Catalytic Reduction of SO2 with CO over LaCoO3 Perovskites Catalysts: Effect of Fe Doping and Pre-Sulfurization
Abstract
1. Introduction
2. Results and Discussion
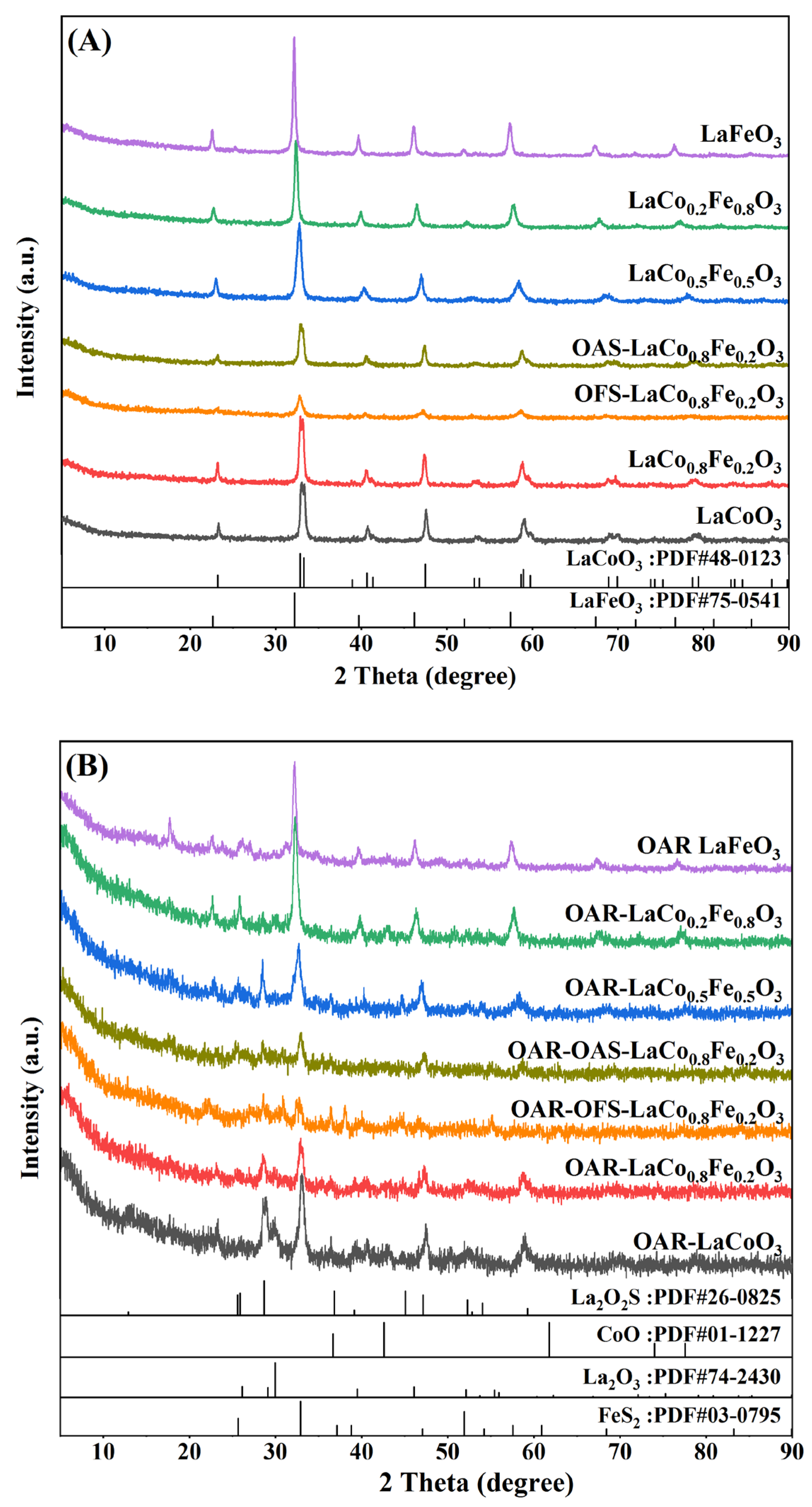
2.1. Physical Properties of XRD for Catalysts
2.2. Physical Properties of BET for Catalysts
2.3. Morphological Characteristics
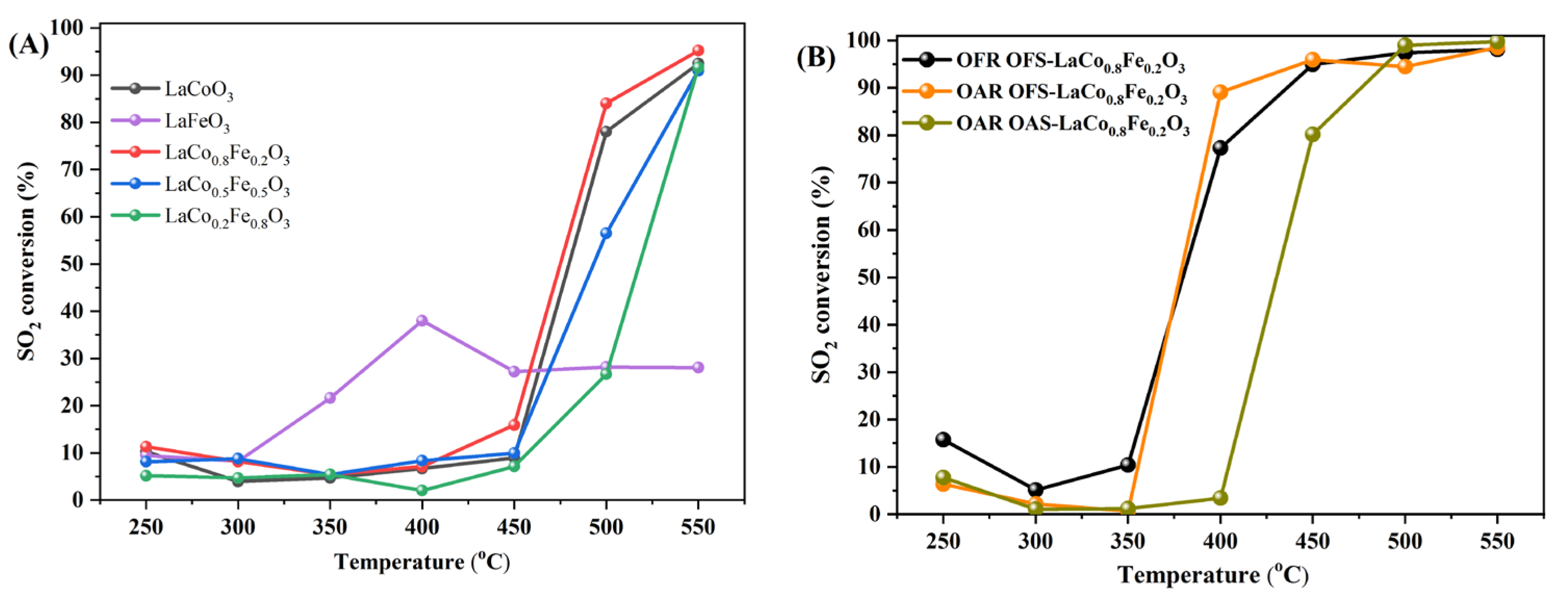
2.4. Catalytic Performance
2.5. TPD Analysis
2.6. XPS Analysis
2.7. Mechanism Explanation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of LaCoxFe1−xO3 Catalysts
3.3. Sulfurization of LaCo0.8Fe0.2O3 Catalyst
3.4. Catalysts Characterization
3.5. Catalytic Performance Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, M.; Chen, L.; Cheng, X.; Meng, X.; Jin, Q.; Zhu, C.; Yang, J.; Xu, H. Reduction of SO2 into sulfur over Ce-based catalyst: Performance optimizations and reaction mechanisms. J. Environ. Chem. Eng. 2024, 12, 114064. [Google Scholar] [CrossRef]
- Wong, P.Y.; Zeng, Y.T.; Su, H.J.; Lung, S.C.C.; Chen, Y.C.; Chen, P.C.; Hsiao, T.C.; Adamkiewicz, G.; Wu, C.D. Effects of feature selection methods in estimating SO2 concentration variations using machine learning and stacking ensemble approach. Environ. Technol. Innov. 2025, 37, 103996. [Google Scholar] [CrossRef]
- Yang, L.; Zhong, W.; Sun, L.; Chen, X.; Shao, Y. Dynamic optimization oriented modeling and nonlinear model predictive control of the wet limestone FGD system. Chin. J. Chem. Eng. 2020, 28, 832–845. [Google Scholar] [CrossRef]
- Behzadi pour, G.; Kamel Oroumiyeh, M.; Fekri aval, L. Rapid SO2 gas removal using MgO/AC/CaCO3/Zeolite nanocomposite at room temperature. Case Stud. Chem. Environ. Eng. 2025, 11, 101042. [Google Scholar] [CrossRef]
- Jin, Q.; Meng, X.; Ji, W.; Wu, P.; Xu, M.; Zhang, Y.; Zhu, C.; Xu, H. SO2 reduction for sulfur production by CO over Ce-Al-Ox composite oxide catalyst. Catal. Commun. 2023, 174, 106587. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Pahlavanzadeh, H.; Khalighi, R.; Khani, M.; Ebrahim, H.A.; Abbasizadeh, S.; Mozaffari, A. Reduction of SO2 to elemental sulfur in flue gas using Copper-Alumina catalysts. J. Nanotechnol. 2023, 2023, 3723612. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, X.; Liu, M.; Zhang, J.; Wang, W.; Song, Z.; Zhao, X. Effect of temperature on the reaction path of pyrite (FeS2)-based catalyst catalyzed CO reduction of SO2 to sulfur. Chemosphere 2023, 340, 139789. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.H.; Lai, S.Y.; Jamaludin, N.F.M.; Mohamed, A.R. A review on dry-based and wet-based catalytic sulphur dioxide (SO2) reduction technologies. J. Hazard. Mater. 2022, 423, 127061. [Google Scholar] [CrossRef] [PubMed]
- Lum, M.M.X.; Ng, K.H.; Lai, S.Y.; Mohamed, A.R.; Alsultan, A.G.; Taufiq-Yap, Y.H.; Koh, M.K.; Mohamed, M.A.; Vo, D.-V.N.; Subramaniam, M.; et al. Sulfur dioxide catalytic reduction for environmental sustainability and circular economy: A review. Process Saf. Environ. Prot. 2023, 176, 580–604. [Google Scholar] [CrossRef]
- Sepehrian, M.; Anbia, M.; Hedayatzadeh, M.H.; Yazdi, F. SO2 dry-based catalytic removal from flue gas leading to elemental sulfur production: A comprehensive review. Process Saf. Environ. Prot. 2024, 182, 456–480. [Google Scholar] [CrossRef]
- Jinyan, C.; Yutao, L.; Tianrun, S.; Qiulin, Z.; Ping, N.; Jianjun, C.; Guocai, T.; Liangtao, Y.; Siyuan, X.; Rongbing, N. The positive role of Ir0 and OV on the reduction of SO2 by CO over Ir/CeO2. Appl. Surf. Sci. 2023, 620, 156826. [Google Scholar]
- Xia, X.; Zhao, X.; Zhou, P.; Feng, T.; Ma, C.; Song, Z. Reduction of SO2 to elemental sulfur with carbon materials through electrical and microwave heating methods. Chem. Eng. Process. 2020, 150, 107877. [Google Scholar]
- Feng, T.; Huo, M.; Zhao, X.; Wang, T.; Xia, X.; Ma, C. Reduction of SO2 to elemental sulfur with H2 and mixed H2/CO gas in an activated carbon bed. Chem. Eng. Res. Des. 2017, 121, 191–199. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, Q.; Ji, W.; Zhu, C.; Xu, M.; Zhu, Y.; Xu, H. Resource utilization of high concentration SO2 for sulfur production over La–Ce-Ox@ZrO2 composite oxide catalyst. J. Rare Earths 2023, 41, 1945–1952. [Google Scholar]
- Li, S.; Feng, T.; Kong, Q.; Li, J.; Liu, P.; Ni, P.; Wang, C. Investigation of iron oxide supported on activated coke for catalytic reduction of sulfur dioxide by carbon monoxide. J. Anal. Appl. Pyrolysis 2024, 179, 106488. [Google Scholar]
- He, L.; Hu, J.; Yang, Y.; Zheng, Z.; Deng, Y.; Xin, Y.; Zou, G.; Hou, H.; Ji, X. Hierarchical cocoon-like Co9S8-N@NC for ambient reduction of sulphur dioxide to sulphur via proton-coupled electron transfer. Appl. Catal. B Environ. 2025, 364, 124860. [Google Scholar]
- AlQahtani, M.S.; Knecht, S.D.; Wang, X.; Bilén, S.G.; Song, C. One-step low-temperature reduction of sulfur dioxide to elemental sulfur by plasma-enhanced catalysis. ACS Catal. 2020, 10, 5272–5277. [Google Scholar]
- Ren, W.; Zhou, P.; Tian, Y.; Wang, W.; Dong, Y.; Wang, T.; Zhang, L.; Ma, C.; Zhao, X. Catalytic performance and reaction mechanism of an iron-loaded catalyst derived from blast furnace slag for the CO-SO2 reaction to produce sulfur. Appl. Catal. A Gen. 2020, 606, 117810. [Google Scholar]
- Teixeira, G.F.; Silva, E., Jr.; Vilela, R.; Zaghete, M.A.; Colmati, F. Perovskite structure associated with precious metals: Influence on feterogenous catalytic process. Catalysts 2019, 9, 721. [Google Scholar]
- Jain, A.; Tamhankar, S.; Jaiswal, Y. Role of La-based perovskite catalysts in environmental pollution remediation. Rev. Chem. Eng. 2024, 40, 193–228. [Google Scholar]
- Chen, K.; Xu, L.; Li, Y.; Xiong, J.; Han, D.; Ma, Y.; Zhang, P.; Guo, H.; Wei, Y. Cerium doping effect in 3DOM perovskite-type La2−xCexCoNiO6 catalysts for boosting soot oxidation. Catalysts 2024, 14, 18. [Google Scholar]
- Fotovat, F.; Beyzaei, M.; Ebrahimi, H.; Mohebolkhames, E. Synthesis, characterization, and attrition resistance of kaolin and boehmite alumina-reinforced La0.7Sr0.3FeO3 perovskite catalysts for chemical looping partial oxidation of methane. Catalysts 2024, 14, 670. [Google Scholar] [CrossRef]
- Heidinger, B.; Royer, S.; Giraudon, J.-M.; Simon, P.; Bion, N.; Alamdari, H.; Lamonier, J.-F. Properties evolution of LaCoO3 perovskite synthesized by reactive grinding-application to the toluene oxidation reaction. J. Environ. Chem. Eng. 2024, 12, 112107. [Google Scholar]
- Moschos, M.; Evdou, A.; Zaspalis, V. (La1−xCax)MnO3−δ (x = 0, 0.2, 0.3, 0.4) perovskites as redox catalysts in chemical looping hydrogen production process: The relation between defect chemistry and redox performance. Catalysts 2024, 14, 431. [Google Scholar] [CrossRef]
- Ji, W.; Jin, Q.; Xu, M.; Chen, Y.; Yang, B.; Li, X.; Shen, Y.; Wang, Y.; Xu, H. Resource utilization of high-concentration SO2 for sulfur production over La-Ce-Ox composite oxide catalyst. Environ. Sci. Pollut. Res. 2023, 30, 21756–21768. [Google Scholar]
- Wang, G.-j.; Qin, Y.-n.; Ma, Z.; Qi, X.-z.; Ding, T. Study on the catalytic reduction mechanism of SO2 by CO over doped copper perovskite catalyst in presence of oxygen. React. Kinet. Catal. Lett. 2006, 89, 229–236. [Google Scholar]
- Zheng, X.; Li, B.; Shen, L.; Cao, Y.; Zhan, Y.; Zheng, S.; Wang, S.; Jiang, L. Oxygen vacancies engineering of Fe doped LaCoO3 perovskite catalysts for efficient H2S selective oxidation. Appl. Catal. B Environ. 2023, 329, 122526. [Google Scholar]
- Hibbert, D.B.; Campbell, R.H. Flue gas desulphurisation: Catalytic removal of sulphur dioxide by carbon monoxide on sulphided La1−xSrxCoO3: II. Reaction of sulphur dioxide and carbon monoxide in a flow system. Appl. Catal. 1988, 41, 289–299. [Google Scholar]
- Ma, J.; Fang, M.; Lau, N. On the synergism between La2O2S and CoS2 in the reduction of SO2 to elemental sulfur by CO. J. Catal. 1996, 158, 251–259. [Google Scholar]
- Wang, T.; Zhang, C.; Wang, J.; Li, H.; Duan, Y.; Liu, Z.; Lee, J.Y.; Hu, X.; Xi, S.; Du, Y.; et al. The interplay between the suprafacial and intrafacial mechanisms for complete methane oxidation on substituted LaCoO3 perovskite oxides. J. Catal. 2020, 390, 1–11. [Google Scholar]
- Ko, Y.; Kim, H.; Kim, S.; Lee, C.; Lee, S.S.; Roh, H.-S.; Shin, J.; Jeon, Y. CO management for hydrogen processes through a catalytic oxidation mechanism on dual-doped perovskites with tuned Co and Ni ratios. Catalysts 2025, 15, 45. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, R.; Feng, J.; Ji, S.; Cao, L. The reaction and poisoning mechanism of SO2 and perovskite LaCoO3 film model catalysts. Appl Catal. A Gen. 2001, 209, 71–77. [Google Scholar]
- Lau, N.; Fang, M.; Chan, C. Reduction of SO2 by CO and COS over La2O2S—A mechanistic study. J. Mol. Catal. A Chem. 2003, 203, 221–229. [Google Scholar] [CrossRef]
- Hibbert, D.B. Reduction of sulfur dioxide on perovskite oxides. Catal. Rev. 1992, 34, 391–408. [Google Scholar]
- Osti, A.; Rizzato, L.; Cavazzani, J.; Meneghello, A.; Glisenti, A. Perovskite oxide catalysts for enhanced CO2 reduction: Embroidering surface decoration with Ni and Cu nanoparticles. Catalysts 2024, 14, 313. [Google Scholar] [CrossRef]
- Zheng, J.-n.; An, K.; Wang, J.-m.; Li, J.; Liu, Y. Direct synthesis of ethanol via CO2 hydrogenation over the Co/La-Ga-O composite oxide catalyst. J. Fuel Chem. Technol. 2019, 47, 697–708. [Google Scholar]
- Ma, L.H.; Gao, X.H.; Zhang, J.L.; Ma, J.J.; Hu, X.D.; Guo, Q.J. Effects of metal doping on the catalytic performance of LaFe-based perovskites for CO2 hydrogenation to light olefins. J. Fuel Chem. Technol. 2023, 51, 101–110. [Google Scholar]
- Wu, S.; Zhan, s.; Dong, F.; Song, X.; Han, W.; Han, W.; Zhang, H.; Dong, X.; Tang, Z. Engineering surface exposed LaCoO3 perovskite nanotubular catalyst for catalytic combustion of toluene through acid etching. J. Mater. Chem. A 2025, 10, 1039. [Google Scholar]
- Teng, F.; Liang, S.; Gaugeu, B.; Zong, R.; Yao, W.; Zhu, Y. Carbon nanotubes-templated assembly of LaCoO3 nanowires at low temperatures and its excellent catalytic properties for CO oxidation. Catal. Commun. 2007, 8, 1748–1754. [Google Scholar]
- Zhang, X.; Cheng, X.; Chen, F.; Xu, L.; Wang, Y.; Qian, J.; Wu, Z.; Zhang, Q. Facile loading carbon dots on Co3O4 as an enhanced oxygen reduction reaction catalyst. Chem. Phys. Lett. 2020, 740, 137058. [Google Scholar]
- Cao, Z.; Li, J.; Zhao, Y.; Mei, Q.; Wang, Q.; Cheng, H. Meta-kaolinite/LaFeCoO3 microsphere catalyst for photocatalytic persulfate activation: Enhanced removal of tetracycline hydrochloride. Chem. Eng. J. 2023, 466, 143076. [Google Scholar]
- He, N.; Yu, Z.; Yang, G.; Tan, Q.; Wang, J.; Chen, Y. Designing with A-site cation defects in LaFeO3: Removal of tetracycline hydrochloride in complex environments using photo-Fenton synergy. Chem. Eng. J. 2024, 484, 149613. [Google Scholar]
- Kostyukhin, E.M.; Kustov, A.L.; Evdokimenko, N.V.; Bazlov, A.I.; Kustov, L.M. Hydrothermal microwave-assisted synthesis of LaFeO3 catalystfor N2O decomposition. J. Am. Ceram. Soc. 2021, 104, 492–503. [Google Scholar]
- Feng, X.; Tian, M.; He, C.; Li, L.; Shi, J.; Yu, Y.; Cheng, J. Yolk-shell-like mesoporous CoCrOx with superior activity and chlorine resistance in dichloromethane destruction. Appl. Catal. B Environ. 2020, 264, 118493. [Google Scholar]
- Lv, Y.; Li, Y.; Ta, N.; Shen, W. Co3O4 nanosheets: Synthesis and catalytic application for CO oxidation at room temperature. Sci. China Chem. 2014, 57, 873–880. [Google Scholar]
- Wen, M.; Dong, F.; Tang, Z.; Zhang, J. Engineering order mesoporous CeCoOx catalyst via in-situ confined encapsulation strategy for VOCs catalytic combustion. Mol. Catal. 2022, 519, 112149. [Google Scholar] [CrossRef]
- Zemlianskii, P.; Morozov, D.; Kapustin, G.; Davshan, N.; Kalmykov, K.; Chernyshev, V.; Kustov, A.; Kustov, L. Correlations between synthetic conditions and catalytic activity of LaMO3 perovskite-like oxide materials (M: Fe, Co, Ni): The key role of glycine. ChemPhysMater 2025, 2, 59. [Google Scholar]







| Samples | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| LaCoO3 | 12.86 | 0.10 | 23.23 |
| LaCo0.8Fe0.2O3 | 14.67 | 0.10 | 21.21 |
| LaCo0.5Fe0.5O3 | 16.04 | 0.10 | 19.05 |
| LaCo0.2Fe0.8O3 | 14.82 | 0.10 | 20.44 |
| LaFeO3 | 14.48 | 0.10 | 21.88 |
| Sample | O (%) | S (%) | Fe (%) | Co (%) | La (%) |
|---|---|---|---|---|---|
| LaCo0.8Fe0.2O3 | 70.31 | 0 | 4.18 | 13.50 | 12.01 |
| OFS-LaCo0.8Fe0.2O3 | 75.45 | 3.85 | 3.48 | 7.91 | 9.31 |
| OAS-LaCo0.8Fe0.2O3 | 71.41 | 1.12 | 3.96 | 11.22 | 12.29 |
| OAR-OFS-LaCo0.8Fe0.2O3 | 73.72 | 12.45 | 1.36 | 5.43 | 7.06 |
| OAR-OAS-LaCo0.8Fe0.2O3 | 39.41 | 31.73 | 2.68 | 10.59 | 15.59 |
| Sample | O (%) | S (%) | Fe (%) | Co (%) | La (%) |
| LaCo0.8Fe0.2O3 | 53.70 | 0 | 3.74 | 6.14 | 12.29 |
| OFS-LaCo0.8Fe0.2O3 | 44.81 | 13.20 | 2.88 | 5.21 | 6.76 |
| OAS-LaCo0.8Fe0.2O3 | 51.32 | 5.77 | 3.13 | 4.23 | 9.22 |
| OAR-OFS-LaCo0.8Fe0.2O3 | 50.03 | 15.90 | 1.93 | 2.78 | 6.31 |
| OAR-OAS-LaCo0.8Fe0.2O3 | 50.57 | 13.14 | 2.14 | 4.47 | 6.39 |
| Sample | Co2+/Co3+ | Fe2+/Fe3+ | Oads/Ototal | Binding Energy (eV) | |||
|---|---|---|---|---|---|---|---|
| Co2+(2p3/2) | Co3+(2p3/2) | Fe2+(2p3/2) | Fe3+(2p3/2) | ||||
| LaCoO3 | 0.80 | - | 0.59 | 781.6 | 780.0 | - | - |
| LaCo0.8Fe0.2O3 | 0.61 | 0.74 | 0.47 | 781.6 | 780.0 | 710.5 | 713.7 |
| OAS-LaCo0.8Fe0.2O3 | 0.82 | 0.84 | 0.59 | 781.4 | 779.8 | 710.6 | 713.8 |
| OFS-LaCo0.8Fe0.2O3 | 1.29 | 1.25 | 0.74 | 780.6 | 779.0 | 710.8 | 714.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Wang, H.; Li, S.; Chen, Y. Catalytic Reduction of SO2 with CO over LaCoO3 Perovskites Catalysts: Effect of Fe Doping and Pre-Sulfurization. Catalysts 2025, 15, 291. https://doi.org/10.3390/catal15030291
Yao L, Wang H, Li S, Chen Y. Catalytic Reduction of SO2 with CO over LaCoO3 Perovskites Catalysts: Effect of Fe Doping and Pre-Sulfurization. Catalysts. 2025; 15(3):291. https://doi.org/10.3390/catal15030291
Chicago/Turabian StyleYao, Liang, Hao Wang, Shuangde Li, and Yunfa Chen. 2025. "Catalytic Reduction of SO2 with CO over LaCoO3 Perovskites Catalysts: Effect of Fe Doping and Pre-Sulfurization" Catalysts 15, no. 3: 291. https://doi.org/10.3390/catal15030291
APA StyleYao, L., Wang, H., Li, S., & Chen, Y. (2025). Catalytic Reduction of SO2 with CO over LaCoO3 Perovskites Catalysts: Effect of Fe Doping and Pre-Sulfurization. Catalysts, 15(3), 291. https://doi.org/10.3390/catal15030291





