The Property–Efficiency Relationship over Rh/GaxNby Catalysts in Photothermal Dry Reforming of CH4
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
2.2. Catalytic Efficiency Evaluation in DRM
2.3. Property–Efficiency Relationship of Rh/GaxNby in DRM
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterizations
3.4. Photoelectrochemical (PEC) Measurement
3.5. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar]
- Kolle, J.M.; Fayaz, M.; Sayari, A. Understanding the effect of water on CO2 adsorption. Chem. Rev. 2021, 121, 7280–7345. [Google Scholar] [PubMed]
- Liu, H.; Song, H.; Zhou, W.; Meng, X.; Ye, J. A promising application of optical hexagonal Tan in photocatalytic reactions. Angew. Chem. Int. Edit. 2018, 57, 16781–16784. [Google Scholar]
- Liu, H.; Meng, X.; Dao, T.D.; Liu, L.; Li, P.; Zhao, G.; Nagao, T.; Yang, L.; Ye, J. Light assisted CO2 reduction with methane over SiO2 encapsulated Ni nanocatalysts for boosted activity and stability. J. Mater. Chem. A 2017, 5, 10567–10573. [Google Scholar]
- Wang, C.; Qiu, Y.; Zhang, X.; Zhang, Y.; Sun, N.; Zhao, Y. Geometric design of a Ni@silica nano-capsule catalyst with superb methane dry reforming stability: Enhanced confinement effect over the nickel site anchoring inside a capsule shell with an appropriate inner cavity. Catal. Sci. Technol. 2018, 8, 4877–4890. [Google Scholar]
- Bu, K.; Deng, J.; Zhang, X.; Kuboon, S.; Yan, T.; Li, H.; Shi, L.; Zhang, D. Promotional effects of B-terminated defective edges of Ni/boron nitride catalysts for coking- and sintering-resistant dry reforming of methane. Appl. Catal. B-Environ. 2020, 267, 118692. [Google Scholar]
- Yao, J.-L.; Zheng, H.-Y.; Bai, P.-W.; Liang, W.-P.; Zhang, Z.-Y.; Li, T.; Xie, T. Design and optimization of solar-dish volumetric reactor for methane dry reforming process with three-dimensional optics-CFD method. Energ. Convers. Manag. 2023, 277, 116663. [Google Scholar]
- Kim, W.Y.; Lee, Y.H.; Park, H.; Choi, Y.H.; Lee, M.H.; Lee, J.S. Coke tolerance of Ni/Al2O3 nanosheet catalyst for dry reforming of methane. Catal. Sci. Technol. 2016, 6, 2060–2064. [Google Scholar]
- Cho, Y.; Shoji, S.; Yamaguchi, A.; Hoshina, T.; Fujita, T.; Abe, H.; Miyauchi, M. Visible-light-driven dry reforming of methane using a semiconductor-supported catalyst. Chem. Commun. 2020, 56, 4611–4614. [Google Scholar]
- Zhang, N.; Han, C.; Fu, X.; Xu, Y.-J. Function-Oriented engineering of Metal-Based nanohybrids for photoredox catalysis: Exerting plasmonic effect and beyond. Chem 2018, 4, 1832–1861. [Google Scholar]
- Liu, H.; Meng, X.; Dao, T.D.; Zhang, H.; Li, P.; Chang, K.; Wang, T.; Li, M.; Nagao, T.; Ye, J. Conversion of carbon dioxide by methane reforming under Visible-Light irradiation: Surface-Plasmon-Mediated nonpolar molecule activation. Angew. Chem. Int. Edit. 2015, 54, 11545–11549. [Google Scholar]
- Song, H.; Meng, X.; Dao, T.D.; Zhou, W.; Liu, H.; Shi, L.; Zhang, H.; Nagao, T.; Kako, T.; Ye, J. Light-Enhanced carbon dioxide activation and conversion by effective plasmonic coupling effect of Pt and Au nanoparticles. ACS Appl. Mater. Inter. 2018, 10, 408–416. [Google Scholar]
- Liu, H.; Li, M.; Dao, T.D.; Liu, Y.; Zhou, W.; Liu, L.; Meng, X.; Nagao, T.; Ye, J. Design of PdAu alloy plasmonic nanoparticles for improved catalytic performance in CO2 reduction with visible light irradiation. Nano Energy 2016, 26, 398–404. [Google Scholar]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-) promoted Ni catalysts. Catal. Sci. Technol. 2006, 31, 555–561. [Google Scholar]
- Hassan, N.E.; Kaydouh, M.N.; Geagea, H.; Zein, H.E.; Jabbour, K.; Casale, S.; Zakhem, H.E.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A-Gen. 2016, 520, 114–121. [Google Scholar]
- Li, Y.; Li, D.; Liu, H.; Lei, Y.; Zhao, R.; He, D.; Zheng, Z.; Luo, H.; Liu, A. In situ fabricating a Rh/Ga2O3 photothermal catalyst for dry reforming of methane. Catal. Sci. Technol. 2024, 14, 2722. [Google Scholar]
- Liu, H.; Meng, X.; Yang, W.; Zhao, G.; He, D.; Ye, J. Photo-thermal CO2 reduction with methane on group VIII metals: In situ reduced WO3 support for enhanced catalytic activity. Chin. J. Catal. 2021, 42, 1976–1982. [Google Scholar]
- Li, Y.; Guo, J.; Liu, H.; Liu, A.; Li, D. In situ generated oxygen vacancy on Nb2O5 for boosted catalytic activities of M/Nb2O5 in photothermal CO2 reforming of CH4. J. CO2 Util. 2023, 67, 102330. [Google Scholar]
- Kim, Y.; Kang, S.; Kang, D.; Lee, K.R.; Song, C.K.; Sung, J.; Kim, J.S.; Lee, H.; Park, J.; Yi, J. Single-Phase formation of Rh2O3 nanoparticles on h-BN support for highly controlled methane partial oxidation to syngas. Angew. Chem. Inter. Edit. 2021, 60, 25411–25418. [Google Scholar]
- Bueno-López, A.; Such-Basáñez, I.; Lecea, C.S.-M.d. Stabilization of active Rh2O3 species for catalytic decomposition of N2O on La-, Pr-doped CeO2. J. Catal. 2006, 244, 102–112. [Google Scholar]
- Suzuki, H.; Takashima, T.; Tomita, O.; Kanazawa, T.; Nozawa, S.; Kato, K.; Yamakata, A.; Nakashima, K.; Saeki, A.; Abe, R. Improved photocatalytic O2 evolution on a Sillén–Aurivillius perovskite oxychloride Bi6NBWO14Cl by Rh2O3 additives and surface modifications. J. Phy Chem. C 2023, 127, 7965–7973. [Google Scholar]
- Nakayama, H.; Nagata, M.; Tomie, T.; Ishitsuka, T.; Matsubayashi, N.; Shimizu, Y. Strong Metal–Support interaction mechanisms of Rh supports in the CO–NO reaction: Rh/Rh2O3 interconversion in promoting NO dissociation and CO2 generation. J. Phy Chem. C 2022, 126, 17904–17912. [Google Scholar]
- Dou, L.; Liu, Y.; Gao, Y.; Li, J.; Hu, X.; Zhang, S.; Ostrikov, K.K.; Shao, T. Disentangling metallic cobalt sites and oxygen vacancy effects in synergistic plasma-catalytic CO2/CH4 conversion into oxygenates. Appl. Catal. B-Environ. 2022, 318, 121830. [Google Scholar]
- Zhang, Q.; Yang, P.; Zhang, H.; Zhao, J.; Shi, H.; Huang, Y.; Yang, H. Oxygen vacancies in Co3O4 promote CO2 photoreduction. Appl. Catal. B-Environ. 2022, 300, 120729. [Google Scholar]
- Xu, Q.; Zhang, S. Fabrication and photoluminescence of Β-Ga2O3 nanorods. Superlattice Microst. 2008, 44, 715–720. [Google Scholar]
- Liu, H.; Wang, Z.; Li, H.; Zhang, X.; Qin, X.; Dai, Y.; Wang, P.; Liu, Y.; Huang, B. Photocatalytic degradation of ethylene by Ga2O3 polymorphs. RSC Adv. 2018, 8, 14328–14334. [Google Scholar]
- Kulkarni, A.K.; Praveen, C.S.; Sethi, Y.A.; Panmand, R.P.; Arbuj, S.S.; Naik, S.D.; Ghule, A.V.; Kale, B.B. Nanostructured N-doped orthorhombic Nb2O5 as an efficient stable photocatalyst for hydrogen generation under visible light. Dalton T. 2017, 46, 14859–14868. [Google Scholar]
- Hao, W.; Wang, Z.; Xie, S.; Wei, L.; Hou, Z.; Liu, Y.; Dai, H.; Deng, J. Catalytic performance of Nb2O5 modified Ru/CeO2 for dichloromethane oxidation. Prog. Nat. Sci. 2023, 33, 486–494. [Google Scholar]
- Hou, H.; Yang, W.; Sun, H.; Zhang, H.; Feng, X.; Kuang, Y. Tailored synthesis of Ga2O3 nanofibers towards enhanced photocatalytic hydrogen evolution. Catal. Lett. 2023, 153, 2950–2958. [Google Scholar]
- Boukha, Z.; Gil-Calvo, M.; Rivas, B.d.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Behaviour of Rh supported on hydroxyapatite catalysts in partial oxidation and steam reforming of methane: On the role of the speciation of the Rh particles. Appl. Catal. A-Gen. 2018, 556, 191–203. [Google Scholar]
- Han, F.; Liu, H.; Cheng, W.; Xu, Q. Highly selective conversion of CO2 to methanol on the CuZnO–ZrO2 solid solution with the assistance of plasma. RSC Adv. 2020, 10, 33620–33627. [Google Scholar] [PubMed]
- Qi, S.-C.; Wu, J.-K.; Lu, J.; Yu, G.-X.; Zhu, R.-R.; Liu, Y.; Liu, X.-Q.; Sun, L.-B. Underlying mchanism of CO2 adsorption onto conjugated Azacyclo-copolymers: N-doped adsorbents capture CO2 chiefly through Acid–Base interaction? J. Mater. Chem. A 2019, 7, 17842–17853. [Google Scholar]
- Shang, B.; Zhao, F.; Choi, C.; Jia, X.; Pauly, M.; Wu, Y.; Wang, H. Monolayer molecular functionalization enabled by Acid–Base interaction for High-Performance photochemical CO2 reduction. ACS Energy Lett. 2022, 7, 2265–2272. [Google Scholar]
- Guo, C.; Tang, Y.; Yang, Z.; Zhao, T.; Liu, J.; Zhao, Y.; Wang, F. Reinforcing the efficiency of photothermal catalytic CO2 methanation through integration of Ru nanoparticles with photothermal MnCo2O4 nanosheets. ACS Nano 2023, 17, 23761–23771. [Google Scholar]
- Yang, Z.; Zhao, T.; Hao, S.; Wang, R.; Zhu, C.; Tang, Y.; Guo, C.; Liu, J.; Wen, X.; Wang, F. Large-Scale synthesis of multifunctional Single-phase Co2C nanomaterials. Adv. Sci. 2023, 10, 2301073. [Google Scholar]
- Tang, Y.; Zhao, T.; Han, H.; Yang, Z.; Liu, J.; Wen, X.; Wang, F. Ir-Coo active centers supported on porous Al2O3 nanosheets as efficient and durable Photo-Thermal catalysts for CO2 conversion. Adv. Sci. 2023, 10, 2300122. [Google Scholar]
- Tang, Y.; Wang, H.; Guo, C.; Yang, Z.; Zhao, T.; Liu, J.; Wang, F. Ruthenium–Cobalt Solid-Solution Alloy nanoparticles for enhanced photopromoted thermocatalytic CO2 hydrogenation to methane. ACS Nano 2024, 18, 11449–11461. [Google Scholar]
- Yang, Z.; Zhao, T.; Tang, Y.; Jiang, Y.; Kitagawa, H.; Wen, X.; Wang, F. Size-Modulated Photo-Thermal catalytic CO2 hydrogenation prformances over Pd nanoparticles. J. Catal. 2023, 424, 22–28. [Google Scholar]
- Lu, Y.; Kang, L.; Guo, D.; Zhao, Y.; Zhao, Y.; Wang, S.; Ma, X. Double-Site doping of a V promoter on Nix-V-Mgal catalysts for the drm reaction: Simultaneous effect on CH4 and CO2 activation. ACS Catal. 2021, 11, 8749–8765. [Google Scholar]
- Li, T.; Liang, Z.; Liu, J.; Zhang, Y.; Zhang, X.; Zhang, G. The role of cerium in cola bimetallic catalysts: Enhancing activation of CH4 and CO2 for improved drm reaction. Int. J. Hydrogen Energy 2024, 61, 611–622. [Google Scholar]
- Shao, L.; Liu, M.; Sang, Y.; Zhan, P.; Chen, J.; Huang, J. Nitrogen-Doped ultrahigh microporous carbons derived from two Nitrogen-containing Post-Cross-Linked polymers for efficient CO2 capture. J. Chem. Eng. Data. 2020, 65, 2238–2250. [Google Scholar]
- Jin, S.; Guan, X.; Zhang, X.; Zhang, C.; Liu, J.; Wang, Y.; Wang, Y.; Li, R.; Li, Z.; Fan, C. CeO2 nanorods with bifunctional oxygen vacancies for promoting Low-Pressure photothermocatalytic CO2 conversion with CH3OH to dimethyl carbonate. J. Environ. Chem. Eng. 2023, 11, 111374. [Google Scholar]
- Ji, G.; Wu, S.; Song, X.; Meng, L.; Jia, Y.; Tian, J. Recent progress in Photo-Thermal synergistic catalysis for methane dry reforming. Int. J. Hydrogen Energy 2024, 57, 696–708. [Google Scholar]
- Yabe, T.; Yamada, K.; Murakami, K.; Toko, K.; Ito, K.; Higo, T.; Ogo, S.; Sekine, Y. Role of electric field and surface protonics on Low-Temperature catalytic dry reforming of methane. ACS Sustain. Chem. Eng. 2019, 7, 5690–5697. [Google Scholar]
- Song, Z.; Wang, Q.; Guo, C.; Li, S.; Yan, W.; Jiao, W.; Qiu, L.; Yan, X.; Li, R. Improved effect of Fe on the stable NiFe/Al2O3 catalyst in Low-Temperature dry reforming of methane. Ind. Eng. Chem. Res. 2020, 59, 17250–17258. [Google Scholar]
- Lorber, K.; Zavašnik, J.; Arčon, I.; Huš, M.; Teržan, J.; Likozar, B.; Djinović, P. CO2 activation over nanoshaped CeO2 decorated with nickel for Low-Temperature methane dry reforming. ACS Appl. Mater. Inter. 2022, 14, 31862–31878. [Google Scholar]
- Ramon, A.P.; Li, X.; Clark, A.H.; Safonova, O.V.; Marcos, F.C.; Assaf, E.M.; Bokhoven, J.A.v.; Artiglia, L.; Assaf, J.M. In Situ study of Low-Temperature dry reforming of methane over La2Ce2O7 and LaNiO3 mixed oxides. Appl. Catal. B-Environ. 2022, 315, 121528. [Google Scholar]
- Ballesteros-Plata, D.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Cauqui, M.A.; Yeste, M.P. Improving noble metal catalytic activity in the dry reforming of methane by adding niobium. Fuel 2022, 308, 121996. [Google Scholar]
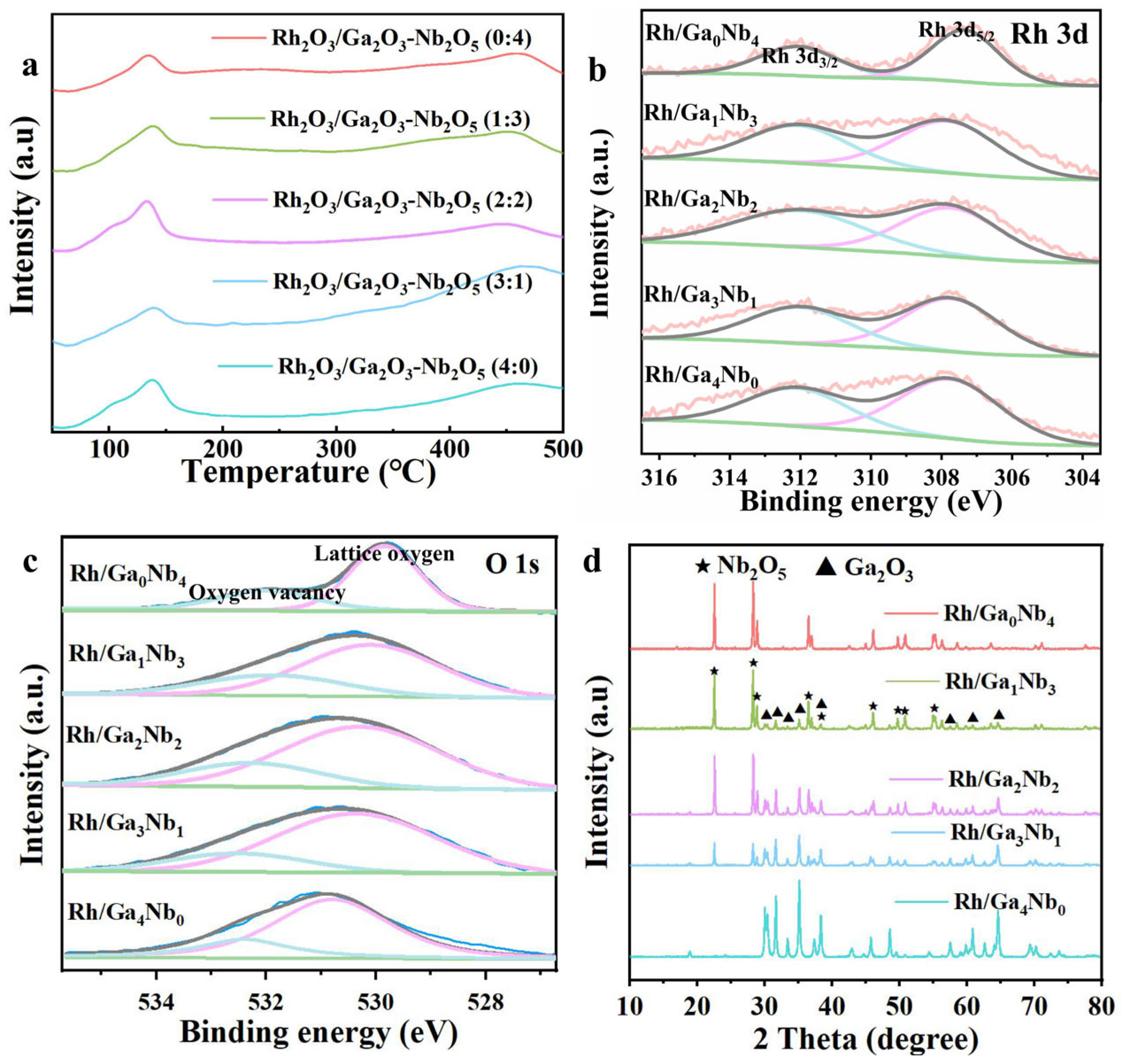
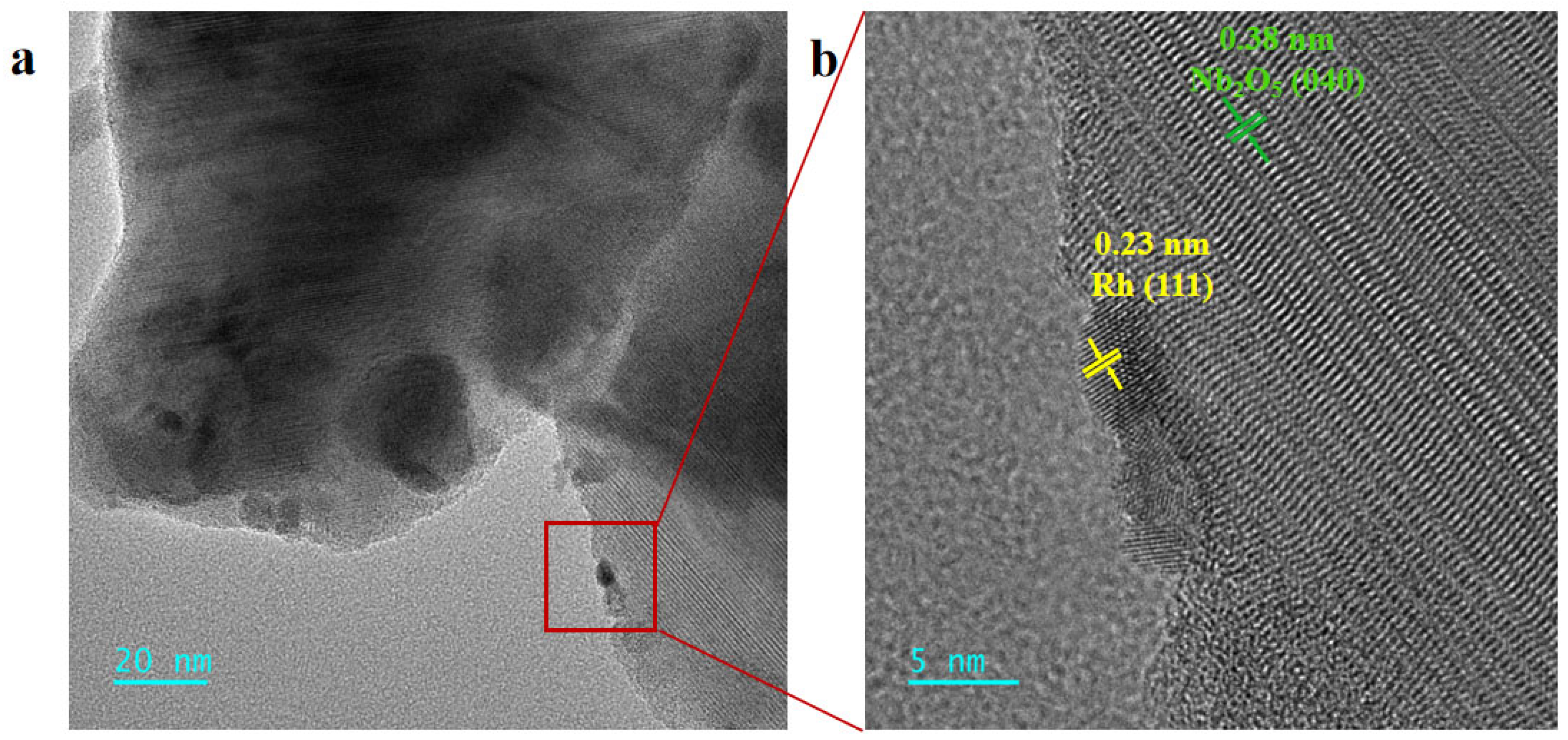

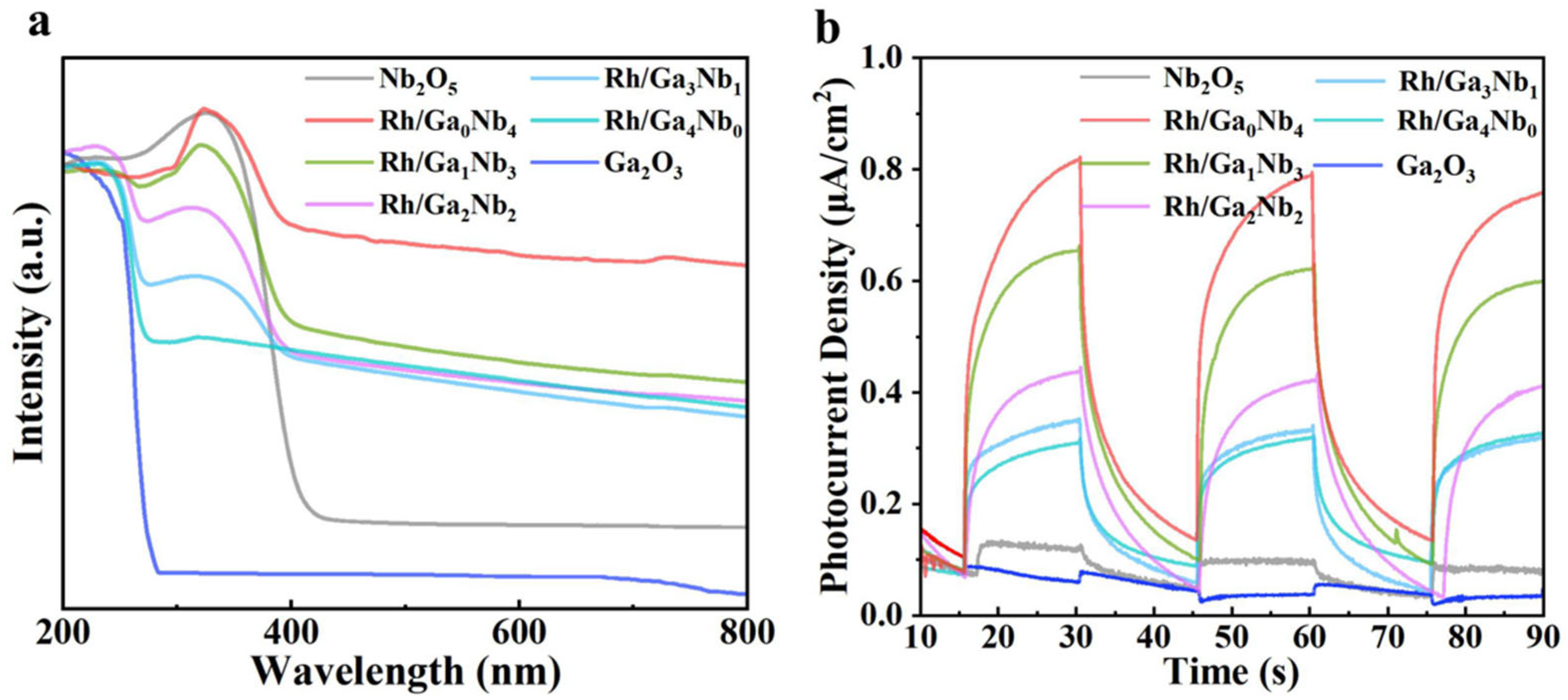
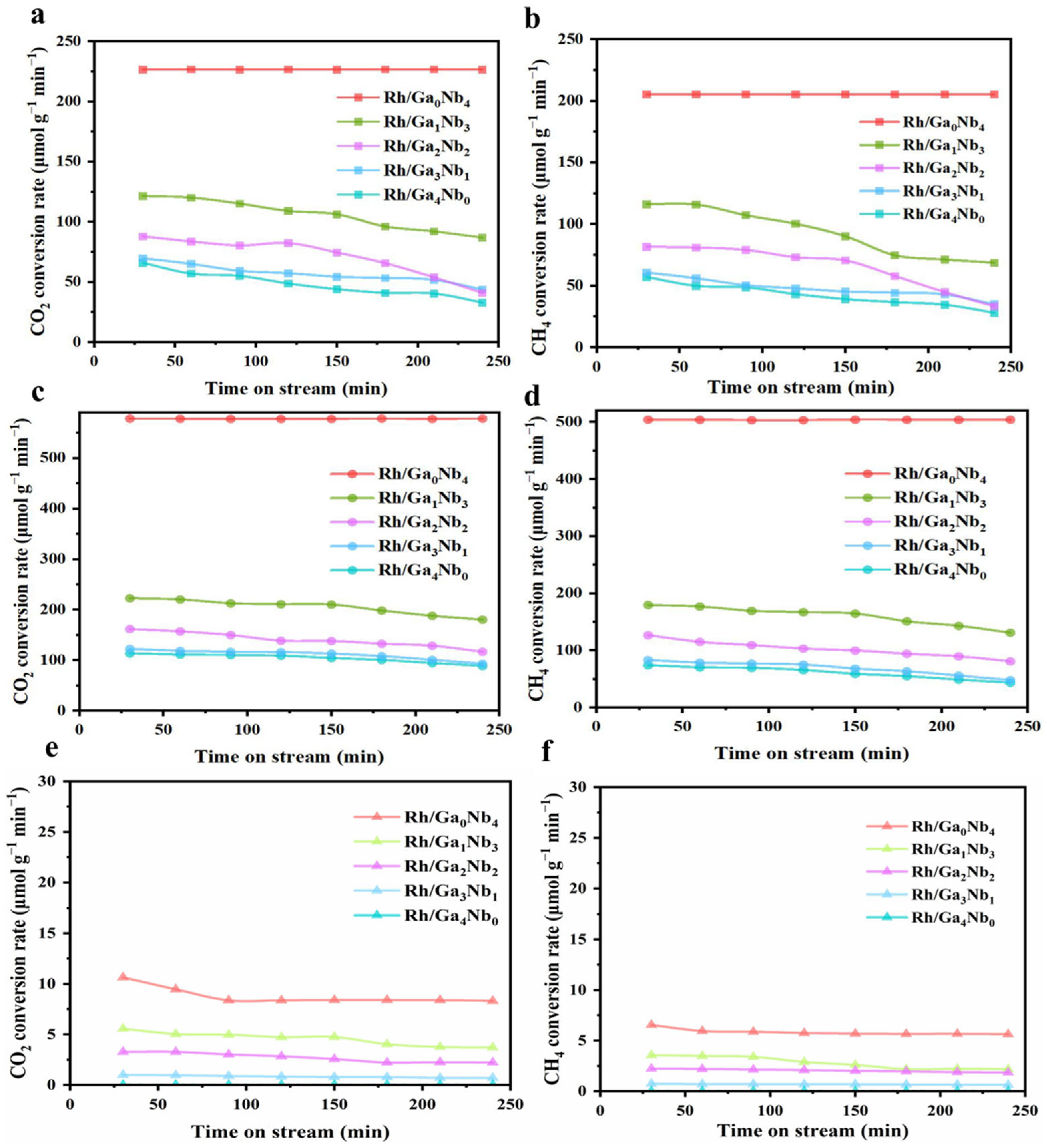
| Spent Catalyst | Lattice Oxygen (%) | Oxygen Vacancy (%) |
|---|---|---|
| Rh/Ga0Nb4 | 70.6 | 29.4 |
| Rh/Ga1Nb3 | 73.8 | 26.2 |
| Rh/Ga2Nb2 | 75.6 | 24.4 |
| Rh/Ga3Nb1 | 79.2 | 20.8 |
| Rh/Ga4Nb0 | 79.5 | 20.5 |
| Catalyst | Specific Surface Area (m2·g−1) | Average Pore Size (nm) |
|---|---|---|
| Nb2O5 | 5.3 | 21.6 |
| Rh/Ga0Nb4 | 6.3 | 23.6 |
| Rh/Ga1Nb3 | 3.6 | 13.3 |
| Rh/Ga2Nb2 | 4.7 | 21.4 |
| Rh/Ga3Nb1 | 3.3 | 28.0 |
| Rh/Ga4Nb0 | 3.3 | 14.8 |
| Ga2O3 | 3.9 | 19.1 |
| Catalyst | Amounts of CO2 Desorption (μmol·g−1) | |||
|---|---|---|---|---|
| α | β | γ | β + γ | |
| Rh/Ga0Nb4 | 3.42 | 25.9 | 27.7 | 53.6 |
| Rh/Ga1Nb3 | 4.07 | 14.8 | 19.4 | 34.2 |
| Rh/Ga2Nb2 | 3.0 | 12.4 | 16.0 | 28.4 |
| Rh/Ga3Nb1 | 4.3 | 9.9 | 5.3 | 15.2 |
| Rh/Ga4Nb0 | 2.4 | 7.9 | 3.5 | 11.4 |
| Sample | CO2 Conversion Rate (μmol·g−1·min−1) | Photocurrent Intensity (μA·cm−1) |
|---|---|---|
| Rh/Ga0Nb4 | 578.01 | 0.84 |
| Rh/Ga1Nb3 | 222.26 | 0.67 |
| Rh/Ga2Nb2 | 161.62 | 0.45 |
| Rh/Ga3Nb1 | 122.15 | 0.35 |
| Rh/Ga4Nb0 | 113.54 | 0.31 |
| Catalysts | Set the Temperature (°C) | Actual Reaction Process Temperature (°C) | |
|---|---|---|---|
| Photothermal | Thermal | ||
| Rh/Ga0Nb4 | 500 | 483.5 | 490.2 |
| Rh/Ga1Nb3 | 500 | 483.3 | 491.3 |
| Rh/Ga2Nb2 | 500 | 484.6 | 490.8 |
| Rh/Ga3Nb1 | 500 | 484.2 | 491.7 |
| Rh/Ga4Nb0 | 500 | 483.9 | 490.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, S.; Li, D.; Liu, H.; Lei, Y. The Property–Efficiency Relationship over Rh/GaxNby Catalysts in Photothermal Dry Reforming of CH4. Catalysts 2025, 15, 312. https://doi.org/10.3390/catal15040312
Li Y, Sun S, Li D, Liu H, Lei Y. The Property–Efficiency Relationship over Rh/GaxNby Catalysts in Photothermal Dry Reforming of CH4. Catalysts. 2025; 15(4):312. https://doi.org/10.3390/catal15040312
Chicago/Turabian StyleLi, Yuqiao, Shaoyuan Sun, Dezheng Li, Huimin Liu, and Yiming Lei. 2025. "The Property–Efficiency Relationship over Rh/GaxNby Catalysts in Photothermal Dry Reforming of CH4" Catalysts 15, no. 4: 312. https://doi.org/10.3390/catal15040312
APA StyleLi, Y., Sun, S., Li, D., Liu, H., & Lei, Y. (2025). The Property–Efficiency Relationship over Rh/GaxNby Catalysts in Photothermal Dry Reforming of CH4. Catalysts, 15(4), 312. https://doi.org/10.3390/catal15040312







