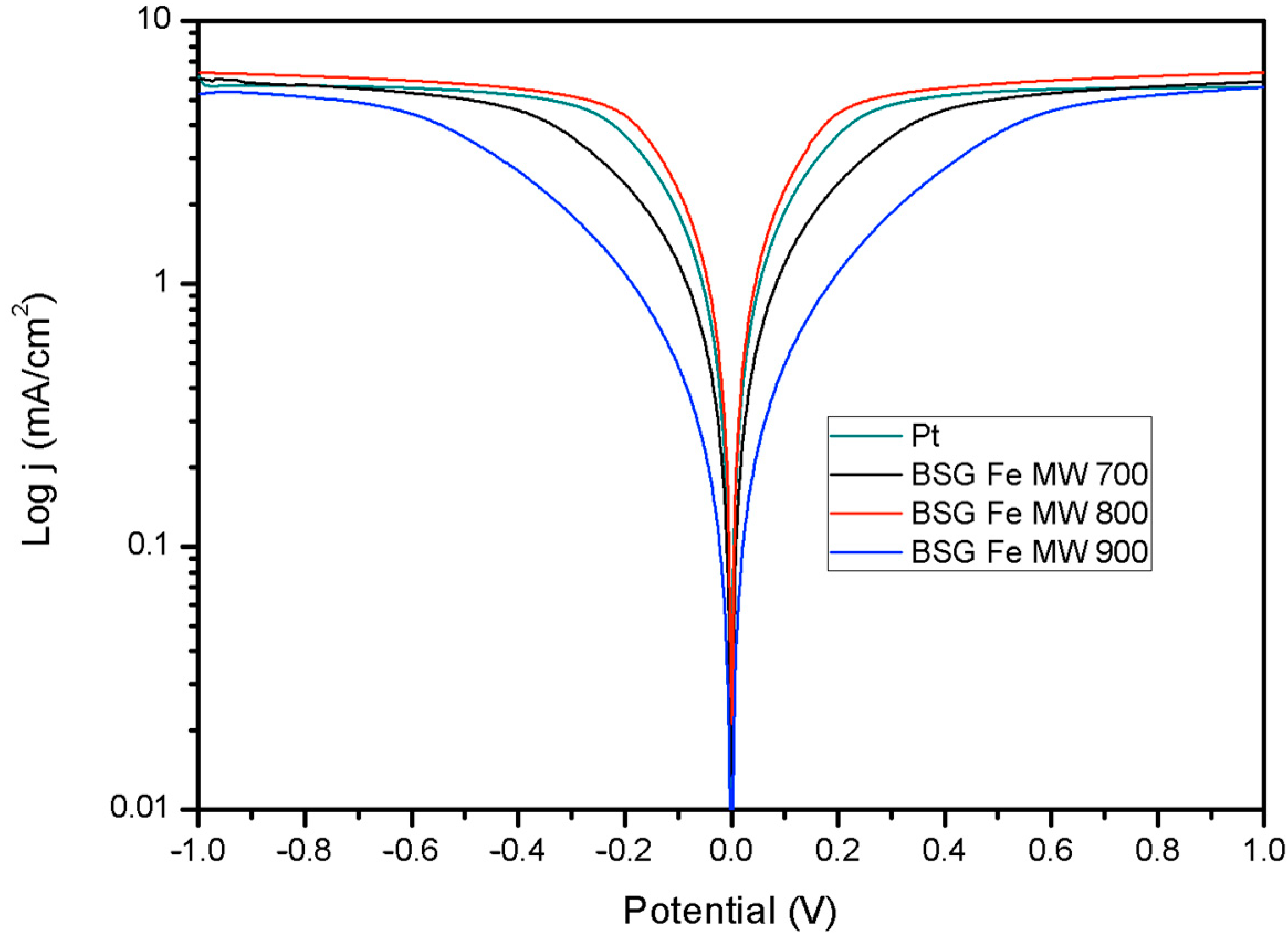
2.2. Electrochemical Characterization of the Counter Electrode
2.3. Characterization of DSSCs
3. Materials and Methods
3.1. Iron Oxide/Biochar Composite Synthesis
3.2. Iron Oxide/Biochar Composite Characterization
3.3. Preparation of Counter Electrodes with Iron Oxide/Biochar Composite
3.4. DSSC Assembly
3.5. Characterization of Counter Electrodes Fabricated with Iron Oxide/Biochar Composite and DSSCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample | 25% | 50% | 75% |
|---|---|---|---|
| BSG Fe MW 700 | 9.69 ± 0.50 | 19.77 ± 0.67 | 40.28 ± 0.66 |
| BSG Fe MW 800 | 10.07 ± 0.80 | 20.01 ± 1.21 | 39.73 ± 1.64 |
| BSG Fe MW 900 | 42.33 ± 3.02 | 68.90 ± 1.81 | 112.42 ± 2.32 |
References
- Kumar, P.; Thokala, S.; Singh, S.P.; Singh, R. Research Progress and Challenges in Extending the Infra-Red Absorption of Perovskite Tandem Solar Cells. Nano Energy 2024, 121, 109175. [Google Scholar] [CrossRef]
- Righini, G.C.; Enrichi, F. Chapter One—Solar Cells’ Evolution and Perspectives: A Short Review. In Solar Cells and Light Management: Materials, Strategies and Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–32. ISBN 9780081027622. [Google Scholar] [CrossRef]
- Gómez-Ortíz, N.M.; Vázquez-Maldonado, I.A.; Pérez-Espadas, A.R.; Mena-Rejón, G.J.; Azamar-Barrios, J.A.; Oskam, G. Dye-Sensitized Solar Cells with Natural Dyes Extracted from Achiote Seeds. Sol. Energy Mater. Sol. Cells 2010, 94, 40–44. [Google Scholar] [CrossRef]
- Gra, M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Recent Progress in Dye Sensitized Solar Cell Materials and Photo-Supercapacitors: A Review. J. Power Sources 2021, 493, 229698. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, X.; He, T. Self-Assembled CoS2 Nanocrystal Film as an Efficient Counter Electrode for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 24877–24883. [Google Scholar] [CrossRef]
- Shahzad, N.; Lutfullah; Perveen, T.; Pugliese, D.; Haq, S.; Fatima, N.; Salman, S.M.; Tagliaferro, A.; Shahzad, M.I. Counter Electrode Materials Based on Carbon Nanotubes for Dye-Sensitized Solar Cells. Renew. Sustain. Energy Rev. 2022, 159, 112196. [Google Scholar] [CrossRef]
- Du, Y.; Yue, G.; Lan, Z.; Gao, Y.; Wu, J.; Tan, F. A Dye-Sensitized Solar Cell Based on Magnetic CoP@FeP4@Carbon Composite Counter Electrode Generated an Efficiency of 9.88%. Inorg. Chem. Front. 2021, 8, 5034–5044. [Google Scholar] [CrossRef]
- Mposa, E.; Sithole, R.K.; Ndala, Z.; Ngubeni, G.N.; Mubiayi, K.P.; Shumbula, P.M.; Machogo-Phao, L.F.E.; Moloto, N. Novel 2D-AuSe Nanostructures as Effective Platinum Replacement Counter Electrodes in Dye-Sensitized Solar Cells. RSC Adv. 2022, 12, 12882–12890. [Google Scholar] [CrossRef]
- Mohanty, S.P.; More, V.; Bhargava, P. Effect of Aging Conditions on the Performance of Dip Coated Platinum Counter Electrode Based Dye Sensitized Solar Cells. RSC Adv. 2015, 5, 18647–18654. [Google Scholar] [CrossRef]
- Nemala, S.S.; Kartikay, P.; Agrawal, R.K.; Bhargava, P.; Mallick, S.; Bohm, S. Few Layers Graphene Based Conductive Composite Inks for Pt Free Stainless Steel Counter Electrodes for DSSC. Sol. Energy 2018, 169, 67–74. [Google Scholar] [CrossRef]
- Akman, E.; Karapinar, H.S. Electrochemically Stable, Cost-Effective and Facile Produced Selenium@activated Carbon Composite Counter Electrodes for Dye-Sensitized Solar Cells. Sol. Energy 2022, 234, 368–376. [Google Scholar] [CrossRef]
- Zatirostami, A. A New Electrochemically Prepared Composite Counter Electrode for Dye-Sensitized Solar Cells. Thin Solid Films 2020, 701, 137926. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.B.; Yoon, O.J.; Kim, H.J. Investigation on the Role of Graphene Oxide Sheet-Platinum Composite Counter Electrode in Dye-Sensitized Solar Cell. Thin Solid Films 2022, 745, 139098. [Google Scholar] [CrossRef]
- Xie, Z.; Guan, W.; Ji, F.; Song, Z.; Zhao, Y. Production of Biologically Activated Carbon from Orange Peel and Landfill Leachate Subsequent Treatment Technology. J. Chem. 2014, 2014, 491912. [Google Scholar] [CrossRef]
- Wang, C.; Yun, S.; Xu, H.; Wang, Z.; Han, F.; Zhang, Y.; Si, Y.; Sun, M. Dual Functional Application of Pomelo Peel-Derived Bio-Based Carbon with Controllable Morphologies: An Efficient Catalyst for Triiodide Reduction and Accelerant for Anaerobic Digestion. Ceram. Int. 2020, 46, 3292–3303. [Google Scholar] [CrossRef]
- Dhillon, K.K.; Patyal, M.; Bhogal, S.; Gupta, N. A Review on Advanced Counter Electrode Materials for High-Efficiency Dye Sensitized Solar Cells. J. Coord. Chem. 2024, 77, 1933–1968. [Google Scholar] [CrossRef]
- Dhonde, M.; Sahu, K.; Das, M.; Yadav, A.; Ghosh, P.; Murty, V.V.S. Review—Recent Advancements in Dye-Sensitized Solar Cells; From Photoelectrode to Counter Electrode. J. Electrochem. Soc. 2022, 169, 066507. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-Efficient Dye-Sensitized Solar Cells with Collaborative Sensitization by Silyl-Anchor and Carboxy-Anchor Dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Safian, M.T.-U.; Umar, K.; Mohamad Ibrahim, M.N. Synthesis and Scalability of Graphene and Its Derivatives: A Journey towards Sustainable and Commercial Material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Chen, B.; Zhou, C.; Ma, X.; Hou, L.; Tang, Y.; Yang, F.; Ning, G.; Zhang, L.; et al. Phosphorus-Doped Porous Graphene Nanosheet as Metal-Free Electrocatalyst for Triiodide Reduction Reaction in Dye-Sensitized Solar Cell. Appl. Surf. Sci. 2017, 405, 308–315. [Google Scholar] [CrossRef]
- Safian, M.T.-U.; Raja, P.B.; Shen, C.Y.; Mohamad Ibrahim, M.N. A Novel Preparation of Bio-Based Graphene from Oil Palm Biomass as a Fluid Loss Additive in Water-Based Drilling Fluid. Geoenergy Sci. Eng. 2023, 231, 212321. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining Biomass Residues for Sustainable Energy and Bio-Products: An Assessment of Technology, Its Importance, and Strategic Applications in Circular Bio-Economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Kumarasinghe, K.D.M.S.P.K.; Kumara, G.R.A.; Rajapakse, R.M.G.; Liyanage, D.N.; Tennakone, K. Activated Coconut Shell Charcoal Based Counter Electrode for Dye-Sensitized Solar Cells. Org. Electron. 2019, 71, 93–97. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.M.; Palanisamy, J.; Ezhil Vilian, A.T. Pumpkin Stem-Derived Activated Carbons as Counter Electrodes for Dye-Sensitized Solar Cells. RSC Adv. 2014, 4, 63917–63921. [Google Scholar] [CrossRef]
- Tiihonen, A.; Siipola, V.; Lahtinen, K.; Pajari, H.; Widsten, P.; Tamminen, T.; Kallio, T.; Miettunen, K. Biocarbon from Brewery Residues as a Counter Electrode Catalyst in Dye Solar Cells. Electrochim. Acta 2021, 368, 137583. [Google Scholar] [CrossRef]
- El Korhani, O.; Zaouk, D.; Cerneaux, S.; Khoury, R.; Khoury, A.; Cornu, D. Synthesis and Performances of Bio-Sourced Nanostructured Carbon Membranes Elaborated by Hydrothermal Conversion of Beer Industry Wastes. Nanoscale Res. Lett. 2013, 8, 121. [Google Scholar] [CrossRef]
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal Conversion of Different Lignocellulosic Biomass Feedstocks—Effect of the Process Conditions on Hydrochar Structures. Fuel 2021, 302, 121166. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Recent Progress in Biochar-Supported Photocatalysts: Synthesis, Role of Biochar, and Applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef]
- Davies, G.; McGregor, J. Hydrothermal Synthesis of Biomass-Derived Magnetic Carbon Composites for Adsorption and Catalysis. ACS Omega 2021, 6, 33000–33009. [Google Scholar] [CrossRef]
- Smith, A.M.; Whittaker, C.; Shield, I.; Ross, A.B. The Potential for Production of High Quality Bio-Coal from Early Harvested Miscanthus by Hydrothermal Carbonisation. Fuel 2018, 220, 546–557. [Google Scholar] [CrossRef]
- Zulkornain, M.F.; Shamsuddin, A.H.; Normanbhay, S.; Md Saad, J.; Zhang, Y.S.; Samsuri, S.; Wan Ab Karim Ghani, W.A. Microwave-Assisted Hydrothermal Carbonization for Solid Biofuel Application: A Brief Review. Carbon Capture Sci. Technol. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P.; Dey, T.K. Effect of Temperature on Dielectric Properties and Penetration Depth of Oil Palm Shell (OPS) and OPS Char Synthesized by Microwave Pyrolysis of OPS. Fuel 2015, 153, 257–266. [Google Scholar] [CrossRef]
- Rodriguez Ortiz, L.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of Pyrolysis Temperature and Bio-Waste Composition on Biochar Characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem.—Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Wang, N.; Chen, Y.; Li, G. Process Characteristics for Microwave Assisted Hydrothermal Carbonization of Cellulose. Bioresour. Technol. 2018, 259, 91–98. [Google Scholar] [CrossRef]
- Samantaray, M.R.; Mondal, A.K.; Murugadoss, G.; Pitchaimuthu, S.; Das, S.; Bahru, R.; Mohamed, M.A. Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-Sensitized Solar Cells: A Review. Materials 2020, 13, 2779. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, R.; Liu, M.; Cao, Y.; Sun, P.; Zhang, Y.; Hao, M.; Zhang, Y.; Zhang, W.; Li, L. Preparation of CoNi@CN Composites Based on Metal-Organic Framework Materials as High Efficiency Counter Electrode Materials for Dye-Sensitized Solar Cells. Sol. Energy 2022, 231, 767–774. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Q.; Liang, Q.; Li, W.; Li, X.; Liu, S.; Shuai, J. α-Fe2O3/Reduced Graphene Oxide Composites as Cost-Effective Counter Electrode for Dye-Sensitized Solar Cells. Catalysts 2022, 12, 645. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter Electrodes in Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2017, 46, 5975–6023. [Google Scholar] [CrossRef]
- Anabalón Fuentes, P.; Kopp Pailañir, M.; Rocha Mella, S.; González Quijón, M.E.; Marzialetti Bernardi, T.; Cea Lemus, M. Development of Bifunctional Biochar/Iron Oxide Composites for Tetracycline Removal from Synthetic Wastewater. J. Water Process Eng. 2024, 64, 105509. [Google Scholar] [CrossRef]
- Lataf, A.; Khalil Awad, A.E.; Joos, B.; Carleer, R.; Yperman, J.; Schreurs, S.; D’Haen, J.; Cuypers, A.; Vandamme, D. Iron Oxide-Activated Carbon Composites for Enhanced Microwave-Assisted Pyrolysis of Hardwood. Environments 2024, 11, 102. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Arauzo, P.J.; Wądrzyk, M.; Kruse, A. Py-GC-MS of Hydrochars Produced from Brewer’s Spent Grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Liu, J.; Liu, C.; Liu, W.; Lin, H. The Effect of Co-Pyrolysis Temperature for Iron-Biochar Composites on Their Adsorption Behavior of Antimonite and Antimonate in Aqueous Solution. Bioresour. Technol. 2022, 347, 126362. [Google Scholar] [CrossRef] [PubMed]
- Zazo, J.A.; Bedia, J.; Fierro, C.M.; Pliego, G.; Casas, J.A.; Rodriguez, J.J. Highly Stable Fe on Activated Carbon Catalysts for CWPO upon FeCl3 Activation of Lignin from Black Liquors. Catal. Today 2012, 187, 115–121. [Google Scholar] [CrossRef]
- Wrogemann, J.M.; Fromm, O.; Deckwirth, F.; Beltrop, K.; Heckmann, A.; Winter, M.; Placke, T. Impact of Degree of Graphitization, Surface Properties and Particle Size Distribution on Electrochemical Performance of Carbon Anodes for Potassium-Ion Batteries. Batter. Supercaps 2022, 5, e202200045. [Google Scholar] [CrossRef]
- Sert, S.; Gultekin, Ş.S.; Gültekin, B.; Duran Kaya, D.; Körlü, A. A Facile Approach to Produce Activated Carbon from Waste Textiles via Self-Purging Microwave Pyrolysis and FeCl3 Activation for Electromagnetic Shielding Applications. Polymers 2024, 16, 915. [Google Scholar] [CrossRef]
- Aslam, J.; Waseem, M.A.; Lu, X.M.; Sun, W.; Wang, Y. From Biochar to Battery Electrodes: A Pathway to Green Lithium and Sodium-Ion Battery Systems. Chem. Eng. J. 2025, 505, 159556. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A Comprehensive Review on Activated Carbon from Pyrolysis of Lignocellulosic Biomass: An Application for Energy and the Environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, N. Activated Carbon Preparation from Eucalyptus Wood Chips Using Continuous Carbonization–Steam Activation Process in a Batch Intermittent Rotary Kiln. Sci. Rep. 2021, 11, 13948. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.M.; Kruse, A. Wet and Dry? Influence of Hydrothermal Carbonization on the Pyrolysis of Spent Grains. J. Clean. Prod. 2020, 260, 121101. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Zhu, M.; Sun, G.; Zhang, T.; Kang, K. Comparative Evaluation of Hydrothermal Carbonization and Low Temperature Pyrolysis of Eucommia Ulmoides Oliver for the Production of Solid Biofuel. Sci. Rep. 2019, 9, 5535. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Labus, K. The Methods of Brewers’ Spent Grain Treatment towards the Recovery of Valuable Ingredients Contained Therein and Comprehensive Management of Its Residues. Chem. Eng. Res. Des. 2022, 183, 494–511. [Google Scholar] [CrossRef]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green Preparation of Reduced Graphene Oxide by Bougainvillea Glabra Flower Extract and Sensing Application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- BhaskaraRao, B.V.; Pabba, D.P.; Aepuru, R.; Akbari-Fakhrabadi, A.; Lokhande, P.; Udayabhaskar, R.; Rosales-Vera, M.; Espinoza-González, R. Fe3O4 Nanoparticles Intercalated Reduced Graphene Oxide Nanosheets for Supercapacitor and Lithium-Ion Battery Anode Performance. J. Mater. Sci. Mater. Electron. 2023, 34, 1910. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Zhang, H.; Cao, Z.; Wu, H.; Jia, B.; Yang, J.J.; Qu, X.; Qin, M. Large-Scale Synthesis of Ultrafine Fe3C Nanoparticles Embedded in Mesoporous Carbon Nanosheets for High-Rate Lithium Storage. RSC Adv. 2022, 12, 6508–6514. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of Lignin Contents from Infrared Spectroscopy: Chemical Digestion and Lignin/Biomass Ratios of Cryptomeria Japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Al-Hazmi, G.H.; Saad, H.A.; Refat, M.S.; Adam, A.M.A. Fe3O4-Carbon-Based Composite Derived from the Charge-Transfer Reaction Using Waste Tea Leaves as the Carbon Precursor for Enhanced Removing of Azocarmine G2, Methyl Violet 2B, Eosin B, and Toluidine Blue from Aqueous Solution. Crystals 2022, 12, 1355. [Google Scholar] [CrossRef]
- Li, J.; Lan, H.; Zhang, W.; An, X.; Liu, H.; Qu, J. Characterization on the Formation Mechanism of Fe0/Fe3C/C Nanostructure and Its Effect on PMS Activation Performance towards BPA Degradation. Chem. Eng. J. 2022, 435, 134709. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Z.; Zhou, Y.; Chen, W.; Zhang, T.; Huang, Y.; Zhang, D. Insights into the Pyrolysis Behavior and Adsorption Properties of Activated Carbon from Waste Cotton Textiles by FeCl3-Activation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123934. [Google Scholar] [CrossRef]
- Shen, Y.; Jing, T.; Ren, W.; Zhang, J.; Jiang, Z.G.; Yu, Z.Z.; Dasari, A. Chemical and Thermal Reduction of Graphene Oxide and Its Electrically Conductive Polylactic Acid Nanocomposites. Compos. Sci. Technol. 2012, 72, 1430–1435. [Google Scholar] [CrossRef]
- Pusceddu, E.; Santilli, S.F.; Fioravanti, G.; Montanaro, A.; Miglietta, F.; Foscolo, P.U. Chemical-Physical Analysis and Exfoliation of Biochar-Carbon Matter: From Agriculture Soil Improver to Starting Material for Advanced Nanotechnologies. Mater Res. Express 2019, 6, 115612. [Google Scholar] [CrossRef]
- Ghosh, D.; Banerjee, R.; Bhaduri, S.N.; Chatterjee, R.; Ghosh, A.B.; Das, S.; Pramanick, I.; Bhaumik, A.; Biswas, P. Fe3C/Fe Decorated N-Doped Carbon Derived from Tetrabutylammonium Tetrachloroferrate Complex as Bifunctional Electrocatalysts for ORR, OER and Zn-Air Batteries in Alkaline Medium. Chem. Asian J. 2024, 19, e202300933. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, G.S.; de Oliveira, H.P.; Larsson, S.H.; Thyrel, M.; Lima, E.C. A Short Review on the Electrochemical Performance of Hierarchical and Nitrogen-Doped Activated Biocarbon-Based Electrodes for Supercapacitors. Nanomaterials 2021, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Sevilla, M.; Magasinski, A.; Fuertes, A.B.; Yushin, G. Sulfur-Containing Activated Carbons with Greatly Reduced Content of Bottle Neck Pores for Double-Layer Capacitors: A Case Study for Pseudocapacitance Detection. Energy Environ. Sci. 2013, 6, 2465–2476. [Google Scholar] [CrossRef]
- Mohammad, F.Z.; Ahmad, I.; Siddiqui, J.J.; Ali, K.M.; Mudsar, M.; Arshad, H. Light Weight RGO/Fe3O4 Nanocomposite for Efficient Electromagnetic Absorption Coating in X-Band. J. Mater. Sci. Mater. Electron. 2018, 29, 19775–19782. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Cao, W.; Han, C.; Yang, H.; Yuan, J.; Cao, M. A Facile Fabrication and Highly Tunable Microwave Absorption of 3D Flower-like Co3O4-RGO Hybrid-Architectures. Chem. Eng. J. 2018, 339, 487–498. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Song, Y.; Yang, H.; Yang, L.; Bai, L.; Wei, D.; Wang, W.; Liang, Y.; Chen, H. Apple Residues Derived Porous Carbon Nanosheets Synthesized with FeCl3 Assisted Hydrothermal Carbonization for Supercapacitors with High Rate Performance. Carbon Lett. 2023, 33, 549–560. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Wang, M.; Du, M.; Wang, Z.; Zou, Y.; Pan, G.; Zhang, J.; Li, H.; Zhang, M.; et al. Balancing Edge Defects and Graphitization in a Pt–Fe/Carbon Electrocatalyst for High-Power-Density and Durable Flow Seawater-Al/Acid Hybrid Fuel Cells and Zn–Air Batteries. Adv. Sci. 2024, 11, 2308923. [Google Scholar] [CrossRef]
- Chai, Y.; Wan, C.; Cheng, W.; Zeng, Q.; Li, X.; Wu, Y. Wood-Derived Pore-Rich Carbon: A Green Catalyst for Counter Electrodes of Dye-Sensitized Solar Cells. Mater. Sci. Eng. B 2023, 296, 116663. [Google Scholar] [CrossRef]
- Chang, B.-Y. The Effective Capacitance of a Constant Phase Element with Resistors in Series and Parallel. SSRN Electron. J. 2022, 13, 479–485. [Google Scholar] [CrossRef]
- Kanjana, N.; Maiaugree, W.; Tontapha, S.; Laokul, P.; Chingsungnoen, A.; Pimanpang, S.; Chaiya, I.; Daengsakul, S.; Amornkitbamrung, V. Effect of Carbonization Temperature on the Electrocatalytic Property and Efficiency of Dye-Sensitized Solar Cells Derived from Corncob and Sugarcane Leaf Agricultural Residues. Biomass Convers. Biorefin. 2023, 13, 8361–8371. [Google Scholar] [CrossRef]
- Chen, M.; Shao, L.L. Review on the Recent Progress of Carbon Counter Electrodes for Dye-Sensitized Solar Cells. Chem. Eng. J. 2016, 304, 629–645. [Google Scholar] [CrossRef]
- Qi, R.; Xu, Z.; Zhou, Y.; Zhang, D.; Sun, Z.; Chen, W.; Xiong, M. Clean Solid Fuel Produced from Cotton Textiles Waste through Hydrothermal Carbonization with FeCl3: Upgrading the Fuel Quality and Combustion Characteristics. Energy 2021, 214, 118926. [Google Scholar] [CrossRef]
- Macdermid-Watts, K.; Adewakun, E.; Norouzi, O.; Abhi, T.D.; Pradhan, R.; Dutta, A. Effects of FeCl3 Catalytic Hydrothermal Carbonization on Chemical Activation of Corn Wet Distillers’ Fiber. ACS Omega 2021, 6, 14875–14886. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Hsu, W.L.; Wang, Y.T.; Ho, C.T.; Chang, P.Z. Enhance the Pyroelectricity of Polyvinylidene Fluoride by Graphene-Oxide Doping. Sensors 2014, 14, 6877–6890. [Google Scholar] [CrossRef]
- Kim, H.; Bin, Y.; Karthick, S.N.; Hemalatha, K.V.; Raj, C.J.; Venkatesan, S.; Park, S.; Vijayakumar, G. Natural Dye Extracted from Rhododendron Species Flowers as a Photosensitizer in Dye Sensitized Solar Cell. Int. J. Electrochem. Sci. 2013, 8, 6734–6743. [Google Scholar] [CrossRef]



| Elemental Analysis (wt.%) | BET/BJH Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Sample | C | H | N | Fe | SBET (m2/g) | VP (cm3/g) | DP (nm) |
| BSG Fe MW 700 | 92.27 ± 1.26 | 2.66 ± 0.22 | 1.03 ± 0.08 | 1.84 ± 0.11 | 110.640 | 0.028 | 1.87 |
| BSG Fe MW 800 | 94.40 ± 0.68 | 2.26 ± 0.04 | 1.15 ± 0.09 | 0.28 ± 0.00 | 13.087 | 0.029 | 1.88 |
| BSG Fe MW 900 | 91.97 ± 1.65 | 1.08 ± 0.34 | 0.78± 0.06 | 0.76 ± 0.37 | 9.561 | 0.037 | 1.86 |
| Sample | D | G | ID/IG |
|---|---|---|---|
| BSG Fe MW 700 | 1333 | 1584 | 0.90 |
| BSG Fe MW 800 | 1338 | 1588 | 0.95 |
| BSG Fe MW 900 | 1348 | 1586 | 0.94 |
| (V) | (mA) | |
|---|---|---|
| Pt | −0.20 ± 0.000 | −3.7 ± 0.0003 |
| BSG Fe MW 700 | −0.79 ± 0.0003 | −6.6 ± 0.0009 |
| BSG Fe MW 800 | −0.54 ± 0.0000 | −5.3 ± 0.0003 |
| BSG Fe MW 900 | −0.84 ± 0.0003 | −5.4± 0.0003 |
| ⋅cm) | ⋅cm) | CPE (F/cm2) | n | |
|---|---|---|---|---|
| Pt | 27.3 | 126 | 14.2 | 0.80 |
| BSG Fe MW 700 | 23.3 | 788 | 15.8 | 0.82 |
| BSG Fe MW 800 | 43.3 | 297 | 26.6 | 0.80 |
| BSG Fe MW 900 | 29.8 | 855 | 18.2 | 0.83 |
| J0 (mA/cm2) | Jlim | |
|---|---|---|
| Pt | 14.670 | 5.607 |
| BSG Fe MW 700 | 11.826 | 5.449 |
| BSG Fe MW 800 | 20.593 | 6.036 |
| BSG Fe MW 900 | 1.1429 | 4.781 |
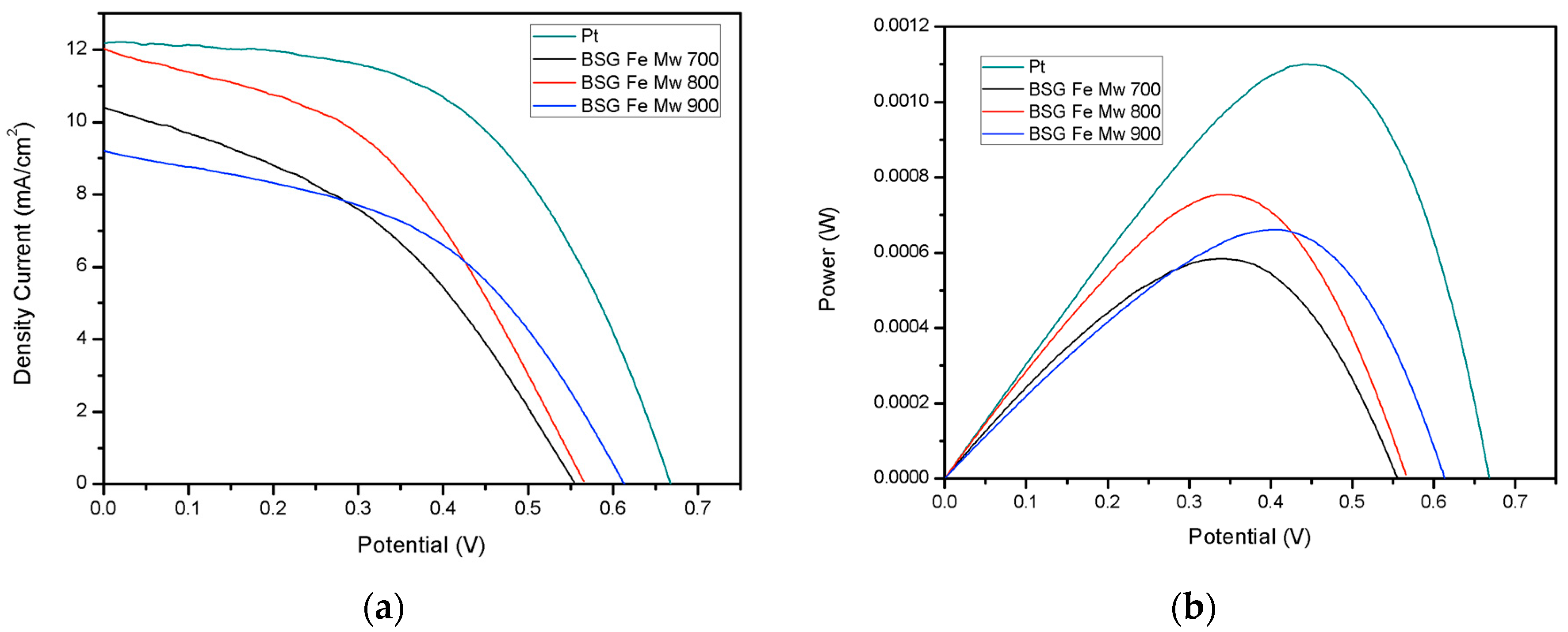
| Counter Electrode | Voc (V) | Jsc (mA/cm2) | Pmáx (mW) | FF | Efficiency (%) |
|---|---|---|---|---|---|
| Pt | 0.668 | 12.26 | 1.108 | 0.54 | 4.43 |
| BSG Fe MW 700 | 0.556 | 10.44 | 0.590 | 0.41 | 2.36 |
| BSG Fe MW 800 | 0.566 | 11.90 | 0.762 | 0.44 | 3.05 |
| BSG Fe MW 900 | 0.614 | 9.187 | 0.666 | 0.47 | 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.; Ramírez, A.M.R.; Pavez, B.; Gonzalez, M.E.; Kopp, M.; Meza, P.; Cea, M. Valorization of Residual Brewery Biomass for the Production of Counter Electrodes for Dye-Sensitized Solar Cells. Catalysts 2025, 15, 433. https://doi.org/10.3390/catal15050433
Silva C, Ramírez AMR, Pavez B, Gonzalez ME, Kopp M, Meza P, Cea M. Valorization of Residual Brewery Biomass for the Production of Counter Electrodes for Dye-Sensitized Solar Cells. Catalysts. 2025; 15(5):433. https://doi.org/10.3390/catal15050433
Chicago/Turabian StyleSilva, Camila, A. M. R. Ramírez, Boris Pavez, María Eugenia Gonzalez, Matías Kopp, Pablo Meza, and Mara Cea. 2025. "Valorization of Residual Brewery Biomass for the Production of Counter Electrodes for Dye-Sensitized Solar Cells" Catalysts 15, no. 5: 433. https://doi.org/10.3390/catal15050433
APA StyleSilva, C., Ramírez, A. M. R., Pavez, B., Gonzalez, M. E., Kopp, M., Meza, P., & Cea, M. (2025). Valorization of Residual Brewery Biomass for the Production of Counter Electrodes for Dye-Sensitized Solar Cells. Catalysts, 15(5), 433. https://doi.org/10.3390/catal15050433








