Production of Hydroxylated Steroid Intermediates at 10-g Scale via the Original Sterol Modification Pathway in Mycolicibacterium neoaurum
Abstract
1. Introduction
2. Results and Discussion
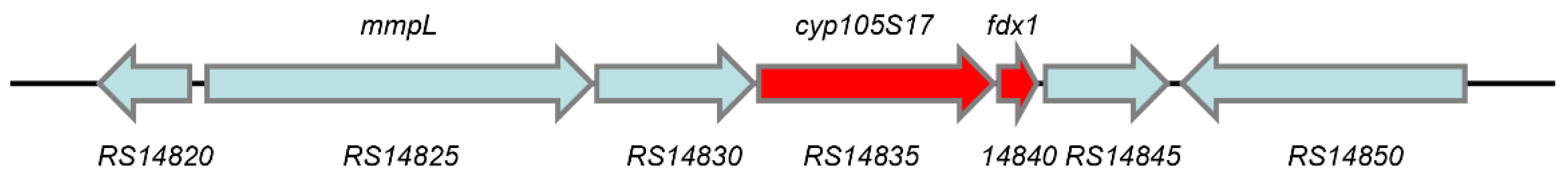
2.1. Screening of cyp450 Genes
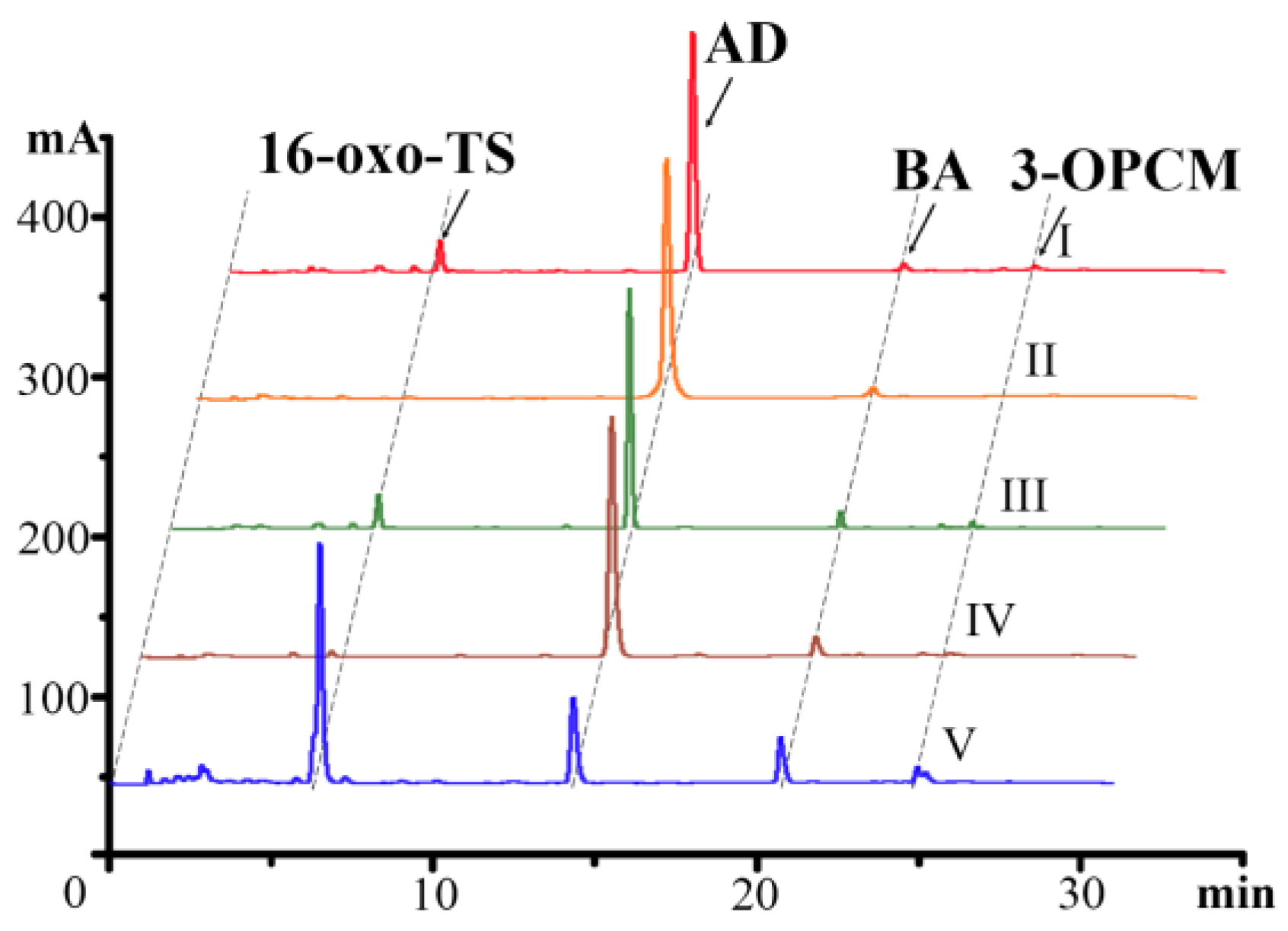
2.2. Analysis of Sterol Transformation Products of Mutant Strains
2.3. P450 Substrate Determination
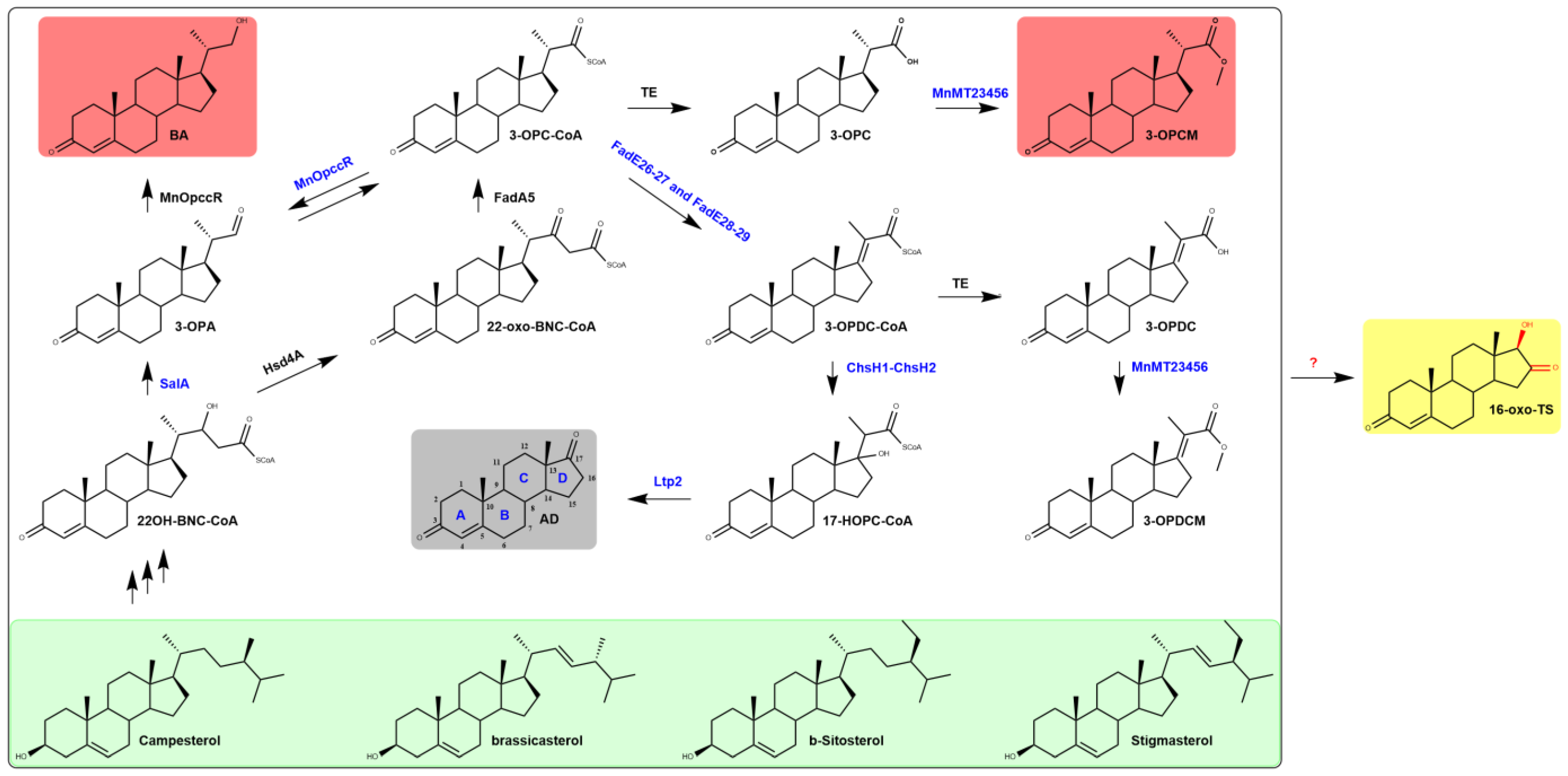
2.4. A Novel Steroid D-Ring Modification Pathway in M. neoaurum
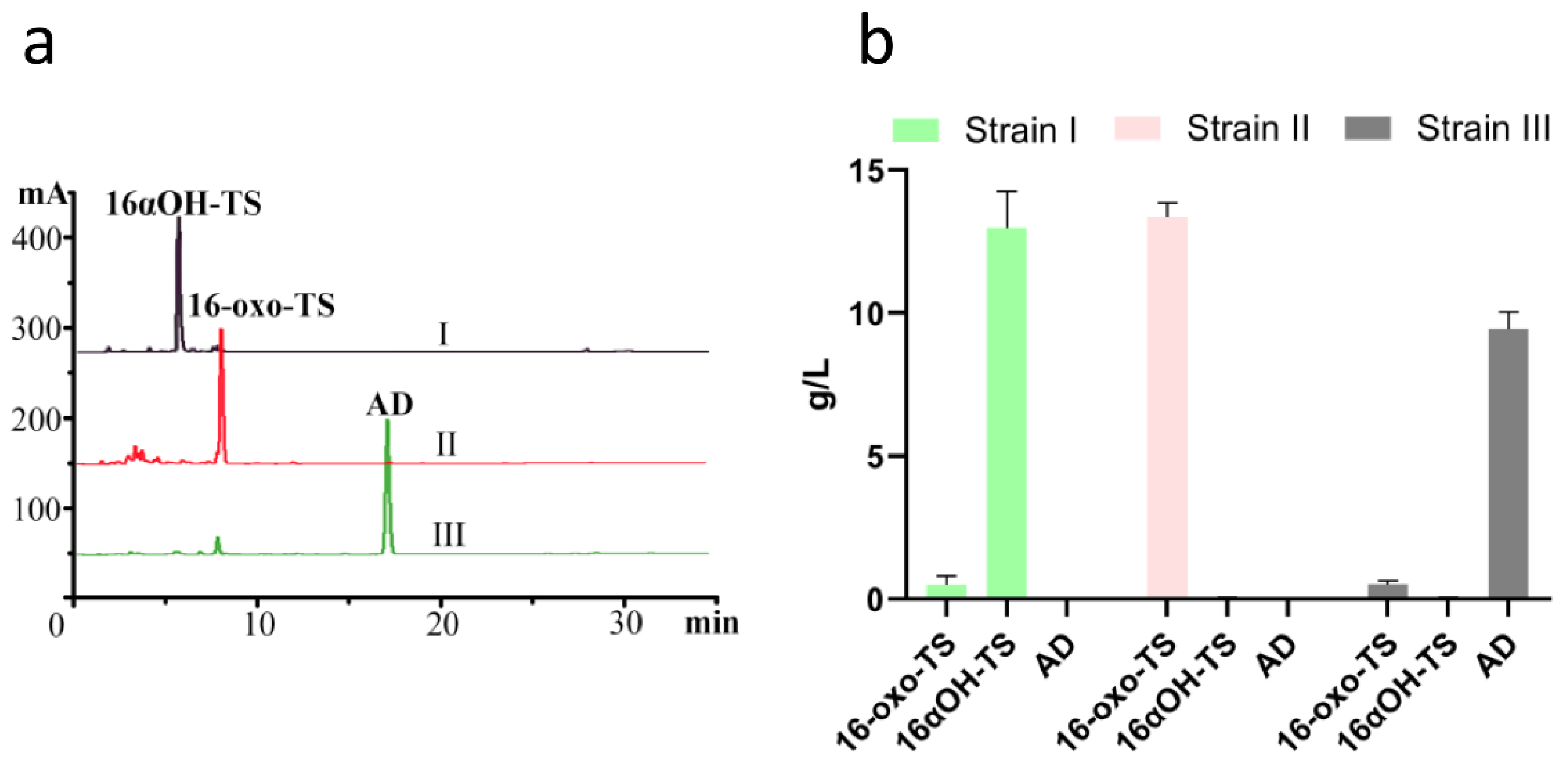
2.5. Construction and Evaluation of a High-Yield 16α-OH-TS Engineered Strain
3. Materials and Methods
3.1. Strains, Plasmids, Reagents, and Culture Conditions
3.2. Gene Manipulation
3.3. Steroid Bioconversion by Mycobacterial Strains
3.4. Steroid Transformation by Resting E. coli Cell
3.5. Analysis of Fermentation Metabolites
3.6. Sequence Alignment and Molecular Docking Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parish, T.; Stoker, N.G. Mycobacteria. In Mycobacteria Protocols; Parish, T., Stoker, N.G., Eds.; Humana Press: Totowa, NJ, USA, 1998; pp. 1–13. [Google Scholar]
- Song, S.; He, J.; Gao, M.; Huang, Y.; Cheng, X.; Su, Z. Loop pathways are responsible for tuning the accumulation of C19- and C22-sterol intermediates in the mycobacterial phytosterol degradation pathway. Microb. Cell Factories 2023, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Song, S.; He, J.; Zhang, J.; Liu, X.; Pena Eliana, L.; Sun, J.; Shi, J.; Su, Z.; Zhang, B. Bioconversion of Phytosterols to 9-Hydroxy-3-Oxo-4,17-Pregadiene-20-Carboxylic Acid Methyl Ester by Enoyl-CoA Deficiency and Modifying Multiple Genes in Mycolicibacterium neoaurum. Appl. Environ. Microbiol. 2022, 88, e01303–e01322. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, X.; Li, Y.; Wang, Z.; Lv, Y.; Liu, J.; Alam, M.A.; Xiong, W.; Xu, J. Mycolicibacterium cell factory for the production of steroid-based drug intermediates. Biotechnol. Adv. 2021, 53, 107860. [Google Scholar] [CrossRef] [PubMed]
- Crowe Adam, M.; Casabon, I.; Brown Kirstin, L.; Liu, J.; Lian, J.; Rogalski Jason, C.; Hurst Timothy, E.; Snieckus, V.; Foster Leonard, J.; Eltis Lindsay, D. Catabolism of the Last Two Steroid Rings in Mycobacterium tuberculosis and Other Bacteria. mBio 2017, 8, e00321-17. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.M.; Krekhno, J.M.C.; Brown, K.L.; Kulkarni, J.A.; Yam, K.C.; Eltis, L.D. The unusual convergence of steroid catabolic pathways in Mycobacterium abscessus. Proc. Natl. Acad. Sci. USA 2022, 119, e2207505119. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wang, F.-Q.; Zhang, H.-C.; Wei, D.-Z. Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab. Eng. 2013, 15, 75–87. [Google Scholar] [CrossRef]
- Bragin, E.Y.; Shtratnikova, V.Y.; Schelkunov, M.I.; Dovbnya, D.V.; Donova, M.V. Genome-wide response on phytosterol in 9-hydroxyandrostenedione-producing strain of Mycobacterium sp. VKM Ac-1817D. BMC Biotechnol. 2019, 19, 39. [Google Scholar] [CrossRef]
- Wang, H.; Song, S.; Peng, F.; Yang, F.; Chen, T.; Li, X.; Cheng, X.; He, Y.; Huang, Y.; Su, Z. Whole-genome and enzymatic analyses of an androstenedione-producing Mycobacterium strain with residual phytosterol-degrading pathways. Microb. Cell Factories 2020, 19, 187. [Google Scholar] [CrossRef]
- Ouellet, H.; Guan, S.; Johnston, J.B.; Chow, E.D.; Kells, P.M.; Burlingame, A.L.; Cox, J.S.; Podust, L.M.; De Montellano, P.R.O. Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest-4-en-3-one. Mol. Microbiol. 2010, 77, 730–742. [Google Scholar] [CrossRef]
- Ghith, A.; Bruning, J.B.; Bell, S.G. The oxidation of cholesterol derivatives by the CYP124 and CYP142 enzymes from Mycobacterium marinum. J. Steroid Biochem. Mol. Biol. 2023, 231, 106317. [Google Scholar] [CrossRef]
- Ghith, A.; Bruning, J.B.; Bell, S.G. The catalytic activity and structure of the lipid metabolizing CYP124 cytochrome P450 enzyme from Mycobacterium marinum. Arch. Biochem. Biophys. 2023, 737, 109554. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.J.; Belcher, J.; Driscoll, M.D.; Fernandez, C.C.; Le Van, D.; Bui, S.; Golovanova, M.; Munro, A.W. The Mycobacterium Tuberculosis Cytochromes P450: Physiology, Biochemistry & Molecular Intervention. Future Med. Chem. 2010, 2, 1339–1353. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Substrate Oxidation by Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 111–176. [Google Scholar]
- Zhang, X.; Peng, Y.; Zhao, J.; Li, Q.; Yu, X.; Acevedo-Rocha, C.G.; Li, A. Bacterial cytochrome P450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresour. Bioprocess. 2020, 7, 2. [Google Scholar] [CrossRef]
- Berrie, J.R.; Williams, R.A.D.; Smith, K.E. Microbial transformations of steroids-XI. Progesterone transformation by Streptomyces roseochromogenes–purification and characterisation of the 16α-hydroxylase system. J. Steroid Biochem. Mol. Biol. 1999, 71, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lobastova, T.G.; Gulevskaya, S.A.; Sukhodolskaya, G.V.; Donova, M.V. Dihydroxylation of dehydroepiandrosterone in positions 7α and 15α by mycelial fungi. Appl. Biochem. Microbiol. 2009, 45, 617–622. [Google Scholar] [CrossRef]
- Milecka-Tronina, N.; Kołek, T.; Świzdor, A.; Panek, A. Hydroxylation of DHEA and its analogues by Absidia coerulea AM93. Can an inducible microbial hydroxylase catalyze 7α- and 7β-hydroxylation of 5-ene and 5α-dihydro C19-steroids? Bioorg. Med. Chem. 2014, 22, 883–891. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Archer, D.F. Gestodene: A review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception 2014, 89, 242–252. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, H.; Tian, W.; Chang, Z. Production of 14α-Hydroxy Progesterone Using a Steroidal Hydroxylase from Cochliobolus lunatus Expressed in Escherichia coli. Catalysts 2024, 14, 247. [Google Scholar] [CrossRef]
- Ke, X.; Dong, H.-D.; Zhao, X.-M.; Wang, X.-X.; Liu, Z.-Q.; Zheng, Y.-G. Functional Expression and Construction of a Self-Sufficient Cytochrome P450 Chimera for Efficient Steroidal C14α Hydroxylation in Escherichia coli. Biotechnol. Bioeng. 2025, 122, 724–735. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, C.; Zhao, J.; Peng, X.-G.; Li, Q.; Li, A. A Designed Chemoenzymatic Route for Efficient Synthesis of 6-Dehydronandrolone Acetate: A Key Precursor in the Synthesis of C7-Functionalized Steroidal Drugs. ACS Catal. 2023, 13, 13111–13116. [Google Scholar] [CrossRef]
- Epiktetov, D.O.; Karpov, M.V.; Donova, M.V. Identification of Electron Transfer in the System of Ferredoxins and Ferredoxin Reductases from Mycolicibacterium smegmatis. Microbiology 2024, 93, 149–153. [Google Scholar] [CrossRef]
- Rank, L.; Herring Laura, E.; Braunstein, M. Evidence for the Mycobacterial Mce4 Transporter Being a Multiprotein Complex. J. Bacteriol. 2021, 203, e00685-20. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Chang, F.; Wang, Q.; Chang, Z.; Xia, H. Functional reconstitution of a steroidal hydroxylase from the fungus Thanatephorus cucumeris in Mycolicibacterium neoaurum for 15α-hydroxylation of progesterone. Biochem. Eng. J. 2023, 193, 108859. [Google Scholar] [CrossRef]
- Wipperman Matthew, F.; Yang, M.; Thomas Suzanne, T.; Sampson Nicole, S. Shrinking the FadE Proteome of Mycobacterium tuberculosis: Insights into Cholesterol Metabolism through Identification of an α2β2 Heterotetrameric Acyl Coenzyme A Dehydrogenase Family. J. Bacteriol. 2013, 195, 4331–4341. [Google Scholar] [CrossRef]
- Yang, M.; Lu, R.; Guja, K.E.; Wipperman, M.F.; St. Clair, J.R.; Bonds, A.C.; Garcia-Diaz, M.; Sampson, N.S. Unraveling Cholesterol Catabolism in Mycobacterium tuberculosis: ChsE4-ChsE5 α2β2 Acyl-CoA Dehydrogenase Initiates β-Oxidation of 3-Oxo-cholest-4-en-26-oyl CoA. ACS Infect. Dis. 2015, 1, 110–125. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Y.; Han, S.; He, B.; Zhai, X.; Lin, W.; Shi, J.; Sun, J.; Zhang, B. Functional analysis of acyl-CoA dehydrogenases and their application to C22 steroid production. Appl. Microbiol. Biotechnol. 2023, 107, 3419–3428. [Google Scholar] [CrossRef]
- Bracco, P.; Janssen, D.B.; Schallmey, A. Selective steroid oxyfunctionalisation by CYP154C5, a bacterial cytochrome P450. Microb. Cell Factories 2013, 12, 95. [Google Scholar] [CrossRef]
- Fan, S.; Qin, M.; Wang, Q.; Jiang, Y.; Cong, Z. Semirationally Engineering an Efficient P450 Peroxygenase for Regio- and Enantioselective Hydroxylation of Steroids. ACS Catal. 2025, 15, 2977–2986. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Q.; Ikeda, H.; Zhang, C.; Xu, L.-H. Hydroxylation of Steroids by a Microbial Substrate-Promiscuous P450 Cytochrome (CYP105D7): Key Arginine Residues for Rational Design. Appl. Environ. Microbiol. 2019, 85, e01530-19. [Google Scholar] [CrossRef]
- Moody, S.C.; Loveridge, E.J. CYP105—Diverse structures, functions and roles in an intriguing family of enzymes in Streptomyces. J. Appl. Microbiol. 2014, 117, 1549–1563. [Google Scholar] [CrossRef]
- Jóźwik, I.K.; Kiss, F.M.; Gricman, Ł.; Abdulmughni, A.; Brill, E.; Zapp, J.; Pleiss, J.; Bernhardt, R.; Thunnissen, A.-M.W.H. Structural basis of steroid binding and oxidation by the cytochrome P450 CYP109E1 from Bacillus megaterium. FEBS J. 2016, 283, 4128–4148. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Medema, M.H.; Kottmann, R.; Lee, S.Y.; Weber, T. The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2016, 45, D555–D559. [Google Scholar] [CrossRef] [PubMed]
- Melly, G.; Purdy, G.E. MmpL Proteins in Physiology and Pathogenesis of M. tuberculosis. Microorganisms 2019, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.L.; Kelly, D.E.; Jackson, C.J.; Warrilow, A.G.S.; Lamb, D.C. The Diversity and Importance of Microbial Cytochromes P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Ortiz de Montellano, P.R., Ed.; Springer: Boston, MA, USA, 2005; pp. 585–617. [Google Scholar]
- Mnguni, F.C.; Padayachee, T.; Chen, W.; Gront, D.; Yu, J.-H.; Nelson, D.R.; Syed, K. More P450s Are Involved in Secondary Metabolite Biosynthesis in Streptomyces Compared to Bacillus, Cyanobacteria, and Mycobacterium. Int. J. Mol. Sci. 2020, 21, 4814. [Google Scholar] [CrossRef]
- McLean, K.J.; Hans, M.; Meijrink, B.; van Scheppingen, W.B.; Vollebregt, A.; Tee, K.L.; van der Laan, J.-M.; Leys, D.; Munro, A.W.; van den Berg, M.A. Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 2015, 112, 2847–2852. [Google Scholar] [CrossRef]
- Yasuda, K.; Sugimoto, H.; Hayashi, K.; Takita, T.; Yasukawa, K.; Ohta, M.; Kamakura, M.; Ikushiro, S.; Shiro, Y.; Sakaki, T. Protein engineering of CYP105s for their industrial uses. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2018, 1866, 23–31. [Google Scholar] [CrossRef]
- Omer, C.A.; Lenstra, R.; Litle, P.J.; Dean, C.; Tepperman, J.M.; Leto, K.J.; Romesser, J.A.; O’Keefe, D.P. Genes for two herbicide-inducible cytochromes P-450 from Streptomyces griseolus. J. Bacteriol. 1990, 172, 3335–3345. [Google Scholar] [CrossRef]
- Kawauchi, H.; Sasaki, J.; Adachi, T.; Hanada, K.; Beppu, T.; Horinouchi, S. Cloning and nucleotide sequence of a bacterial cytochrome P-450VD25 gene encoding vitamin D-3 25-hydroxylase. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1994, 1219, 179–183. [Google Scholar] [CrossRef]
- Horii, M.; Ishizaki, T.; Paik, S.Y.; Manome, T.; Murooka, Y. An operon containing the genes for cholesterol oxidase and a cytochrome P-450-like protein from a Streptomyces sp. J. Bacteriol. 1990, 172, 3644–3653. [Google Scholar] [CrossRef]
- Mohamed, H.; Child, S.A.; Doherty, D.Z.; Bruning, J.B.; Bell, S.G. Structural determination and characterisation of the CYP105Q4 cytochrome P450 enzyme from Mycobacterium marinum. Arch. Biochem. Biophys. 2024, 754, 109950. [Google Scholar] [CrossRef]
- Jungmann, V.; Molnár, I.; Hammer Philip, E.; Hill, D.S.; Zirkle, R.; Buckel Thomas, G.; Buckel, D.; Ligon James, M.; Pachlatko, J.P. Biocatalytic Conversion of Avermectin to 4″-Oxo-Avermectin: Characterization of Biocatalytically Active Bacterial Strains and of Cytochrome P450 Monooxygenase Enzymes and Their Genes. Appl. Environ. Microbiol. 2005, 71, 6968–6976. [Google Scholar] [CrossRef]
- Nagano, S.; Poulos, T.L. Crystallographic Study on the Dioxygen Complex of Wild-type and Mutant Cytochrome P450cam: IMPLICATIONS FOR THE DIOXYGEN ACTIVATION MECHANISM. J. Biol. Chem. 2005, 280, 31659–31663. [Google Scholar] [CrossRef]
- Kieslich, K.; Sebek, O.K. Microbial Transformations of Steroids. In Annual Reports on Fermentation Processes; Perlman, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1979; Volume 3, pp. 275–304. [Google Scholar]
- Yamashita, H.; Shibata, K.; Yamakoshi, N.; Kurosawa, Y.; Mori, H. Microbial 16β-Hydroxylation of Steroids with Aspergillus niger. Agric. Biol. Chem. 1976, 40, 505–509. [Google Scholar] [CrossRef]
- Szklarz, G.D.; Halpert, J.R. Use of homology modeling in conjunction with site-directed mutagenesis for analysis of structure-function relationships of Mammalian cytochromes P450. Life Sci. 1997, 61, 2507–2520. [Google Scholar] [CrossRef]
- Lonsdale, R.; Harvey, J.N.; Mulholland, A.J. Inclusion of Dispersion Effects Significantly Improves Accuracy of Calculated Reaction Barriers for Cytochrome P450 Catalyzed Reactions. J. Phys. Chem. Lett. 2010, 1, 3232–3237. [Google Scholar] [CrossRef]
- Eppstein, S.H.; Meister, P.D.; Murray, H.C.; Peterson, D.H. Microbiological Transformations of Steroids and Their Applications to the Synthesis of Hormones. In Vitamins & Hormones; Harris, R.S., Marrian, G.F., Thimann, K.V., Eds.; Academic Press: Hoboken, NJ, USA, 1956; Volume 14, pp. 359–432. [Google Scholar]
- Gustafsson, J.-A. The formation of 16,17-dihydroxylated C19 steroids from 10-dehydro C19 steroids in liver microsomes from male and female rats. Biochim. Biophys. Acta (Bba)-Lipids Lipid Metab. 1973, 296, 179–188. [Google Scholar] [CrossRef]
- Rane, A.; Gustafsson, J.-Å. Formation of a 16,17-trans-glycolic metabolite from a 16-dehydro-androgen in human fetal liver microsomes. Clin. Pharmacol. Ther. 1973, 14, 833–839. [Google Scholar] [CrossRef]
- Siekmann, L.; Disse, B.; Breuer, H. Biosynthesis and metabolism of 16α,17-epoxy-C21-steroids in rat liver microsomes. J. Steroid Biochem. 1980, 13, 1181–1205. [Google Scholar] [CrossRef]
- Wood, A.; Swinney, D.; Thomas, P.; Ryan, D.; Hall, P.; Levin, W.; Garland, W. Mechanism of androstenedione formation from testosterone and epitestosterone catalyzed by purified cytochrome P-450b. J. Biol. Chem. 1988, 263, 17322–17332. [Google Scholar] [CrossRef]
- Peart, P.; Reynolds, W.; Reese, P. The facile bioconversion of testosterone by alginate-immobilised filamentous fungi. J. Mol. Catal. B Enzym. 2013, 95, 70–81. [Google Scholar] [CrossRef]
- Ke, X.; Cui, J.-H.; Ren, Q.-J.; Zheng, T.; Wang, X.-X.; Liu, Z.-Q.; Zheng, Y.-G. Rerouting phytosterol degradation pathway for directed androst-1,4-diene-3,17-dione microbial bioconversion. Appl. Microbiol. Biotechnol. 2024, 108, 186. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ke, X.; Zhao, X.; Ren, Q.; Cui, J.; Liu, Z.; Zheng, Y. Aldolase SalA dominates C24 steroidal side-chain-cleavage in the phytosterol degradation from Mycobacterium neoaurum. Process Biochem. 2023, 131, 217–225. [Google Scholar] [CrossRef]
- Su, L.; Xu, S.; Shen, Y.; Xia, M.; Ren, X.; Wang, L.; Shang, Z.; Wang, M. The Sterol Carrier Hydroxypropyl-β-Cyclodextrin Enhances the Metabolism of Phytosterols by Mycobacterium neoaurum. Appl. Environ. Microbiol. 2020, 86, e00441-20. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hu, Z.; Zhou, L.; Liu, B.; Huang, X.; Deng, Z.; Qu, X. A large conserved family of small-molecule carboxyl methyltransferases identified from microorganisms. Proc. Natl. Acad. Sci. USA 2023, 120, e2301389120. [Google Scholar] [CrossRef]
- Pelicic, V.; Reyrat, J.-M.; Gicquel, B. Generation of unmarked directed mutations in mycobacteria, using sucrose counter-selectable suicide vectors. Mol. Microbiol. 1996, 20, 919–925. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, L.; Li, X.; Sun, X.; Chang, S.; Chang, Z. Production of Hydroxylated Steroid Intermediates at 10-g Scale via the Original Sterol Modification Pathway in Mycolicibacterium neoaurum. Catalysts 2025, 15, 423. https://doi.org/10.3390/catal15050423
Zou L, Li X, Sun X, Chang S, Chang Z. Production of Hydroxylated Steroid Intermediates at 10-g Scale via the Original Sterol Modification Pathway in Mycolicibacterium neoaurum. Catalysts. 2025; 15(5):423. https://doi.org/10.3390/catal15050423
Chicago/Turabian StyleZou, Lei, Xue Li, Xue Sun, Shangfeng Chang, and Zunxue Chang. 2025. "Production of Hydroxylated Steroid Intermediates at 10-g Scale via the Original Sterol Modification Pathway in Mycolicibacterium neoaurum" Catalysts 15, no. 5: 423. https://doi.org/10.3390/catal15050423
APA StyleZou, L., Li, X., Sun, X., Chang, S., & Chang, Z. (2025). Production of Hydroxylated Steroid Intermediates at 10-g Scale via the Original Sterol Modification Pathway in Mycolicibacterium neoaurum. Catalysts, 15(5), 423. https://doi.org/10.3390/catal15050423




