Geometric Matching Effect Induced High Dispersion of Na2WO4 Nanocluster on Cristobalite Support for Efficient Methyl Chloride-to-Vinyl Chloride Conversion
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization
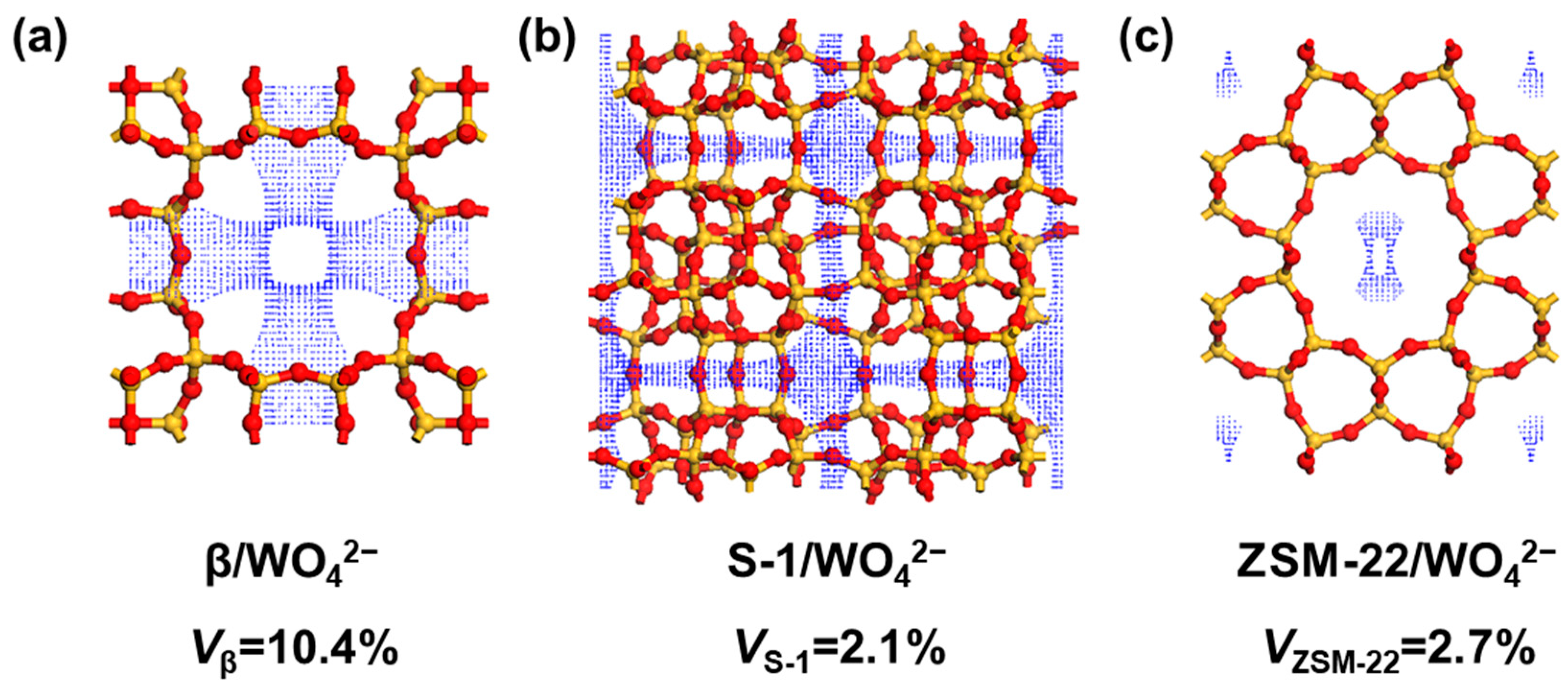
2.2. Zeolite Characterization and Calculation
2.3. Catalytic Performance
3. Materials and Methods
3.1. Precursor Material Synthesis
3.2. Synthesis of Catalysts
3.3. Material Characterization Methods
3.4. Catalytic Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, R.; Amrute, A.P.; Pérez-Ramírez, J. Halogen-mediated conversion of hydrocarbons to commodities. Chem. Rev. 2017, 117, 4182–4247. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, N.; Li, W.; Dai, B. Progress on cleaner production of vinyl chloride monomers over non-mercury catalysts. Front. Chem. Sci. Eng. 2011, 5, 514–520. [Google Scholar] [CrossRef]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Davies, C.J.; Lu, L.; Dawson, S.; Thetford, A.; Gibson, E.K.; Morgan, D.J.; Jones, W.; et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 2017, 355, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, J.; Xu, J.; Ni, J.; Zhang, T.; Xu, X.; Li, X. Activated-carbon-supported gold-cesium(I) as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride. ChemPlusChem 2015, 80, 196–201. [Google Scholar] [CrossRef]
- Medrano-García, J.D.; Giulimondi, V.; Ceruti, A.; Zichittella, G.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Economic and environmental competitiveness of ethane-based technologies for vinyl chloride synthesis. ACS Sustain. Chem. Eng. 2023, 11, 13062–13069. [Google Scholar] [CrossRef] [PubMed]
- Leofanti, G.; Marsella, A.; Cremaschi, B.; Garilli, M.; Zecchina, A.; Spoto, G.; Bordiga, S.; Fisicaro, P.; Prestipino, C.; Villain, F.; et al. Alumina-supported copper chloride: 4. Effect of Exposure to O2 and HCl. J. Catal. 2002, 205, 375–381. [Google Scholar] [CrossRef]
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef]
- Hutchings, G.; Grady, D. Hydrochlorination of acetylene: The effect of mercuric chloride concentration on catalyst life. Appl. Catal. 1985, 17, 155–160. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Fako, E.; Manzocchi, G.; Krumeich, F.; Hauert, R.; Clark, A.H.; Safonova, O.V.; López, N.; Pérez-Ramírez, J. Nanostructuring unlocks high performance of platinum single-atom catalysts for stable vinyl chloride production. Nat. Catal. 2020, 3, 376–385. [Google Scholar] [CrossRef]
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination. J. Am. Chem. Soc. 2015, 137, 1454–14557. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Zhao, Z.; Luo, G.; Miller, J.T.; Wong, M.S.; Wei, F. Synergistic gold bismuth catalysis for non-mercury hydrochlorination of acetylene to vinyl chloride monomer. ACS Catal. 2014, 4, 3112–3116. [Google Scholar] [CrossRef]
- Zichittella, G.; Pérez-Ramírez, J. Ethane-based catalytic process for vinyl chloride manufacture. Angew. Chem. Int. Ed. 2021, 60, 24089–24095. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, D.; Bao, X. Catalysis for selected C1 chemistry. Chem 2020, 6, 2497–2514. [Google Scholar] [CrossRef]
- Singla, M.; Rasmussen, M.L.; Hashemi, H.; Wu, H.; Glarborg, P.; Pelucchi, M.; Faravelli, T.; Marshall, P. Ab initio calculations and kinetic modeling of thermal conversion of methyl chloride: Implications for gasification of biomass. Phys. Chem. Chem. Phys. 2018, 20, 10741–10752. [Google Scholar] [CrossRef] [PubMed]
- Terlingen, B.; Oord, R.; Ahr, M.; Hutter, E.; van Lare, C.; Weckhuysen, B.M. Mechanistic insights into the lanthanide-catalyzed oxychlorination of methane as revealed by operando spectroscopy. ACS Catal. 2021, 11, 10574–10588. [Google Scholar] [CrossRef]
- Pelucchi, M.; Cavallotti, C.; Frassoldati, A.; Ranzi, E.; Glarborg, P.; Faravelli, T. Theoretical and kinetic modeling study of chloromethane (CH3Cl) pyrolysis and oxidation. Int. J. Chem. Kinet. 2021, 53, 403–418. [Google Scholar] [CrossRef]
- Valecillos, J.; Manzano, H.; Aguayo, A.T.; Bilbao, J.; Castaño, P. Kinetic and deactivation differences among methanol, dimethyl ether and chloromethane as stock for hydrocarbons. ChemCatChem 2019, 11, 5444–5456. [Google Scholar] [CrossRef]
- Ibáñez, M.; Gamero, M.; Ruiz-Martínez, J.; Weckhuysen, B.M.; Aguayo, A.T.; Bilbao, J.; Castaño, P. Simultaneous coking and dealumination of zeolite H-ZSM-5 during the transformation of chloromethane into olefins. Catal. Sci. Technol. 2016, 6, 296–306. [Google Scholar] [CrossRef]
- Olsbye, U.; Saure, O.V.; Muddada, N.B.; Bordiga, S.; Lamberti, C.; Nilsen, M.H.; Lillerud, K.P.; Svelle, S. Methane conversion to light olefins-how does the methyl halide route differ from the methanol-to-olefins (MTO) route? Catal. Today 2011, 171, 211–220. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, D.; Liu, Z.; Su, B.-L. Highly efficient catalytic conversion of chloromethane to light olefins over HSAPO-34 as studied by catalytic testing and in situ FTIR. J. Catal. 2006, 238, 46–57. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, D.; Xu, L.; Liu, Z.; Su, B.-L. New route for light olefins production from chloromethane over HSAPO-34 molecular sieve. Catal. Today 2005, 106, 84–89. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, S.; Nabera, A.; Chen, X.; Pan, Y.; Wei, K.; Bao, Y.; Hu, J.; Zhao, Y.; Liu, C.; et al. C1-based route for vinyl chloride synthesis with environmental and economic benefits. J. Am. Chem. Soc. 2025, 147, 7757–7764. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Y.; Qi, Y.; Rout, K.R.; Chen, D. Critical review of catalysis for ethylene oxychlorination. ACS Catal. 2020, 10, 9299–9319. [Google Scholar] [CrossRef]
- Ma, H.; Ma, G.; Qi, Y.; Wang, Y.; Chen, Q.; Rout, K.R.; Fuglerud, T.; Chen, D. Nitrogen-doped carbon-assisted one-pot tandem reaction for vinyl chloride production via ethylene oxychlorination. Angew. Chem. Int. Ed. 2020, 59, 22080–22085. [Google Scholar] [CrossRef]
- Hassan, H.M.; Alruwaili, H.A.; Alhumaimess, M.S.; Alanazi, A.H.; El-Aassar, M.; Alshammari, M.S.; Hussein, M.F.; Alsohaimi, I.H. Sustainable nitrophenol reduction using ce-mof-808-supported bimetallic nanoparticles optimized by response surface methodology. Environ. Res. 2025, 264 Pt 1, 120340. [Google Scholar] [CrossRef]
- Hassan, H.M.; Alruwaili, M.S.; Alsohaimi, I.H.; El-Hashemy, M.A.; Alqadami, A.A.; Alshammari, K.; Alali, I.K.; Alanazi, S.J.; El-Aassar, M.R. Electrospun polyacrylonitrile nanofiber composites integrated with Al-MOF/mesoporous carbon for superior CO2 capture and VOC removal. Diam. Relat. Mater. 2024, 149, 111649. [Google Scholar] [CrossRef]
- Zou, S.; Li, Z.; Zhou, Q.; Pan, Y.; Yuan, W.; He, L.; Wang, S.; Wen, W.; Liu, J.; Wang, Y.; et al. Surface coupling of methyl radicals for efficient low-temperature oxidative coupling of methane. Chin. J. Catal. 2021, 42, 1117–1125. [Google Scholar] [CrossRef]
- Qajar, A.; Peer, M.; Rajagopalan, R.; Foley, H.C. Characterization of micro- and mesoporous materials using accelerated dynamics adsorption. Langmuir 2013, 29, 12400–12409. [Google Scholar] [CrossRef]
- Santiso, E.E.; George, A.M.; Turner, C.H.; Kostov, M.K.; Gubbins, K.E.; Buongiorno-Nardelli, M.; Sliwinska-Bartkowiak, M. Adsorption and catalysis: The effect of confinement on chemical reactions. Appl. Surf. Sci. 2005, 252, 766–777. [Google Scholar] [CrossRef]
- Holbrook, B.P.M.; Rajagopalan, R.; Dronvajjala, K.; Choudhary, Y.K.; Foley, H.C. Molecular sieving carbon catalysts for liquid phase reactions: Study of alkene hydrogenation using platinum embedded nanoporous carbon. J. Mol. Catal. A-Chem. 2013, 367, 61. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, D.; Zhang, X.; Zhang, L.; Ma, X.; Zhang, Y.; Cai, M. Sonochemical incorporation of nanosized TiO2 inside mesoporous silica with high photocatalytic performance. Micropor. Mesopor. Mat. 2009, 126, 20–25. [Google Scholar] [CrossRef]
- Tong, M.; Zhang, D.; Fan, W.; Xu, J.; Zhu, L.; Guo, W.; Yan, W.; Yu, J.; Qiu, S.; Wang, J.; et al. Synthesis of chiral polymorph A-enriched zeolite Beta with an extremely concentrated fluoride route. Sci. Rep. 2015, 5, 11521. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, J.; Chen, J.; Zhang, J.; Wang, G.; Shi, Z.; Yang, F.; Zhao, J.; Zhuo, R.; Wang, R. Re-dispersion of active Zn species on modified S-1 zeolite as an efficient catalyst for direct dehydrogenation of propane. Micropor. Mesopor. Mat. 2022, 348, 112402. [Google Scholar] [CrossRef]
- Jamil, A.K.; Muraza, O. Facile control of nanosized ZSM-22 crystals using dynamic crystallization technique. Micropor. Mesopor. Mat. 2016, 227, 16–22. [Google Scholar] [CrossRef]
- Fan, J.; Yu, C.; Gao, F.; Lei, J.; Tian, B.; Wang, L.; Luo, Q.; Tu, B.; Zhou, W.; Zhao, D. Cubic mesoporous silica with large controllable entrance sizes and advanced adsorption properties. Angew. Chem. Int. Ed. 2003, 115, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Lei, X.-Q.; Sun, H.-M.; Zhang, H.-W.; Cui, C.-S.; Yue, Y.-Y.; Liu, H.-Y.; Bao, X.-J.; Wang, T.-H. Effects of pore size, mesostructure and aluminum modification on FDU-12 supported NiMo catalysts for hydrodesulfurization. Petrol. Sci. 2020, 17, 15. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q. Optimization of roasting parameters for recovery of vanadium and tungsten from spent SCR catalyst with composite roasting. Processes 2021, 9, 1923. [Google Scholar] [CrossRef]
- Kovacheva, P.; Predoeva, A.; Arishtirova, K.; Vassilev, S. Oxidative methylation of toluene with methane using X zeolite catalyst modified with alkali earth oxides. Appl. Catal. A Gen. 2002, 223, 121–128. [Google Scholar] [CrossRef]
- Hajjar, R.; Millot, Y.; Man, P.P.; Che, M.; Dzwigaj, S. Two Kinds of framework Al sites studied in BEA zeolite by X-ray diffraction, fourier transform infrared spectroscopy, NMR techniques, and V Probe. J. Phys. Chem. C 2008, 112, 20167–20175. [Google Scholar] [CrossRef]
- Yildiz, M.; Aksu, Y.; Simon, U.; Kailasam, K.; Goerke, O.; Rosowski, F.; Schomäcker, R.; Thomas, A.; Arndt, S. Enhanced catalytic performance of MnxOy-Na2WO4/SiO2 for the oxidative coupling of methane using an ordered mesoporous silica support. Chem. Commun. 2014, 50, 14440–14442. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Wachs, I.E.; Baltrusaitis, J. Synthesis and molecular structure of model silica-supported tungsten oxide catalysts for oxidative coupling of methane (OCM). Catal. Sci. Technol. 2020, 10, 3334–3345. [Google Scholar] [CrossRef]
- Connolly, M.L. Analytical molecular surface calculation. J. Appl. Crystallogr. 1983, 16, 548–558. [Google Scholar] [CrossRef]
- Connolly, M.L. Solvent-accessible surfaces of proteins and nucleic acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, K. Size-Sieving Separation of hard-sphere mixtures through cylindrical pores. Soft Matter. 2021, 17, 10025–10031. [Google Scholar] [CrossRef] [PubMed]




| Catalyst | T (°C) | CH3Cl Conv. (%) | Sel. (%) | C2H3Cl Yield (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H4 | C2H2 | C3H3Cl | C2 | COx | ||||
| 10W/β-C | 650 | 27.5 | 5.9 | 3.1 | 2.9 | 35.1 | 41.1 | 16.8 | 9.6 |
| 675 | 55.9 | 1.3 | 3.3 | 1.1 | 41.5 | 44.9 | 20.2 | 23.2 | |
| 700 | 75.5 | 1.2 | 3.5 | 2.9 | 47.2 | 53.6 | 21.4 | 35.6 | |
| 725 | 92.1 | 0.9 | 2.5 | 6.2 | 37.6 | 46.3 | 48.1 | 34.6 | |
| 0W/β-C | 700 | 66.1 | 1.3 | 0.5 | 0.6 | 2.6 | 3.7 | 93.3 | 1.7 |
| Na2WO4 | 700 | 10.2 | 0.7 | 1.9 | 0.2 | 24.3 | 26.4 | 40.2 | 2.5 |
| 10W/C | 700 | 82.2 | 0.7 | 1.4 | 1.5 | 22.3 | 25.2 | 68.2 | 18.3 |
| 10W/FDU-C | 700 | 79.8 | 1.1 | 1.7 | 2.3 | 22.3 | 26.3 | 60.8 | 13.8 |
| 10W/S1-C | 700 | 77.5 | 0.8 | 1.6 | 1.3 | 21.0 | 23.9 | 69.8 | 16.3 |
| 10W/Z22-C | 700 | 76.1 | 0.7 | 1.4 | 1.7 | 16.9 | 20.0 | 72.3 | 12.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Shi, Y.; Ren, Y.; Wang, Y.; Sun, X.; Wei, Z.; Chen, X.; Fan, J. Geometric Matching Effect Induced High Dispersion of Na2WO4 Nanocluster on Cristobalite Support for Efficient Methyl Chloride-to-Vinyl Chloride Conversion. Catalysts 2025, 15, 382. https://doi.org/10.3390/catal15040382
Lu N, Shi Y, Ren Y, Wang Y, Sun X, Wei Z, Chen X, Fan J. Geometric Matching Effect Induced High Dispersion of Na2WO4 Nanocluster on Cristobalite Support for Efficient Methyl Chloride-to-Vinyl Chloride Conversion. Catalysts. 2025; 15(4):382. https://doi.org/10.3390/catal15040382
Chicago/Turabian StyleLu, Nan, Yifeng Shi, Yutao Ren, Yue Wang, Xinyi Sun, Zejing Wei, Xutao Chen, and Jie Fan. 2025. "Geometric Matching Effect Induced High Dispersion of Na2WO4 Nanocluster on Cristobalite Support for Efficient Methyl Chloride-to-Vinyl Chloride Conversion" Catalysts 15, no. 4: 382. https://doi.org/10.3390/catal15040382
APA StyleLu, N., Shi, Y., Ren, Y., Wang, Y., Sun, X., Wei, Z., Chen, X., & Fan, J. (2025). Geometric Matching Effect Induced High Dispersion of Na2WO4 Nanocluster on Cristobalite Support for Efficient Methyl Chloride-to-Vinyl Chloride Conversion. Catalysts, 15(4), 382. https://doi.org/10.3390/catal15040382








