Engineering Electron Transport Pathways in Cobalt-Doped g-C3N4 Photocatalysts: Enhanced Tetracycline Degradation Through Interlayer Bridging
Abstract
1. Introduction
2. Results and Discussion
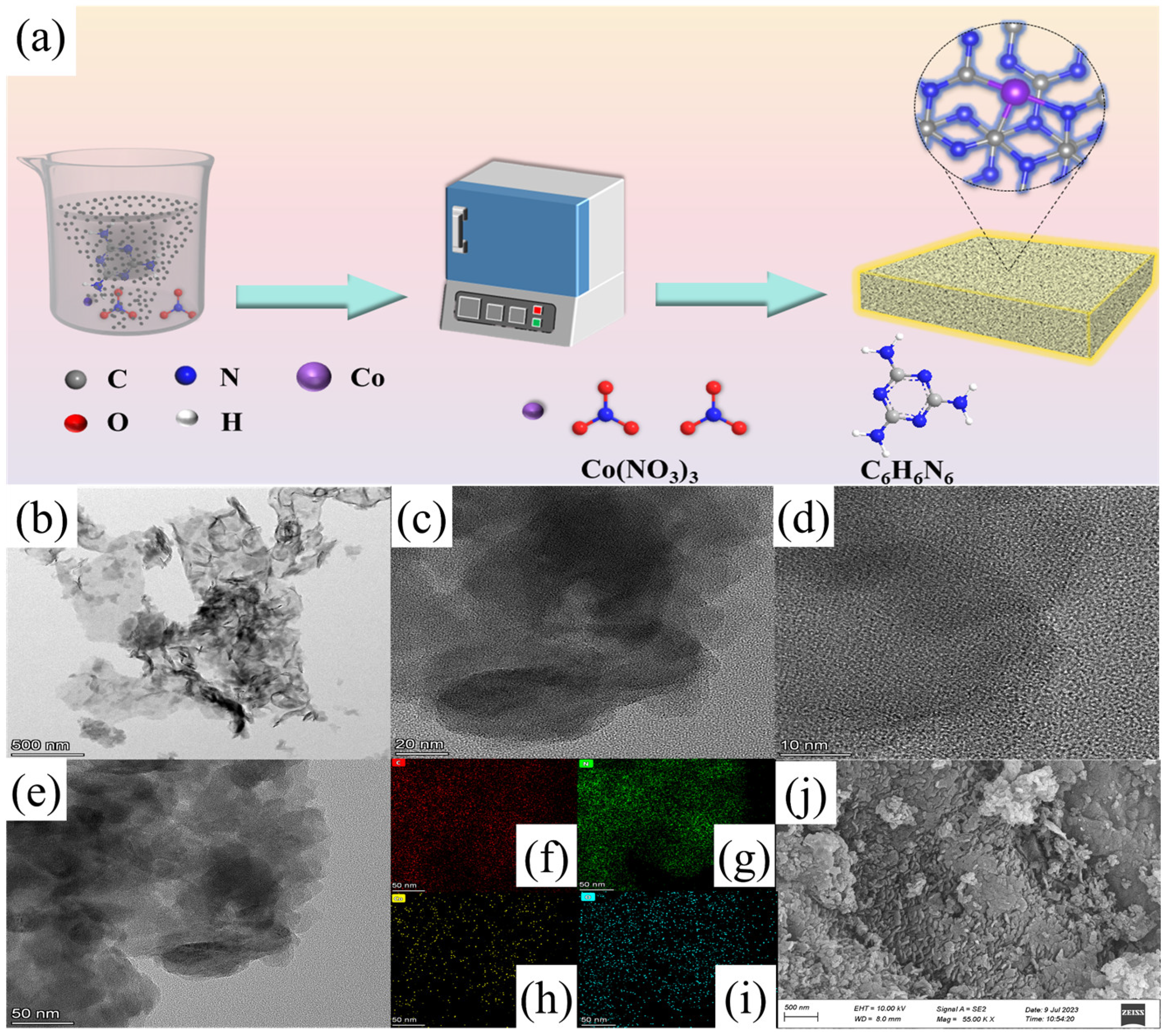
2.1. Synthesis and Characterization of Catalysts
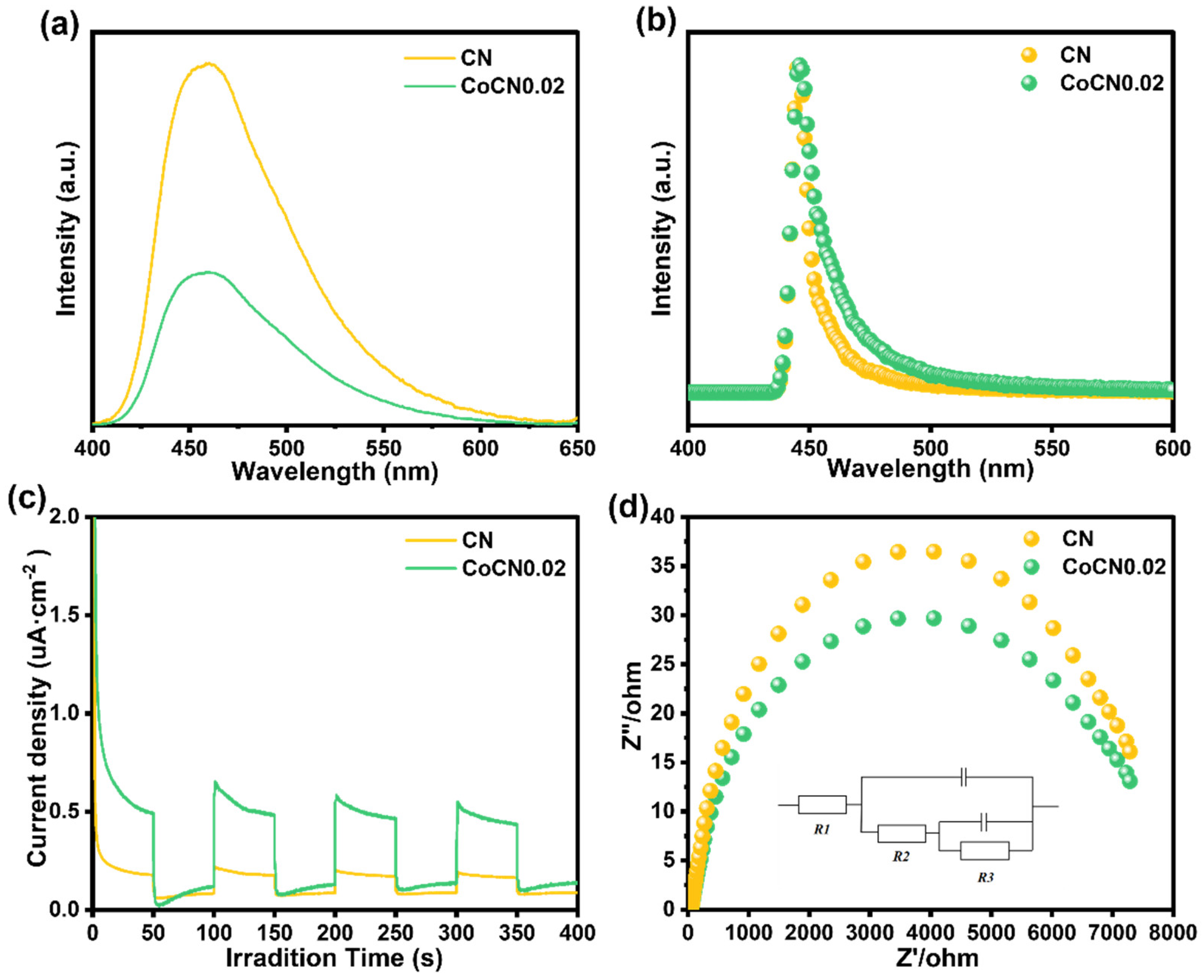
2.2. Surface Area and Optical Properties
2.3. Photoelectric Properties of the Photocatalyst
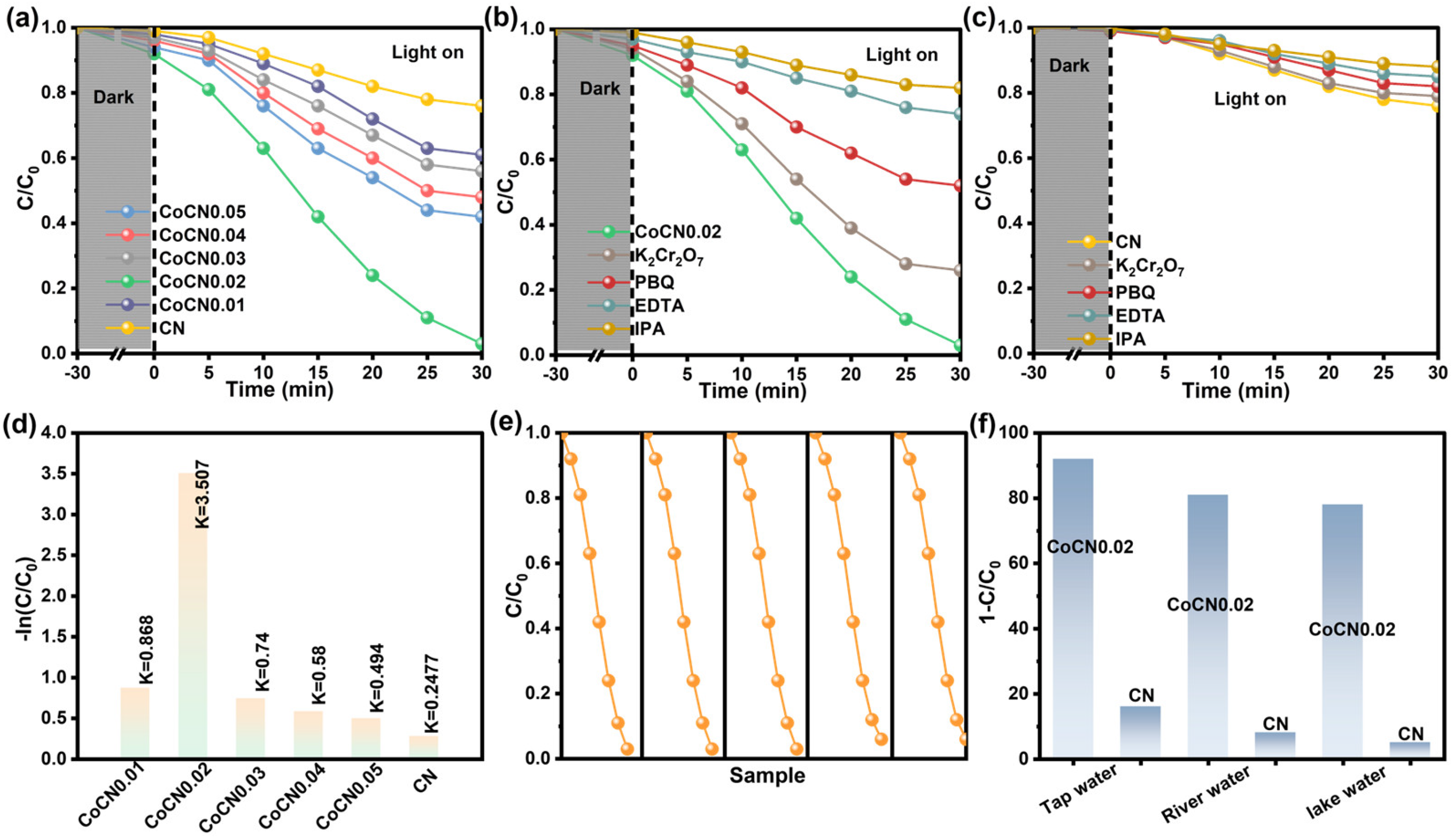
2.4. Photocatalytic TC Degradation Performance
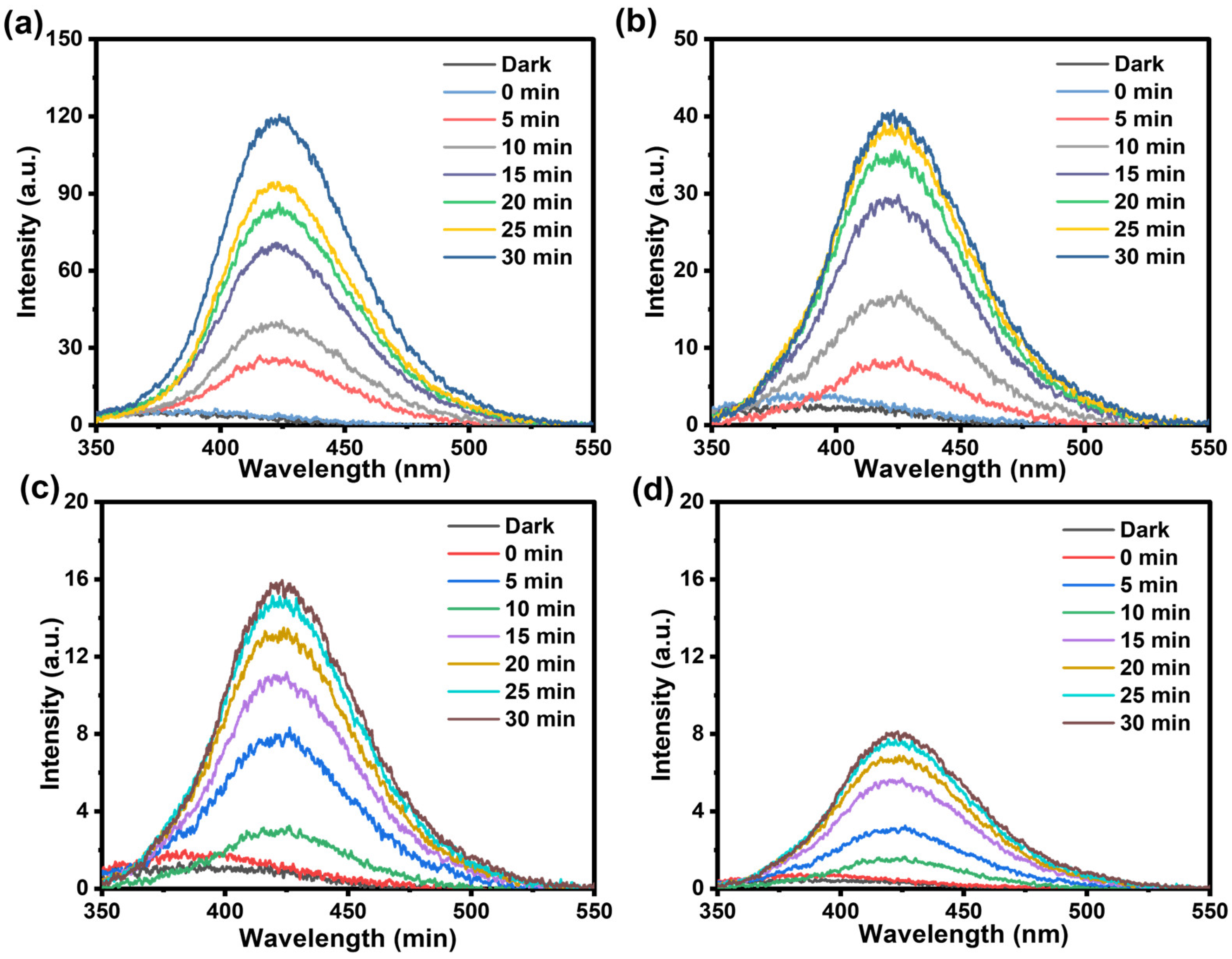
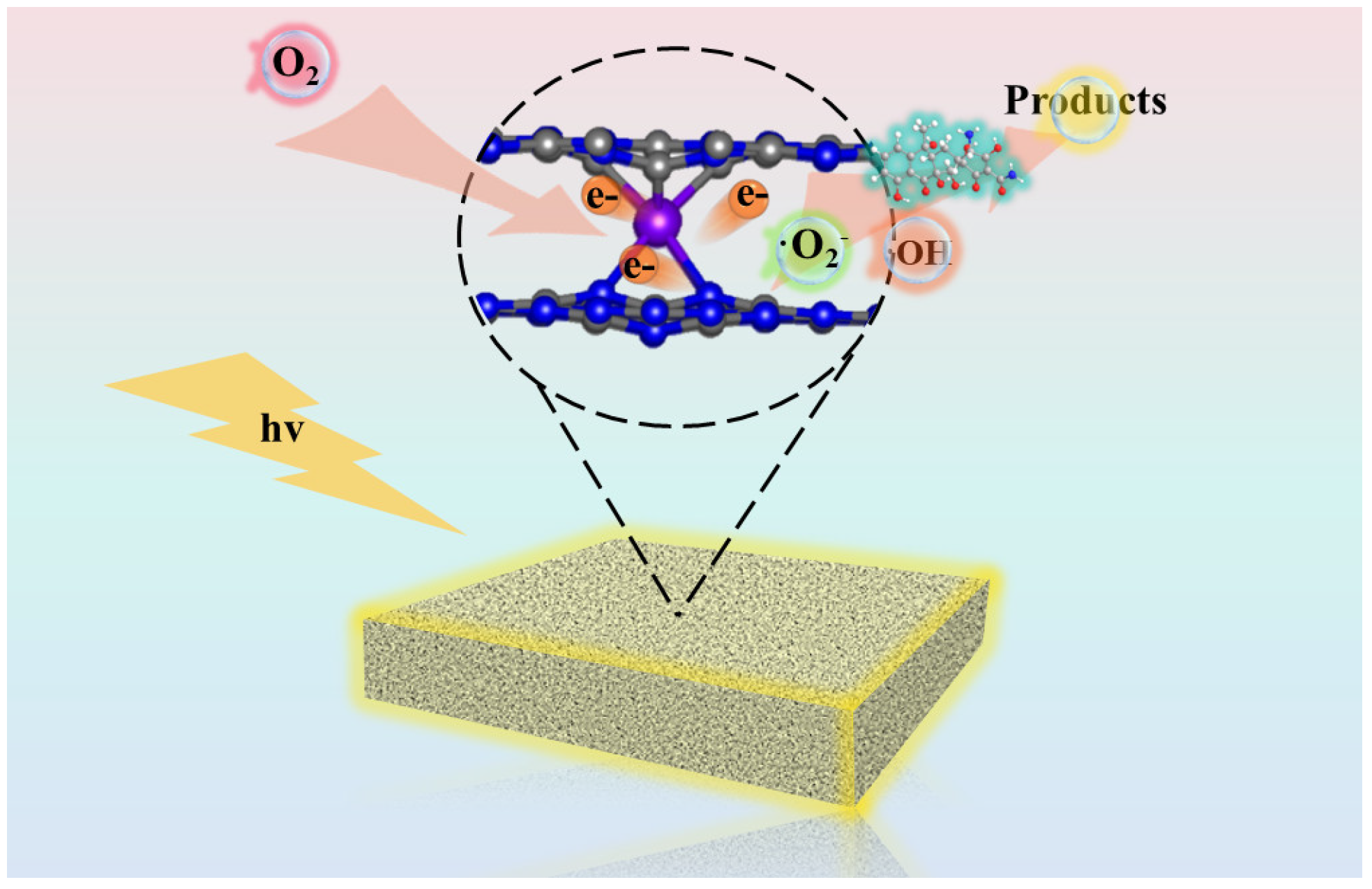
2.5. Mechanism of TC Degradation by Photocatalysis
3. Experiment
3.1. Materials
3.2. Synthesis of Catalysts
3.3. Characterization of the Catalysts
3.4. Photoelectrochemical Experiment
3.5. Photocatalytic Degradation Efficiency for TC
3.6. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Hu, X.; Li, M.; Fang, J.; Leng, C.; Zhu, X.; Xu, W.; Qin, J.; Yao, L.; Liu, Z.; et al. Constructing mesoporous Zr-doped SiO2 onto efficient Z-scheme TiO2/g-C3N4 heterojunction for antibiotic degradation via adsorption-photocatalysis and mechanism insight. Environ. Res. 2022, 214, 114189. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, Q.; Zhu, Z.; Wu, W.; Liu, L.; Zhang, J.; Gao, C.; Yang, T. Construction of a photocatalytic fuel cell using a novel Z-scheme MoS2/rGO/Bi2S3 as electrode degraded antibiotic wastewater. Sep. Purif. Technol. 2021, 277, 119276. [Google Scholar] [CrossRef]
- Zhong, C.; Zhao, H.; Han, Q.; Cao, H.; Duan, F.; Shen, J.; Xie, Y.; Guo, W.; Sun, S. Coupling-oxidation process promoted ring-opening degradation of 2-mecapto-5-methyl-1,3,4-thiadizaole in wastewater. Water Res. 2020, 186, 116362. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, N.; Yu, H.; Chen, S.; Quan, X. Degradation of aqueous bisphenol A in the CoCN/Vis/PMS system: Catalyst design, reaction kinetic and mechanism analysis. Chem. Eng. J. 2021, 407, 127228. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, W.; Zhao, Z.; Liu, W.; Ye, J.; Tong, M.; Li, Y. Different degradation mechanisms of carbamazepine and diclofenac by single-atom Barium embedded g-C3N4: The role of photosensitation-like mechanism. J. Hazard. Mater. 2021, 416, 125936. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Yue, C.; Liu, S.; Lin, Y.; Xie, T.; Dong, S. Efficient photoactivation of peroxymonosulfate by Z-scheme nitrogen-defect-rich NiCo2O4/g-C3N4 for rapid emerging pollutants degradation. J. Hazard. Mater. 2021, 414, 125528. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Jin, X.; Zheng, X.; Wang, Y.; Wei, D.; Zhang, Q.; Feng, Y.; Xie, Z.; Chen, P.; et al. Highly active metal-free carbon dots/g-C3N4 hollow porous nanospheres for solar-light-driven PPCPs remediation: Mechanism insights, kinetics and effects of natural water matrices. Water Res. 2020, 172, 115492. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Zhao, Z.; Zhong, H.; Zhu, Z.; Chen, W. In situ stable growth of β-FeOOH on g-C3N4 for deep oxidation of emerging contaminants by photocatalytic activation of peroxymonosulfate under solar irradiation. Chem. Eng. J. 2020, 400, 125872. [Google Scholar] [CrossRef]
- Kim, H.; Choong, C.E.; Han, I.; Park, C.M.; Nah, I.W.; Kim, J.R.; Jeon, B.-H.; Yoon, Y.; Jang, M. Insight into the role of charge carrier mediation zone for singlet oxygen production over rod-shape graphitic carbon nitride: Batch and continuous-flow reactor. J. Hazard. Mater. 2022, 424, 127652. [Google Scholar] [CrossRef]
- Zhan, R.; Zhou, Y.; Liu, C.; Wang, X.; Sun, X.; Zhu, Y.; Niu, J. Insights into mechanism of Fe-dominated active sites via phosphorus bridging in Fe-Ni bimetal single atom photocatalysts. Sep. Purif. Technol. 2022, 286, 120443. [Google Scholar] [CrossRef]
- Choong, C.E.; Park, C.M.; Chang, Y.-Y.; Yang, J.-k.; Kim, J.R.; Oh, S.-E.; Jeon, B.-H.; Choi, E.H.; Yoon, Y.; Jang, M. Interfacial coupling perovskite CeFeO3 on layered graphitic carbon nitride as a multifunctional Z-scheme photocatalyst for boosting nitrogen fixation and organic pollutants demineralization. Chem. Eng. J. 2022, 427, 131406. [Google Scholar] [CrossRef]
- Niu, B.; Cai, J.; Song, W.; Zhao, G. Intermediate accumulation and toxicity reduction during the selective photoelectrochemical process of atrazine in complex water bodies. Water Res. 2021, 205, 117663. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Han, H.; Wang, X.; Lai, Y.; Liu, B.; Zhong, R.; Zhang, Y.; Zhang, S.; Wang, L. Constructing oxygen absorption and activation sites in Ce-doped g-C3N4 photocatalyst for effective removal of amoxicillin: Performance, mechanism and degradation pathways. Appl. Surf. Sci. 2023, 611, 155808. [Google Scholar] [CrossRef]
- Han, H.; Wang, X.; Qiao, Y.; Lai, Y.; Liu, B.; Zhang, Y.; Luo, J.; Toan, S.; Wang, L. Construction of S-scheme heterojunction for enhanced photocatalytic conversation of NO over dual-defect CeO2−x/g-C3N4−x. J. Alloys Compd. 2023, 933, 167819. [Google Scholar] [CrossRef]
- Zhu, Q.; Hailili, R.; Xin, Y.; Zhou, Y.; Huang, Y.; Pang, X.; Zhang, K.; Robertson, P.K.J.; Bahnemann, D.W.; Wang, C. Efficient full spectrum responsive photocatalytic NO conversion at Bi2Ti2O7: Co-effect of plasmonic Bi and oxygen vacancies. Appl. Catal. B Environ. 2022, 319, 121888. [Google Scholar] [CrossRef]
- Luo, J.; Han, H.; Wu, J.; Wang, X.; Feng, J.; Toan, S.; Wang, L.; Lai, Y. Excellent photocatalytic activity of MoO3-adorned g-C3N4 systems: Construction of S-scheme heterojunction. Appl. Surf. Sci. 2022, 604, 154512. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, K.; Li, D.; Li, N.; Xu, J.; Bahnemann, D.W.; Wang, C. Polarization-enhanced photocatalytic activity in non-centrosymmetric materials based photocatalysis: A review. Chem. Eng. J. 2021, 426, 131681. [Google Scholar] [CrossRef]
- Luo, J.; Han, H.; Wang, X.; Qiu, X.; Liu, B.; Lai, Y.; Chen, X.; Zhong, R.; Wang, L.; Wang, C. Single-atom Nb anchored on graphitic carbon nitride for boosting electron transfer towards improved photocatalytic performance. Appl. Catal. B Environ. 2023, 328, 122495. [Google Scholar] [CrossRef]
- Hu, Y.; Nie, M.; Hong, P.; He, J.; Li, Y.; Zhang, K.; Yang, D.; Jiang, L.; Liu, J.; Kong, L. Defect-engineered WO3-x@MoS2 hollow tube exhibiting enhanced Fenton-like and photocatalytic activities via electric field rearrangement and band alignment. Appl. Catal. B Environ. 2023, 320, 122013. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, X.; Yi, Z.; Xu, X.; Yang, J.; Zhu, M. Enhanced reactive oxidation species generation by ligand-to-metal-charge transfer between oxygen vacancy-rich ZnO mesocrystal with ciprofloxacin pollutants. Appl. Catal. B Environ. 2023, 321, 122033. [Google Scholar] [CrossRef]
- Zhou, Y.; Sha, T.; Liu, D.; Liao, B.; Li, K. Molecularly imprinted ratiometric fluorescence detection of tetracycline based on its fluorescence enhancement effect caused by tungsten trioxide quantum dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Meng, C.; Zhang, Z. Nanoconfined catalytic membranes assembled by cobalt-functionalized graphitic carbon nitride nanosheets for rapid degradation of pollutants. Appl. Catal. B Environ. 2023, 322, 122098. [Google Scholar] [CrossRef]
- Zhu, Q.; Dar, A.A.; Zhou, Y.; Zhang, K.; Qin, J.; Pan, B.; Lin, J.; Patrocinio, A.O.T.; Wang, C. Oxygen Vacancies Promoted Piezoelectricity toward Piezo-Photocatalytic Decomposition of Tetracycline over SrBi4Ti4O15. ACS EST Eng. 2022, 2, 1365–1375. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Zhang, B.; Wang, Y.; Bi, Y.; Yang, Q.; Liu, Y.; Liu, T.; Wang, Z.; Ren, H.; et al. Synergistic integration of energy storage catalysis: A multifunctional catalytic material for round-the-clock environmental cleaning. Appl. Catal. B Environ. 2023, 321, 122052. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, L.; Chu, H.; Wang, J.-F.; Wang, T.; Ma, Y.; Wang, C.-C. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@N-C-x: Singlet oxygen evolution and electron-transfer mechanisms. Appl. Catal. B Environ. 2023, 321, 122034. [Google Scholar] [CrossRef]
- Guo, C.; Wang, L.; Lang, D.; Qian, Q.; Wang, W.; Wu, R.; Wang, J. UV and chemical aging alter the adsorption behavior of microplastics for tetracycline. Environ. Pollut. 2023, 318, 120859. [Google Scholar] [CrossRef]
- Dong, C.; Zheng, Z.; Wang, Z.; He, J.; Ye, Z.; Gong, X.; Lo, I.M.C. N-doped graphitic C3N4 nanosheets decorated with CoP nanoparticles: A highly efficient activator in singlet oxygen dominated visible-light-driven peroxymonosulfate activation for degradation of pharmaceuticals and personal care products. J. Hazard. Mater. 2021, 416, 125891. [Google Scholar] [CrossRef]
- Qian, M.; Yang, F.; Li, N.; Gao, J.; Chen, X.; Xu, T.; Zhu, Z.; Lu, W.; Chen, W. A novel biodegradable porous graphitic carbon nitride/poly(lactic acid) fiber photocatalyst for efficient elimination of carbamazepine under solar irradiation. Chem. Eng. J. 2021, 414, 128845. [Google Scholar] [CrossRef]
- Feng, C.; Ouyang, X.; Deng, Y.; Wang, J.; Tang, L. A novel g-C3N4/g-C3N4−x homojunction with efficient interfacial charge transfer for photocatalytic degradation of atrazine and tetracycline. J. Hazard. Mater. 2023, 441, 129845. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, Z.; Zeng, H.; Tang, R.; Li, L.; Wang, J.; Feng, C.; Gong, D.; Tang, L.; Huang, Y. Phosphorus and kalium co-doped g-C3N4 with multiple-locus synergies to degrade atrazine: Insights into the depth analysis of the generation and role of singlet oxygen. Appl. Catal. B Environ. 2023, 320, 121942. [Google Scholar] [CrossRef]
- Xu, L.; Liu, L. Piezo-photocatalytic fuel cell with atomic Fe@MoS2 on CFC helical electrode has enhanced peroxymonosulfate activation, pollutant degradation and power generation. Appl. Catal. B Environ. 2022, 304, 120953. [Google Scholar] [CrossRef]
- Shang, Y.; Jiang, N.; Liu, Z.; Li, C.; Sun, H.; Guo, H.; Peng, B.; Li, J. Pulsed discharge plasma assisted with Z-scheme graphene-TiO2-MnFe2O4 for simultaneous removal of atrazine and Cr (VI): Performance and mechanism. Chem. Eng. J. 2023, 452, 139342. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.-L.; Chen, S.-W.; Yang, T.C.K. Rational synthesis of BixFe1−xVO4 heterostructures impregnated sulfur-doped g-C3N4: A visible-light-driven type-II heterojunction photo(electro)catalyst for efficient photodegradation of roxarsone and photoelectrochemical OER reactions. Appl. Catal. B Environ. 2022, 304, 120852. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, B.; Shan, C.; Zhang, W.; Dionysiou, D.D.; Pan, B. Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: Quantitative relationship and first-principles investigation. Appl. Catal. B Environ. 2021, 292, 120155. [Google Scholar] [CrossRef]
- Feng, Z.; Tian, Q.; Yang, Q.; Zhou, Y.; Zhao, H.; Zhao, G. Selectively photoelectrocatalytic reduction of oxygen to hydroxyl radical and singlet oxygen: Mechanism and validation in coal wastewater. Appl. Catal. B Environ. 2021, 286, 119908. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Single atom cobalt catalyst derived from co-pyrolysis of vitamin B12 and graphitic carbon nitride for PMS activation to degrade emerging pollutants. Appl. Catal. B Environ. 2023, 321, 122051. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, W.; Fang, J.; Chen, D.; Pan, T.; Feng, W.; Liang, Y.; Fang, Z. Soft-template assisted construction of superstructure TiO2/SiO2/g-C3N4 hybrid as efficient visible-light photocatalysts to degrade berberine in seawater via an adsorption-photocatalysis synergy and mechanism insight. Appl. Catal. B Environ. 2020, 268, 118751. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, K.; Toe, C.Y.; Amal, R.; Zhang, X.; McCarthy, D.T.; Deletic, A. Stormwater herbicides removal with a solar-driven advanced oxidation process: A feasibility investigation. Water Res. 2021, 190, 116783. [Google Scholar] [CrossRef]
- Cui, M.; Cui, K.; Liu, X.; Shi, J.; Chen, X.; Chen, Y. Synergistic effect of mesoporous graphitic carbon nitride and peroxydisulfate in visible light-induced degradation of atenolol: A combined experimental and theoretical study. Chem. Eng. J. 2021, 412, 127979. [Google Scholar] [CrossRef]
- Tang, R.; Zeng, H.; Gong, D.; Deng, Y.; Xiong, S.; Li, L.; Zhou, Z.; Wang, J.; Feng, C.; Tang, L. Thin-walled vesicular Triazole-CN-PDI with electronic n→π* excitation and directional movement for enhanced atrazine photodegradation. Chem. Eng. J. 2023, 451, 138445. [Google Scholar] [CrossRef]
- Lumbaque, E.C.; Lüdtke, D.S.; Dionysiou, D.D.; Vilar, V.J.P.; Sirtori, C. Tube-in-tube membrane photoreactor as a new technology to boost sulfate radical advanced oxidation processes. Water Res. 2021, 191, 116815. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Gong, D.; Zhou, Y.; Deng, Y.; Feng, C.; Xiong, S.; Huang, Y.; Peng, G.; Li, L. Unique g-C3N4/PDI-g-C3N4 homojunction with synergistic piezo-photocatalytic effect for aquatic contaminant control and H2O2 generation under visible light. Appl. Catal. B Environ. 2022, 303, 120929. [Google Scholar] [CrossRef]
- Ding, P.; Ji, H.; Li, P.; Liu, Q.; Wu, Y.; Guo, M.; Zhou, Z.; Gao, S.; Xu, W.; Liu, W.; et al. Visible-light degradation of antibiotics catalyzed by titania/zirconia/graphitic carbon nitride ternary nanocomposites: A combined experimental and theoretical study. Appl. Catal. B Environ. 2022, 300, 120633. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Deng, J.; Li, L.; Zhou, Z.; Zheng, J.; Su, L.; Yang, L. π-π Stacked step-scheme PDI/g-C3N4/TiO2@Ti3C2 photocatalyst with enhanced visible photocatalytic degradation towards atrazine via peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 131809. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, S.-Z.; Liu, L.-Y.; Zhang, Z.-F.; Ma, W.-L. Bi modified oxidized tubular carbon nitride with high-yield singlet oxygen for propylparaben degradation: Implication for a novel oxygen activation mechanism. Appl. Catal. B Environ. 2023, 321, 122025. [Google Scholar] [CrossRef]
- Gao, K.; Hou, L.-a.; An, X.; Huang, D.; Yang, Y. BiOBr/MXene/g-C3N4 Z-scheme heterostructure photocatalysts mediated by oxygen vacancies and MXene quantum dots for tetracycline degradation: Process, mechanism and toxicity analysis. Appl. Catal. B Environ. 2023, 323, 122150. [Google Scholar] [CrossRef]
- Xu, L.; Li, L.; Hu, Z.; Yu, J.C. Boosting alkaline photocatalytic H2O2 generation by incorporating pyrophosphate on g-C3N4 for effective proton shuttle and oxygen activation. Appl. Catal. B Environ. 2023, 328, 122490. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Y.; Zhang, H.; Liu, S.; Wei, H.; Yuan, H.; Wen, H.; Wu, X.; Mehdi, S.; Liu, T.; et al. Coupling oxygen vacancy and hetero-phase junction for boosting catalytic activity of Pd toward hydrogen generation. Appl. Catal. B Environ. 2023, 328, 122484. [Google Scholar] [CrossRef]
- Miao, Y.; Li, Z.; Song, Y.; Fan, K.; Guo, J.; Li, R.; Shao, M. Surface active oxygen engineering of photoanodes to boost photoelectrochemical water and alcohol oxidation coupled with hydrogen production. Appl. Catal. B Environ. 2023, 323, 122147. [Google Scholar] [CrossRef]
- Bagheri, S.; TermehYousefi, A.; Do, T.-O. Photocatalytic pathway toward degradation of environmental pharmaceutical pollutants: Structure, kinetics and mechanism approach. Catal. Sci. Technol. 2017, 7, 4548–4569. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Spray-Deposited TiO2 Layers on Aluminum Foil for Sustainable Water Remediation. Crystals 2024, 14, 875. [Google Scholar] [CrossRef]
- Li, G.; Sun, X.; Chen, P.; Song, M.; Zhao, T.; Liu, F.; Yin, S.-F. Insights into spin polarization regulated exciton dissociation and charge separation of C3N4 for efficient hydrogen evolution and simultaneous benzylamine oxidation. Nano Res. 2023, 16, 8845–8852. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, N.; Liu, J.; Wang, X.; Robertson, P.K.J.; Chen, X.; Chen, C.; Luo, J.; Wang, C. Full-spectrum Bi@SrTiO3 for bi-directional promotion effects on photocatalytic redox reaction: Insight into intermediates and mechanism. J. Rare Earths 2024, 42, 917–929. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Li, W.; Lv, K.; Zhu, L.; Zhang, Y.; Wang, L.; Li, Y.; Luo, J.; Huang, Z. Engineering Electron Transport Pathways in Cobalt-Doped g-C3N4 Photocatalysts: Enhanced Tetracycline Degradation Through Interlayer Bridging. Catalysts 2025, 15, 366. https://doi.org/10.3390/catal15040366
Zhang S, Li W, Lv K, Zhu L, Zhang Y, Wang L, Li Y, Luo J, Huang Z. Engineering Electron Transport Pathways in Cobalt-Doped g-C3N4 Photocatalysts: Enhanced Tetracycline Degradation Through Interlayer Bridging. Catalysts. 2025; 15(4):366. https://doi.org/10.3390/catal15040366
Chicago/Turabian StyleZhang, Suna, Wenqin Li, Kangle Lv, Luping Zhu, Yaxin Zhang, Lijun Wang, Yuhan Li, Jianmin Luo, and Zeai Huang. 2025. "Engineering Electron Transport Pathways in Cobalt-Doped g-C3N4 Photocatalysts: Enhanced Tetracycline Degradation Through Interlayer Bridging" Catalysts 15, no. 4: 366. https://doi.org/10.3390/catal15040366
APA StyleZhang, S., Li, W., Lv, K., Zhu, L., Zhang, Y., Wang, L., Li, Y., Luo, J., & Huang, Z. (2025). Engineering Electron Transport Pathways in Cobalt-Doped g-C3N4 Photocatalysts: Enhanced Tetracycline Degradation Through Interlayer Bridging. Catalysts, 15(4), 366. https://doi.org/10.3390/catal15040366








