Investigation of Electrocatalytic Applications of Various Advanced Nanostructured Alloys—An Overview
Abstract
:1. Introduction
2. Advantages of Using Nanostructured Alloys as Modifiers
3. Fabrication of Nanostructured Alloys by Ball-Milling Method
4. Preparation of Nanostructured-Alloys-Modified Carbon Paste Electrodes (NsA-MCPE)
5. Duplex Stainless Steel-Modified Carbon Paste Electrode (DS-MCPE)
5.1. Introduction to Duplex Stainless Steel (DS)
5.2. Electrochemical Determination of Dopamine
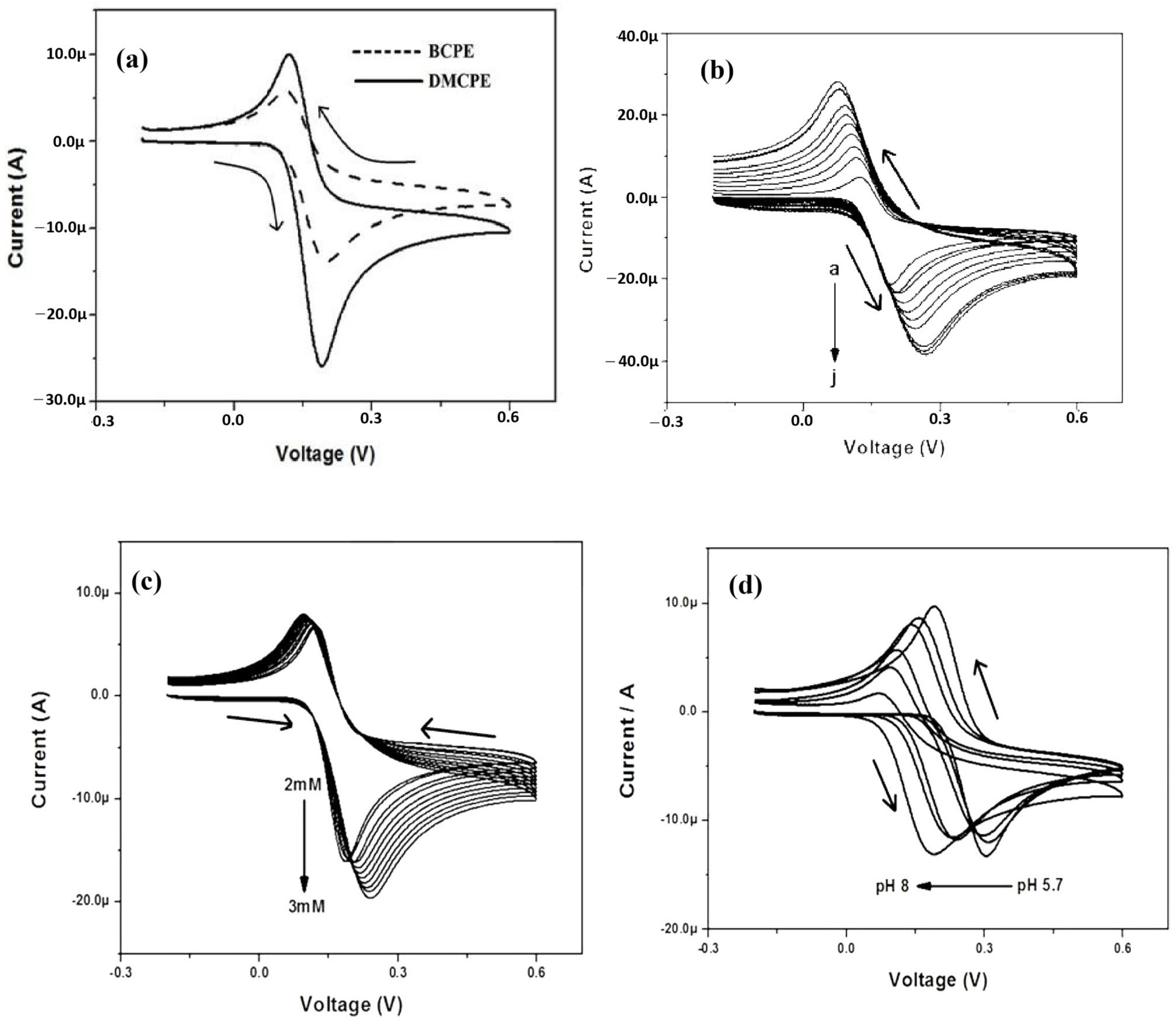
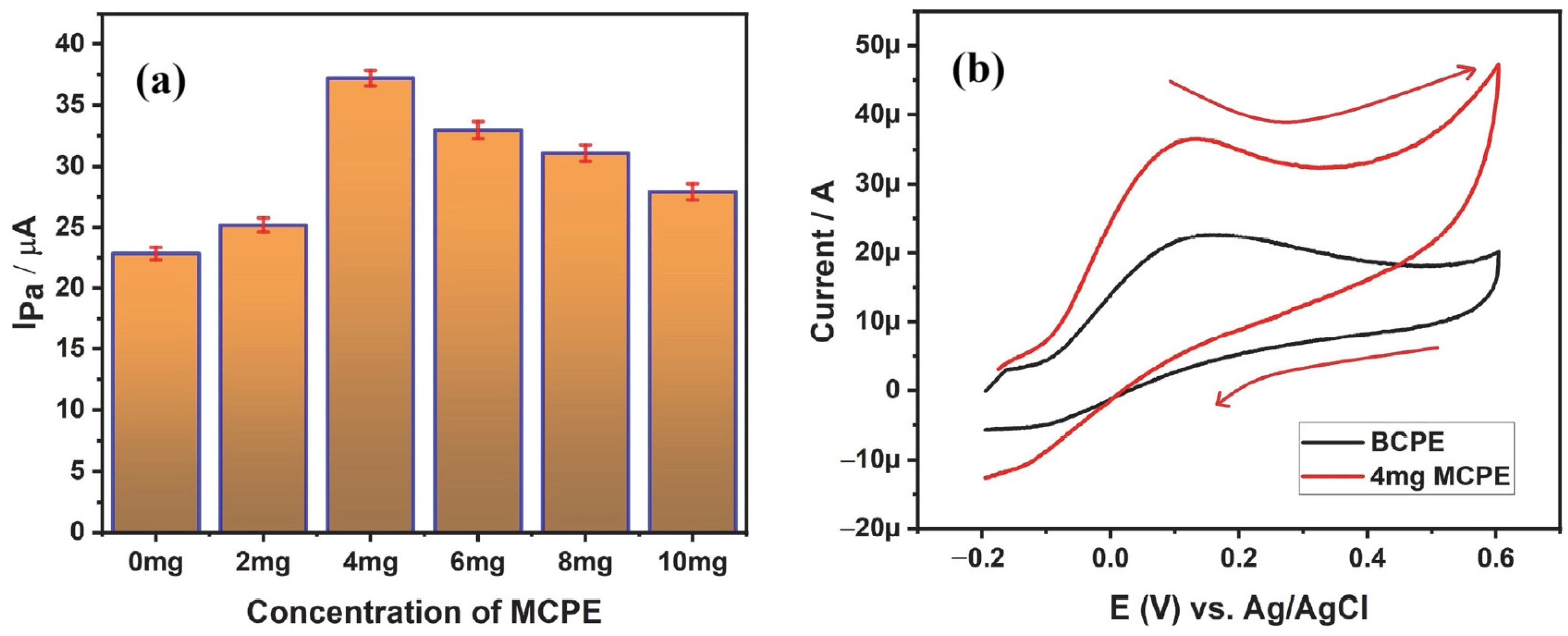
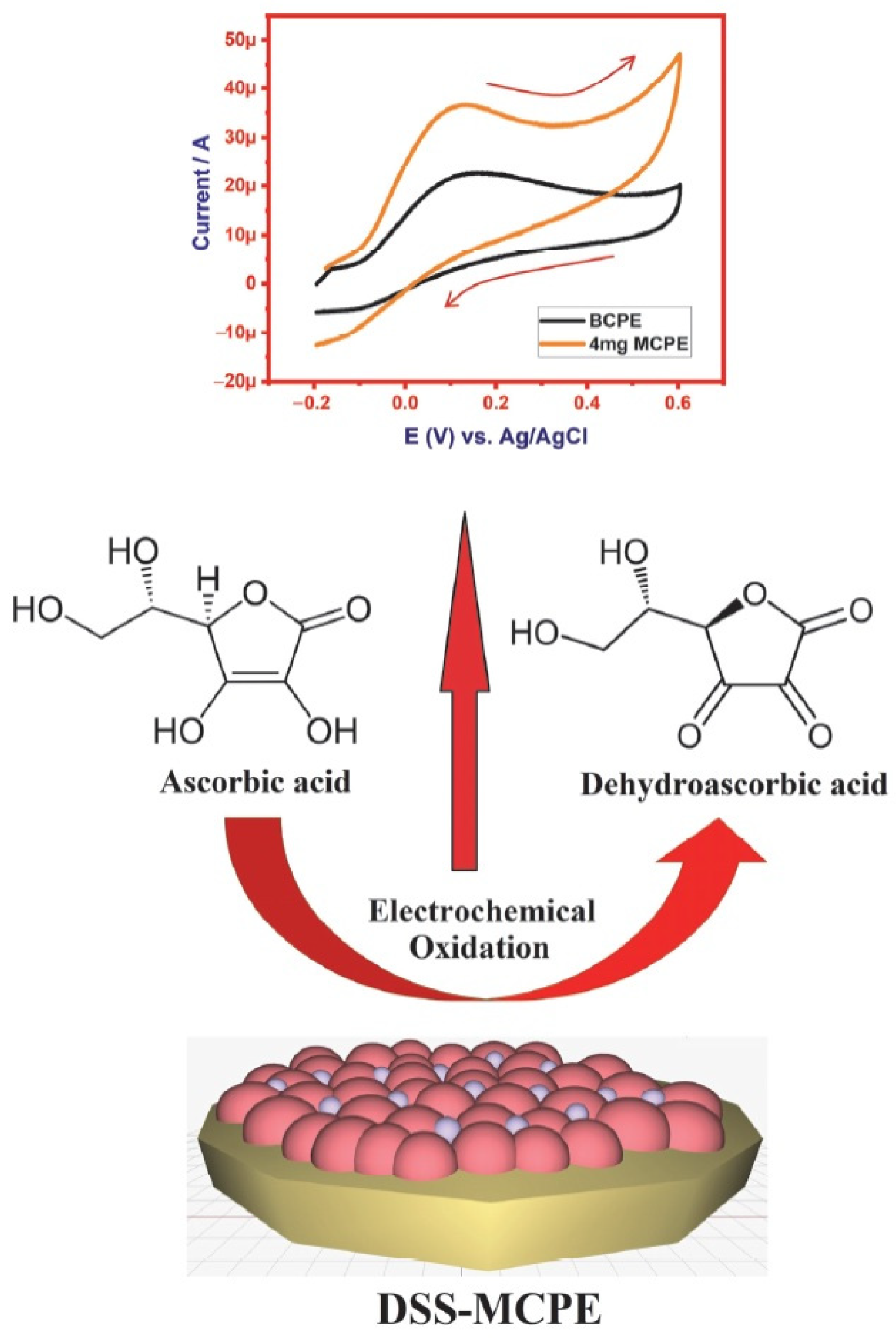
5.3. Electrochemical Determination of AA

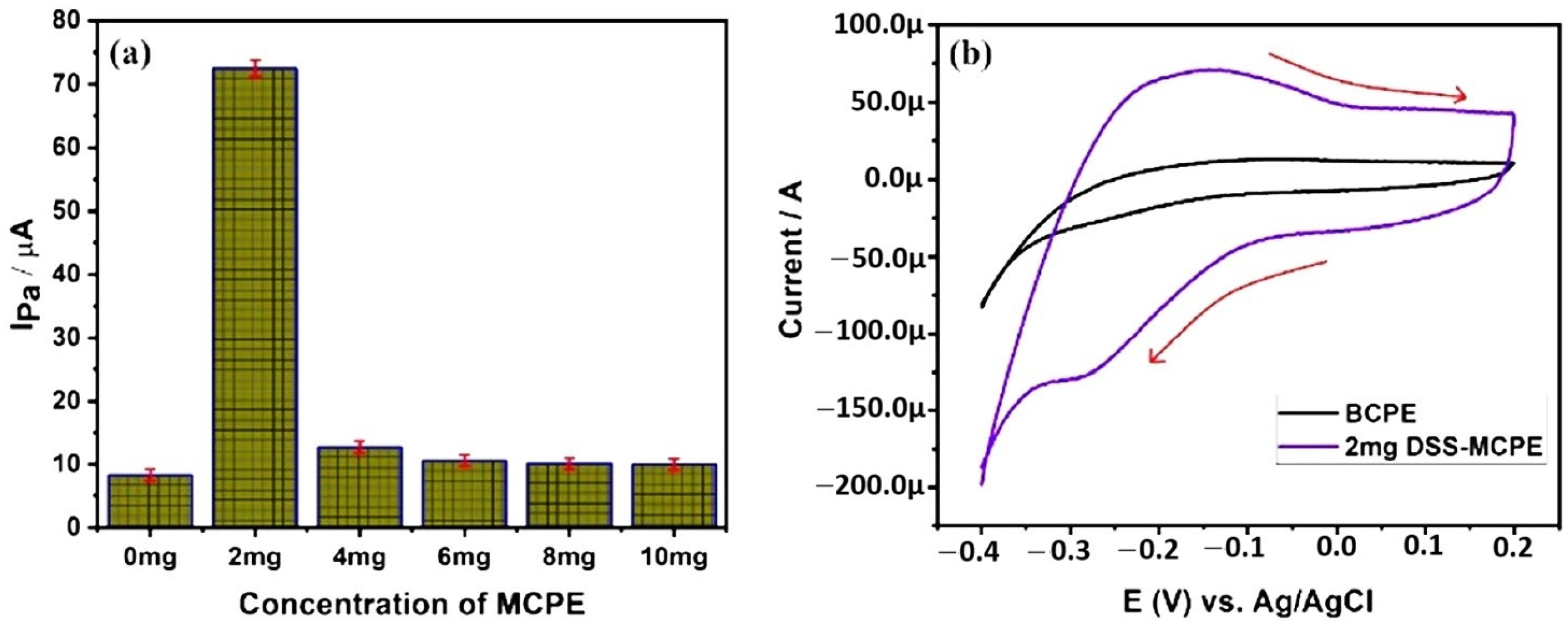
5.4. Electrochemical Determination of MB (MB)
5.5. Electrochemical Determination of Rhodamine B (Rh B)
5.6. Electrochemical Determination of Folic Acid (FA)
6. High-Entropy-Alloy-Modified Carbon Paste Electrode (HEA-MCPE)
6.1. Introduction to High-Entropy Alloys (HEAs)
6.2. Electrochemical Determination of AA
6.3. Electrochemical Determination of Methyl Orange
6.4. Electrochemical Determination of Methylene Blue
7. Shape-Memory-Alloy-Modified Carbon Paste Electrode (SMA-MCPE)
7.1. Introduction to Shape-Memory Alloys (SMAs)
7.2. Preparation of Shape-Memory Alloys (SMAs) by Mechanical Alloying
7.3. Electrochemical Determination of Dopamine
8. Challenges and Future Perspectives
- ➢
- Reproducibility: Because of differences in how nanostructured alloys were prepared and how the nanoparticles were distributed in the past, it might be difficult to achieve similar performance across several sensors.
- ➢
- Interference from Biological Matrices: Measurement accuracy may be impacted by the presence of interferents in complex biological samples, such as blood or urine. To reduce these interferences, sophisticated sensor designs and adjustments are required.
- ➢
- Long-term Stability: Although nanostructured alloys are generally durable, oxidation and other causes may cause them to perform worse over time, which could compromise the sensors’ long-term dependability.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, H.; Miyazaki, M. Enzyme-Immobilized Microfluidic Devices for Biomolecule Detection. TrAC Trends Anal. Chem. 2022, 159, 116908. [Google Scholar] [CrossRef]
- Ziani, I.; Bouakline, H.; Guerraf, A.E.; Bachiri, A.E.; Fauconnier, N.M.-L.; Sher, F. Integrating AI and Advanced Spectroscopic Techniques for Precision Food Safety and Quality Control. Trends Food Sci. Technol. 2024, 156, 104850. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Advances in Surfactant Biosensor and Sensor Technologies; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G.G.; Hareesha, N.; Prinith, S.N.; Ravishankar, D.K.; Arpitha, H.J. An Overview of Recent Development in Carbon-Based Sensors for Neurotransmitter Detection. Comb. Chem. High Throughput Screen. 2023, 26, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Hareesha, N.; Manjunatha, J.G.; Girish, T.; ALOthman, Z.A. Analysis of Catechol as an Environmental Pollutant Based on Electropolymerised Methyl Orange Modified Carbon Paste Sensor. Int. J. Environ. Anal. Chem. 2023, 104, 9186–9198. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Babu, K.J.; Kumar, G.G.; Kim, A.R.; Yoo, D.J. Flexible Electrospun PVDF-HFP/NI/CO Membranes for Efficient and Highly Selective Enzyme Free Glucose Detection. Ind. Eng. Chem. Res. 2014, 53, 10347–10357. [Google Scholar] [CrossRef]
- Tamilarasi, S.; Kumar, R.S.; Cho, K.-B.; Kim, C.-J.; Yoo, D.J. High-Performance Electrochemical Detection of Glucose in Human Blood Serum Using a Hierarchical NiO2 Nanostructure Supported on Phosphorus Doped Graphene. Mater. Today Chem. 2023, 34, 101765. [Google Scholar] [CrossRef]
- Nadar, N.R.; Deepak, J.; Sharma, S.C.; Krushna, B.R.R.; Puneeth, N.; Sowjanya, R.; Varalakshmi, V.S.; Sahu, S.; Sargunam, B.; Nagabhushana, H.; et al. Graphene Oxide Based Gd2O3:Eu3+ Nanocomposites: A Multifaceted Approach to Advanced Energy Storage and Bio Sensing Applications. Inorg. Chem. Commun. 2024, 165, 112515. [Google Scholar] [CrossRef]
- Vaibhav, N.; Swamy, B.E.K.; Manjunatha, L.S.; Manjunatha, K.G.; Sharma, S.C. Electrochemical Determination of Uric Acid in Presence of FA Using Synthesized Cobalt Oxide Modified Carbon Paste Electrode. Inorg. Chem. Commun. 2024, 165, 112469. [Google Scholar] [CrossRef]
- Nadar, N.R.; Deepak, J.; Sharma, S.C.; Krushna, B.R.R.; Puneeth, N.; Sowjanya, R.; Varalakshmi, V.S.; Sahu, S.; Sargunam, B.; Nagabhushana, H.; et al. Synergistic Doping Strategies Boosting Electrochemical Performance: GO-Y2O3: Eu3+/Li+ Nanocomposites for Supercapacitor and Biosensor Applications. Inorg. Chem. Commun. 2024, 164, 112397. [Google Scholar] [CrossRef]
- Atta, N.F.; Gawad, S.A.A.; Galal, A.; Razik, A.A.; El-Gohary, A.R. Efficient Electrochemical Sensor for Determination of H2O2 in Human Serum Based on Nano Iron-nickel Alloy/Carbon Nanotubes/Ionic Liquid Crystal Composite. J. Electroanal. Chem. 2020, 881, 114953. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Martinez, T.; Llanos, J.; Bertotti, M. A Cu-NPG/SPE Sensor for Non-Enzymatic and Non-Invasive Electrochemical Glucose Detection. Microchem. J. 2020, 160, 105629. [Google Scholar] [CrossRef]
- Hajmalek, S.; Jahani, S.; Foroughi, M.M. Simultaneous Voltammetric Determination of Tramadol and Paracetamol Exploiting Glassy Carbon Electrode Modified with FeNi3 Nanoalloy in Biological and Pharmaceutical Media. ChemistrySelect 2021, 6, 8797–8808. [Google Scholar] [CrossRef]
- Halfadji, A.; Naous, M.; Rajendrachari, S.; Ceylan, Y.; Ceylan, K.B.; Shekar, P.V.R. Effective Investigation of Electro-Catalytic, Photocatalytic, and Antimicrobial Properties of Porous CuO Nanoparticles Green Synthesized Using Leaves of Cupressocyparis Leylandii. J. Mol. Struct. 2023, 1301, 137318. [Google Scholar] [CrossRef]
- Halfadji, A.; Naous, M.; Kharroubi, K.N.; Belmehdi, F.E.Z.; Rajendrachari, S. An Ultrasonic-Assisted Synthesis, Characterization, and Application of Nano-Fe3O4/TiO2 as Nano-Catalyst for the Removal of Organic Dye by Like-Photo-Fenton Reactions. Inorg. Chem. Commun. 2023, 158, 111686. [Google Scholar] [CrossRef]
- Halfadji, A.; Chougui, A.; Djeradi, R.; Ouabad, F.Z.; Aoudia, H.; Rajendrachari, S. TiO2-Decorated by Nano-γ-Fe2O3 as a Catalyst for Efficient Photocatalytic Degradation of Orange G Dye under Eco-Friendly White LED Irradiation. ACS Omega 2023, 8, 39907–39916. [Google Scholar] [CrossRef]
- Rajendrachari Shashanka, B.N.; M Avar, B.S.P.; Kumar, R.M.; Baek, K.H. Assessing the Food Quality Using Carbon Nanomaterial Based Electrodes by Voltammetric Techniques. Biosensors 2022, 12, 1173. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Jayaprakash, G.K.; Pandith, A.; Karaoglanli, A.C.; Uzun, O. Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles. Catalysts 2022, 12, 994. [Google Scholar] [CrossRef]
- Mahale, R.S.; Shamanth, V.K.; Hemanth, K.; Shashanka, R.; Sharath, P.C.; Sreekanth, N.V. Electrochemical Sensor Applications of Nanoparticle Modified Carbon Paste Electrodes to Detect Various Neurotransmitters: A Review. Appl. Mech. Mater. 2022, 908, 69–88. [Google Scholar] [CrossRef]
- Shashanka, R.; Jayaprakash, G.K.; BG, P.; Kumar, M.; Swamy, B.E.K. Electrocatalytic Determination of AA Using a Green Synthesised Magnetite Nano-Flake Modified Carbon Paste Electrode by Cyclic Voltammetric Method. Mater. Res. Innov. 2021, 26, 229–239. [Google Scholar] [CrossRef]
- Pavitra, V.; Praveen, B.M.; Nagaraju, G.; Shashanka, R. Energy Storage, Photocatalytic and Electrochemical Nitrite Sensing of Ultrasound-Assisted Stable TA2O5 Nanoparticles. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Ramakrishna, D. Functionalized Nanomaterial-Based Electrochemical Sensors: ASensitive Sensor Platform. In Elsevier eBooks; Woodhead Publishing: Cambridge, UK, 2022; pp. 3–25. [Google Scholar]
- Rajendrachari, S.; Taslimi, P.; Karaoglanli, A.C.; Uzun, O.; Alp, E.; Jayaprakash, G.K. Photocatalytic Degradation of Rhodamine B (RhB) Dye in Waste Water and Enzymatic Inhibition Study Using Cauliflower Shaped ZnO Nanoparticles Synthesized by a Novel One-Pot Green Synthesis Method. Arab. J. Chem. 2021, 14, 103180. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D.; Swamy, B.E.K. Fabrication of yttria dispersed duplex stainless steel electrode to determine dopamine, ascorbic and uric acid electrochemically by using cyclic voltammetry. Int. J. Sci. Eng. Res. 2016, 7, 1275–1285. [Google Scholar]
- Shashanka, R.; Chaira, D.; Swamy, B.E.K. Electrochemical investigation of duplex stainless steel at carbon paste electrode and its application to the detection of dopamine, ascorbic and uric acid. Int. J. Sci. Eng. Res. 2015, 6, 1863–1871. [Google Scholar]
- Mahale, R.S.; Vasanth, S.; Chikkegouda, S.P.; Rajendrachari, S.; Narsimhachary, D.; Basavegowda, N. Electrochemical Determination of AA by Mechanically Alloyed Super Duplex Stainless Steel Powders. Metals 2023, 13, 1430. [Google Scholar] [CrossRef]
- Mahale, R.S.; Rajashekar, V.; Vasanth, S.; Chikkegowda, S.P.; Rajendrachari, S.; Mahesh, V. Fabrication of Mechanically Alloyed Super Duplex Stainless Steel Powder-Modified Carbon Paste Electrode for the Determination of MBby the Cyclic Voltammetry Technique. ACS Omega 2024, 9, 10660–10670. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Vinaykumar, R.; Katti, R.; Koujalagi, B.G.; Jayaprakash, G.K.; Ramkumar, R. Cyclic Voltammetric Determination of Rhodamine B Dye Using a Gas-Atomized Duplex Stainless Steel Powder-Modified Carbon Paste Electrode. Top. Catal. 2024. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D.; Swamy, B.E.K. Electrocatalytic Response of Duplex and Yittria Dispersed Duplex Stainless Steel Modified Carbon Paste Electrode in Detecting FA Using Cyclic Voltammetry. Int. J. Electrochem. Sci. 2015, 10, 5586–5598. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Adimule, V.; Gulen, M.; Khosravi, F.; Somashekharappa, K.K. Synthesis and Characterization of High Entropy Alloy 23Fe-21Cr-18Ni-20Ti-18Mn for Electrochemical Sensor Applications. Materials 2022, 15, 7591. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Mahale, R.S.; Adimule, V.; Gulen, M.; Mahesh, V.; Basavegowda, N. Fabrication of Mechanically Alloyed High Entropy Alloys (20-Fe-20Cr-20Ni-20Mn-20Ti) Modified Carbon Paste Electrode Sensor for the Cyclic Voltammetric Determination of Methyl Orange. Inorg. Chem. Commun. 2024, 164, 112428. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Adimule, V.M.; Jayaprakash, G.K.; Pandith, A. Electrochemical Oxidation of MB Dye in Wastewater Using Mechanically Alloyed High Entropy Alloy Modified Carbon Paste Electrode Using Cyclic Voltammetry. Mater. Res. Express 2023, 10, 054003. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Altaş, E.; Erdogan, A.; Küçük, Y.; Gök, M.S.; Khosravi, F. Electrochemical Determination of Dopamine by Poly (Methyl Orange) Shape Memory Alloy Modified Carbon Paste Electrode. Inorg. Chem. Commun. 2024, 167, 112826. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C. Nanocrystalline Materials. Int. Mater. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The Science and Technology of Mechanical Alloying. Mater. Sci. Eng. A 2001, 304–306, 151–158. [Google Scholar] [CrossRef]
- Prabhu, B.; Suryanarayana, C.; An, L.; Vaidyanathan, R. Synthesis and Characterization of High Volume Fraction Al–Al2O3 Nanocomposite Powders by High-Energy Milling. Mater. Sci. Eng. A 2006, 425, 192–200. [Google Scholar] [CrossRef]
- Suryanarayana, C. Structure and Properties of Nanocrystalline Materials. Bull. Mater. Sci. 1994, 17, 307–346. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Optimization of Milling Parameters for the Synthesis of Nano-Structured Duplex and Ferritic Stainless Steel Powders by High Energy Planetary Milling. Powder Technol. 2015, 278, 35–45. [Google Scholar] [CrossRef]
- Chaira, D. Development of Nano-Structured Duplex and Ferritic Stainless Steels by Pulverisette Planetary Milling Followed by Pressureless Sintering. Mater. Charact. 2014, 99, 220–229. [Google Scholar] [CrossRef]
- Chaira, D. Phase Transformation and Microstructure Study of Nano-Structured Austenitic and Ferritic Stainless Steel Powders Prepared by Planetary Milling. Powder Technol. 2014, 259, 125–136. [Google Scholar] [CrossRef]
- Hocevar, S.; Ogorevc, B. Preparation and Characterization of Carbon Paste Micro-Electrode Based on Carbon Nano-Particles. Talanta 2007, 74, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.; Apetrei, I.M.; De Saja, J.A.; Rodriguez-Mendez, M.L. Carbon Paste Electrodes Made from Different Carbonaceous Materials: Application in the Study of Antioxidants. Sensors 2011, 11, 1328–1344. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A. Chemically Modified Carbon Paste Electrode for Determination of Copper(II) by Potentiometric Method. Talanta 2002, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Koirala, K.; Santos, J.H.; Tan, A.L.; Ali, M.A.; Mirza, A.H. Chemically Modified Carbon Paste Electrode for the Detection of Lead, Cadmium and Zinc Ions. Sens. Rev. 2016, 36, 339–346. [Google Scholar] [CrossRef]
- Nesakumar, N.; Berchmans, S.; Alwarappan, S. Chemically Modified Carbon Based Electrodes for the Detection of Reduced Glutathione. Sens. Actuators B Chem. 2018, 264, 448–466. [Google Scholar] [CrossRef]
- Ghassab, N.; Soleymanpour, A.; Shafaatian, B. Development of an Ultrasensitive Chemically Modified Carbon Paste Electrode for Selective Determination Trace Amount of Sulfate Ion. Measurement 2022, 205, 112231. [Google Scholar] [CrossRef]
- Soleymanpour, A.; Shafaatian, B.; Mirfakhraei, H.S.; Rezaeifard, A. Development of a New Chemically Modified Carbon Paste Electrode for Selective Determination of Urinary and Serum Oxalate Concentration. Talanta 2013, 116, 427–433. [Google Scholar] [CrossRef]
- Wang, H.; Qian, D.; Xiao, X.; Deng, C.; Liao, L.; Deng, J.; Lin, Y.-W. Preparation and Application of a Carbon Paste Electrode Modified with Multi-Walled Carbon Nanotubes and Boron-Embedded Molecularly Imprinted Composite Membranes. Bioelectrochemistry 2018, 121, 115–124. [Google Scholar] [CrossRef]
- Hamidi, H.; Shams, E.; Yadollahi, B.; Esfahani, F.K. Fabrication of Bulk-Modified Carbon Paste Electrode Containing α-PW12O403−Polyanion Supported on Modified Silica Gel: Preparation, Electrochemistry and Electrocatalysis. Talanta 2007, 74, 909–914. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G. Determination of Mn(II) Ion by a Modified Carbon Paste Electrode Based on Multi-Walled Carbon Nanotubes (MWCNTs) in Different Water Samples. Sens. Actuators B Chem. 2014, 202, 699–707. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M. Electrochemical Evaluation of Biologically Modified Carbon Paste Electrode with Cyclic Voltammetric Technique. Port. Electrochim. Acta 2019, 37, 285–294. [Google Scholar] [CrossRef]
- Francis, R.; Byrne, G. Duplex Stainless Steels—Alloys for the 21st Century. Metals 2021, 11, 836. [Google Scholar] [CrossRef]
- Straße, A.; Gumenyuk, A.; Rethmeier, M. Study on Duplex Stainless Steel Powder Compositions for the Coating of Thick Plates for Laser Beam Welding. Adv. Eng. Mater. 2022, 24, 2101327. [Google Scholar] [CrossRef]
- Pezzato, L.; Calliari, I. Advances in Duplex Stainless Steels. Materials 2022, 15, 7132. [Google Scholar] [CrossRef]
- Omiogbemi, I.M.-B.; Yawas, D.S.; Das, A.; Afolayan, M.O.; Dauda, E.T.; Kumar, R.; Gorja, S.R.; Chowdhury, S.G. Mechanical Properties and Corrosion Behaviour of Duplex Stainless Steel Weldment Using Novel Electrodes. Sci. Rep. 2022, 12, 22405. [Google Scholar] [CrossRef]
- Alvarez-Armas, I. Duplex Stainless Steels: Brief History and Some Recent Alloys. Recent Pat. Mech. Eng. 2008, 1, 51–57. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.-H.; Wu, C.-B.; Zhao, Y.; Zu, G.-Q.; Zhu, W.-W.; Ran, X. A Short Review on the Role of Alloying Elements in Duplex Stainless Steels. Tungsten 2022, 5, 419–439. [Google Scholar] [CrossRef]
- Besharatloo, H.; Carpio, M.; Cabrera, J.-M.; Mateo, A.M.; Fargas, G.; Wheeler, J.M.; Roa, J.J.; Llanes, L. Novel Mechanical Characterization of Austenite and Ferrite Phases within Duplex Stainless Steel. Metals 2020, 10, 1352. [Google Scholar] [CrossRef]
- Duplex Stainless Steels. In ASM International eBooks; ASM: Almere, The Netherlands, 2008; pp. 91–107.
- Olguín, H.J.; Guzmán, D.C.; García, E.H.; Mejía, G.B. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2016, 9730467. [Google Scholar] [CrossRef]
- Speranza, L.; Di Porzio, U.; Viggiano, D.; De Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y. The Role of Dopamine Neurotransmitters in Neurological Diseases: New Sight. Int. J. Mol. Sci. 2024, 25, 7529. [Google Scholar] [CrossRef] [PubMed]
- Meder, D.; Herz, D.M.; Rowe, J.B.; Lehéricy, S.; Siebner, H.R. The Role of Dopamine in the Brain-Lessons Learned from Parkinson’s Disease. NeuroImage 2018, 190, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jahangeer, M.; Razia, D.M.; Ashiq, M.; Ghaffar, A.; Akram, M.; Allam, A.E.; Bouyahya, A.; Garipova, L.; Shariati, M.A.; et al. Dopamine in Parkinson’s Disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Cramb, K.M.L.; Beccano-Kelly, D.; Cragg, S.J.; Wade-Martins, R. Impaired Dopamine Release in Parkinson’s Disease. Brain 2023, 146, 3117–3132. [Google Scholar] [CrossRef]
- Falamaş, A.; Petran, A.; Hada, A.-M.; Bende, A. Dopamine Photochemical Behaviour under UV Irradiation. Int. J. Mol. Sci. 2022, 23, 5483. [Google Scholar] [CrossRef]
- Amare, M. Electrochemical Determination of AA in Pharmaceutical Tablets Using Carbon Paste Electrode. Org. Med. Chem. Int. J. 2019, 8, 124–132. [Google Scholar] [CrossRef]
- Hamam, A.; Maiza, M. Voltammetric Determination of AA Using Cu Foil Electrode. Int. J. Electrochem. Sci. 2021, 16, 210426. [Google Scholar] [CrossRef]
- Alemu, T.; Zelalem, B.; Amare, N. Voltammetric Determination of AA Content in Cabbage Using Anthraquinone Modified Carbon Paste Electrode. J. Chem. 2022, 2022, 7154170. [Google Scholar] [CrossRef]
- Parkook, F.; Shahvandi, S.K.; Ghaedi, M.; Javadian, H.; Parkook, A. Determination of AA in Biological Samples Using an Electrochemical Sensor Modified with Au-Cu2O/MWCNTs Nanocomposite. Diam. Relat. Mater. 2024, 144, 110954. [Google Scholar] [CrossRef]
- Rončević, I.Š.; Skroza, D.; Vrca, I.; Kondža, A.M.; Vladislavić, N. Development and Optimization of Electrochemical Method for Determination of Vitamin C. Chemosensors 2022, 10, 283. [Google Scholar] [CrossRef]
- Gülen, M.; Taş, R.; Dünya, H.; Rajendrachari, S. Enlargement of the Surface Area of High-Entropy Alloys with Acid Treatment as Positive Electrode for High Specific Capacitance Supercapacitors. ChemistryOpen 2025. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Mlcek, J.; Sochor, J.; Baron, M.; Kynicky, J.; Jurikova, T. Determination of AA by Electrochemical Techniques and Other Methods. Int. J. Electrochem. Sci. 2015, 10, 2421–2431. [Google Scholar] [CrossRef]
- Thbayh, D.K.; Reizer, E.; Kahaly, M.U.; Viskolcz, B.; Fiser, B. Antioxidant Potential of Santowhite as Synthetic and AA as Natural Polymer Additives. Polymers 2022, 14, 3518. [Google Scholar] [CrossRef]
- Ramkumar, R.; Veerakumar, P.; Rajendrachari, S.; Dhakal, G.; Yun, J.; Shim, J.-J.; Kim, W.K. Copper Aerogel Frameworks—Electrochemical Detection of Dopamine and Catalytic Reduction of 4-Nitrophenol. Microchem. J. 2024, 208, 112486. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Bužga, M.; Machytka, E.; Dvořáčková, E.; Švagera, Z.; Stejskal, D.; Máca, J.; Král, J. Methylene Blue: A Controversial Diagnostic Acid and Medication? Toxicol. Res. 2022, 11, 711–717. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Melnik, E.; Kuzina, V.; Klochko, M.; Reshetov, I.; Shiryaev, A.; Loschenov, V.; Ramenskaya, G. MBin Anticancer Photodynamic Therapy: Systematic Review of Preclinical Studies. Front. Pharmacol. 2023, 14, 1264961. [Google Scholar] [CrossRef]
- Xiong, Z.-M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of MBfor Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Photocatalytic Degradation of MBunder Natural Sunlight Using Iron Titanate Nanoparticles Prepared by a Modified Sol–Gel Method. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of MBfrom Textile Industrial Wastewater Using Activated Carbon Developed from Rumex Abyssinicus Plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef] [PubMed]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. MBDye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-U.; Ahn, Y.; Son, C.T.; et al. Recent and Emerging Trends in Remediation of MBDye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749. [Google Scholar] [CrossRef]
- Mulushewa, Z.; Dinbore, W.T.; Ayele, Y. Removal of MBfrom Textile Waste Water Using Kaolin and Zeolite-x Synthesized from Ethiopian Kaolin. Environ. Anal. Health Toxicol. 2021, 36, e2021007. [Google Scholar] [CrossRef]
- Vutskits, L.; Briner, A.; Klauser, P.; Gascon, E.; Dayer, A.G.; Kiss, J.Z.; Muller, D.; Licker, M.J.; Morel, D.R. Adverse Effects of MBon the Central Nervous System. Anesthesiology 2008, 108, 684–692. [Google Scholar] [CrossRef]
- Albert, M.; Lessin, M.S.; Gilchrist, B.F. Methylene Blue: Dangerous Dye for Neonates. J. Pediatr. Surg. 2003, 38, 1244–1245. [Google Scholar] [CrossRef]
- Auerbach, S.S.; Bristol, D.W.; Peckham, J.C.; Travlos, G.S.; Hébert, C.D.; Chhabra, R.S. Toxicity and Carcinogenicity Studies of MBTrihydrate in F344N Rats and B6C3F1 Mice. Food Chem. Toxicol. 2009, 48, 169–177. [Google Scholar] [CrossRef]
- Silva, M.; Caro, V.; Guzmán, C.; Perry, G.; Areche, C.; Cornejo, A. α-Synuclein and Tau, Two Targets for Dementia. Stud. Nat. Prod. Chem. 2020, 67, 1–25. [Google Scholar]
- Saigl, Z.M. Various Adsorbents for Removal of Rhodamine B Dye: A Review. Indones. J. Chem. 2021, 21, 1039. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Bhatt, U.; Soni, V. Toxic Effects of Rhodamine B on Antioxidant System and Photosynthesis of Hydrilla Verticillata. J. Hazard. Mater. Lett. 2022, 3, 100069. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Kumar, P.S.; Mohamed, R.M.S.R.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of Rhodamine B from Textile Wastewater Using Nanoparticle Photocatalysts: A Review for Sustainable Approaches. Chemosphere 2021, 287, 132162. [Google Scholar] [CrossRef] [PubMed]
- Khubiev, O.M.; Egorov, A.R.; Semenkova, D.I.; Salokho, D.S.; Golubev, R.A.; Sikaona, N.D.; Lobanov, N.N.; Kritchenkov, I.S.; Tskhovrebov, A.G.; Kirichuk, A.A.; et al. Rhodamine B-Containing Chitosan-Based Films: Preparation, Luminescent, Antibacterial, and Antioxidant Properties. Polymers 2024, 16, 755. [Google Scholar] [CrossRef] [PubMed]
- Chadelaud, T.; Zeghioud, H.; De La Garza, A.R.; Fuerte, O.; Benítez-Rico, A.; Revel, M.; Chávez-Miyauchi, T.E.; Djelal, H. Comparative Study of Rhodamine B Treatment: Assessing of Efficiency Processes and Ecotoxicity of By-Products. Processes 2023, 11, 2671. [Google Scholar] [CrossRef]
- Kapoor, K.; Bala, M.; Choudhary, P.; Mridula, D.; Singh, R.K. Development of Method for Detection of Rhodamine B Dye in Chilli Powder (Capsicum annuum L.). Asian J. Dairy Food Res. 2022, 43, 539–543. [Google Scholar] [CrossRef]
- Vizuete, G.; Santana-Romo, F.; Almeida-Naranjo, C.E. Virtual Screening of Fluorescent Heterocyclic Molecules and Advanced Oxidation Degradation of Rhodamine B in Synthetic Solutions. Water 2024, 16, 2141. [Google Scholar] [CrossRef]
- Smith, A.D.; Kim, Y.-I.; Refsum, H. Is Folic Acid Good for Everyone? Am. J. Clin. Nutr. 2008, 87, 517–533. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of FA in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Pamunuwa, G.; Nilakshi, H.; Rajapaksha, G.; Shakoor, F.; Karunaratne, D.N. Sensory and Physicochemical Properties and Stability of FA in a Pineapple Ready-to-Serve Beverage Fortified with Encapsulated FA. J. Food Qual. 2021, 2021, 9913884. [Google Scholar] [CrossRef]
- Pertiwi, H.; Mahendra, M.Y.N.; Kamaludeen, J. FA: Sources, Chemistry, Absorption, Metabolism, Beneficial Effects on Poultry Performance and Health. Vet. Med. Int. 2022, 2022, 2163756. [Google Scholar] [CrossRef]
- Ismail, S.; Eljazzar, S.; Ganji, V. Intended and Unintended Benefits of FA Fortification—A Narrative Review. Foods 2023, 12, 1612. [Google Scholar] [CrossRef]
- Ajeigbe, K.; Aibangbee, K.; Saeed, S.; Ajeigbe, O.; Onifade, A. FA Protects and Heals Gastric Mucosa: Role of Acid Output, Inflammatory Cytokines, Angiogenic and Growth Factors. J. Basic Appl. Zool. 2022, 83, 15. [Google Scholar] [CrossRef]
- Pérez-Carreón, K.; Martínez, L.M.; Videa, M.; Cruz-Angeles, J.; Gómez, J.; Ramírez, E. Effect of Basic Amino Acids on FA Solubility. Pharmaceutics 2023, 15, 2544. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.A.; Noble, N.; Radhika, N.; Saleh, B. A Comprehensive Review on Advances in High Entropy Alloys: Fabrication and Surface Modification Methods, Properties, Applications, and Future Prospects. J. Manuf. Process. 2023, 109, 583–606. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. Mechanically Alloyed High Entropy Alloys: Existing Challenges and Opportunities. J. Mater. Res. Technol. 2022, 17, 2431–2456. [Google Scholar] [CrossRef]
- Xie, X.; Li, N.; Liu, W.; Huang, S.; He, X.; Yu, Q.; Xiong, H.; Wang, E.; Hou, X. Research Progress of Refractory High Entropy Alloys: A Review. Chin. J. Mech. Eng. 2022, 35, 142. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-Entropy Alloys: Emerging Materials for Advanced Functional Applications. J. Mater. Chem. A 2020, 9, 663–701. [Google Scholar] [CrossRef]
- Rajendrachari, S. An Overview of High-Entropy Alloys Prepared by Mechanical Alloying Followed by the Characterization of Their Microstructure and Various Properties. Alloys 2022, 1, 116–132. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-Entropy Alloys by Mechanical Alloying: A Review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Custodio, A.G.D.; Saha, G.C.; Aranas, C. Mechanical Alloying of AlCoCrFe and AlCoCrFeNi: Design and Experimental Evaluation of Medium- and High-Entropy Alloy Particles for Cold Spraying. Powder Technol. 2024, 437, 119556. [Google Scholar] [CrossRef]
- Santiago-Andrades, L.; Vidal-Crespo, A.; Blázquez, J.S.; Ipus, J.J.; Conde, C.F. Mechanical Alloying as a Way to Produce Metastable Single-Phase High-Entropy Alloys beyond the Stability Criteria. Nanomaterials 2023, 14, 27. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. A Critical Review on Mechanically Alloyed High Entropy Alloys: Processing Challenges and Properties. Mater. Res. Express 2022, 9, 052001. [Google Scholar] [CrossRef]
- Tsukamoto, H. Mechanical Behavior of Shape Memory Alloy Fiber Reinforced Aluminum Matrix Composites. Mater. Today Commun. 2021, 29, 102750. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Srimath, R.; Vignesh, L.; Rajesh, S. Application of Shape Memory Alloys in Engineering—A Review. J. Phys. Conf. Ser. 2021, 2054, 012078. [Google Scholar] [CrossRef]
- Yavuzer, B.; Bicakci, Ü.; Simsek, D.; Ozyurek, D. Effect of Alloying Time and Heat Treatment on Microstructure and Tribological Properties of Mechanical Alloyed CU-AL-NI Shape Memory Alloy. J. Mater. Eng. Perform. 2023, 33, 11176–11187. [Google Scholar] [CrossRef]
- Salwa, P.; Goryczka, T. Crystallization of Mechanically Alloyed NI50TI50 and TI50NI25CU25 Shape Memory Alloys. J. Mater. Eng. Perform. 2020, 29, 2848–2852. [Google Scholar] [CrossRef]
- GöGebakan, M.; Soguksu, A.K.; Uzun, O.; Dogan, A. Production of Cu-Al-Ni Shape Memory Alloys by Mechanical Alloy. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2007; Volume 899, p. 654. [Google Scholar] [CrossRef]
- Lee, T.-J.; Kim, W.-J. Effect of Severe Plastic Deformation and Post-Deformation Heat Treatment on the Microstructure and Superelastic Properties of TI-50.8 at.% Ni Alloy. Materials 2022, 15, 7822. [Google Scholar] [CrossRef]
- Manjunatha, J.G.; Kanthappa, B.; Hareesha, N.; Raril, C.; Tighezza, A.M.; Albaqami, M.D. Enhanced Electrochemical Detection of Rutin Using Poly (Methyl Orange) Modified Carbon Paste Electrode as a Responsive Electrochemical Sensor. Chem. Afr. 2023, 7, 1141–1150. [Google Scholar] [CrossRef]







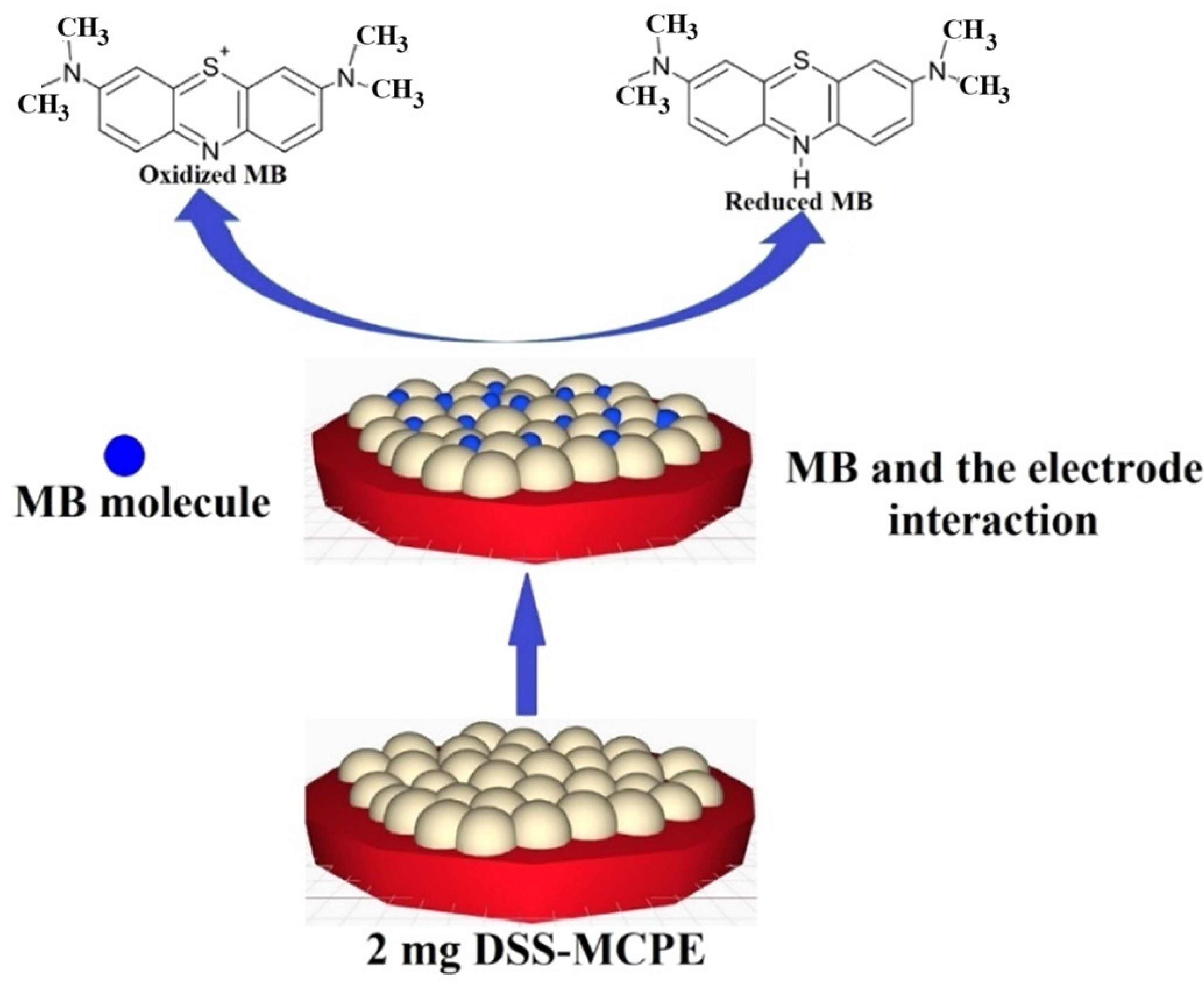
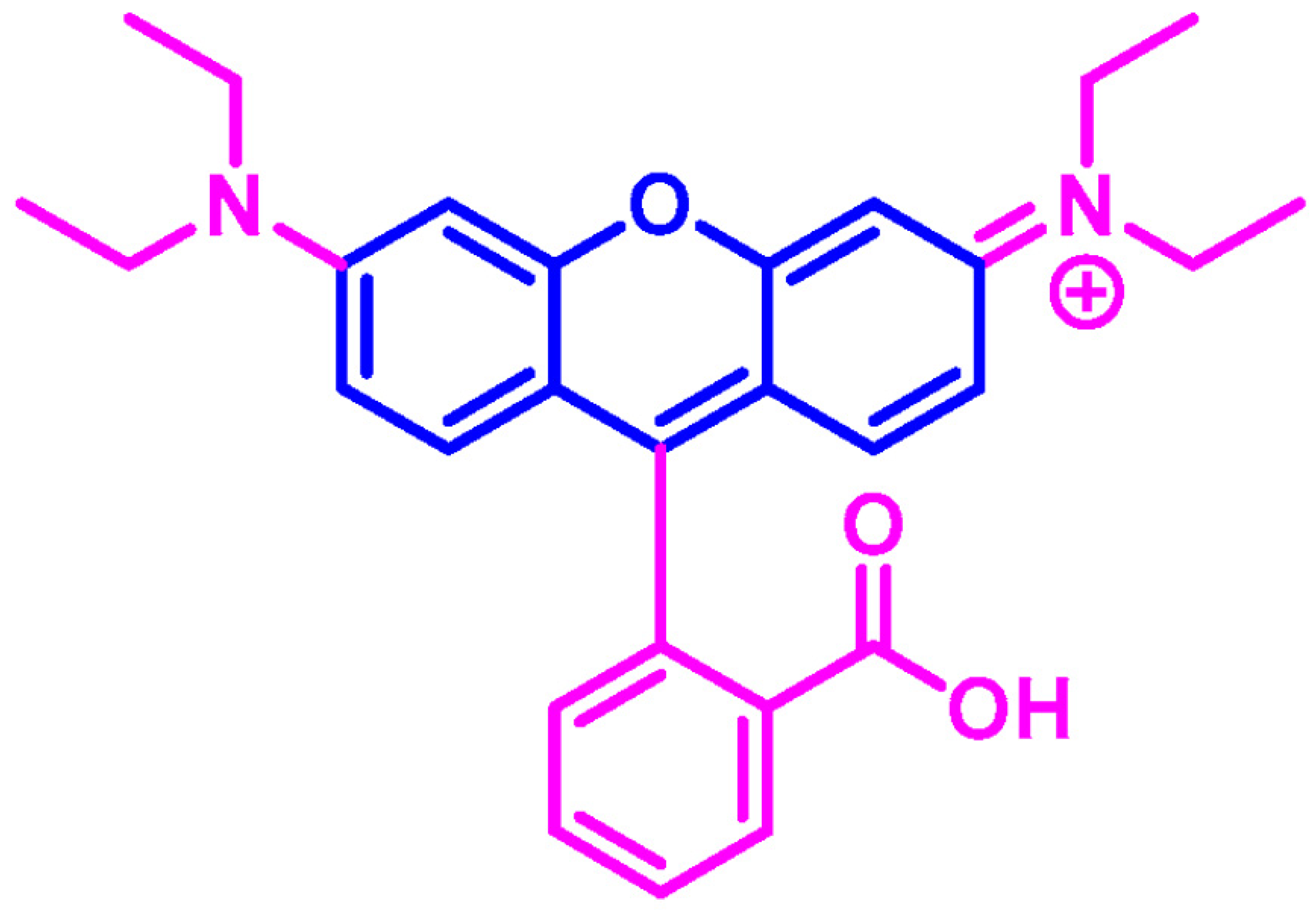
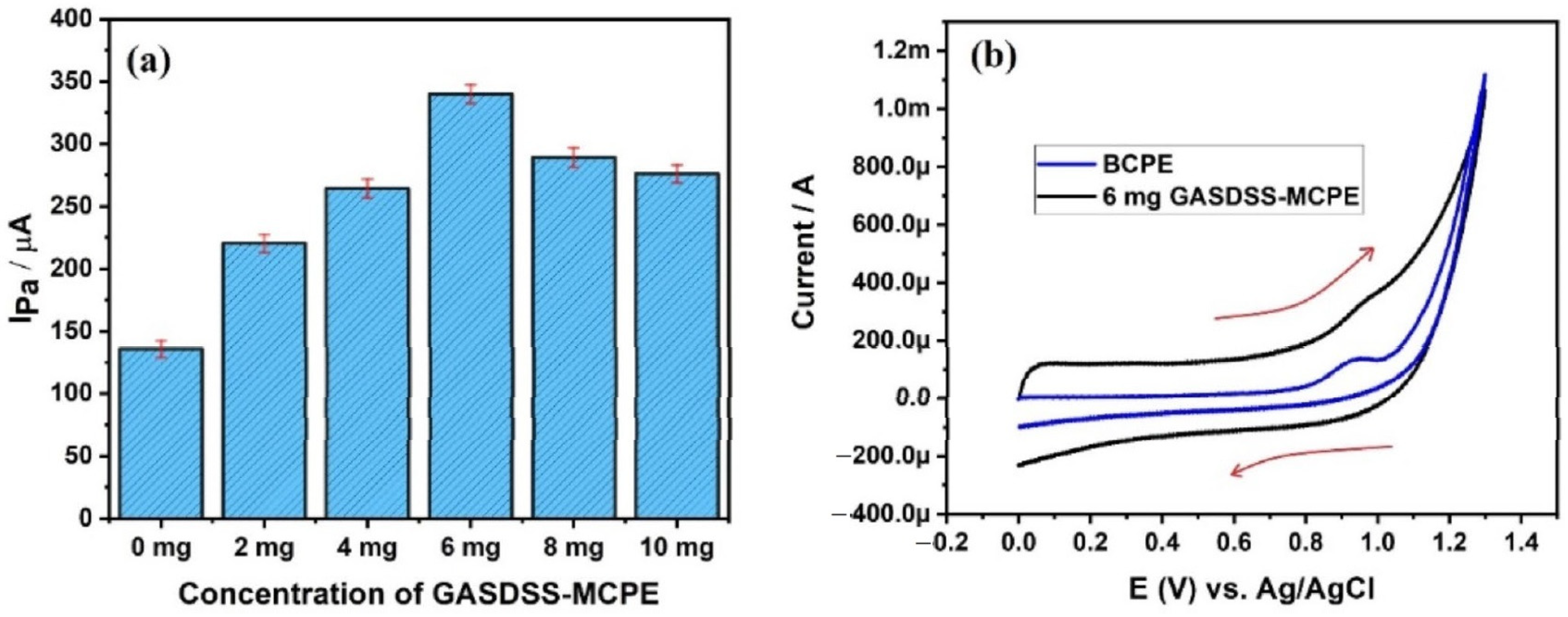

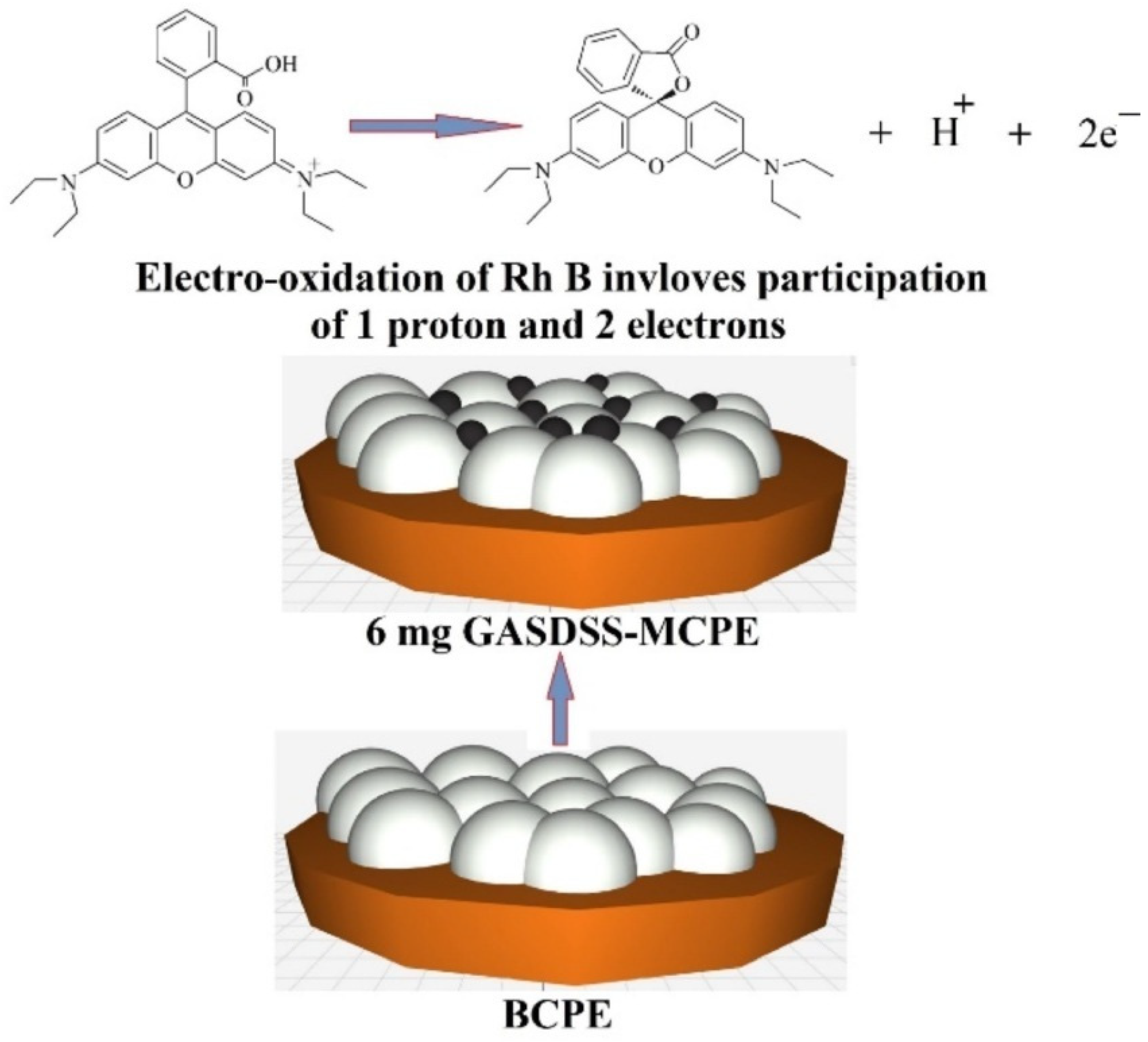
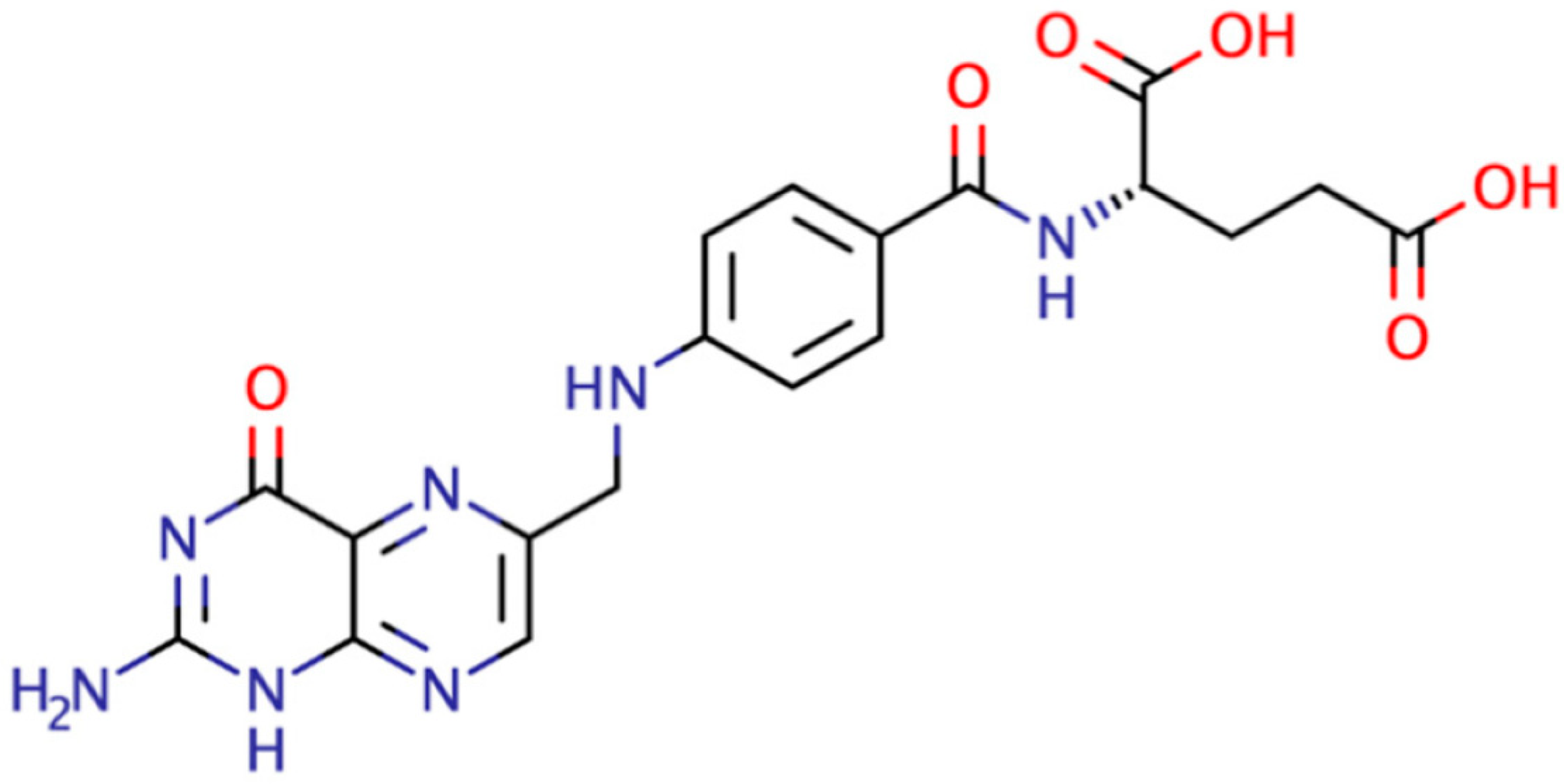


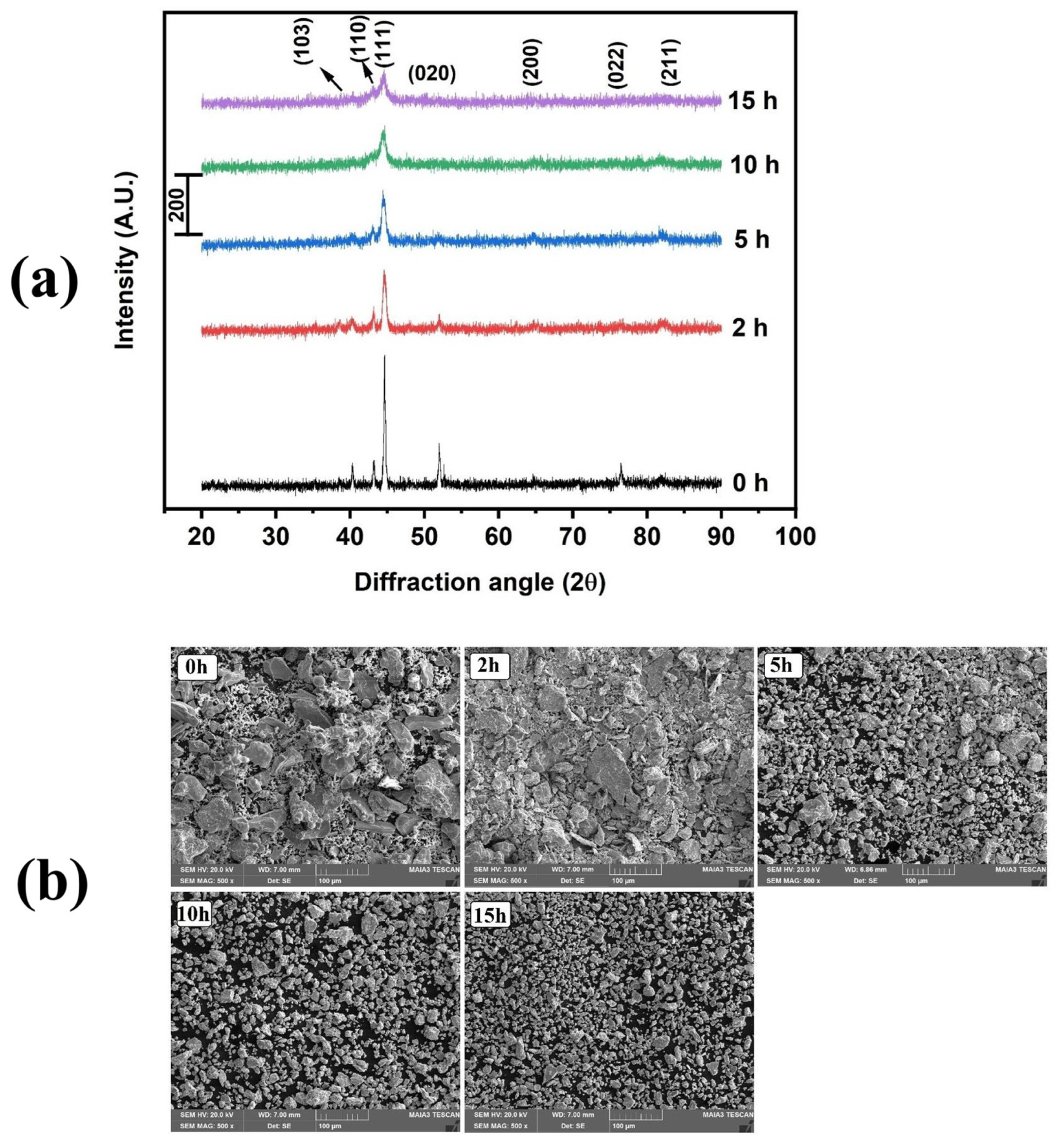


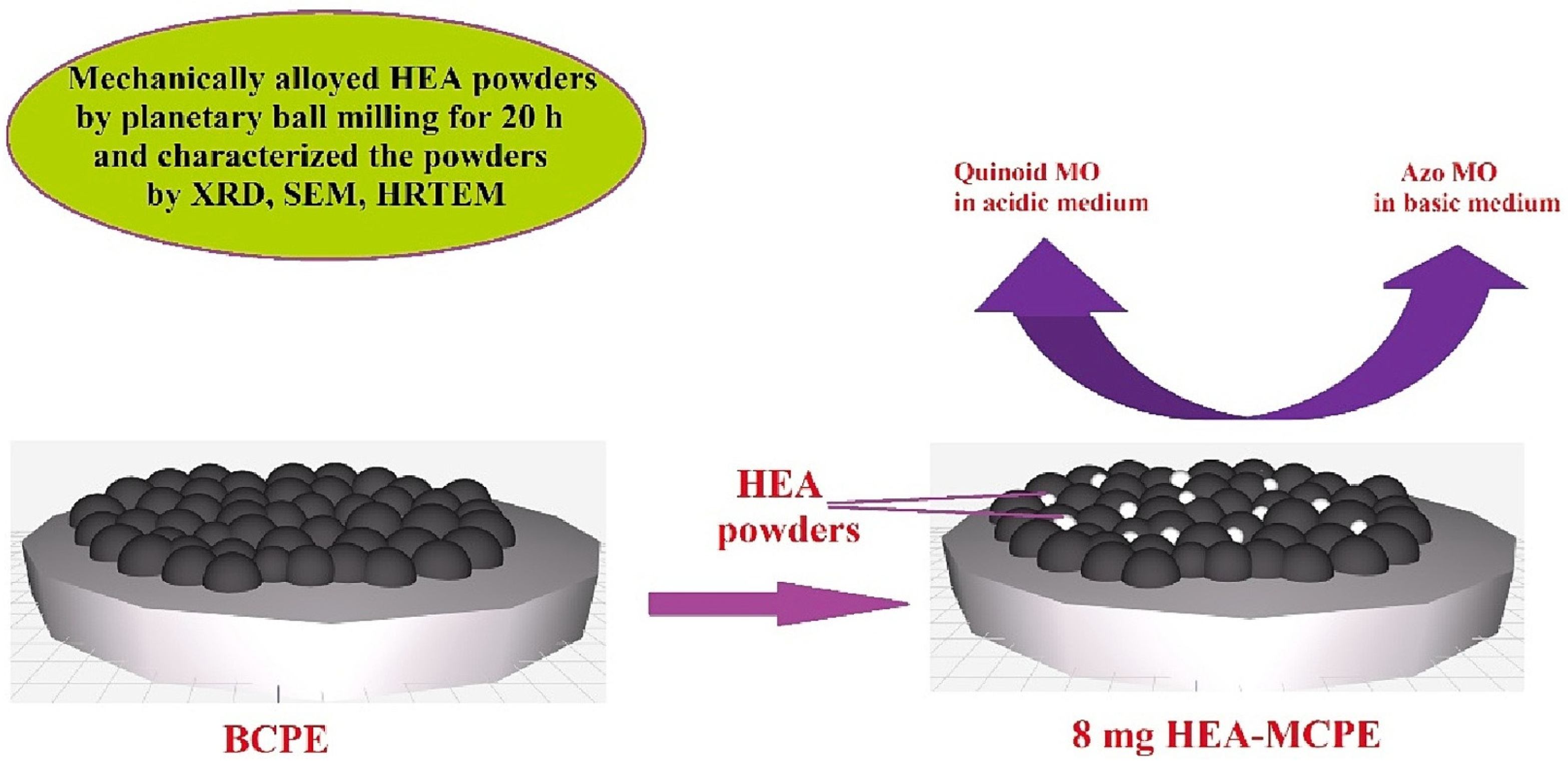

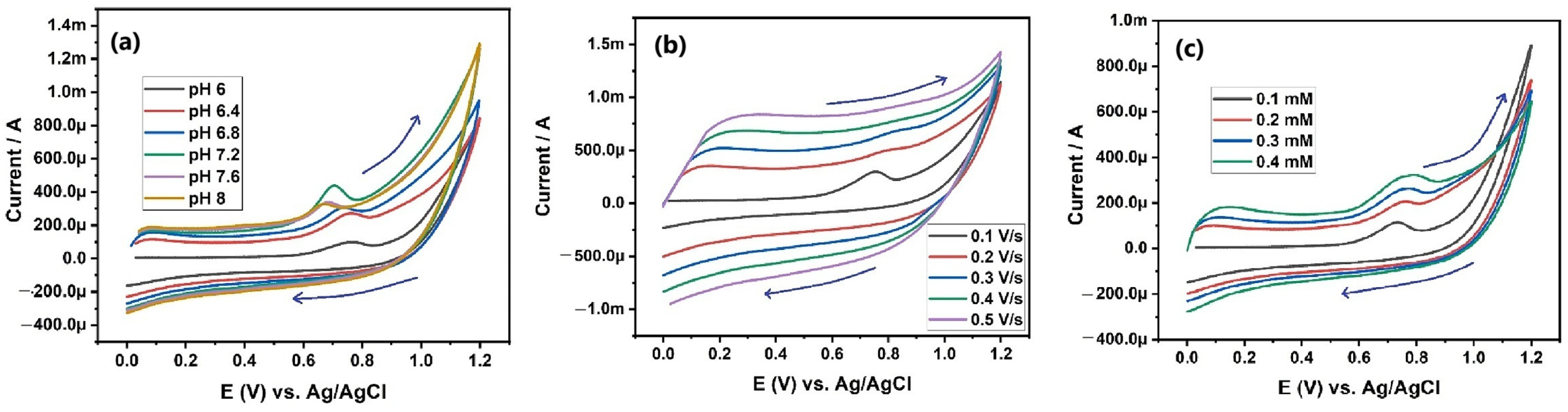



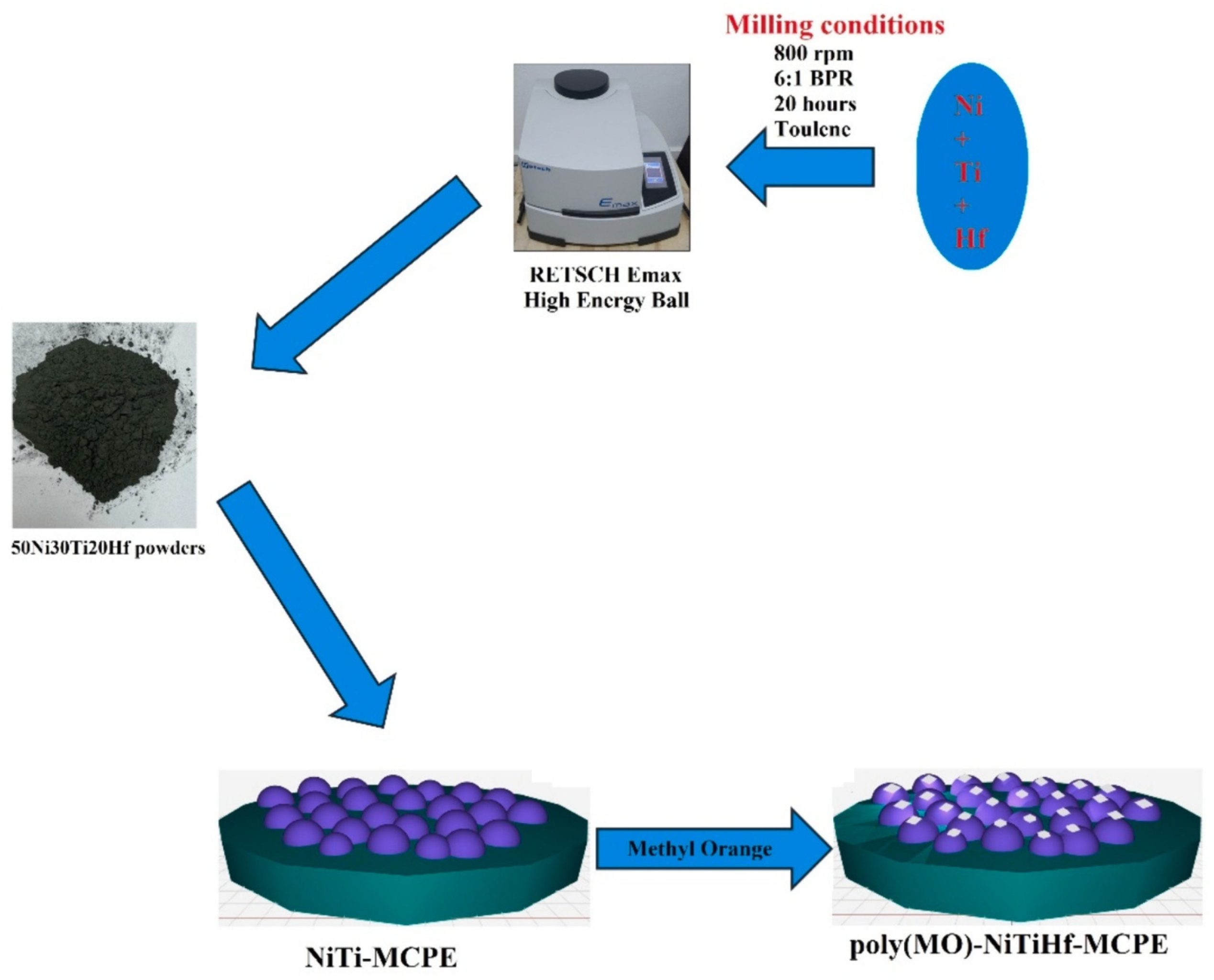
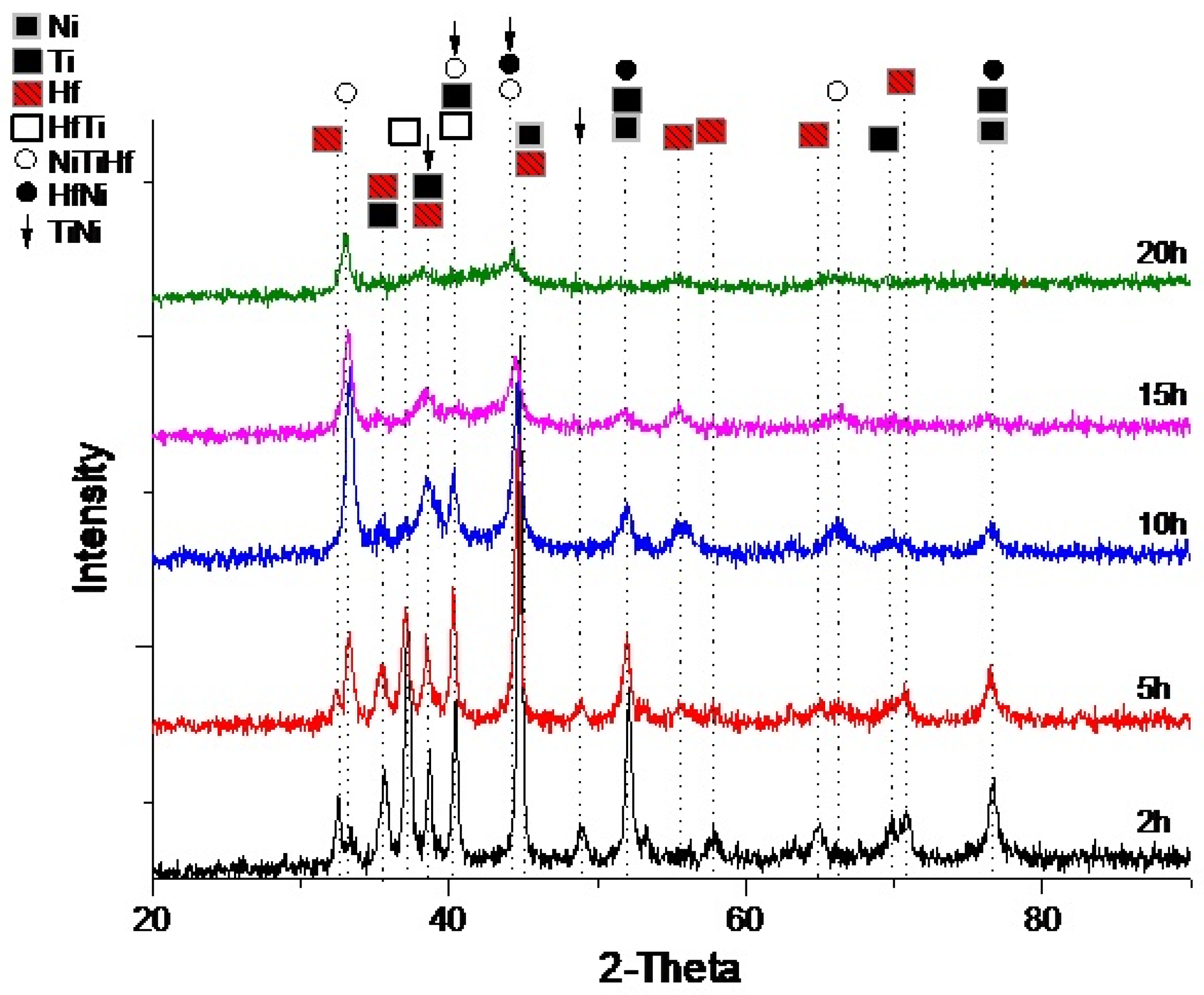
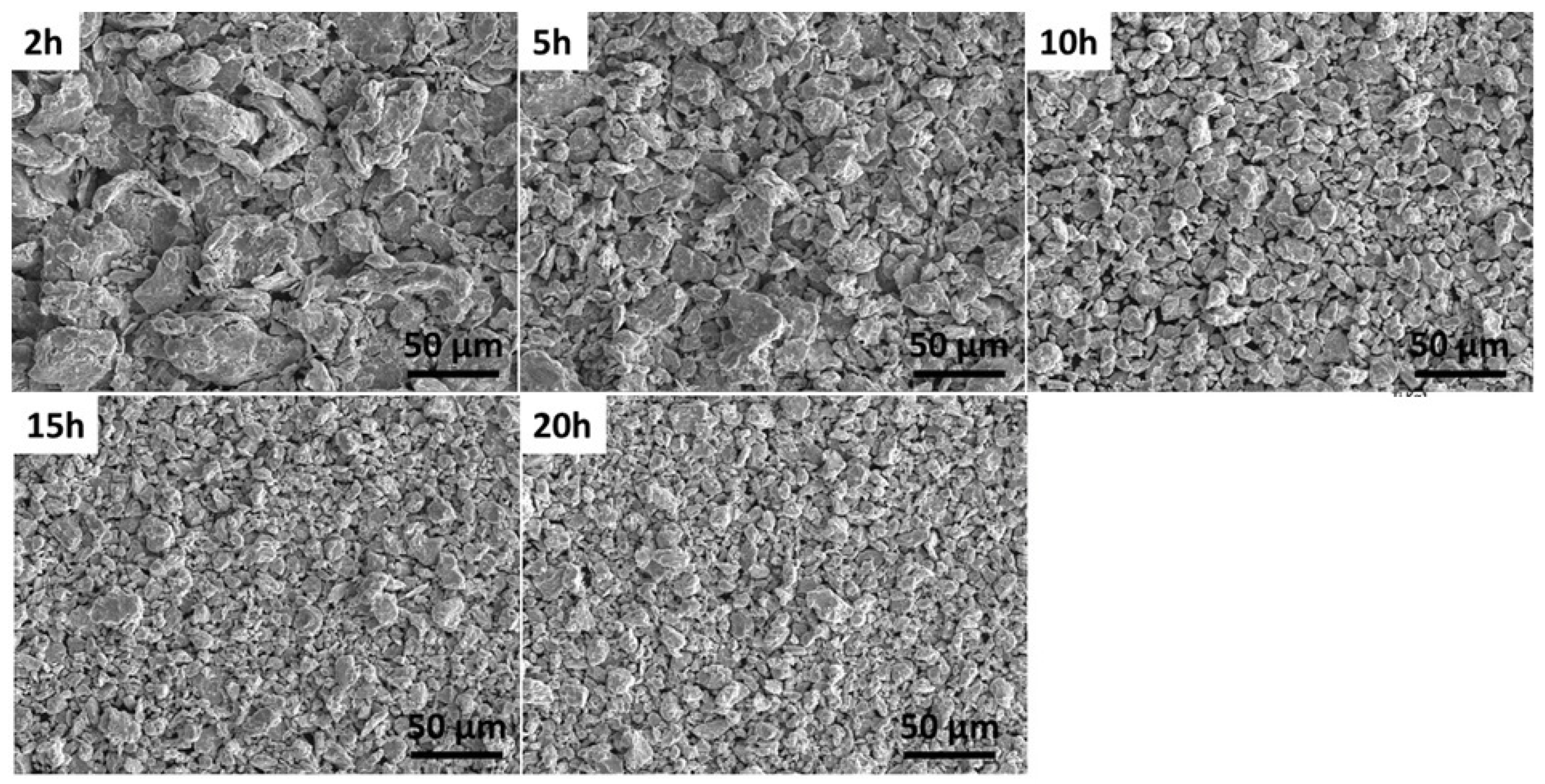
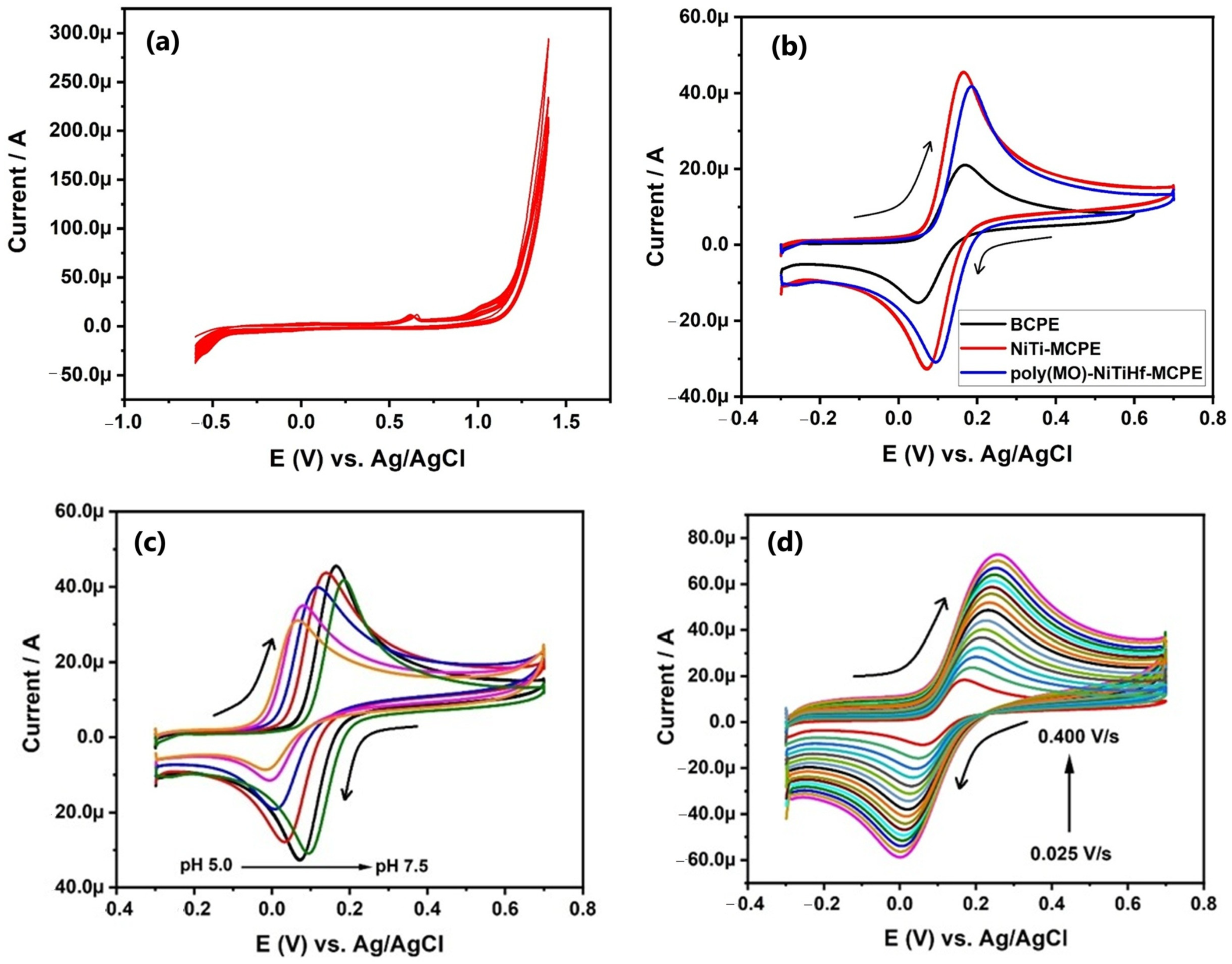

| Modifiers Used | Electrochemical Technique | Analyte Determined | Important Findings | References |
|---|---|---|---|---|
| Fe-18Cr-13Ni (stainless steel) | CV | Folic Acid | Electrode reactions were adsorption controlled. Current sensitivity of 17.32 µA was recorded. | [29] |
| Duplex stainless steel | CV | Ascorbic Acid | The stainless steel showed good current sensitivity of 143.52 µA. | [25] |
| Duplex stainless steel | CV | Uric Acid | The stainless steel showed good current sensitivity of 19.36 µA. | [25] |
| Duplex stainless steel | CV | Dopamine | The stainless steel showed good current sensitivity of 25.61 µA. | [25] |
| Yttria dispersed Fe-18Cr-13Ni | CV | Ascorbic Acid | The stainless steel showed good current sensitivity of 370 mV. | [24] |
| Yttria dispersed duplex stainless steel | CV | Uric Acid | The stainless steel showed good current sensitivity of 31.01 µA. | [24] |
| Yttria dispersed duplex stainless steel | CV | Dopamine | The stainless steel showed good current sensitivity of 28.48 µA. | [24] |
| 23Fe-21Cr-18Ni-20Ti-18Mn (HEA) | CV | Ascorbic Acid | For the concentration of 8 mg modifier, a maximum peak current of 104.07 µA was measured. For the high-entropy-alloy-modified carbon paste electrode and the bare carbon paste electrode, the active surface areas for the electron transfer process of ascorbic acid are calculated to be 0.0014 cm2 and 0.0027 cm2, respectively. | [30] |
| 25Fe-19Cr-19Ni-18Ti- 19Mn high-entropy alloy | CV | Methylene Blue | The anodic peak current of 508.4 µA was displayed by the 4 mg high-entropy-alloy-modified carbon paste electrode, while only 99.74 µA was displayed by the bare carbon paste electrode. This significant anodic peak current difference between the two different electrodes have demonstrated the value of the the modifier in enhancing the electrode sensor’s sensitivity, robustness, and selectivity. | [32] |
| 2507 super duplex stainless steel | CV | Ascorbic Acid | BCPE has shown an anodic peak current of 22.5 µA and 4 mg SDSS-MCPE has recorded 37.2 µA of anodic peak current during the electro-oxidation of 1 mM AA. LOD and LOQ were calculated to be 2.06 nM and 6.8 nM, respectively. | [26] |
| poly (methyl orange) shape memory alloy | CV | Dopamine | The calculated active surface area for BCPE, NiTiHf-MCPE, and the poly(MO)- NiTiHf-MCPE were found to be 0.044, 0.089, and 0.098 cm2, respectively. LOD and LOQ were calculated to be 5.55 μM and 18.45 μM, respectively. | [33] |
| 23Fe-21Cr-18Ni-20Ti-18Mn | CV | Methyl Orange | MO oxidizes at 700 mV. The calculated electrode surface area of BCPE and HEAMCPE was found to be 0.0546 and 0.4439 cm2, respectively. The LOD obtained is 0.080 μM. | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendrachari, S.; Chalageri, G.R.; Mahale, R.S.; Altas, E.; Chapke, Y.; Adimule, V. Investigation of Electrocatalytic Applications of Various Advanced Nanostructured Alloys—An Overview. Catalysts 2025, 15, 259. https://doi.org/10.3390/catal15030259
Rajendrachari S, Chalageri GR, Mahale RS, Altas E, Chapke Y, Adimule V. Investigation of Electrocatalytic Applications of Various Advanced Nanostructured Alloys—An Overview. Catalysts. 2025; 15(3):259. https://doi.org/10.3390/catal15030259
Chicago/Turabian StyleRajendrachari, Shashanka, Gireesha R. Chalageri, Rayappa Shrinivas Mahale, Emre Altas, Yashwant Chapke, and Vinayak Adimule. 2025. "Investigation of Electrocatalytic Applications of Various Advanced Nanostructured Alloys—An Overview" Catalysts 15, no. 3: 259. https://doi.org/10.3390/catal15030259
APA StyleRajendrachari, S., Chalageri, G. R., Mahale, R. S., Altas, E., Chapke, Y., & Adimule, V. (2025). Investigation of Electrocatalytic Applications of Various Advanced Nanostructured Alloys—An Overview. Catalysts, 15(3), 259. https://doi.org/10.3390/catal15030259








