Advanced Degradation of Aniline in Secondary Effluent from a Chemical Industry Park by Cobalt Ferrite/Peracetic Acid System
Abstract
1. Introduction
2. Results and Discussion
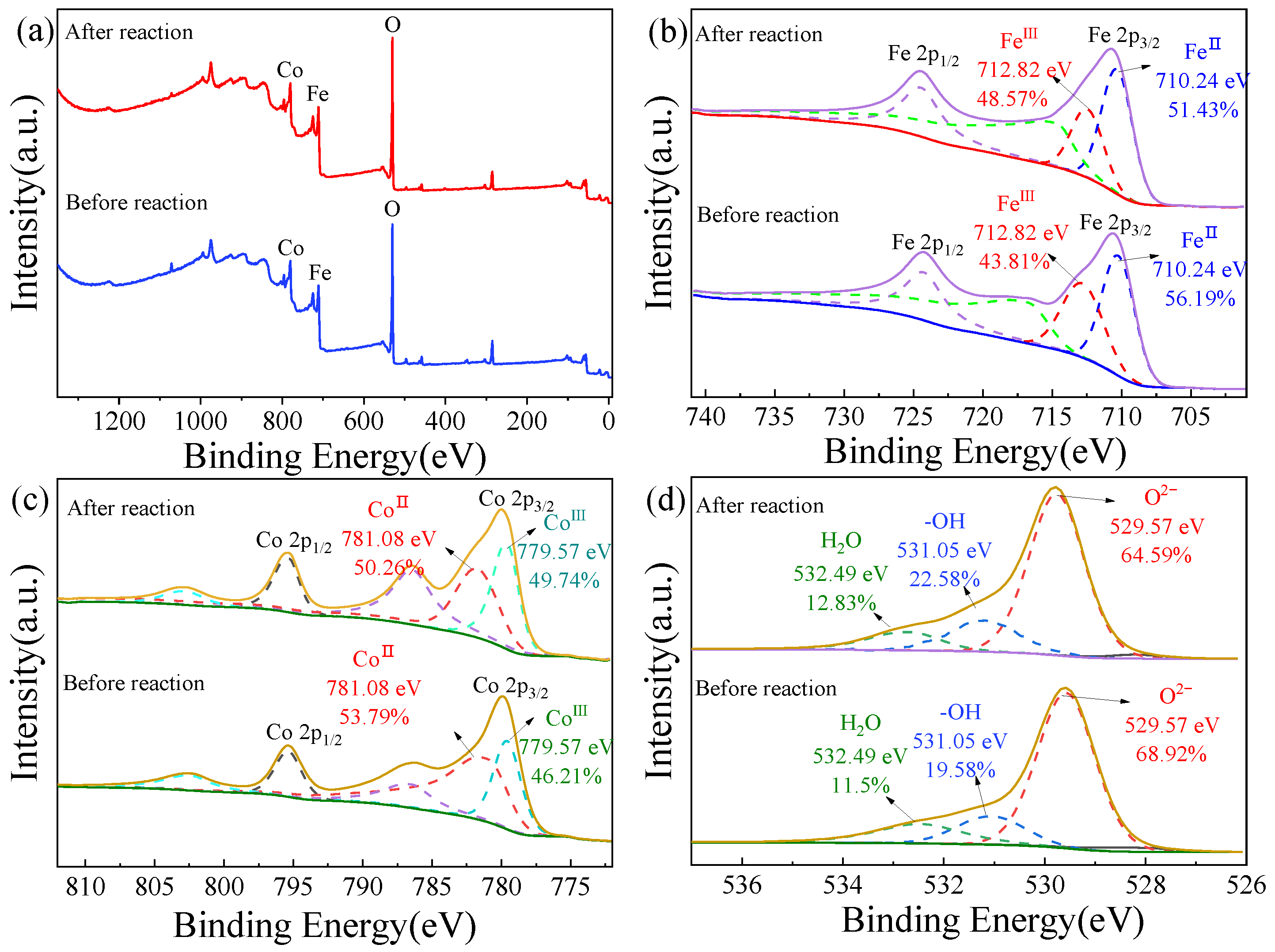
2.1. Characterizations of the Catalysts
2.2. Performance of CoFe2O4-Activated PAA Oxidation for the Degradation of Aniline
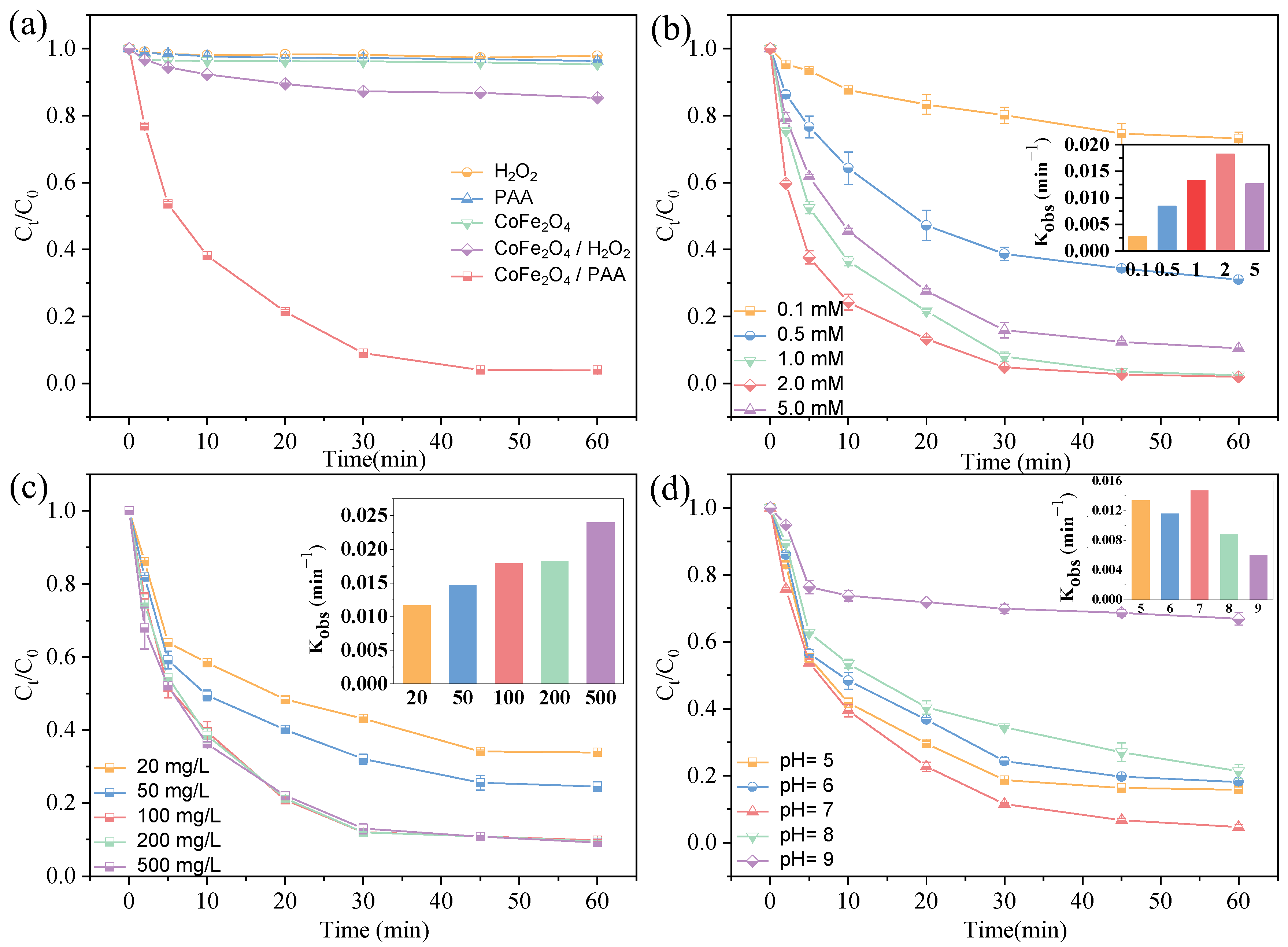
2.2.1. Aniline Degradation by CoFe2O4/PAA System Degradation
2.2.2. Effects of Reaction Factors
2.3. Influence of Water Matrix
2.4. Identification and Analysis of Reactive Species
2.5. Activation Mechanisms
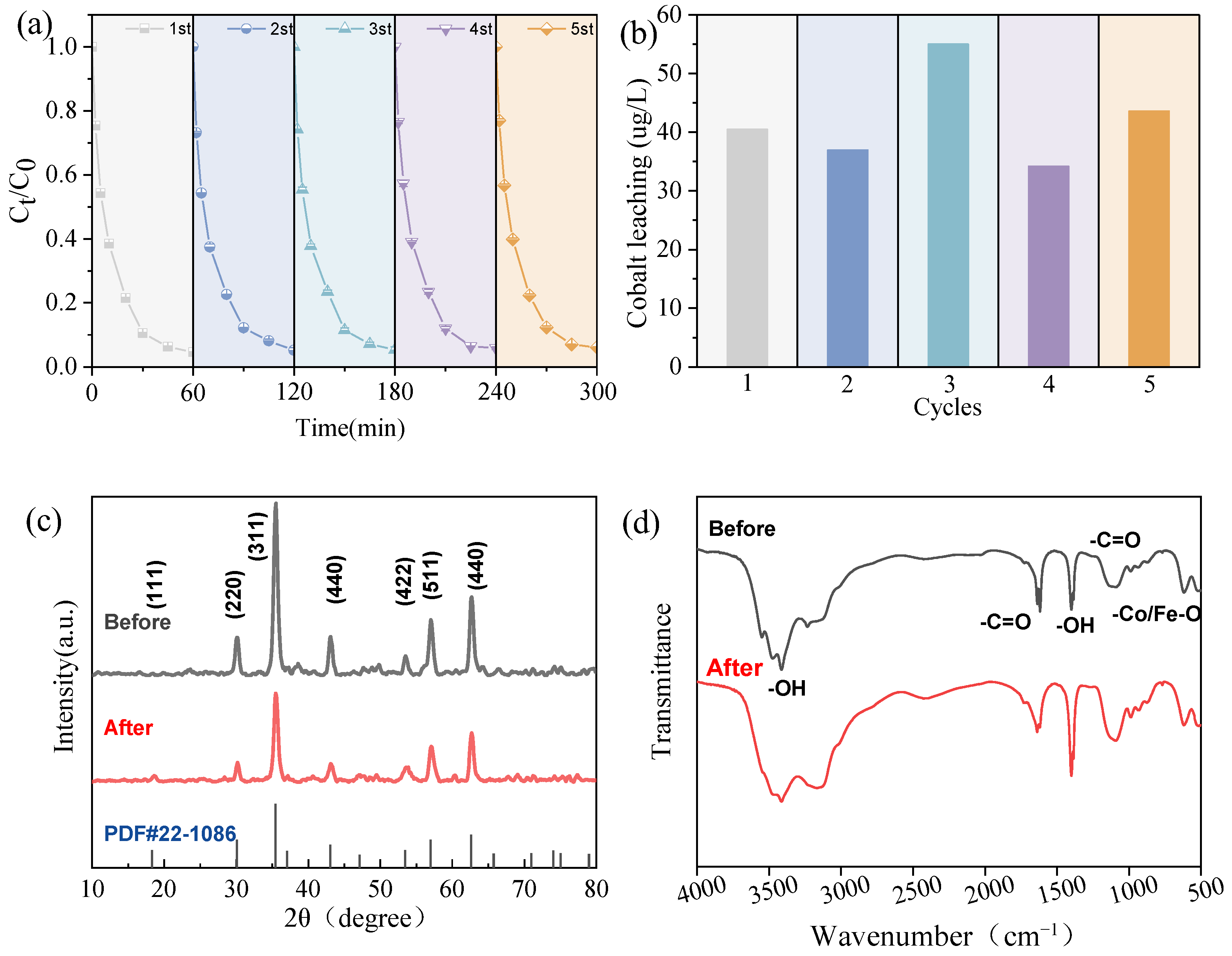
2.6. Reusability and Stability of the CoFe2O4
2.7. Practical Application
2.7.1. Degradation of Different Organic Pollutants by CoFe2O4/PAA
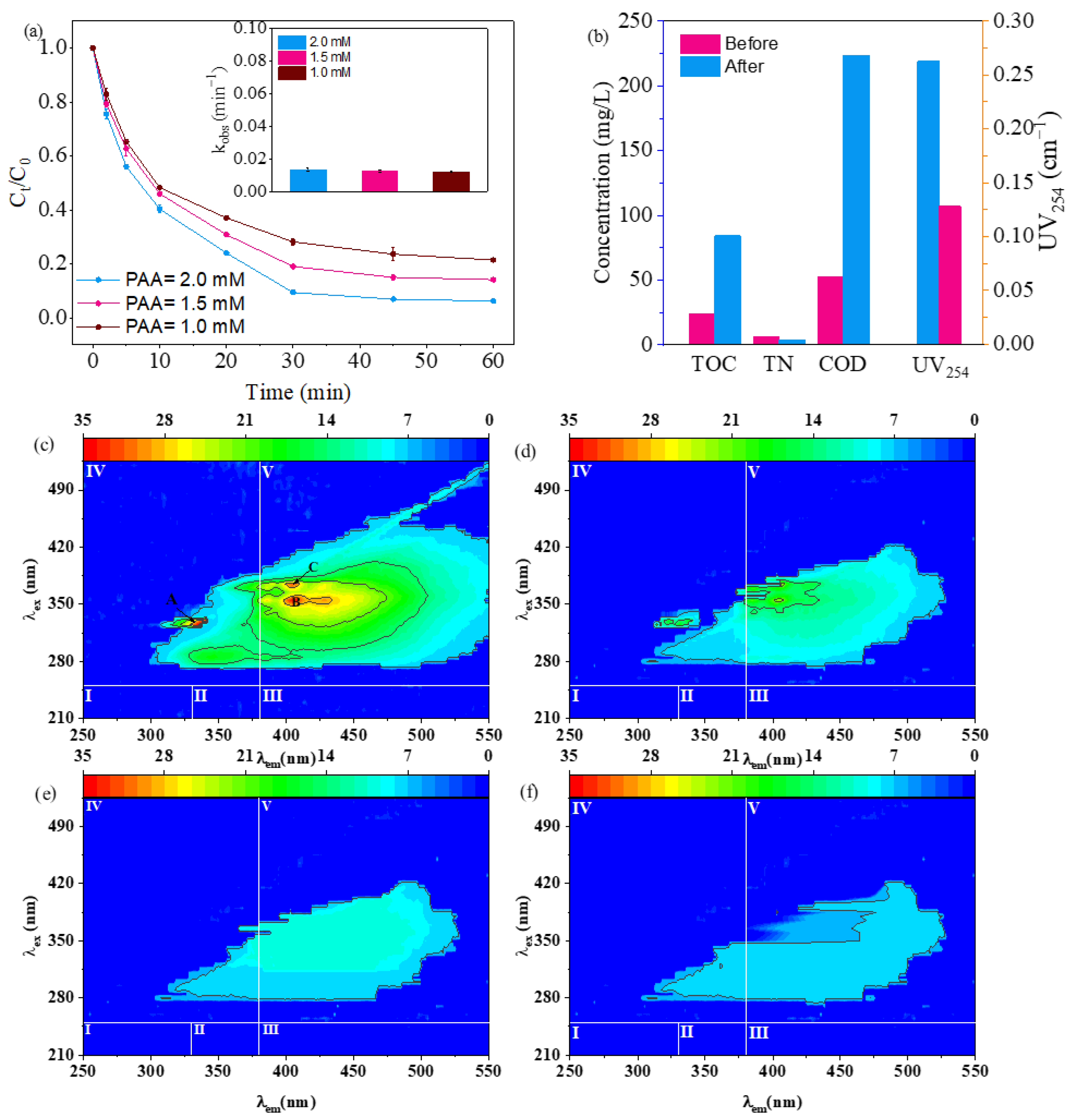
2.7.2. Degradation of Aniline in Secondary Effluent from the Chemical Industry Park
3. Materials and Methods
3.1. Chemicals and Reagents
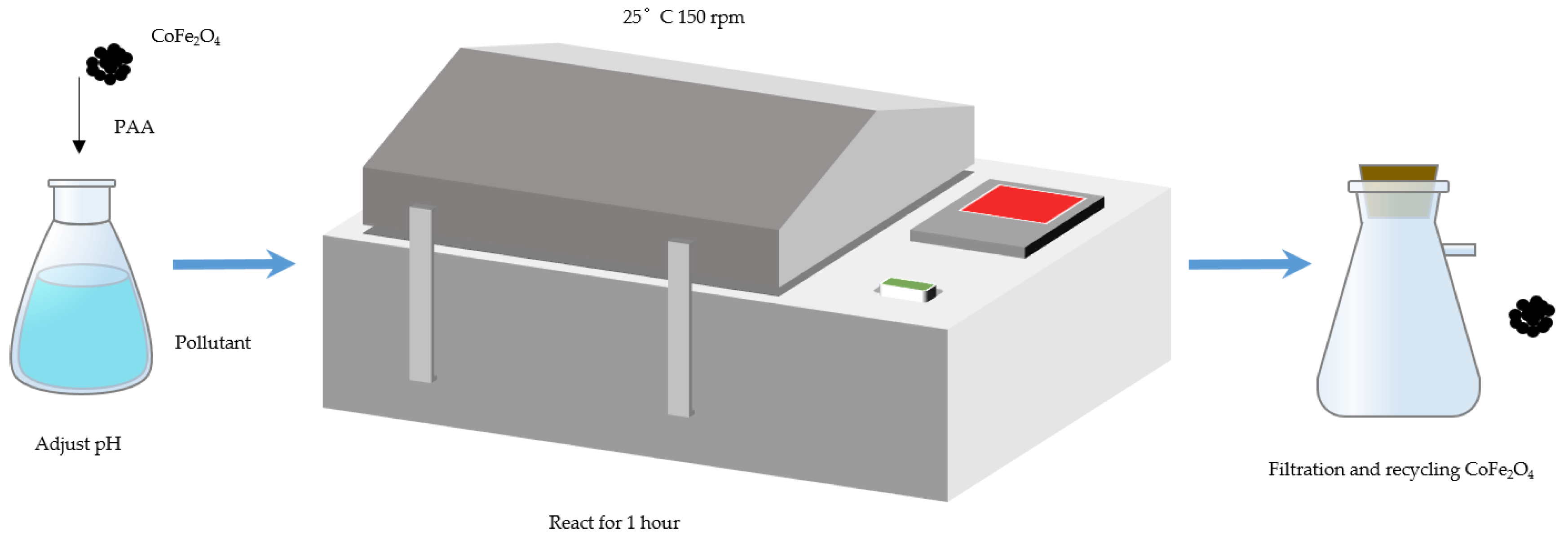
3.2. Degradation Experiments
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anjalin, M.; Kanagathara; Suganthi, B. A brief review on aniline and its derivatives. Mater. Today Proc. 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, Y.; Kong, Q.; Cheng, F.; Fan, M.; Deng, Y.; Zhao, Q.; Qiu, J.; Wang, P.; Yang, X. o-Semiquinone Radical and o-Benzoquinone Selectively Degrade Aniline Contaminants in the Periodate-Mediated Advanced Oxidation Process. Environ. Sci. Technol. 2024, 58, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, H.; Xue, G.; Liu, Y.; Chen, S.; Jia, C. A critical review of the aniline transformation fate in azo dye wastewater treatment. J. Clean. Prod. 2021, 321, 128971. [Google Scholar] [CrossRef]
- Soumya, G.; Alhadji, M.; Charné, B.; Amina, O.; Christian, O.; Kashitarash, E.Z.; Ahmad, K.W.; Shabnam, A.; Hadi, D.M. Novel green adsorbents for removal of aniline from industrial effluents: A review. J. Mol. Liq. 2022, 345, 118167. [Google Scholar] [CrossRef]
- Deng, X.; Liu, X.; Xia, S.; Zhao, H.; Liu, Y.; Ding, Q.; Zhang, H. Selective oxidation of anilines to azobenzenes by an Ag nanoparticles photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132352. [Google Scholar] [CrossRef]
- Tao, N.; Zhang, W.; Si, L.; Zhang, R.; Wang, D.; Guo, C. Effects of aniline on growth, oxidative and DNA damage of rice (Oryza sativa L.) seedlings. Environ. Technol. Innov. 2022, 28, 102583. [Google Scholar] [CrossRef]
- Boya, M.; Wenjing, L.; Jinying, L.; Chunwei, Y.; Qian, T.; Dong, W. Promotion removal of aniline with electro-Fenton processes utilizing carbon nanotube 3D morphology modification of an Ag-loaded copper foam cathode. J. Water Process Eng. 2021, 43, 102295. [Google Scholar] [CrossRef]
- Yuan, Y.; Luo, T.; Xu, J.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Enhanced oxidation of aniline using Fe(III)-S(IV) system: Role of different oxysulfur radicals. Chem. Eng. J. 2019, 362, 183–189. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, Y.; Yang, C.; Wang, D. Magnetic γ-Fe2O3/ZnO@CNTs synthesized by a green precipitation method for the degradation of aniline through photocatalysis coupling catalytic ozonation. Appl. Surf. Sci. 2024, 659, 159866. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Tan, B.; Li, M.; Peng, H.; He, J.; Zhang, Y.; Su, J. Microbial community and metabolic characteristics evaluation in start-up stage of electro-enhanced SBR for aniline wastewater treatment. J. Water Process Eng. 2022, 45, 102489. [Google Scholar] [CrossRef]
- Henao, L.D.; Turolla, A.; Antonelli, M. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, W.; Cao, H.; Zhao, H.; Huang, C.-H. Oxidation of amino acids by peracetic acid: Reaction kinetics, pathways and theoretical calculations. Water Res. X 2018, 1, 100002. [Google Scholar] [CrossRef]
- Ao, X.-W.; Eloranta, J.; Huang, C.-H.; Santoro, D.; Sun, W.-J.; Lu, Z.-D.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Zhang, Y.; Xu, Y.; Zheng, T.; Zhou, X. Highly efficient activation of peracetic acid by nano-CuO for carbamazepine degradation in wastewater: The significant role of H2O2 and evidence of acetylperoxy radical contribution. Water Res. 2022, 216, 118322. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, B.; Ma, X.; Huang, H.; Zou, C.; Liu, J.; Luo, Q.; Wang, C.; Liang, J. Recent advances in peracetic acid-based advanced oxidation processes for emerging pollutants elimination: A review. J. Environ. Chem. Eng. 2024, 12, 112927. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced oxidation processes in microreactors for water and wastewater treatment: Development, challenges, and opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xiong, B.; Bai, F.; Wang, S.; Wan, Y.; Zhang, L.; Xie, P.; Wiesner, M.R. Application of Cobalt/Peracetic Acid to Degrade Sulfamethoxazole at Neutral Condition: Efficiency and Mechanisms. Environ. Sci. Technol. 2020, 54, 464–475. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Shi, Q.; Deng, S.; Zheng, Y.; Du, Y.; Li, L.; Yang, S.; Zhang, G.; Du, L.; Wang, G.; Cheng, M.; et al. The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ. Res. 2022, 212, 113340. [Google Scholar] [CrossRef]
- Yu, T.; Chen, H.; Hu, T.; Feng, J.; Xing, W.; Tang, L.; Tang, W. Recent advances in the applications of encapsulated transition-metal nanoparticles in advanced oxidation processes for degradation of organic pollutants: A critical review. Appl. Catal. B-Environ. 2024, 342, 123401. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Zheng, T.; Xu, Y.; Liu, T.; Yin, W.; Zhang, Y.; Zhou, X. Co-Mn spinel oxides trigger peracetic acid activation for ultrafast degradation of sulfonamide antibiotics: Unveiling critical role of Mn species in boosting Co activity. Water Res. 2023, 229, 119462. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Makarova, K.; Lahtinen, M.; Golovina, E.A.; Van As, H.; Virkutyte, J. Ultrasound-assisted MnO2 catalyzed homolysis of peracetic acid for phenol degradation: The assessment of process chemistry and kinetics. Chem. Eng. J. 2013, 221, 476–486. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B-Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, B.; Miao, L.; Wang, S.; Xie, P.; Wang, Z.; Ma, J. Applying a novel advanced oxidation process of activated peracetic acid by CoFe2O4 to efficiently degrade sulfamethoxazole. Appl. Catal. B Environ. 2021, 280, 119422. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Z.; Man, J.H.K.; Lo, I.M.C. Regulating charge transfer for enhanced PAA activation over sulfur-doped magnetic CoFe2O4: A novel strategy for simultaneous micropollutants degradation and bacteria inactivation. Water Res. 2024, 256, 121595. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lu, X.; Gao, J.; Chen, Y.; Pan, S. Activation of Peracetic Acid by CoFe2O4 for Efficient Degradation of Ofloxacin: Reactive Species and Mechanism. Molecules 2023, 28, 7906. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Li, C.; Wang, D.; Jia, C.; Zhao, J.; Liu, Z.; Zhang, X.; Geng, L. Electronic modulation of fiber-shaped-CoFe2O4 via Mg doping for improved PMS activation and sustainable degradation of organic pollutants. Appl. Surf. Sci. 2022, 605, 154732. [Google Scholar] [CrossRef]
- Liang, M.; Jiayue, D.; Jing, C.; Lei, L.; Qingguo, H.; Junhe, L. Activation of peracetic acid by spinel CoFe2O4 nanoparticles for the degradation of sulfamethoxazole. Chem. Eng. J. 2023, 456, 141084. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C.-H. Advanced Oxidation Process with Peracetic Acid and Fe(II) for Contaminant Degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Liu, Y.; Fu, Y. Effective degradation of sulfamethoxazole with Fe2+-zeolite/peracetic acid. Sep. Purif. Technol. 2020, 233, 115973. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C.-H. UV/Peracetic Acid for Degradation of Pharmaceuticals and Reactive Species Evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Wu, W.; Tian, D.; Liu, T.; Chen, J.; Huang, T.; Zhou, X.; Zhang, Y. Degradation of organic compounds by peracetic acid activated with Co3O4: A novel advanced oxidation process and organic radical contribution. Chem. Eng. J. 2020, 394, 124938. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Liang, J.; Wang, L.; Fang, J. Roles of nitrogen dioxide radical (•NO2) in the transformation of aniline by sulfate radical and hydroxyl radical systems with the presence of nitrite. Chem. Eng. J. 2023, 451, 138755. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Shang, Y.; Yang, K. Simultaneous removal of aniline, nitrogen and phosphorus in aniline-containing wastewater treatment by using sequencing batch reactor. Bioresour. Technol. 2016, 207, 422–429. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B-Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Chen, Y.-d.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.-q.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Xu, A.; Li, X.; Ye, S.; Yin, G.; Zeng, Q. Catalyzed oxidative degradation of methylene blue by in situ generated cobalt (II)-bicarbonate complexes with hydrogen peroxide. Appl. Catal. B Environ. 2011, 102, 37–43. [Google Scholar] [CrossRef]
- Shen, P.; Hou, K.; Chen, F.; Pi, Z.; He, L.; Chen, S.; Li, X.; Yang, Q. Ultra-rapid and long-lasting activation of peracetic acid by Cu-Co spinel oxides for eliminating organic contamination: Role of radical and non-radical catalytic oxidation. Chem. Eng. J. 2023, 463, 142344. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Zheng, H.; Zheng, Y.; He, C.-S.; Lai, B.; Ma, J.; Nan, J. Introduction of oxygen vacancy to manganese ferrite by Co substitution for enhanced peracetic acid activation and 1O2 dominated tetracycline hydrochloride degradation under microwave irradiation. Water Res. 2022, 225, 110176. [Google Scholar] [CrossRef]
- Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the commonly used quenching method really evaluate the role of reactive oxygen species in pollutant abatement during catalytic ozonation? Water Res. 2022, 215, 118275. [Google Scholar] [CrossRef]
- Gao, Q.; Li, L.; Zhang, Y.; Zhou, H.; Jiang, J.; Wei, L.; Wang, G.; Ding, J.; Zhao, Q. Advanced oxidation processes (AOPs)-based sludge pretreatment techniques for enhanced short-chain fatty acids production: A critical review. Chem. Eng. J. 2024, 489, 151496. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Jia, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Novel Nonradical Oxidation of Sulfonamide Antibiotics with Co(II)-Doped g-C3N4-Activated Peracetic Acid: Role of High-Valent Cobalt-Oxo Species. Environ. Sci. Technol. 2021, 55, 12640–12651. [Google Scholar] [CrossRef]
- Xie, Z.-H.; He, C.-S.; He, Y.-L.; Yang, S.-R.; Yu, S.-Y.; Xiong, Z.; Du, Y.; Liu, Y.; Pan, Z.-C.; Yao, G.; et al. Peracetic acid activation via the synergic effect of Co and Fe in CoFe-LDH for efficient degradation of pharmaceuticals in hospital wastewater. Water Res. 2023, 232, 119666. [Google Scholar] [CrossRef]
- Chen, L.; Duan, J.; Du, P.; Sun, W.; Lai, B.; Liu, W. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 2022, 221, 118747. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- He, C.-S.; Ding, R.-R.; Chen, J.-Q.; Li, W.-Q.; Li, Q.; Mu, Y. Interactions between nanoscale zero valent iron and extracellular polymeric substances of anaerobic sludge. Water Res. 2020, 178, 115817. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yang, P.; Zhu, M.; Zhou, H.; Pan, S. Advanced Degradation of Aniline in Secondary Effluent from a Chemical Industry Park by Cobalt Ferrite/Peracetic Acid System. Catalysts 2025, 15, 410. https://doi.org/10.3390/catal15050410
Gao J, Yang P, Zhu M, Zhou H, Pan S. Advanced Degradation of Aniline in Secondary Effluent from a Chemical Industry Park by Cobalt Ferrite/Peracetic Acid System. Catalysts. 2025; 15(5):410. https://doi.org/10.3390/catal15050410
Chicago/Turabian StyleGao, Jinxiang, Peishan Yang, Mingxin Zhu, Hua Zhou, and Shunlong Pan. 2025. "Advanced Degradation of Aniline in Secondary Effluent from a Chemical Industry Park by Cobalt Ferrite/Peracetic Acid System" Catalysts 15, no. 5: 410. https://doi.org/10.3390/catal15050410
APA StyleGao, J., Yang, P., Zhu, M., Zhou, H., & Pan, S. (2025). Advanced Degradation of Aniline in Secondary Effluent from a Chemical Industry Park by Cobalt Ferrite/Peracetic Acid System. Catalysts, 15(5), 410. https://doi.org/10.3390/catal15050410










