Development of Electrochemical Water Splitting with Highly Active Nanostructured NiFe Layered Double Hydroxide Catalysts: A Comprehensive Review
Abstract
1. Introduction
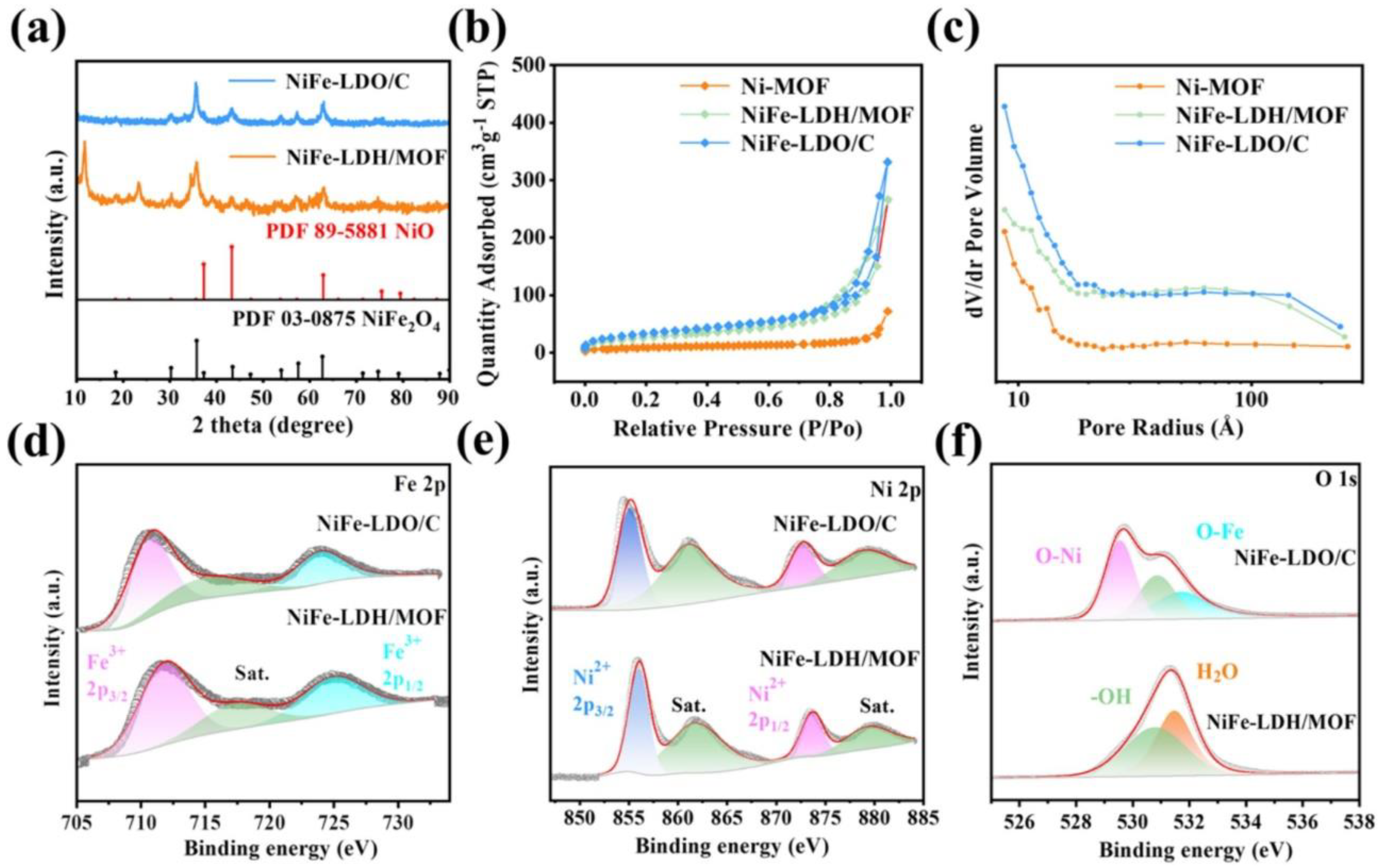
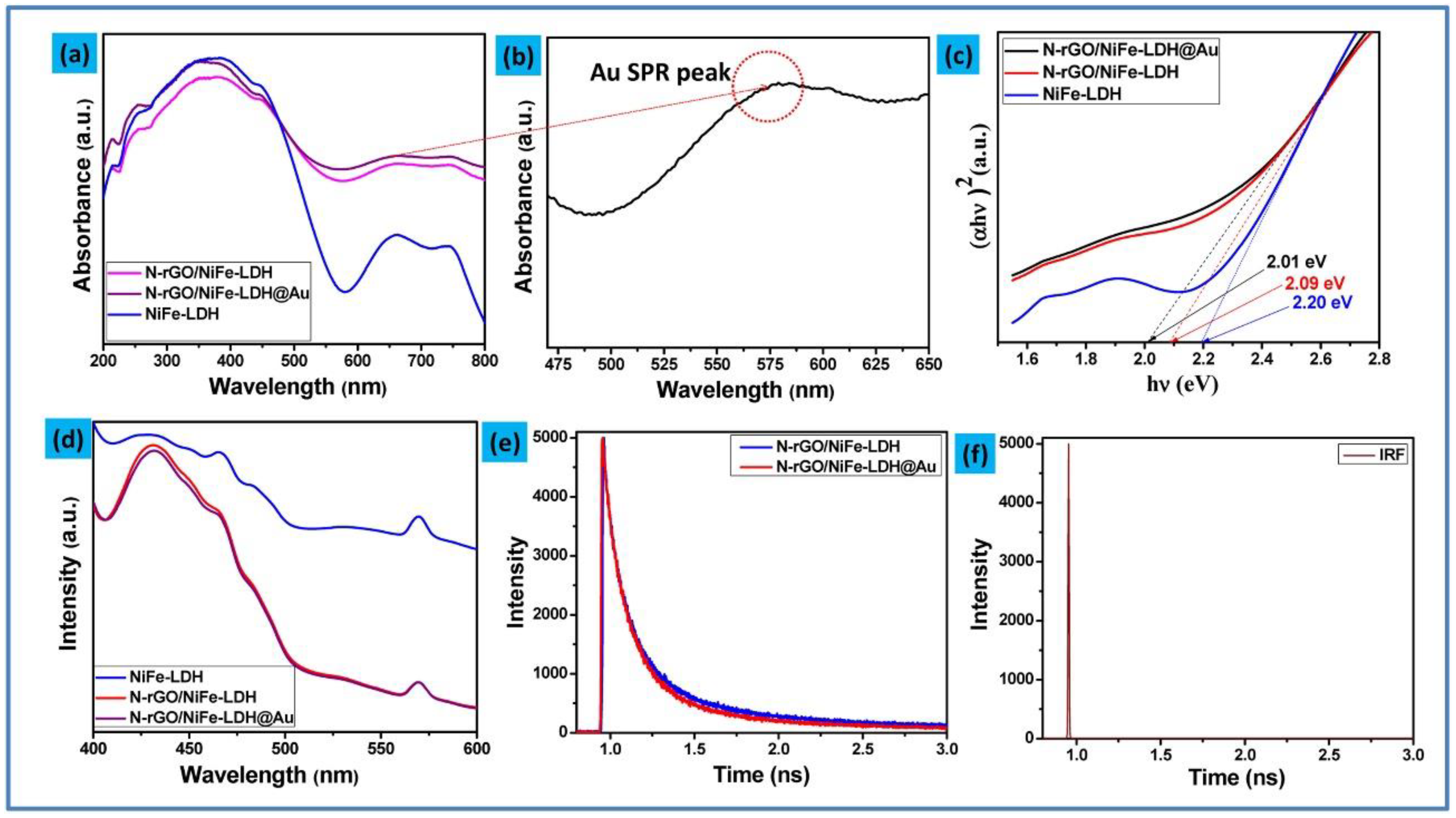
2. Morphology, Structural, Optical, and Elemental Studies
3. Catalytic Water Splitting
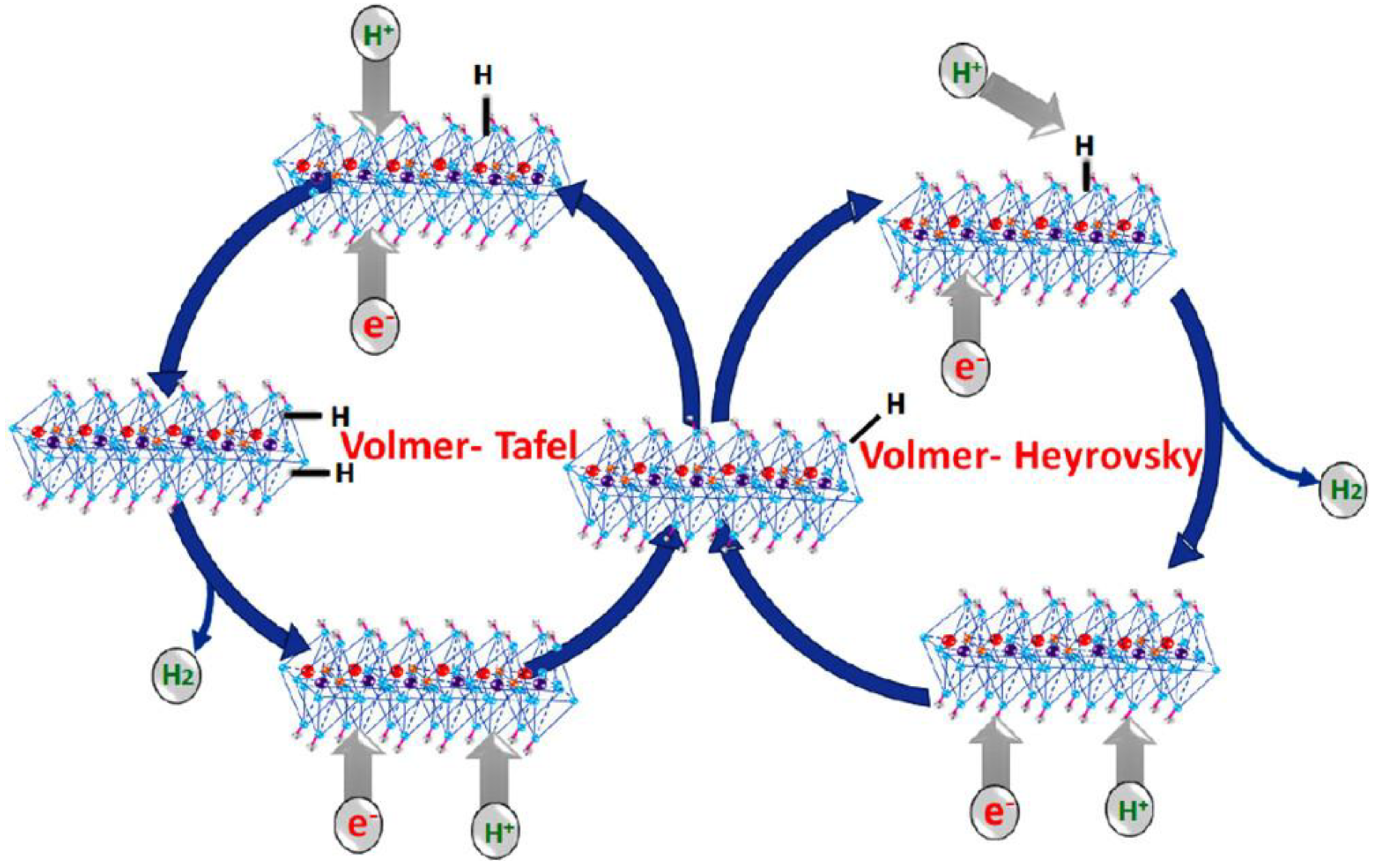
3.1. Hydrogen Evolution Reaction
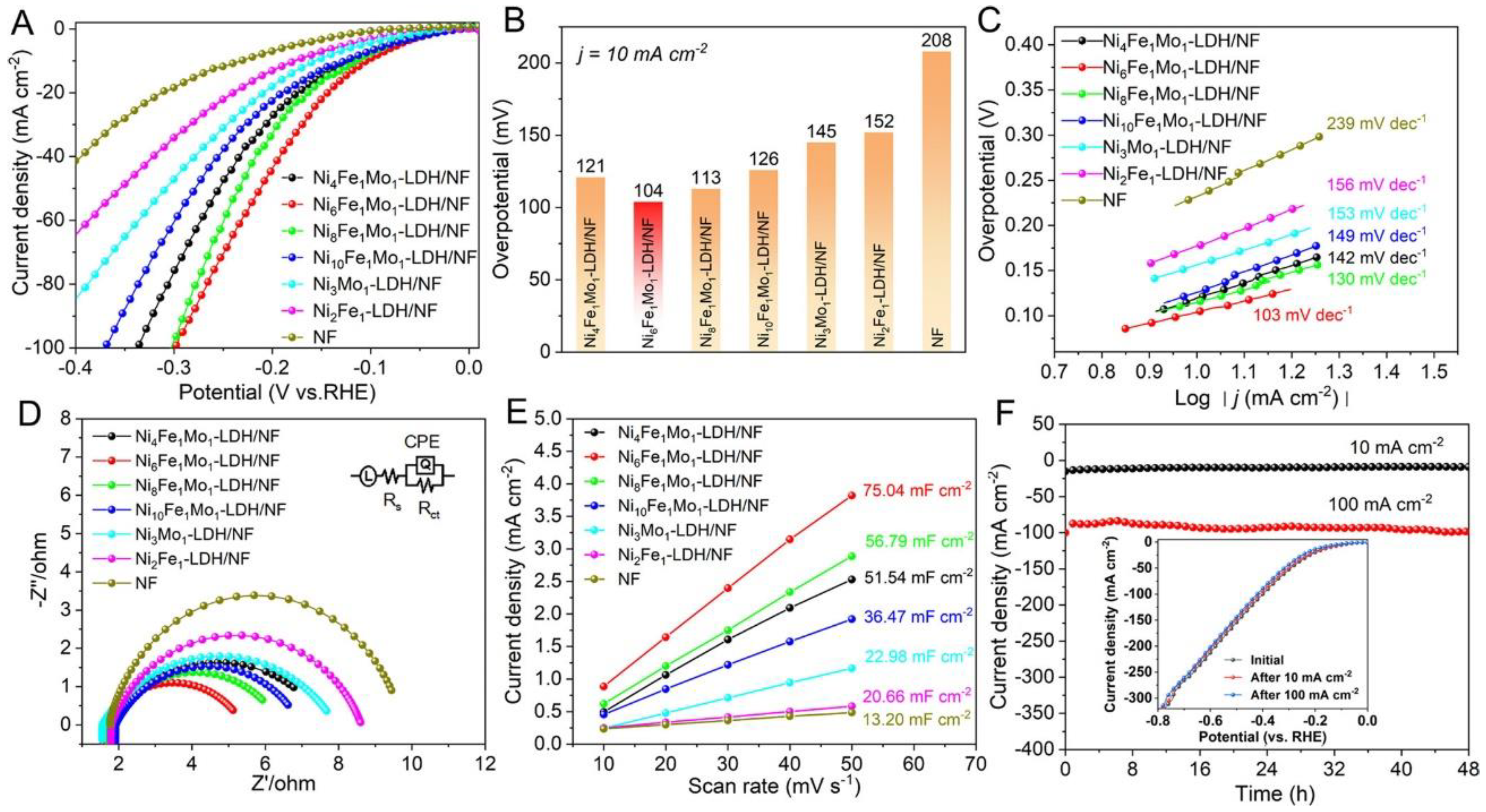
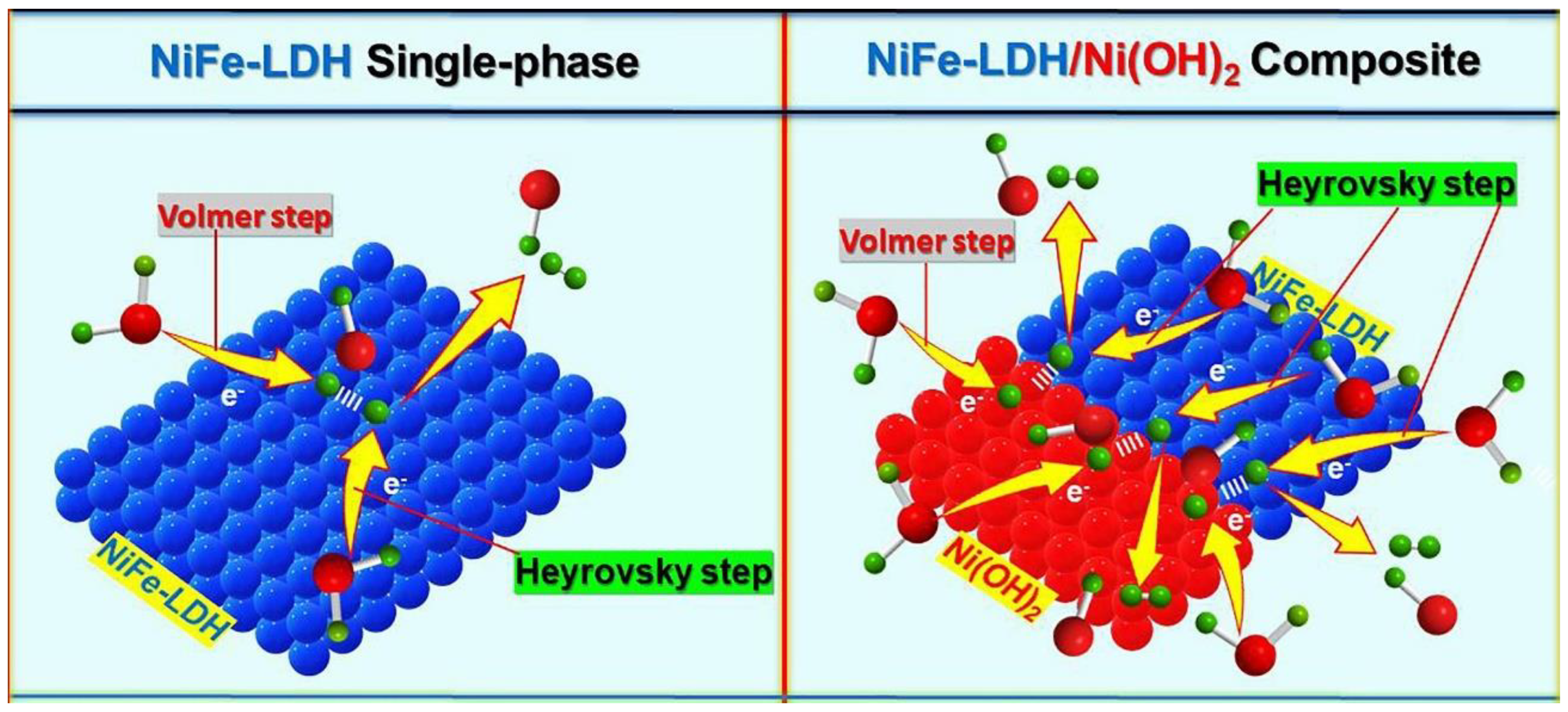
3.2. Oxygen Evolution Reaction
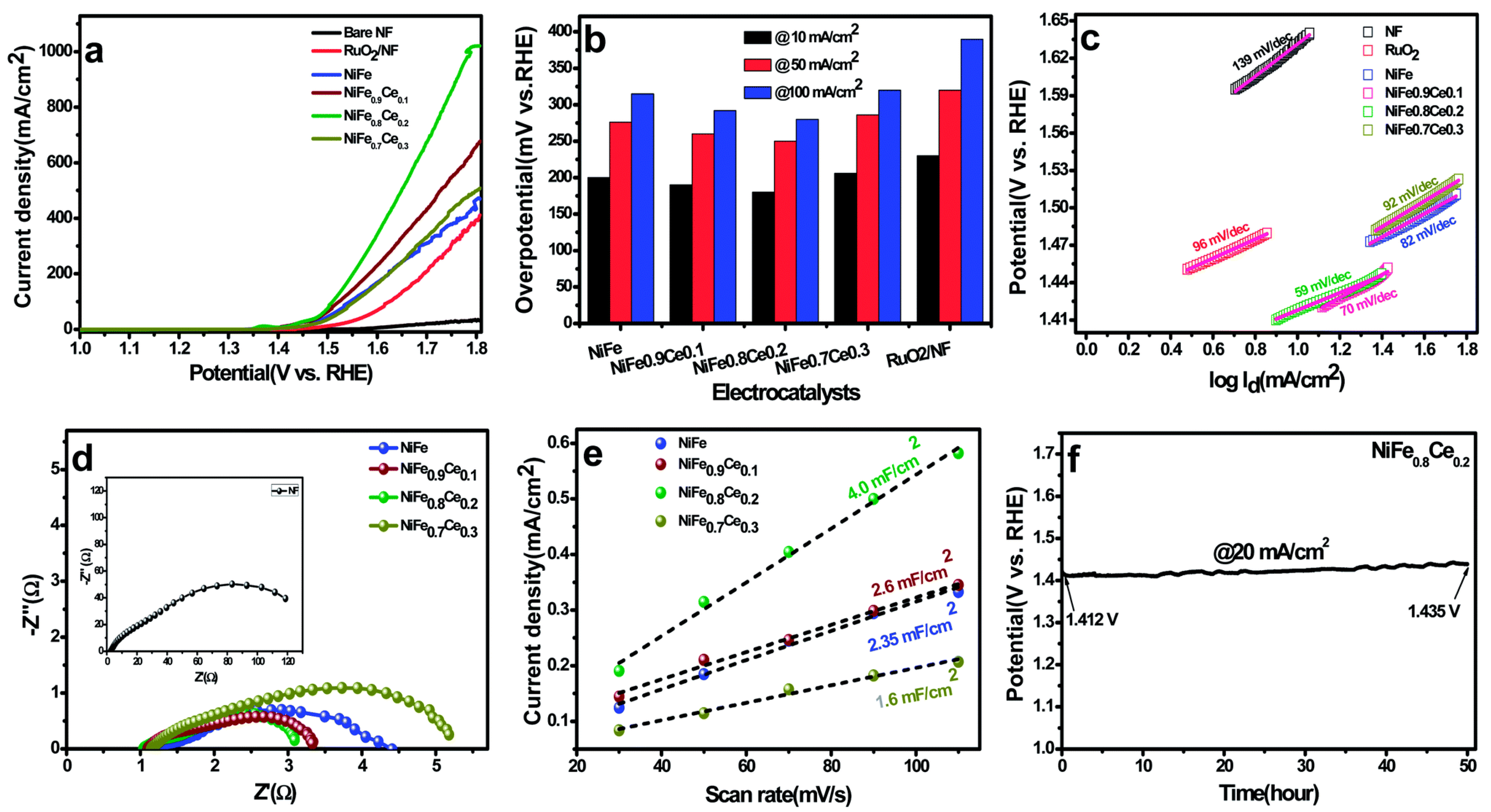
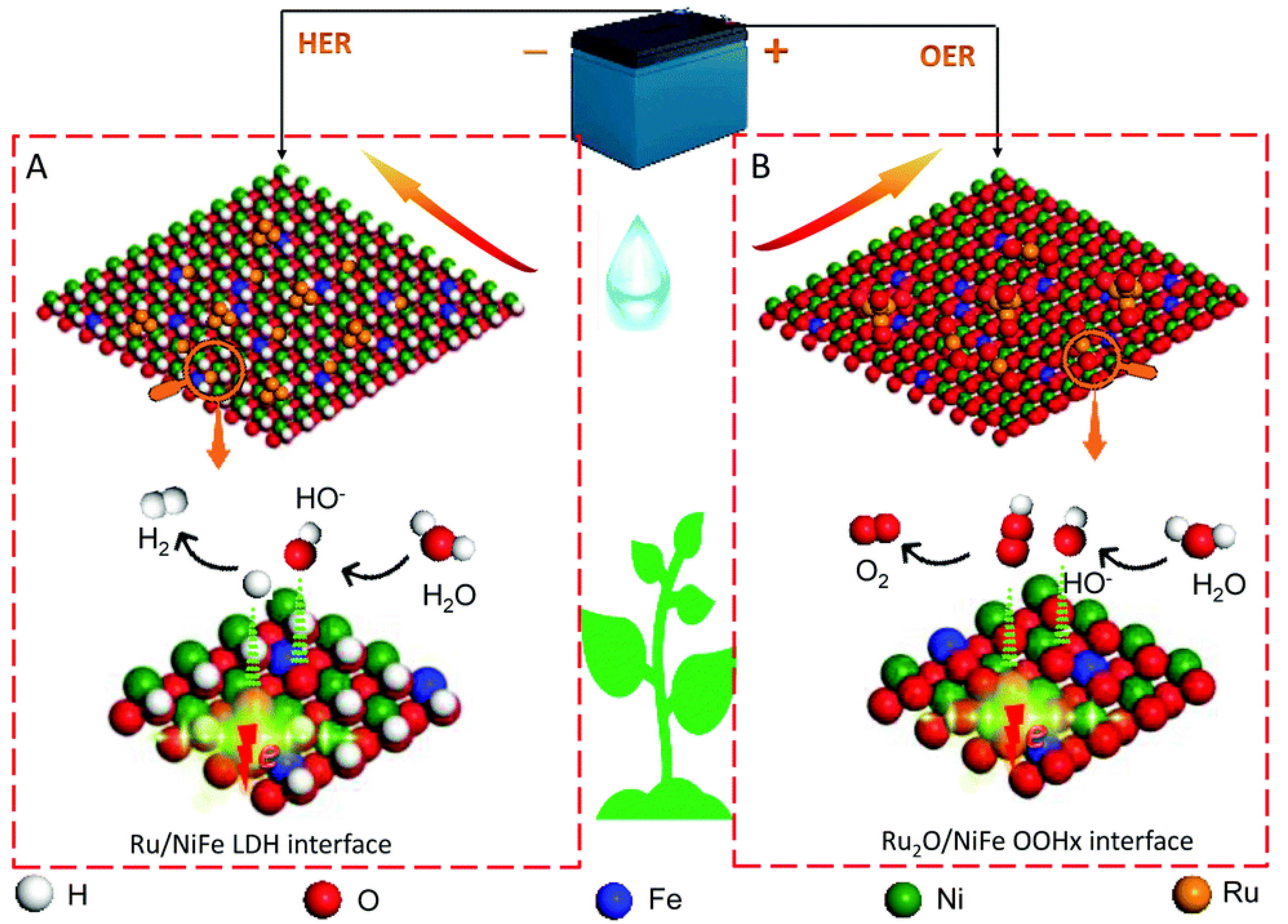
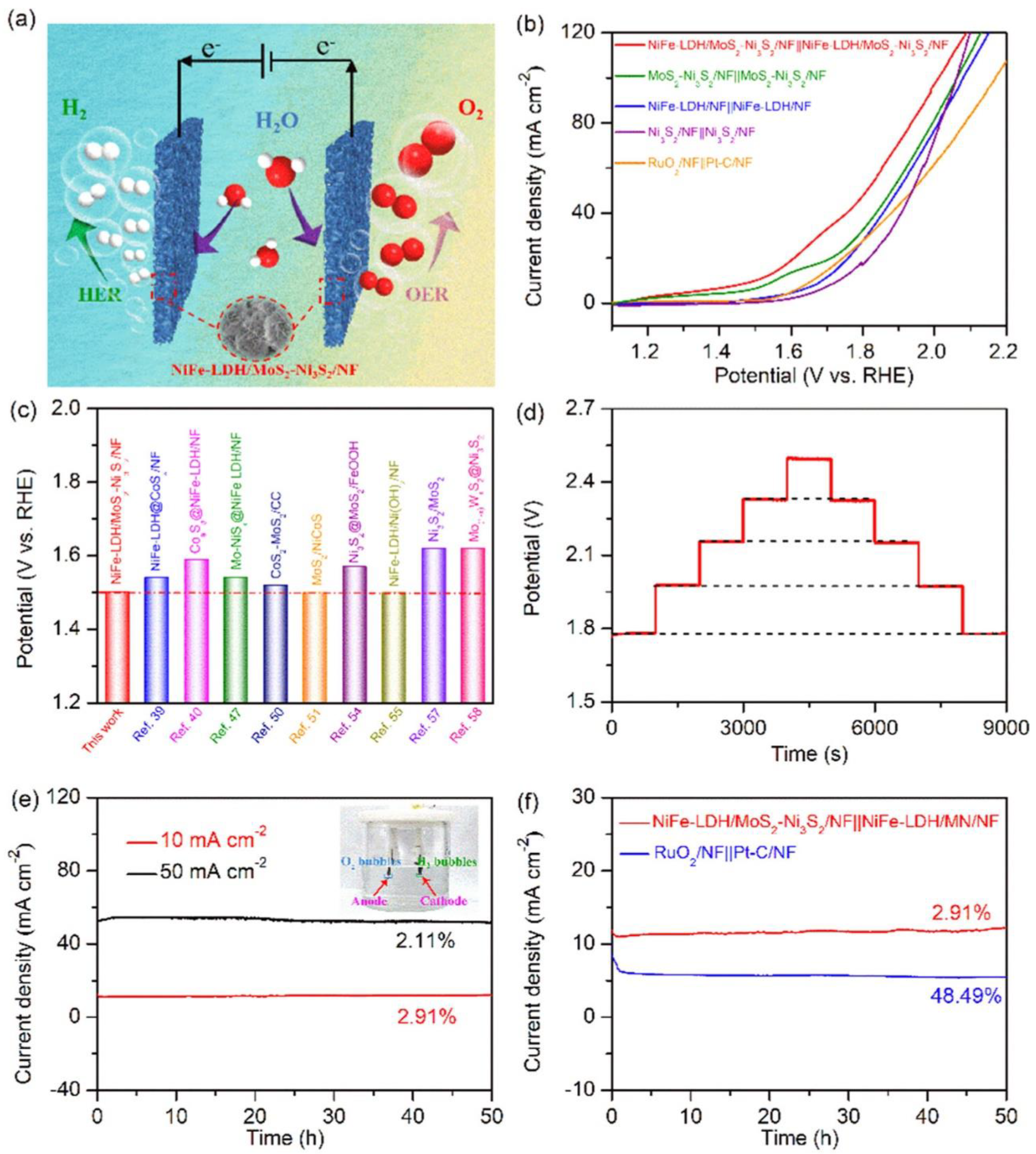
3.3. Overall Water Splitting
3.4. Key Parameters for the Evaluation of Catalytic Activities
3.4.1. Reversible Hydrogen Electrode (RHE)
3.4.2. Overpotential (η)
3.4.3. Tafel Slopes (b)
3.4.4. Specific Capacitance (CS)
3.4.5. Double-Layer Capacitance (DLC)
3.4.6. Electrochemical Active Surface Area (ECSA)
3.4.7. Roughness Factor (RF)
3.4.8. Mass Activity (MA)
3.4.9. Active Site (AS)
3.4.10. Turnover Frequency (TOF)
3.4.11. Faradaic Efficiency (FE)
3.4.12. Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen Energy Systems: Technologies, Trends, and Future Prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, D.; Sun, K.; Yang, S.; Peng, F.; Zhang, K.; Guo, G.; Si, Y. Towards a Future Hydrogen Supply Chain: A Review of Technologies and Challenges. Sustainability 2024, 16, 1890. [Google Scholar] [CrossRef]
- Habib, M.A.; Abdulrahman, G.A.Q.; Alquaity, A.B.S.; Qasem, N.A.A. Hydrogen Combustion, Production, and Applications: A Review. Alex. Eng. J. 2024, 100, 182–207. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology Control and Electronic Tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Evro, S.; Oni, B.A.; Tomomewo, O.S. Carbon Neutrality and Hydrogen Energy Systems. Int. J. Hydrogen Energy 2024, 78, 1449–1467. [Google Scholar] [CrossRef]
- Khalid, N.; Rehman, A.; Pervaiz, E. Electrochemical Pathway to Enhanced Water Splitting Efficiency through Cobalt Phosphide-MOFs Hybrid. Int. J. Hydrogen Energy 2024, 73, 231–256. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Juarez-García, M.; Sánchez-Ramírez, E. Green Hydrogen Production for Sustainable Development: A Critical Examination of Barriers and Strategic Opportunities. RSC Sustain. 2024, 3, 134–157. [Google Scholar] [CrossRef]
- Ozcan, H.; El-Emam, R.S.; Celik, S.; Amini Horri, B. Recent Advances, Challenges, and Prospects of Electrochemical Water-Splitting Technologies for Net-Zero Transition. Clean. Chem. Eng. 2023, 8, 100115. [Google Scholar] [CrossRef]
- Vazhayil, A.; Vazhayal, L.; Thomas, J.; Ashok, C.S.; Thomas, N. A Comprehensive Review on the Recent Developments in Transition Metal-Based Electrocatalysts for Oxygen Evolution Reaction. Appl. Surf. Sci. Adv. 2021, 6, 100184. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. Recent Advances in Noble Metal (Pt, Ru, and Ir)-Based Electrocatalysts for Efficient Hydrogen Evolution Reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Gao, G.; Sun, Z.; Chen, X.; Zhu, G.; Sun, B.; Yamauchi, Y.; Liu, S. Recent Advances in Ru/Ir-Based Electrocatalysts for Acidic Oxygen Evolution Reaction. Appl. Catal. B 2024, 343, 123584. [Google Scholar] [CrossRef]
- Verma, J.; Goel, S. Cost-Effective Electrocatalysts for Hydrogen Evolution Reactions (HER): Challenges and Prospects. Int. J. Hydrogen Energy 2022, 47, 38964–38982. [Google Scholar] [CrossRef]
- Fan, K.; Xu, P.; Li, Z.; Shao, M.; Duan, X. Layered Double Hydroxides: Next Promising Materials for Energy Storage and Conversion. Next Mater. 2023, 1, 100040. [Google Scholar] [CrossRef]
- Sreenivasulu, M.; Hiremath, N.K.V.; Alshehri, M.A.; Shetti, N.P. A Green Solvent-Free Approach Synthesis for Rational Designing of a NiFe Layered Double Hydroxide [NiFe LDH] Electrocatalyst for Hydrogen Generation. Energy Fuels 2024, 38, 20791–20806. [Google Scholar] [CrossRef]
- Nejati, K.; Davari, S.; Akbari, A.; Asadpour-Zeynali, K.; Rezvani, Z. A Highly Active Oxygen Evolution Electrocatalyst: Ni-Fe-Layered Double Hydroxide Intercalated with the Molybdate and Vanadate Anions. Int. J. Hydrogen Energy 2019, 44, 14842–14852. [Google Scholar] [CrossRef]
- Thenuwara, A.C.; Attanayake, N.H.; Yu, J.; Perdew, J.P.; Elzinga, E.J.; Yan, Q.; Strongin, D.R. Cobalt Intercalated Layered NiFe Double Hydroxides for the Oxygen Evolution Reaction. J. Phys. Chem. B 2018, 122, 847–854. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Ma, J.; He, H. Effect of Interlayer Anions on NiFe Layered Double Hydroxides for Catalytic Ozone Decomposition. Environ. Sci. Technol. 2024, 58, 8597–8606. [Google Scholar] [CrossRef]
- Tang, L.; Xie, X.; Li, C.; Xu, Y.; Zhu, W.; Wang, L. Regulation of Structure and Anion-Exchange Performance of Layered Double Hydroxide: Function of the Metal Cation Composition of a Brucite-like Layer. Materials 2022, 15, 7983. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.; Duan, X. Layered Double Hydroxide-Based Electrocatalysts for the Oxygen Evolution Reaction: Identification and Tailoring of Active Sites, and Superaerophobic Nanoarray Electrode Assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Azam, A.; Liu, Y.; Yang, J.; Wang, D.; Sorrell, C.C.; Zhao, C.; Li, S. Unlocking Efficiency: Minimizing Energy Loss in Electrocatalysts for Water Splitting. Adv. Mater. 2024, 36, 2404658. [Google Scholar] [CrossRef]
- Alves, D.; Moral, R.A.; Jayakumari, D.; Dempsey, E.; Breslin, C.B. Factorial Optimization of CoCuFe-LDH/Graphene Ternary Composites as Electrocatalysts for Water Splitting. ACS Appl. Mater. Interfaces 2024, 16, 50846–50858. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, W.; Lu, Y.; Li, W.; He, J.; Zhou, D.; Hu, W.; Zhong, X. Mo-Doping and Construction of the Heterostructure between NiFe LDH and NiSx Co-Trigger the Activity Enhancement for Overall Water Splitting. J. Colloid Interface Sci. 2024, 664, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, T.; Wang, X.; Deng, Q.; Yang, J.; Mao, Y.; Wang, G. Intercalation and Elimination of Carbonate Ions of NiCo Layered Double Hydroxide for Enhanced Oxygen Evolution Catalysis. Int. J. Hydrogen Energy 2020, 45, 12629–12640. [Google Scholar] [CrossRef]
- Zhai, X.J.; Lv, Q.X.; Xie, J.Y.; Zhang, Y.X.; Chai, Y.M.; Dong, B. Advances in the Design of Highly Stable NiFe LDH Electrocatalysts for Oxygen Evolution in Seawater. Chem. Eng. J. 2024, 496, 153187. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Zhou, Q.; Li, X.; Zhao, D.; Ding, B.; Wang, S. Ti3C2 Mediates the NiFe LDH Layered Electrocatalyst to Enhance the OER Performance for Water Splitting. Heliyon 2024, 10, e30966. [Google Scholar] [CrossRef]
- Liao, F.; Zhao, X.; Yang, G.; Cheng, Q.; Mao, L.; Chen, L. Recent Advances on Two-Dimensional NiFe LDHs and Their Composites for Electrochemical Energy Conversion and Storage. J. Alloys Compd. 2021, 872, 159649. [Google Scholar] [CrossRef]
- Nair, K.M.; Ajith, S.; Paul, F.; Pallilavalappil, S.; Thomas, N.; Hinder, S.J.; Manjakkal, L.; Pillai, S.C. Spindle-Shaped Ni-Fe-Layered Double Hydroxide: Effect of Etching Time on Flexible Energy Storage. Small 2025, 21, 2409959. [Google Scholar] [CrossRef]
- Guo, P.F.; Yang, Y.; Wang, W.J.; Zhu, B.; Wang, W.T.; Wang, Z.Y.; Wang, J.L.; Wang, K.; He, Z.H.; Liu, Z.T. Stable and Active NiFeW Layered Double Hydroxide for Enhanced Electrocatalytic Oxygen Evolution Reaction. Chem. Eng. J. 2021, 426, 130768. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning Synergy Between Nickel and Iron in Ruddlesden–Popper Perovskites Through Controllable Crystal Dimensionalities Towards Enhanced Oxygen-Evolving Activity and Stability. Carbon Energy 2024, 6, e465. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Gray, H.B.; Müller, A.M. Effect of Interlayer Anions on [NiFe]-LDH Nanosheet Water Oxidation Activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef]
- Bin Jiang, D.; Jing, C.; Yuan, Y.; Feng, L.; Liu, X.; Dong, F.; Dong, B.; Zhang, Y.X. 2D-2D Growth of NiFe LDH Nanoflakes on Montmorillonite for Cationic and Anionic Dye Adsorption Performance. J. Colloid Interface Sci. 2019, 540, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Senthil, R.A.; Min, A.; Kumar, A.; Moon, C.J.; Jeong, G.H.; Kim, T.W.; Choi, M.Y. Exploring Ir-Doped NiFe LDH Nanosheets via a Pulsed Laser for Oxygen Evolution Kinetics: In Situ Raman and DFT Insights. J. Mater. Chem. A Mater. 2024, 12, 8694–8706. [Google Scholar] [CrossRef]
- Tang, F.; Liu, T.; Jiang, W.; Gan, L. Windowless Thin Layer Electrochemical Raman Spectroscopy of Ni-Fe Oxide Electrocatalysts during Oxygen Evolution Reaction. J. Electroanal. Chem. 2020, 871, 114282. [Google Scholar] [CrossRef]
- Hu, J.; Xi, W.; Zhang, Y.; Wang, R.; Wang, H.; Gong, Y.; He, B.; Jin, J. Rational Design of LDH-Derived NiFe Layered Double Oxides as Capacitive Deionization Anode for Efficient Chlorine Ion Storage with a “Memory Effect”. Appl. Surf. Sci. 2025, 687, 162289. [Google Scholar] [CrossRef]
- Que, R.; Liu, S.; He, P.; Yang, Y.; Pan, Y.Y. Hierarchical Heterostructure CoCO3@NiFe LDH Nanowires Array as Outstanding Bifunctional Electrocatalysts for Overall Water Splitting. Mater. Lett. 2020, 277, 128285. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Sun, C.; Song, C.; Wang, D. One-Step Ionothermal Accompanied Thermolysis Strategy for N-Doped Carbon Quantum Dots Hybridized NiFe LDH Ultrathin Nanosheets for Electrocatalytic Water Oxidation. Electrochim. Acta 2021, 391, 138932. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Liang, X.; Ma, F.X.; Xiong, Y.X.; Zhang, G.; Chen, G.; Zhen, L.; Xu, C.Y. Decoration of NiFe LDH Nanodots Endows Lower Fe-d Band Center of Fe1-N-C Hollow Nanorods as Bifunctional Oxygen Electrocatalysts with Small Overpotential Gap. Adv. Energy Mater. 2023, 13, 2203609. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu Nanowires Shelled with NiFe Layered Double Hydroxide Nanosheets as Bifunctional Electrocatalysts for Overall Water Splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Nayak, S.; Mohapatra, L.; Parida, K. Visible Light-Driven Novel g-C3N4/NiFe LDH Composite Photocatalyst with Enhanced Photocatalytic Activity towards Water Oxidation and Reduction Reaction. J. Mater. Chem. A Mater. 2015, 3, 18622–18635. [Google Scholar] [CrossRef]
- Valencia-Lopez, C.D.; Zafra-Calvo, M.; de Vidales, M.J.M.; Blanco-Gutierrez, V.; Atanes-Sanchez, E.; Merayo, N.; Fernandez-Martinez, F.; Nieto-Marquez, A.; Dos santos-Garcia, A.J. Synthesis of NiFe2O4-LDH Composites with High Adsorption and Photocatalytic Activity for Methyl Orange Degradation. Inorganics 2018, 6, 98. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.A.; Vázquez-Samperio, J.; Manzo-Robledo, A.; González-Huerta, R.D.G.; Flores-Moreno, J.L.; Ramírez-Rosales, D.; Guzmán-Vargas, A. An Approach to Understanding the Electrocatalytic Activity Enhancement by Superexchange Interaction toward OER in Alkaline Media of Ni-Fe LDH. J. Phys. Chem. C 2014, 118, 22432–22438. [Google Scholar] [CrossRef]
- Tyndall, D.; Craig, M.J.; Gannon, L.; McGuinness, C.; McEvoy, N.; Roy, A.; García-Melchor, M.; Browne, M.P.; Nicolosi, V. Demonstrating the Source of Inherent Instability in NiFe LDH-Based OER Electrocatalysts. J. Mater. Chem. A 2023, 11, 4067–4077. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.A.; Reddy, K.A.J.; Gopannagari, M.; Kim, Y.; Rangappa, A.P.; Kumar, D.P.; Kim, T.K. Exposure of NiFe LDH Active Sites by Cation–Exchange to Promote Photoelectrochemical Water Splitting Performance. Appl. Surf. Sci. 2021, 570, 151134. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K.M. Deciphering Z-Scheme Charge Transfer Dynamics in Heterostructure NiFe LDH/N-RGO/g-C3N4 Nanocomposite for Photocatalytic Pollutant Removal and Water Splitting Reactions. Sci. Rep. 2019, 9, 2458. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Plasmon-Induced Electron Transportation in Heterostructure N-RGO/NiFe LDH@Au with Enhanced Photoelectrochemical Water Oxidation. Electrochim. Acta 2024, 500, 144724. [Google Scholar] [CrossRef]
- Lee, E.B.; Jo, S.G.; Park, G.R.; Lee, J.; Kim, C.S.; Kim, S.J.; Lee, J.W. Facile and Fast Route of Electrodepositing Chloride Ion-Modified NiFePt Layered Double Hydroxides for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2024, 662, 160112. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, B.; Qiao, Z.; Ji, D.; Yuan, D.; Li, Z.; Wu, H. Electronic Structure Engineering of CNTs@NiFe LDH Composite via Heteroatom Doping for Efficient Seawater Electrolysis. Int. J. Hydrogen Energy 2024, 83, 803–810. [Google Scholar] [CrossRef]
- Messaoudi, Y.; Belhadj, H.; Khelladi, M.R.; Azizi, A. Rational Design of NiFe Alloys for Efficient Electrochemical Hydrogen Evolution Reaction: Effects of Ni/Fe Molar Ratios. RSC Adv. 2022, 12, 29143–29150. [Google Scholar] [CrossRef]
- Li, X.P.; Han, W.K.; Xiao, K.; Ouyang, T.; Li, N.; Peng, F.; Liu, Z.Q. Enhancing Hydrogen Evolution Reaction through Modulating Electronic Structure of Self-Supported NiFe LDH. Catal. Sci. Technol. 2020, 10, 4184–4190. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Dong, L.; Bin Wu, H.; Yu, X.-Y. Activating the Hydrogen Evolution and Overall Water Splitting Performance of NiFe LDH by Cation Doping and Plasma Reduction. Appl. Catal. B 2020, 266, 118627. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.Q.; Xi, C.; Cheng, C.Q.; Kuai, C.G.; Zheng, X.L.; Zhang, R.; Xie, Y.M.; Dong, C.K.; Chen, Y.J.; et al. Valence-State Effect of Iridium Dopant in NiFe(OH)2 Catalyst for Hydrogen Evolution Reaction. Small 2021, 17, 2100203. [Google Scholar] [CrossRef]
- Guo, J.; Wang, K.; Zhang, H.; Zhang, H. Enhanced Electrocatalytic Activity of Mo-Doped NiFe Layered Double Hydroxide Nanosheet Arrays for the Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2023, 6, 379–389. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Hsu, C.N.; Kuo, D.H. Activating Nickel Iron Layer Double Hydroxide for Alkaline Hydrogen Evolution Reaction and Overall Water Splitting by Electrodepositing Nickel Hydroxide. Chem. Eng. J. 2021, 419, 129608. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, G.; Zhang, B.; Liao, W.; Zhao, N.; Xu, X. Built-in Electric Field Control of Electron Redistribution (NiFeBased Electrocatalyst) with Efficient Overall Water Splitting at Industrial Temperature. J. Colloid Interface Sci. 2025, 677, 68–78. [Google Scholar] [CrossRef]
- Abdelraouf, A.A.; Abdelrahim, A.M.; Abd El-Moghny, M.G.; El-Deab, M.S. Interface Engineering: Enhancing the Electrocatalytic Activity of Heterostructure NiFeBased Alloy over Valorized Carbon Waste towards Water Splitting. Int. J. Hydrogen Energy 2025, 101, 556–567. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, W.; Wei, S.; Zhang, K.; Wang, H.; Liu, G.; Li, J. Magnesium-Promoted Rapid Self-Reconstruction of NiFeBased Electrocatalysts toward Efficient Oxygen Evolution. J. Colloid Interface Sci. 2025, 677, 208–216. [Google Scholar] [CrossRef]
- Tang, X.; Yang, N.; Li, Z.; Cai, Z.; Dai, Q.; Wang, H.; He, X.; Yao, Y.; Li, T.; Guo, J.; et al. NiFeBased Arrays with Manganese Dioxide Enhance Chloride Blocking for Durable Alkaline Seawater Oxidation. J. Colloid Interface Sci. 2025, 684, 64–72. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, Y.; Li, S.; Ding, J.; Ren, Y.; Tang, K.; Wang, J.; Li, C.; Shi, Z.; Sun, Z.; et al. In Situ Synthesis of Ternary Ni-Fe-Mo Nanosheet Arrays for OER in Water Electrolysis. Molecules 2025, 30, 177. [Google Scholar] [CrossRef]
- Dai, B.; Wei, X.; Chen, L.; Bao, X.; Zhong, Q.; Qu, H. Introducing Different Long-Chain Flexible Ligands to Regulate the Transformation Behavior of NiFeMOF and as Bifunctional Catalysts for the HER/OER. J. Colloid Interface Sci. 2025, 682, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Ma, H.; Wang, J.; Xu, R.; Li, G.; Yang, L.; Guo, H. Electronic Structure Engineering of NiFe Hydroxide Nanosheets via Ion Doping for Efficient OER Electrocatalysis. Chem. Eng. J. 2024, 499, 156430. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Lu, B.; Zhang, R.R.; Zhou, F.; Wang, Q.; Li, J.; Yang, Z.; Lei, Z. In-Situ Growth Strategy to Synthesize Porous Nano-Coral-like Structure OER Catalyst Based on Nife Foam Alloy Substrate. J. Alloys Compd. 2024, 1006, 176310. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Lu, Z.; Xie, J.; Huang, J.; Hu, J.; Cao, Y. Multielectronic Synergetic Hf-Doped NiFe LDH/NF for Efficient Bifunctional Electrocatalytic OER/UOR. Int. J. Hydrogen Energy 2024, 82, 724–732. [Google Scholar] [CrossRef]
- Cao, Y.-B.; Dong, W.-W.; Ye, Y.; Jiang, J.; Zhang, L.; Ding, Y.; Wang, X.; Hu, L.; Shi, L. Microflower-like NiFe Layered Double Hydroxide @ g-C3N4 Electrocatalysts for Enhanced Photo-Assisted OER and Overall Water Splitting Performance. J. Alloys Compd. 2024, 996, 174752. [Google Scholar] [CrossRef]
- He, L.; Wang, N.; Xiang, M.; Zhong, L.; Komarneni, S.; Hu, W. S-Vacancy-Rich NiFeS Nanosheets Based on a Fully Electrochemical Strategy for Large-Scale and Quasi-Industrial OER Catalysts. Appl. Catal. B 2024, 345, 123686. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Desalegan, B.Z.; Seo, J.G. An Advanced and Highly Efficient Ce Assisted NiFe LDH Electrocatalyst for Overall Water Splitting. Sustain. Energy Fuels 2019, 4, 312–323. [Google Scholar] [CrossRef]
- Sreenivasulu, M.; Hadrihalli, A.; Alshehri, M.A.; Shetti, N.P. Rational Designing of Nickel-Iron Containing Layered Double Hydroxide [NiFe@LDH] Electrocatalysts for Effective Water Splitting. Energy Fuels 2024, 38, 12888–12899. [Google Scholar] [CrossRef]
- Liang, J.; Shen, H.; Ma, Y.; Liu, D.; Li, M.; Kong, J.; Tang, Y.; Ding, S. Autogenous Growth of the Hierarchical V-Doped NiFe Layer Double Metal Hydroxide Electrodes for an Enhanced Overall Water Splitting. Dalton Trans. 2020, 49, 11217–11225. [Google Scholar] [CrossRef]
- Wang, B.; Jiao, S.; Wang, Z.; Lu, M.; Chen, D.; Kang, Y.; Pang, G.; Feng, S. Rational Design of NiFe LDH@Ni3N Nano/Microsheet Arrays as a Bifunctional Electrocatalyst for Overall Water Splitting. J. Mater. Chem. A Mater. 2020, 8, 17202–17211. [Google Scholar] [CrossRef]
- Liu, F.; Guo, X.; Hou, Y.; Wang, F.; Zou, C.; Yang, H. Hydrothermal Combined with Electrodeposition Construction of a Stable Co9S8/Ni3S2@NiFe LDH Heterostructure Electrocatalyst for Overall Water Splitting. Sustain. Energy Fuels 2021, 5, 1429–1438. [Google Scholar] [CrossRef]
- Shi, H.; Yang, K.; Wang, F.; Ni, Y.; Zhai, M. Hierarchical MnCo2O4nanowire@NiFe Layered Double Hydroxide Nanosheet Heterostructures on Ni Foam for Overall Water Splitting. CrystEngComm 2021, 23, 7141–7150. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhang, Y.; Qiu, F.; Sun, Y.; Ning, W.; Tao, Q.; Li, W.; Miao, S. Coupling Thulium 4f Orbitals with Ni3Fe LDH Loaded with Pt to Form an Electronic Buffer Band for Catalyzing Alkaline Overall Water Splitting. J. Mater. Chem. A Mater. 2024, 12, 17574–17585. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Cao, D.; Gong, Y. Hierarchical Self-Assembly of NiFe LDH Nanosheets on CoFe2O4@Co3S4 Nanowires for Enhanced Overall Water Splitting. Sustain. Energy Fuels 2020, 4, 1933–1944. [Google Scholar] [CrossRef]
- Wu, W.; Min, B.; Li, H.; Liu, F.; Zheng, M.; Ding, K.; Lu, S.; Liu, M. NiFe LDH Coated NiSe/Ni Foam as a Bifunctional Electrocatalyst for Overall Water Splitting. React. Chem. Eng. 2023, 8, 1711–1718. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.; Li, M.; Li, Y.; Zhang, X.; Chen, J.; Fang, X.; Liu, Y.; Yuan, X.; Dai, X.; et al. Interfacial Synergy between Dispersed Ru Sub-Nanoclusters and Porous NiFe Layered Double Hydroxide on Accelerated Overall Water Splitting by Intermediate Modulation. Nanoscale 2020, 12, 9669–9679. [Google Scholar] [CrossRef]
- Wang, S.; Ning, X.; Cao, Y.; Chen, R.; Lu, Z.; Hu, J.; Xie, J.; Hao, A. Construction of an Advanced NiFe LDH/MoS2-Ni3S2/NF Heterostructure Catalyst toward Efficient Electrocatalytic Overall Water Splitting. Inorg. Chem. 2023, 62, 6428–6438. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J. Hierarchical NiCo2S4@NiFe LDH Heterostructures Supported on Nickel Foam for Enhanced Overall-Water-Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 15364–15372. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Zhang, Y.; Zhang, H.; Zhao, J.; Song, R. Hollow Mo-Doped NiSx Nanoarrays Decorated with NiFe Layered Double-Hydroxides for Efficient and Stable Overall Water Splitting. J. Mater. Chem. A Mater. 2022, 10, 18989–18999. [Google Scholar] [CrossRef]
- Heijden, O.v.d.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.M. Tafel Slope Plot as a Tool to Analyze Electrocatalytic Reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.S.; Zhang, Z.; Baranova, E.A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Jian, J.; Li, Y.; Bi, H.; Wang, X.; Wu, X.; Qin, W. Aluminum Decoration on MoS2 Ultrathin Nanosheets for HighlyEfficient Hydrogen Evolution. ACS Sustain. Chem. Eng. 2020, 8, 4547–4554. [Google Scholar] [CrossRef]
- Akbar, K.; Jeon, J.H.; Kim, M.; Jeong, J.; Yi, Y.; Chun, S.-H. Bifunctional Electrodeposited 3D NiCoSe2/Nickle Foam Electrocatalysts for Its Applications in Enhanced Oxygen Evolution Reaction and for Hydrazine Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 7735–7742. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Shin, H.H.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Cobalt Iron Hydroxide as a Precious Metal-Free Bifunctional Electrocatalyst for Efficient Overall Water Splitting. Small 2018, 14, 1702568. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teli, A.M.; Mane, S.M.; Beknalkar, S.A.; Mishra, R.K.; Jeon, W.; Shin, J.C. Development of Electrochemical Water Splitting with Highly Active Nanostructured NiFe Layered Double Hydroxide Catalysts: A Comprehensive Review. Catalysts 2025, 15, 293. https://doi.org/10.3390/catal15030293
Teli AM, Mane SM, Beknalkar SA, Mishra RK, Jeon W, Shin JC. Development of Electrochemical Water Splitting with Highly Active Nanostructured NiFe Layered Double Hydroxide Catalysts: A Comprehensive Review. Catalysts. 2025; 15(3):293. https://doi.org/10.3390/catal15030293
Chicago/Turabian StyleTeli, Aviraj M., Sagar M. Mane, Sonali A. Beknalkar, Rajneesh Kumar Mishra, Wookhee Jeon, and Jae Cheol Shin. 2025. "Development of Electrochemical Water Splitting with Highly Active Nanostructured NiFe Layered Double Hydroxide Catalysts: A Comprehensive Review" Catalysts 15, no. 3: 293. https://doi.org/10.3390/catal15030293
APA StyleTeli, A. M., Mane, S. M., Beknalkar, S. A., Mishra, R. K., Jeon, W., & Shin, J. C. (2025). Development of Electrochemical Water Splitting with Highly Active Nanostructured NiFe Layered Double Hydroxide Catalysts: A Comprehensive Review. Catalysts, 15(3), 293. https://doi.org/10.3390/catal15030293







