Enhancing Levofloxacin Degradation in Contaminated Water: Catalytic Performance of Pegmatite in a Sodium Percarbonate/Ultrasound System
Abstract
1. Introduction
2. Results and Discussion
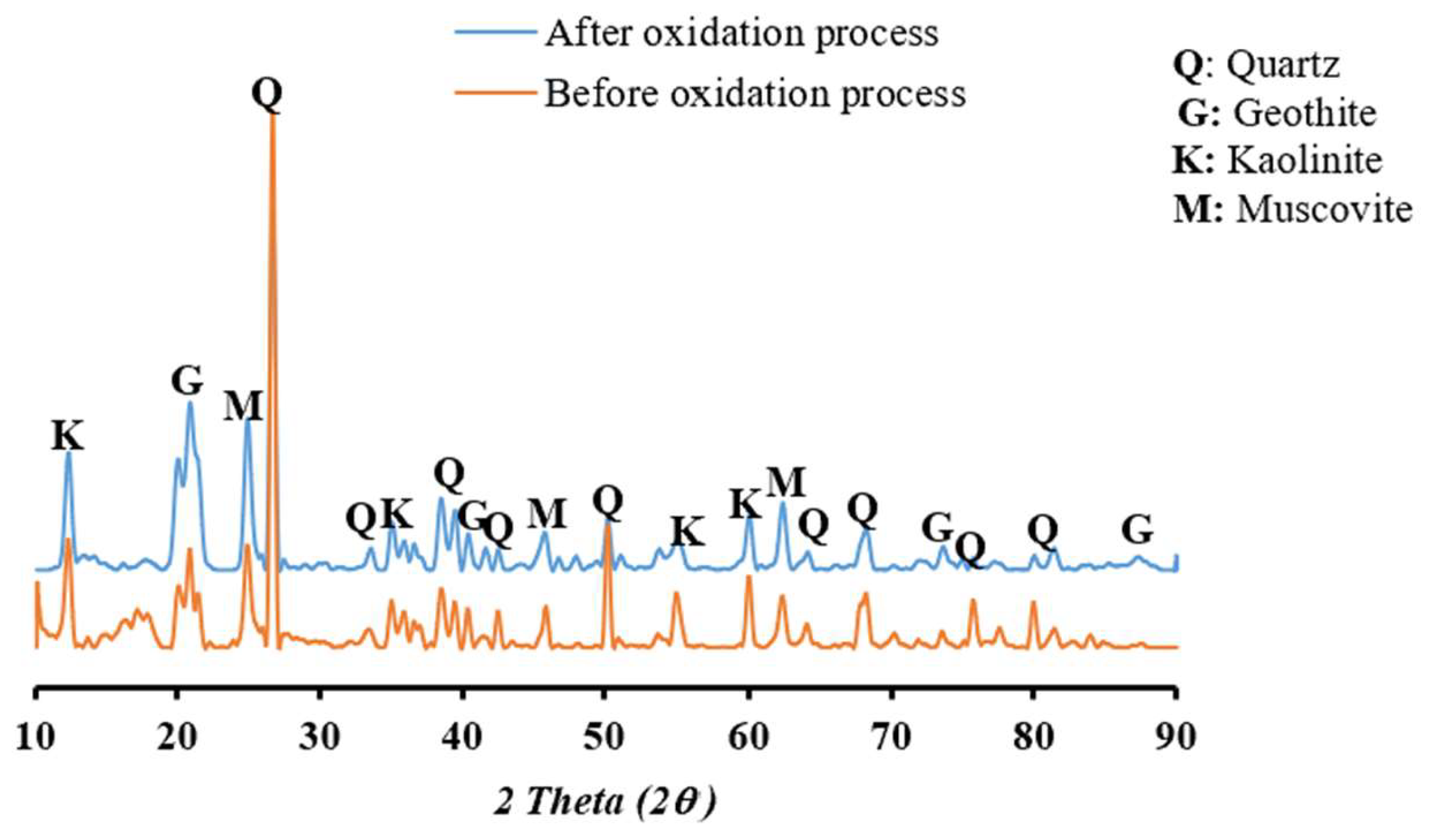
2.1. Pegmatite Soil Characterization
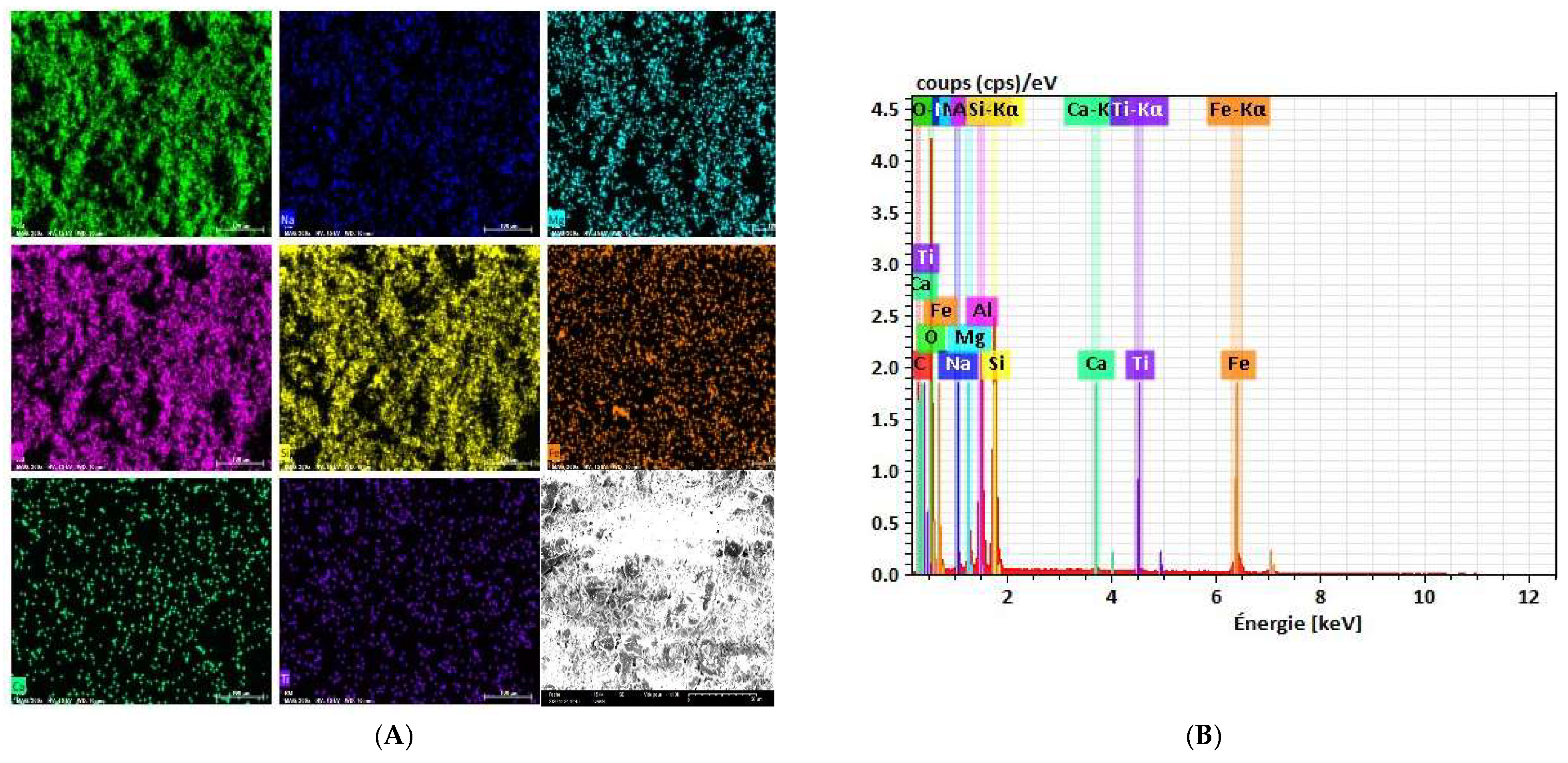
2.1.1. Morphology and Chemical Characterizations
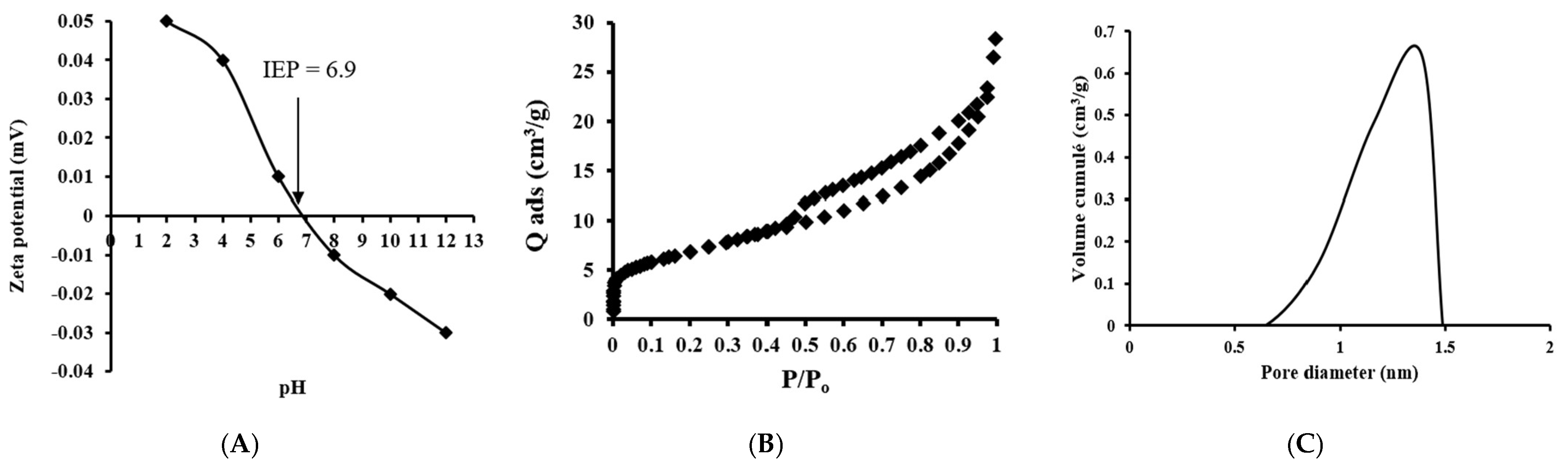
2.1.2. Surface Characteristics
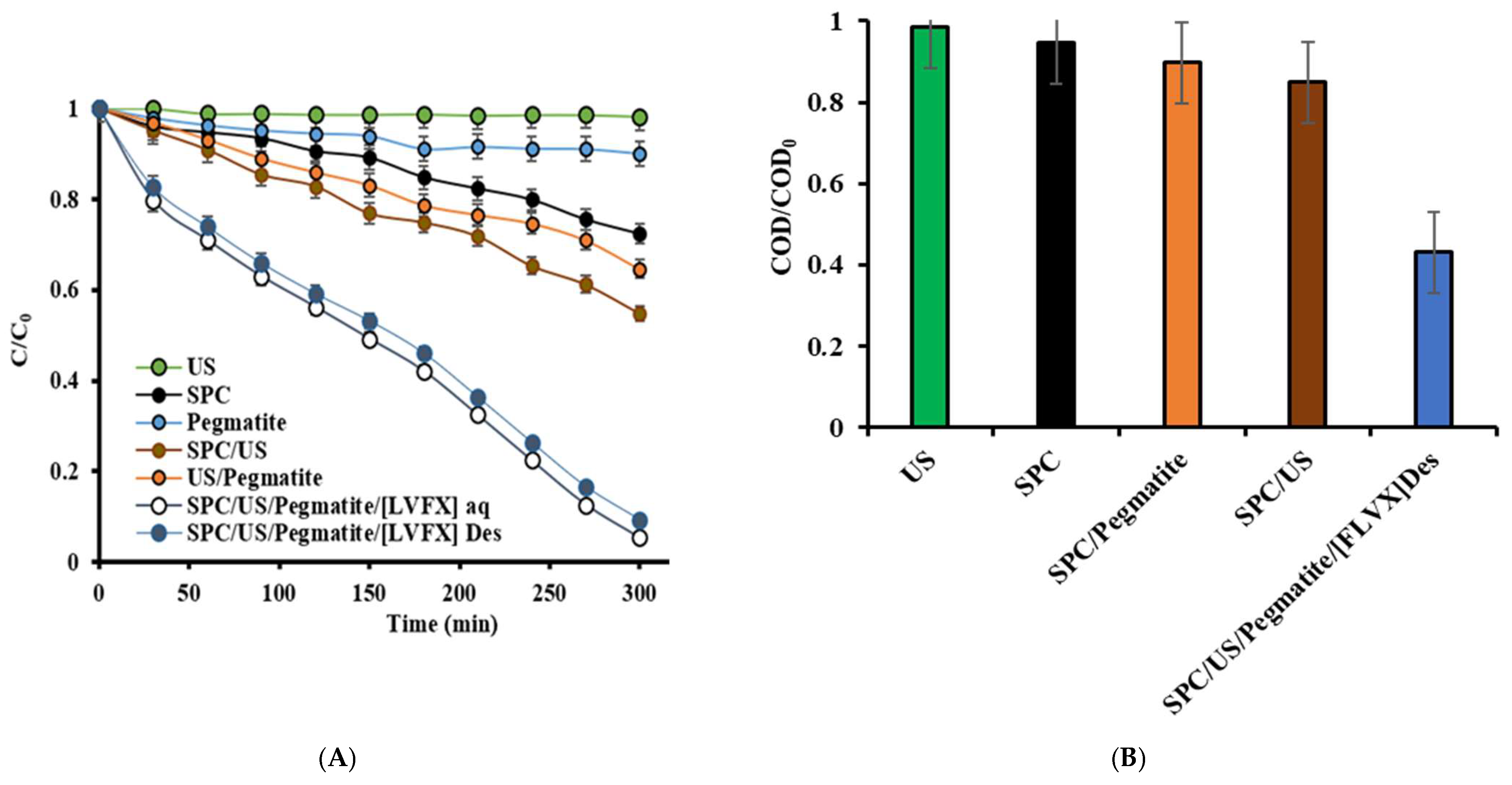
2.2. Removal Capacity of Pegmatite
2.3. Optimization of Operating Process Conditions
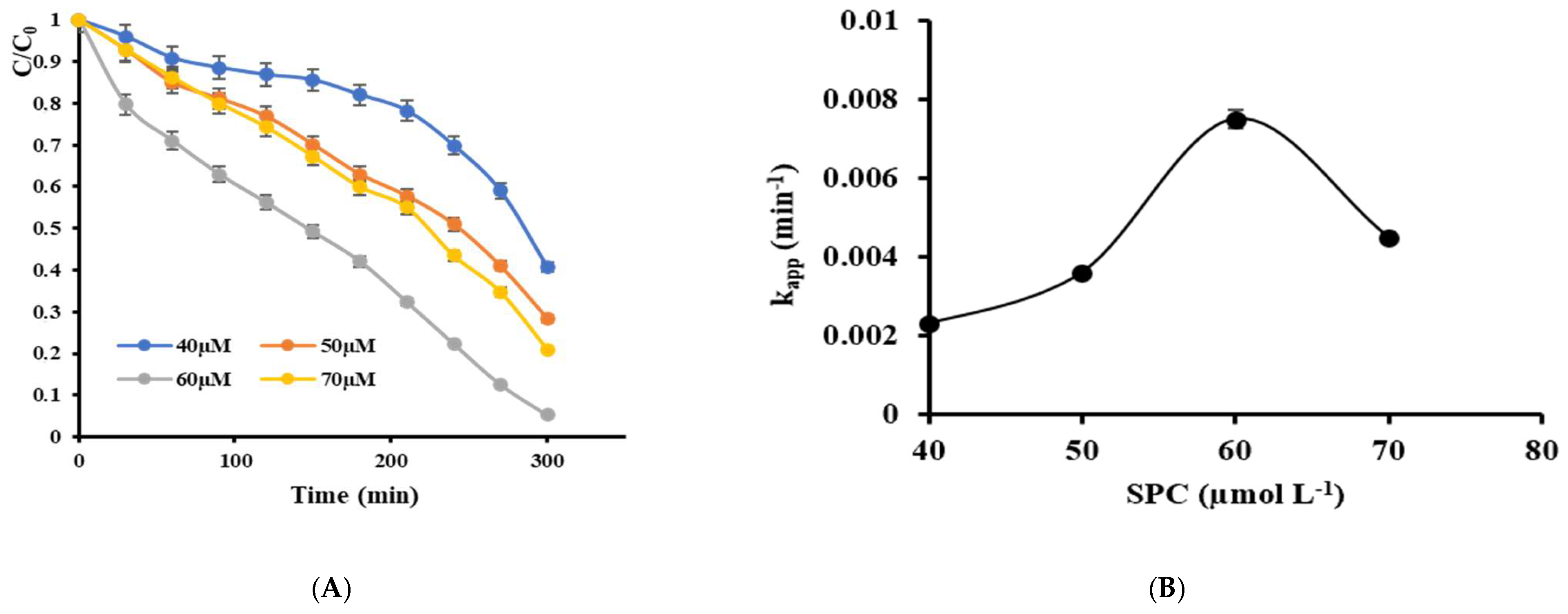
2.3.1. Effect of Oxidant SPC Concentrations
2.3.2. Effect of Catalyst (Pegmatite) Dosage
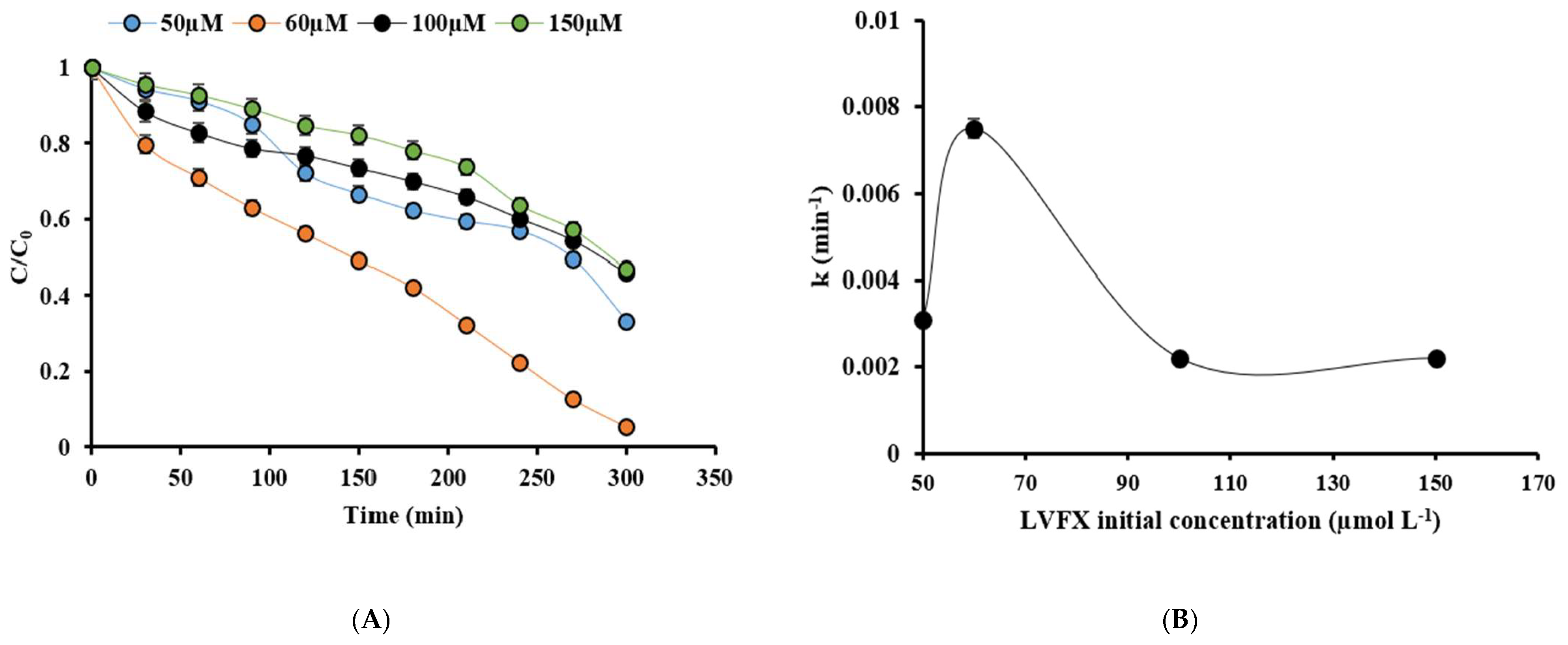
2.3.3. Effect of Levofloxacin Concentrations
2.4. Identification of Radical Species in the Degradation Process
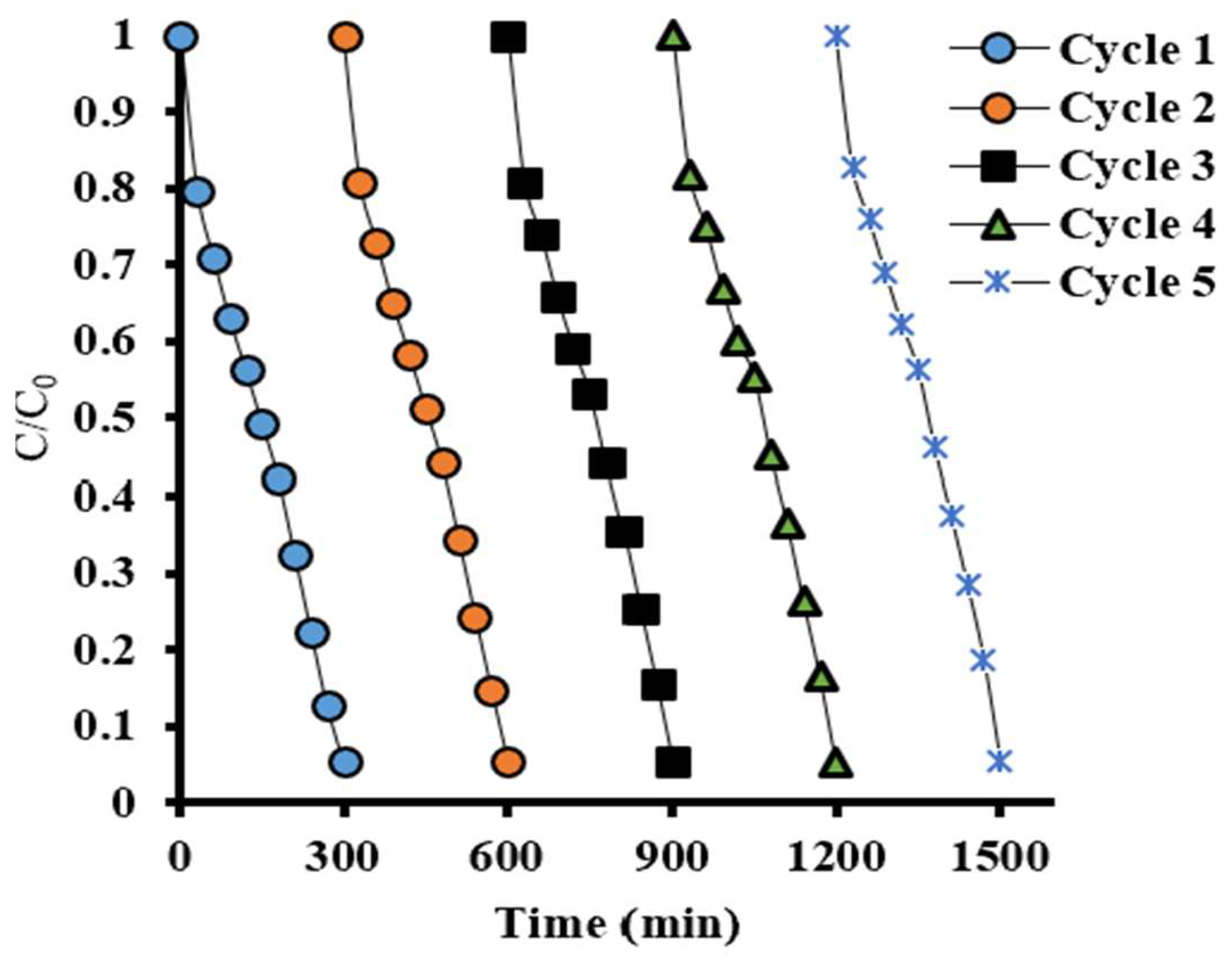
2.5. Assessment of Pegmatite Catalytic Ability
3. Materials and Methods
3.1. Chemicals
3.2. The Soil and Characterization Methods
3.3. Ultrasonic Degradation Tests and Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yun, X.; Wang, J.; Li, Q.; Wang, Y. A review on the ecotoxicological effect of sulphonamides on aquatic organisms. Toxicol. Rep. 2022, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Chukwudozie, K.I.; Nyaruaba, R.; Ita, R.E.; Oladipo, A.; Ejeromedoghene, O.; Atakpa, E.O.; Agu, C.V.; Okoye, C.O. Antibiotic resistance in aquaculture and aquatic organisms: A review of current nanotechnology applications for sustainable management. Environ. Sci. Pollut. Res. 2022, 29, 69241–69274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, Y.; Fung-Yee Tam, N.; Ruan, W.; Yang, Y.; Yang, Y.; Tao, R.; Zhang, J. Specific metabolism related to sulfonamide tolerance and uptake in wetland plants. Chemosphere 2019, 227, 496–504. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Liu, Y.; Zhang, C.; Wan, J.; Hu, L.; Zhou, C.; Xiong, W. Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS/H2O2 Fenton-like system. Water Res. 2018, 138, 7–18. [Google Scholar] [CrossRef]
- Assadi, A.A.; Palau, J.; Bouzaza, A.; Wolbert, D. Modeling of a continuous photocatalytic reactor for isovaleraldehyde oxidation: Effect of different operating parameters and chemical degradation pathway. Chem. Eng. Res. Des. 2013, 91, 1307–1316. [Google Scholar]
- Giraldo, A.L.; Erazo-Erazo, E.D.; Flórez-Acosta, O.A.; Serna-Galvis, E.A.; Torres-Palma, R.A. Degradation of the antibiotic oxacillin in water by anodic oxidation with Ti/IrO2 anodes: Evaluation of degradation routes, organic by-products and effects of water matrix components. Chem. Eng. J. 2015, 279, 103–114. [Google Scholar] [CrossRef]
- Kamagate, M.; Amin Assadi, A.; Kone, T.; Coulibaly, L.; Hanna, K. Activation of persulfate by irradiated laterite for removal of fluoroquinolones in multi-component systems. J. Hazard. Mater. 2018, 346, 159–166. [Google Scholar] [CrossRef]
- Brahimi, B.; Mekatel, E.; Kadmi, Y.; Mellal, M.; Baaloudj, O.; Belmedani, M.; Trari, M. Removal of basic blue 41 dye from water by the hetero-system NiCo2O4/ZnO using a stirred reactor: Kinetics, mechanism and energy diagram. Optik 2022, 258, 168933. [Google Scholar] [CrossRef]
- Baaloudj, O.; Badawi, A.K.; Kenfoud, H.; Benrighi, Y.; Hassan, R.; Nasrallah, N.; Assadi, A.A. Techno-economic studies for a pilot-scale Bi12TiO20 based photocatalytic system for pharmaceutical wastewater treatment: From laboratory studies to commercial-scale applications. J. Water Process. Eng. 2022, 48, 102847. [Google Scholar] [CrossRef]
- Wang, H.; Meng, S.; Zhang, Z.; Zhou, C.; Zhang, H.; Liu, Y.; He, C.-s.; Zhou, P.; Lai, B. Mechanical agitation and ultrasound constructed in situ Fenton system: The role of atomic hydrogen for Fe(III) reduction. Sep. Purif. Technol. 2024, 340, 126859. [Google Scholar] [CrossRef]
- Li, L.; Niu, X.; Zhang, D.; Ye, X.; Zhang, Z.; Liu, Q.; Ding, L.; Chen, K.; Chen, Y.; Chen, K. A systematic review on percarbonate-based advanced oxidation processes in wastewater remediation: From theoretical understandings to practical applications. Water Res. 2024, 259, 121842. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, H.R.; Brown, M.T.; Boyer, T.H. Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources. Chemosphere 2014, 105, 112–118. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q. Insights into a novel CuS/percarbonate/tetraacetylethylenediamine process for sulfamethazine degradation in alkaline medium. J. Hazard. Mater. 2022, 435, 128999. [Google Scholar] [CrossRef]
- Farooq, U.; Wang, F.; Shang, J.; Zeeshan Shahid, M.; Akram, W.; Wang, X. Heightening effects of cysteine on degradation of trichloroethylene in Fe3+/SPC process. Chem. Eng. J. 2023, 454, 139996. [Google Scholar] [CrossRef]
- Yan, P.; Sui, Q.; Lyu, S.; Hao, H.; Schröder, H.F.; Gebhardt, W. Elucidation of the oxidation mechanisms and pathways of sulfamethoxazole degradation under Fe(II) activated percarbonate treatment. Sci. Total Environ. 2018, 640–641, 973–980. [Google Scholar] [CrossRef]
- Fu, X.; Gu, X.; Lu, S.; Sharma, V.K.; Brusseau, M.L.; Xue, Y.; Danish, M.; Fu, G.Y.; Qiu, Z.; Sui, Q. Benzene oxidation by Fe(III)-activated percarbonate: Matrix-constituent effects and degradation pathways. Chem. Eng. J. 2017, 309, 22–29. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Xu, G.; Li, H.; Yu, Y. Photocatalytic activation of sodium percarbonate by C3N5/C2N3 homojunction for rapid water purification: Efficient electron transfer of built-in electric field. Chem. Eng. J. 2024, 491, 151885. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D.; Petit, P. Isovaleraldehyde elimination by UV/TiO2 photocatalysis: Comparative study of the process at different reactors configurations and scales. Environ. Sci. Pollut. Res. 2014, 21, 11178–11188. [Google Scholar] [CrossRef]
- Rao, M.; Xia, H.; Xu, Y.; Jiang, G.; Zhang, Q.; Yuan, Y.; Zhang, L. Study on ultrasonic assisted intensive leaching of germanium from germanium concentrate using HCl/NaOCl. Hydrometallurgy 2024, 230, 106385. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, L.; Jin, S.; Zou, W.; Wang, H.; Xie, Y.; Hou, C.; Zhai, Y.; Luo, P. Treatment of sludge hydrothermal carbonization wastewater by ferrous/sodium percarbonate system: Effect of wastewater composition and role of coagulation and oxidation. Water Res. 2024, 267, 122531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tong, M.; Yuan, S.; Qian, A.; Liu, H. Interplay between iron species transformation and hydroxyl radicals production in soils and sediments during anoxic-oxic cycles. Geoderma 2020, 370, 114351. [Google Scholar] [CrossRef]
- Kamagate, M.; Assadi, A.A.; Kone, T.; Giraudet, S.; Coulibaly, L.; Hanna, K. Use of laterite as a sustainable catalyst for removal of fluoroquinolone antibiotics from contaminated water. Chemosphere 2018, 195, 847–853. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; He, J.; Liu, T.; Zhang, K.; Huang, X.; Kong, L.; Liu, J. EDTA-Fe(III) Fenton-like oxidation for the degradation of malachite green. J. Environ. Manag. 2018, 226, 256–263. [Google Scholar]
- Qi, H.; Chen, Y.; Li, L.; Li, X.; Li, Y. Degradation of Levofloxacin via Fenton Oxidation Combined with Ultrasonic Treatment in Water. Pol. J. Environ. Stud. 2024, 33, 6305–6321. [Google Scholar] [CrossRef]
- Mechakra, H.; Sehili, T.; Kribeche, M.A.; Ayachi, A.A.; Rossignol, S.; George, C. Use of natural iron oxide as heterogeneous catalyst in photo-Fenton-like oxidation of chlorophenylurea herbicide in aqueous solution: Reaction monitoring and degradation pathways. J. Photochem. Photobiol. A Chem. 2016, 317, 140–150. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, T.; Liu, H.; Li, X.; Zhao, M.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Enhancing ferric ion/sodium percarbonate Fenton-like reaction with tungsten disulfide cocatalyst for metronidazole decomposition over wide pH range. J. Chem. Eng. 2023, 452, 139245. [Google Scholar] [CrossRef]
- Yu, X.; Kamali, M.; Aken, P.V.; Appels, L.; Van der Bruggen, B.; Dewil, R. Synergistic effects of the combined use of ozone and sodium percarbonate for the oxidative degradation of dichlorvos. J. Water Process. Eng. 2020, 39, 101721. [Google Scholar] [CrossRef]
- Rusevova Crincoli, K.; Huling, S.G. Hydroxyl radical scavenging by solid mineral surfaces in oxidative treatment systems: Rate constants and implications. Water Res. 2020, 169, 115240. [Google Scholar] [CrossRef]
- Miller, C.M.; Valentine, R.L. Mechanistic studies of surface catalyzed H2O2 decomposition and contaminant degradation in the presence of sand. Water Res. 1999, 33, 2805–2816. [Google Scholar] [CrossRef]
- Ren, H.; Quan, Y.; Liu, S.; Hao, J. Effectiveness of ultrasound (US) and slightly acidic electrolyzed water (SAEW) treatments for removing Listeria monocytogenes biofilms. Ultrason. Sonochem. 2025, 112, 107190. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Lucena-Mendoza, E.; Zoltan, T.; Torres, Y.; León, M.; Angulo, B.; Tovar, G.I. Photochemical and Sonochemical Strategies in Advanced Oxidation Processes for Micropollutants’ Treatments. In Advanced Oxidation Processes for Micropollutant Remediation; CRC Press: Boca Raton, FL, USA, 2023; pp. 65–98. [Google Scholar] [CrossRef]
- Mandal, S. Reaction Rate Constants of Hydroxyl Radicals with Micropollutants and Their Significance in Advanced Oxidation Processes. J. Adv. Oxid. Technol. 2018, 21, 178–195. [Google Scholar] [CrossRef]
- Hayon, E.; McGarvey, J.J. Flash photolysis in the vacuum ultraviolet region of sulfate, carbonate, and hydroxyl ions in aqueous solutions. J. Phys. Chem. 1967, 71, 1472–1477. [Google Scholar] [CrossRef]
- Assadi, A.A. Efficient Photocatalytic Luminous Textile for Simulated Real Water Purification: Advancing Economical and Compact Reactors. Materials 2024, 17, 296. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamagate, M.; Coulibaly, N.G.; Koffi, A.P.M.; Zie, O.; Coulibaly, L.; Assadi, A.A.; Elfalleh, W.; Hjiri, M.; Khezami, L.; Tahraoui, H.; et al. Enhancing Levofloxacin Degradation in Contaminated Water: Catalytic Performance of Pegmatite in a Sodium Percarbonate/Ultrasound System. Catalysts 2025, 15, 363. https://doi.org/10.3390/catal15040363
Kamagate M, Coulibaly NG, Koffi APM, Zie O, Coulibaly L, Assadi AA, Elfalleh W, Hjiri M, Khezami L, Tahraoui H, et al. Enhancing Levofloxacin Degradation in Contaminated Water: Catalytic Performance of Pegmatite in a Sodium Percarbonate/Ultrasound System. Catalysts. 2025; 15(4):363. https://doi.org/10.3390/catal15040363
Chicago/Turabian StyleKamagate, Mahamadou, Nina G. Coulibaly, Adingra Pohn Martial Koffi, Ouattara Zie, Lacina Coulibaly, Amine Aymen Assadi, Walid Elfalleh, Mokhtar Hjiri, Lotfi Khezami, Hichem Tahraoui, and et al. 2025. "Enhancing Levofloxacin Degradation in Contaminated Water: Catalytic Performance of Pegmatite in a Sodium Percarbonate/Ultrasound System" Catalysts 15, no. 4: 363. https://doi.org/10.3390/catal15040363
APA StyleKamagate, M., Coulibaly, N. G., Koffi, A. P. M., Zie, O., Coulibaly, L., Assadi, A. A., Elfalleh, W., Hjiri, M., Khezami, L., Tahraoui, H., Zhang, J., & Amrane, A. (2025). Enhancing Levofloxacin Degradation in Contaminated Water: Catalytic Performance of Pegmatite in a Sodium Percarbonate/Ultrasound System. Catalysts, 15(4), 363. https://doi.org/10.3390/catal15040363











