Catalytic Applications of Natural Iron Oxides and Hydroxides: A Review
Abstract
1. Introduction
Geological Abundance of Iron Oxide and Hydroxide Minerals
2. Most Prevalent Iron Oxides and Hydroxides
2.1. Magnetite (Fe3O4)
2.2. Hematite (α-Fe2O3)
2.3. Goethite (α-FeOOH)
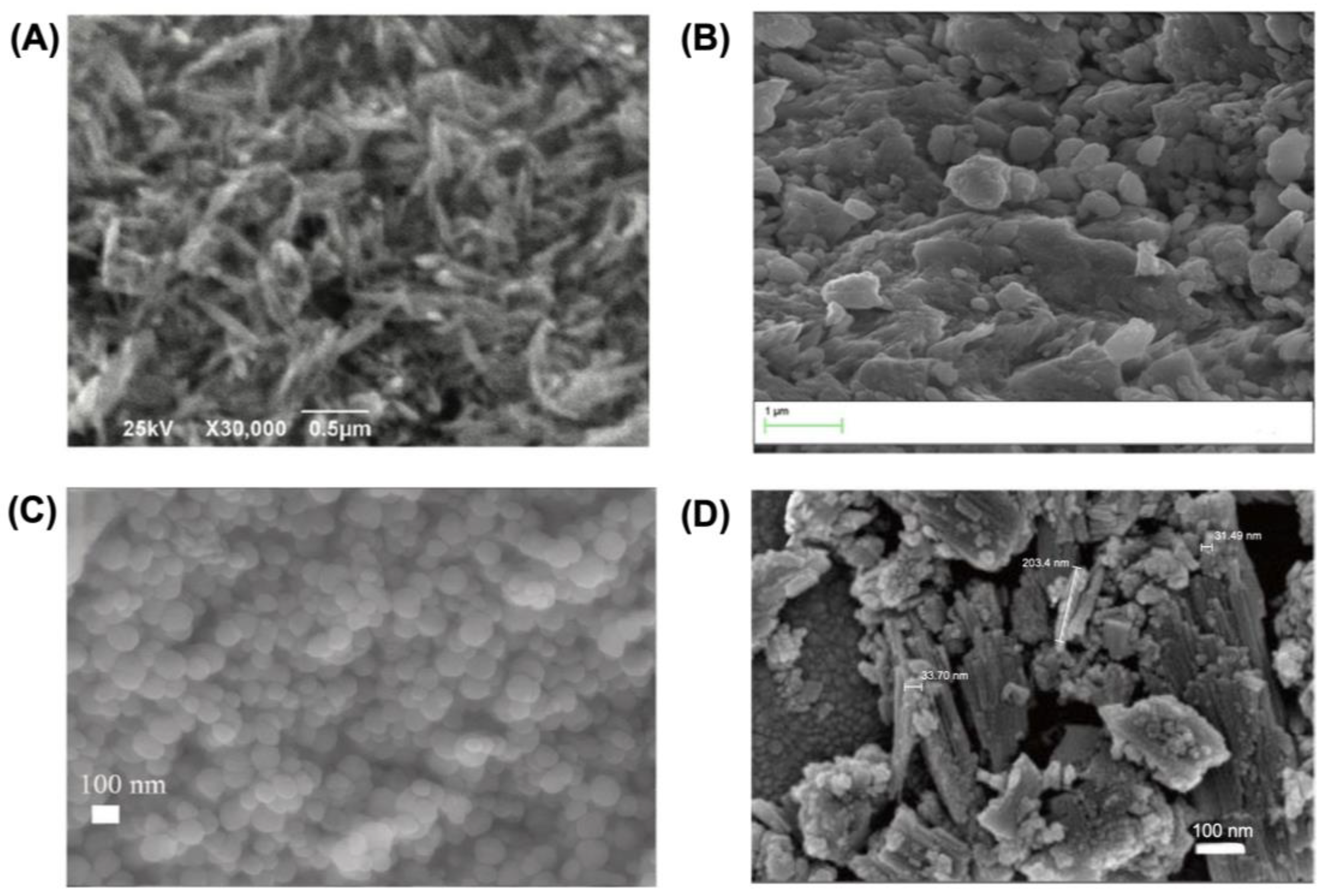
3. Less Abundant but Widely Distributed Fe-OH
3.1. Maghemite (γ-Fe2O3)
3.2. Lepidocrocite (γ-FeOOH)
3.3. Ferrihydrite (Fe8.2O8.5(OH)7.4 + 3H2O)
4. Rare Iron Oxides and Hydroxides
4.1. Wüstite (FeO)
4.2. Akaganéite (β-FeOOH)
4.3. Feroxyhyte (δ′-FeOOH)
4.4. Bernalite (Fe(OH)3)
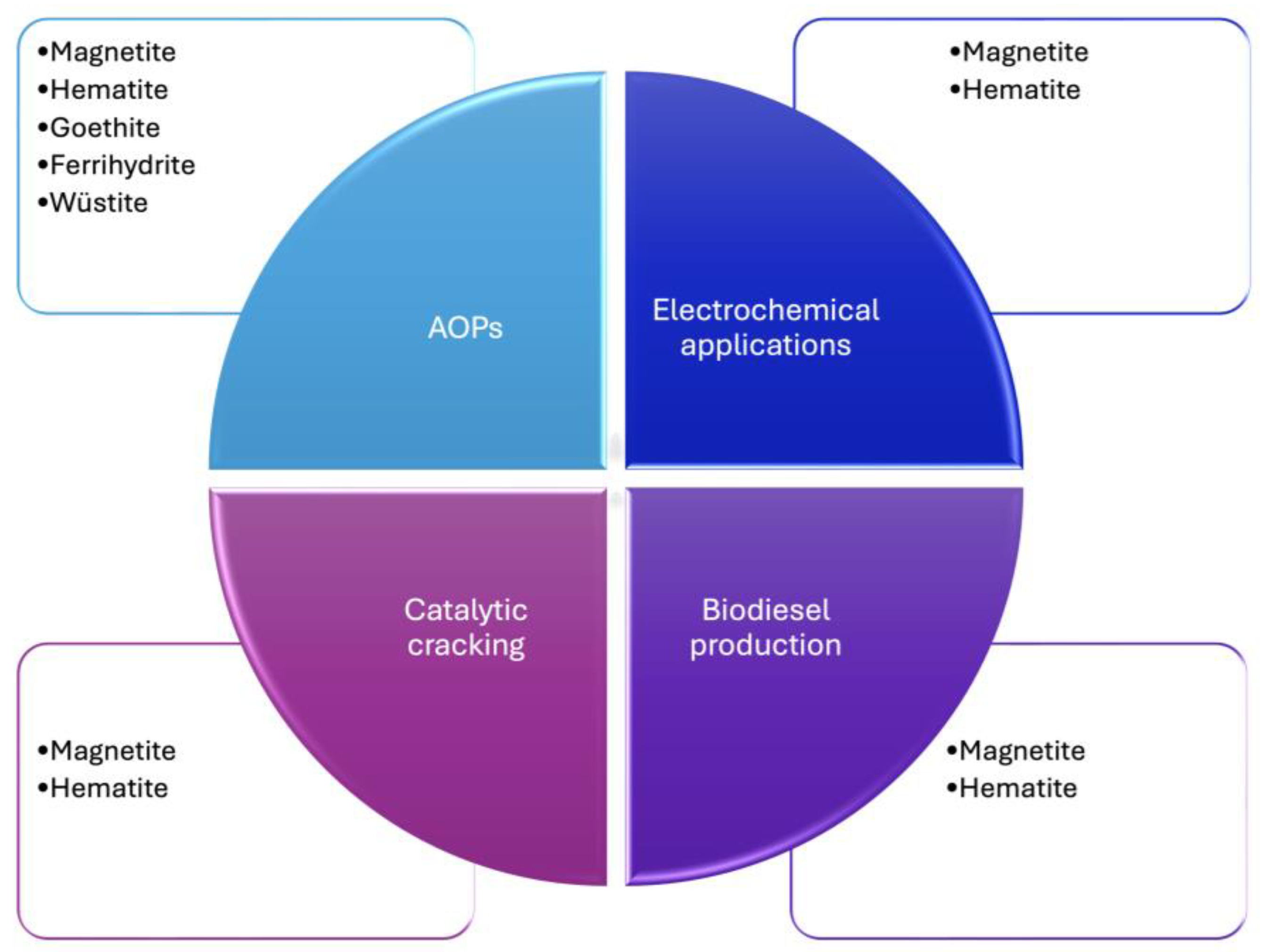
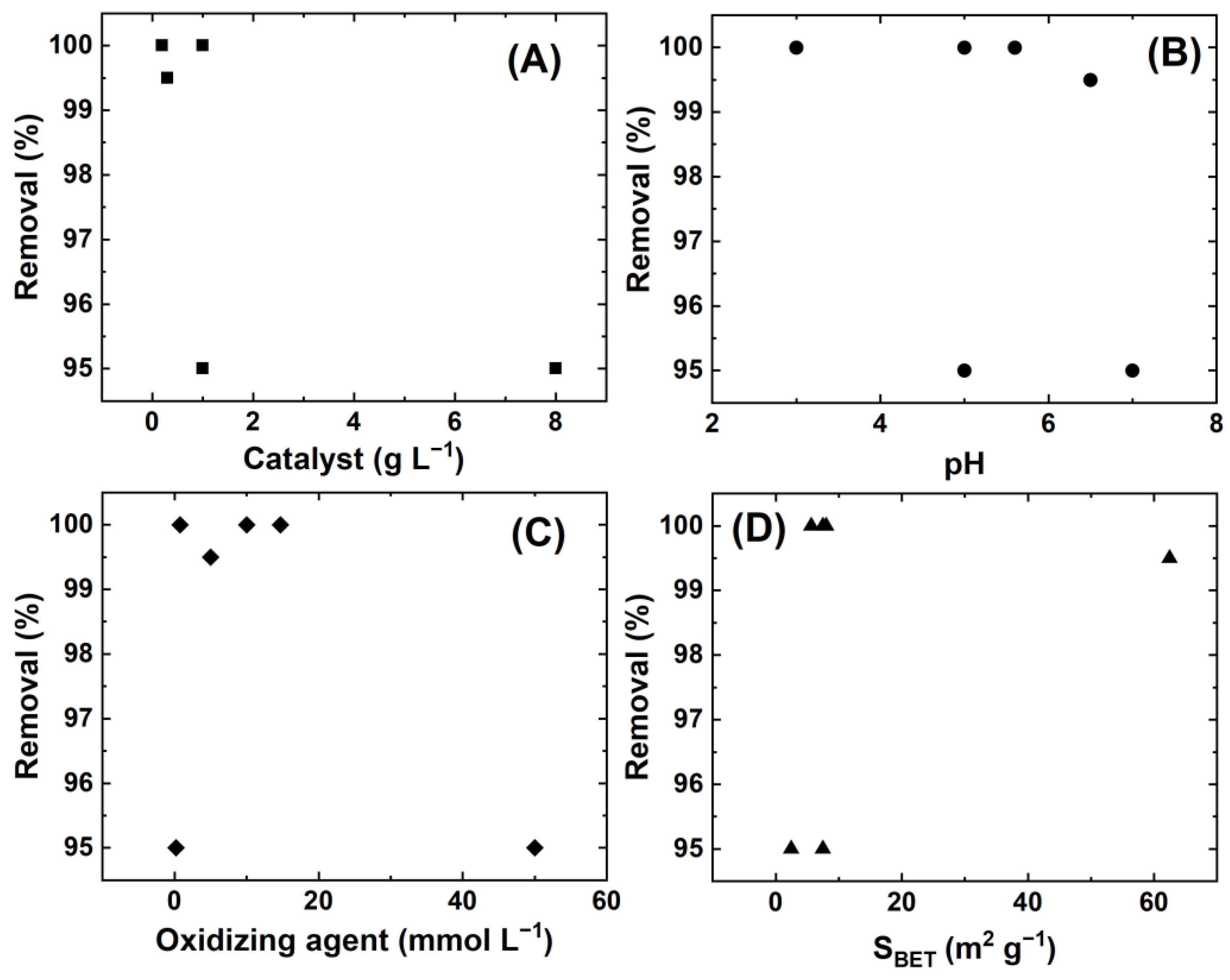
5. Future Directions and Perspectives on Catalytic Systems
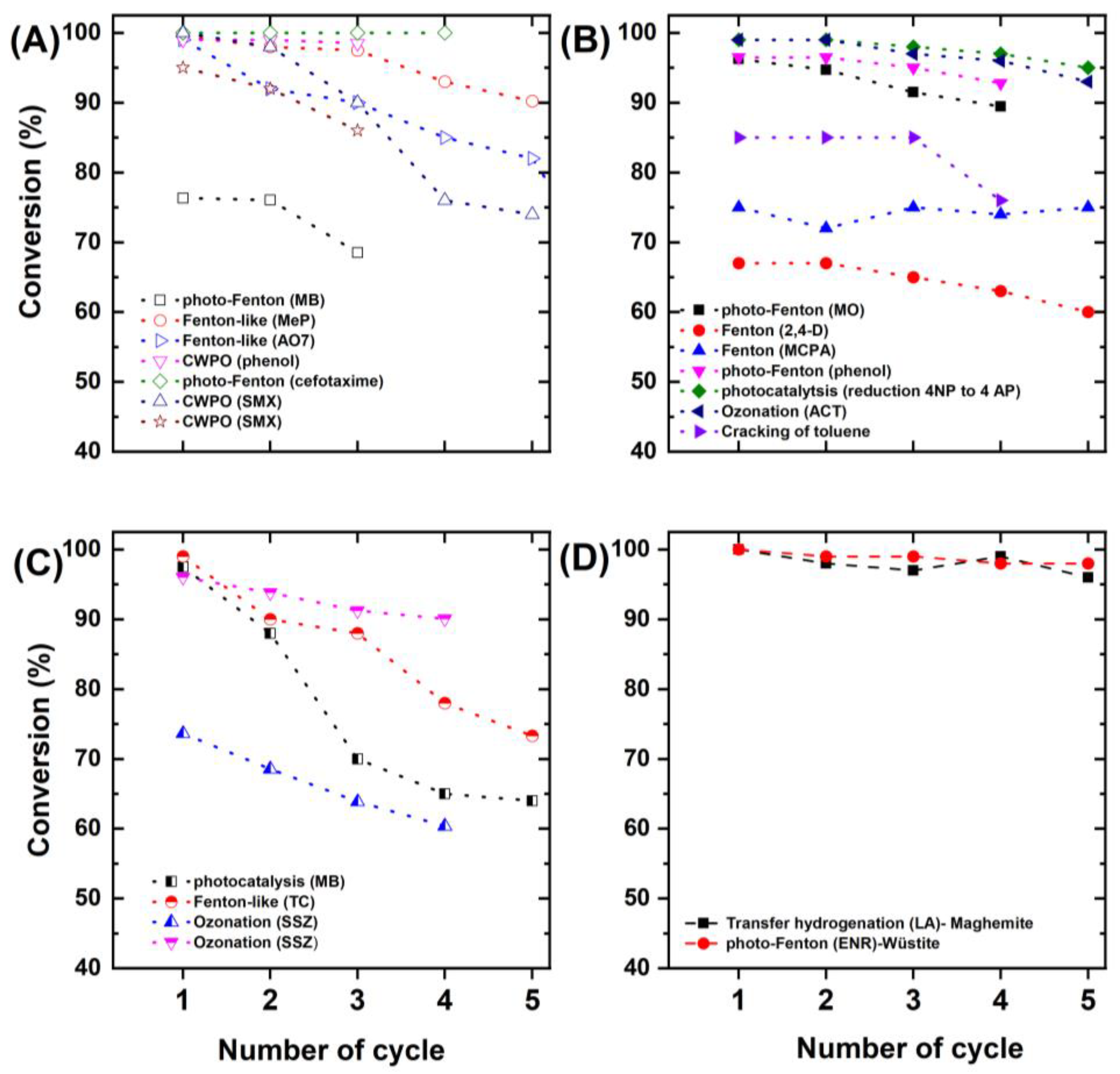
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| 4AP | 4-aminophenol |
| 4-NP | 4-nitrophenol |
| ACT | Acetaminophen |
| AO7 | Acid orange 7 |
| AOP | Advanced oxidation process |
| ATR-FTIR | Attenuated total reflection–Fourier transform infrared spectroscopy |
| BIFs | Banded iron formations |
| BSA | 5-bromosalicylic acid |
| CAP | Chloramphenicol |
| CGW | Contaminated groundwater |
| COD | Chemical oxygen demand |
| CWPO | Catalytic wet peroxide oxidation |
| DCF | Diclofenac |
| DFT | Density functional theory |
| DTA | Differential thermal analysis |
| EDTA | Ethylenediaminetetracetic acid |
| ENR | Enrofloxacin |
| FBR | Fixed-bed reactor |
| Fe-OH | Iron oxides and iron hydroxides |
| GC/MS | Gas chromatography–mass spectrometry |
| GHSV | Gas hourly space velocity |
| GVL | Gamma-valerolactone |
| Hc | Coercivity |
| HER | Hydrogen evolution reaction |
| HRS | Hormuz red soil |
| HTCT | High-temperature catalytic treatment |
| IC | Indigo carmine |
| IMA | International Mineralogical Association |
| IR | Infrared spectroscopy |
| LA | Levulinic acid |
| LEV | Levofloxacin |
| MB | Methylene blue |
| MC-LR | Microcystin-LR |
| MCPA | 2-methyl-4-chlorophenoxyacetic acid |
| MeP | Methylparaben |
| MFCs | Microbial fuel cells |
| MM | Monodentate mononuclear |
| MO | Methyl orange |
| Mr | Remanent magnetization |
| NPs | Nanoparticles |
| OCs | Oxygen carriers |
| OER | Oxygen evolution reaction |
| ORR | Oxygen reduction reaction |
| OG | Orange G |
| PANI | Polyaniline |
| p-NP | p-nitrophenol |
| PS | Persulfate |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| SMX | Sulfamethoxazole |
| SPS | Persulfate |
| SSZ | Sulfasalazine antibiotic |
| TC | Tetracycline |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| TM | Temperature of Morin transition |
| WFFDBD | Water falling film dielectric barrier discharge |
| WWTP | Wastewater treatment plant |
| XANES | X-ray absorption near-edge structure |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural Magnetite: An Efficient Catalyst for the Degradation of Organic Contaminant. Sci. Rep. 2015, 5, 10139. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527302741. [Google Scholar]
- Lagroix, F.; Banerjee, S.K.; Jackson, M.J. Geological Occurrences and Relevance of Iron Oxides. In Iron Oxides; Faivre, D., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 7–30. [Google Scholar]
- Jambor, J.L.; Dutrizac, J.E. Occurrence and Constitution of Natural and Synthetic Ferrihydrite, a Widespread Iron Oxyhydroxide. Chem. Rev. 1998, 98, 2549–2586. [Google Scholar] [CrossRef] [PubMed]
- Misra, K.C. Understanding Mineral Deposits; Springer: Dordrecht, The Netherlands, 2000; ISBN 978-94-010-5752-3. [Google Scholar]
- Schwertmann, U.; Fitzpatrick, R. Iron Minerals in Surface Environments. Catena Suppl. 1992, 21, 7–30. [Google Scholar]
- Dowlath, M.J.H.; Musthafa, S.A.; Mohamed Khalith, S.B.; Varjani, S.; Karuppannan, S.K.; Ramanujam, G.M.; Arunachalam, A.M.; Arunachalam, K.D.; Chandrasekaran, M.; Chang, S.W.; et al. Comparison of Characteristics and Biocompatibility of Green Synthesized Iron Oxide Nanoparticles with Chemical Synthesized Nanoparticles. Environ. Res. 2021, 201, 111585. [Google Scholar] [CrossRef]
- Patil, R.M.; Shete, P.B.; Thorat, N.D.; Otari, S.V.; Barick, K.C.; Prasad, A.; Ningthoujam, R.S.; Tiwale, B.M.; Pawar, S.H. Superparamagnetic Iron Oxide/Chitosan Core/Shells for Hyperthermia Application: Improved Colloidal Stability and Biocompatibility. J. Magn. Magn. Mater. 2014, 355, 22–30. [Google Scholar] [CrossRef]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and Design of Biologically Inspired Biocompatible Iron Oxide Nanoparticles for Biomedical Applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, L.C.A.; Murad, E. Iron Oxide Catalysts: Fenton and Fentonlike Reactions—A Review. Clay Min. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Raman, A.A.; Wan Daud, W.M.A. Review on the Application of Modified Iron Oxides as Heterogeneous Catalysts in Fenton Reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Wu, Q.; Siddique, M.S.; Wang, H.; Cui, L.; Wang, H.; Pan, M.; Yan, J. Visible-Light-Driven Iron-Based Heterogeneous Photo-Fenton Catalysts for Wastewater Decontamination: A Review of Recent Advances. Chemosphere 2023, 313, 137509. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Hubáček, J.; Tomas, M.; Bačiak, M.; Vakili, M. Production of Light Olefins via Fischer-Tropsch Process Using Iron-Based Catalysts: A Review. Catalysts 2022, 12, 174. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Mubenesha, S. The Use of Iron Ore as a Catalyst in Fischer–Tropsch Synthesis—A Review. Crystals 2022, 12, 1349. [Google Scholar] [CrossRef]
- Ramadhani, B.; Kivevele, T.; Kihedu, J.H.; Jande, Y.A.C. Catalytic Tar Conversion and the Prospective Use of Iron-Based Catalyst in the Future Development of Biomass Gasification: A Review. Biomass Convers. Biorefinery 2022, 12, 1369–1392. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High-Temperature Water–Gas Shift (HT-WGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, X.; Zhao, Y.; Wang, H. In-Situ Soil Remediation via Heterogeneous Iron-Based Catalysts Activated Persulfate Process: A Review. Chem. Eng. J. 2022, 431, 133833. [Google Scholar] [CrossRef]
- Cui, B.; Tian, T.; Duan, L.; Rong, H.; Chen, Z.; Luo, S.; Guo, D.; Naidu, R. Towards Advanced Removal of Organics in Persulfate Solution by Heterogeneous Iron-Based Catalyst: A Review. J. Environ. Sci. 2024, 146, 163–175. [Google Scholar] [CrossRef]
- Sang, W.; Xu, X.; Zhan, C.; Lu, W.; Jia, D.; Wang, C.; Zhang, Q.; Gan, F.; Li, M. Recent Advances of Antibiotics Degradation in Different Environment by Iron-Based Catalysts Activated Persulfate: A Review. J. Water Process Eng. 2022, 49, 103075. [Google Scholar] [CrossRef]
- Lai, L.; He, Y.; Zhou, H.; Huang, B.; Yao, G.; Lai, B. Critical Review of Natural Iron-Based Minerals Used as Heterogeneous Catalysts in Peroxide Activation Processes: Characteristics, Applications and Mechanisms. J. Hazard. Mater. 2021, 416, 125809. [Google Scholar] [CrossRef]
- Roy, S.D.; Das, K.C.; Dhar, S.S. Conventional to Green Synthesis of Magnetic Iron Oxide Nanoparticles; Its Application as Catalyst, Photocatalyst and Toxicity: A Short Review. Inorg. Chem. Commun. 2021, 134, 109050. [Google Scholar] [CrossRef]
- Ruan, Y.; Kong, L.; Zhong, Y.; Diao, Z.; Shih, K.; Hou, L.; Wang, S.; Chen, D. Review on the Synthesis and Activity of Iron-Based Catalyst in Catalytic Oxidation of Refractory Organic Pollutants in Wastewater. J. Clean. Prod. 2021, 321, 128924. [Google Scholar] [CrossRef]
- Azfar Shaida, M.; Verma, S.; Talukdar, S.; Kumar, N.; Salim Mahtab, M.; Naushad, M.; Haq Farooqi, I. Critical Analysis of the Role of Various Iron-Based Heterogeneous Catalysts for Advanced Oxidation Processes: A State of the Art Review. J. Mol. Liq. 2023, 374, 121259. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of Magnetite-Based Catalysts and Their Application in Heterogeneous Fenton Oxidation—A Review. Appl. Catal. B 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Buthelezi, A.S.; Tucker, C.L.; Heeres, H.J.; Shozi, M.L.; van de Bovenkamp, H.H.; Ntola, P. Fischer-Tropsch Synthesis Using Promoted, Unsupported, Supported, Bimetallic and Spray-Dried Iron Catalysts: A Review. Results Chem. 2024, 9, 101623. [Google Scholar] [CrossRef]
- Jia, J.-Y.; Shan, Y.-L.; Tuo, Y.-X.; Yan, H.; Feng, X.; Chen, D. Review of Iron-Based Catalysts for Carbon Dioxide Fischer–Tropsch Synthesis. Trans. Tianjin Univ. 2024, 30, 178–197. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Tian, X.; Dai, L.; Wang, G.; Lei, Z.; Ma, G.; Zuo, Q.; Li, M.; Zhao, M.; et al. A Review of Iron-Based Catalysts for Persulfate Activation to Remove PFAS in Water: Catalytic Effects of Various Iron Species, Influencing Factors and Reaction Pathways. Water Air Soil. Pollut. 2025, 236, 42. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Gao, M.; Chen, Y.; Ren, S.; Yang, J.; Liu, Q. Deactivation Mechanisms and Strategies to Mitigate Deactivation of Iron-Based Catalysts in NH3-SCR for NO Reduction: A Comprehensive Review. Sep. Purif. Technol. 2025, 358, 130268. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Newair, E.F.; Han, C. The Development of Iron-Based Catalysts for the Reduction of Nitrogen and per- and Polyfluoroalkyl Substances in Wastewater Treatment: A Review. Curr. Opin. Chem. Eng. 2024, 44, 101024. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Z.; Wan, Z.; An, Z.; Wang, X. Recent Advances and Prospects of Iron-Based Noble Metal-Free Catalysts for Oxygen Reduction Reaction in Acidic Environment: A Mini Review. Int. J. Hydrogen Energy 2024, 59, 697–714. [Google Scholar] [CrossRef]
- Tarek, M.; Santos, J.S.; Márquez, V.; Fereidooni, M.; Yazdanpanah, M.; Praserthdam, S.; Praserthdam, P. A Critical Review towards the Causes of the Iron-Based Catalysts Deactivation Mechanisms in the Selective Oxidation of Hydrogen Sulfide to Elemental Sulfur from Biogas. J. Energy Chem. 2024, 90, 388–411. [Google Scholar] [CrossRef]
- Peng, H.; Hou, L.; Sun, C.; Zou, H.; Wang, T.-R.; Ma, Z.-Z. Geochemistry of Magnetite from the Hongniu–Hongshan Cu Skarn Deposit in Yunnan Province, SW China. Ore Geol. Rev. 2021, 136, 104237. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of Hydrothermal Magnetite. Contrib. Mineral. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef] [PubMed]
- Kozlenko, D.P.; Dubrovinsky, L.S.; Kichanov, S.E.; Lukin, E.V.; Cerantola, V.; Chumakov, A.I.; Savenko, B.N. Magnetic and Electronic Properties of Magnetite across the High Pressure Anomaly. Sci. Rep. 2019, 9, 4464. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-Magnetite (Fe3O4) as a Support for Recyclable Catalysts in the Development of Sustainable Methodologies. Chem. Soc. Rev. 2013, 42, 3371. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef]
- Nengsih, S.; Madjid, S.N.; Mursal; Jalil, Z. Synthesis and Characterization of Magnetite Particles from Syiah Kuala Iron Sand Prepared by Co-Precipitation Method. J. Phys. Conf. Ser. 2023, 2582, 012005. [Google Scholar] [CrossRef]
- Safitri, I.; Wibowo, Y.G.; Rosarina, D.; Sudibyo. Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles from Iron Sand in Batanghari Beach. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012020. [Google Scholar] [CrossRef]
- Heryanto, H.; Tahir, D. The Correlations between Structural and Optical Properties of Magnetite Nanoparticles Synthesised from Natural Iron Sand. Ceram. Int. 2021, 47, 16820–16827. [Google Scholar] [CrossRef]
- Darvina, Y.; Yulfriska, N.; Rifai, H.; Dwiridal, L.; Ramli, R. Synthesis of Magnetite Nanoparticles from Iron Sand by Ball-Milling. J. Phys. Conf. Ser. 2019, 1185, 012017. [Google Scholar] [CrossRef]
- Morel, M.; Martínez, F.; Mosquera, E. Synthesis and Characterization of Magnetite Nanoparticles from Mineral Magnetite. J. Magn. Magn. Mater. 2013, 343, 76–81. [Google Scholar] [CrossRef]
- Amiruddin, E.; Awaluddin, A.; Hariani, I.; Sihombing, R.; Angraini, R. The Influence of Milling Ball Size on the Structural, Morphological and Catalytic Properties of Magnetite (Fe3O4) Nanoparticles toward Methylene Blue Degradation. J. Phys. Conf. Ser. 2020, 1655, 012006. [Google Scholar] [CrossRef]
- Khan, R.; Ahmad, I.; Khan, H.; Ismail, M.; Gul, K.; Yasin, A.; Ahmad, W. Production of Diesel-like Fuel from Spent Engine Oil by Catalytic Pyrolysis over Natural Magnetite. J. Anal. Appl. Pyrolysis 2016, 120, 493–500. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, R. Facile Synthesis of Highly Efficient and Cost-Effective Photo-Fenton Catalyst by Ball Milling Commercial TiO2 and Natural Magnetite. J. Alloys Compd. 2021, 862, 158670. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced Oxidation Processes: Performance, Advantages, and Scale-up of Emerging Technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Vafaei Molamahmood, H.; Geng, W.; Wei, Y.; Miao, J.; Yu, S.; Shahi, A.; Chen, C.; Long, M. Catalyzed H2O2 Decomposition over Iron Oxides and Oxyhydroxides: Insights from Oxygen Production and Organic Degradation. Chemosphere 2022, 291, 133037. [Google Scholar] [CrossRef]
- Aghdasinia, H.; Arehjani, P.; Vahid, B.; Khataee, A. Fluidized-Bed Fenton-like Oxidation of a Textile Dye Using Natural Magnetite. Res. Chem. Intermed. 2016, 42, 8083–8095. [Google Scholar] [CrossRef]
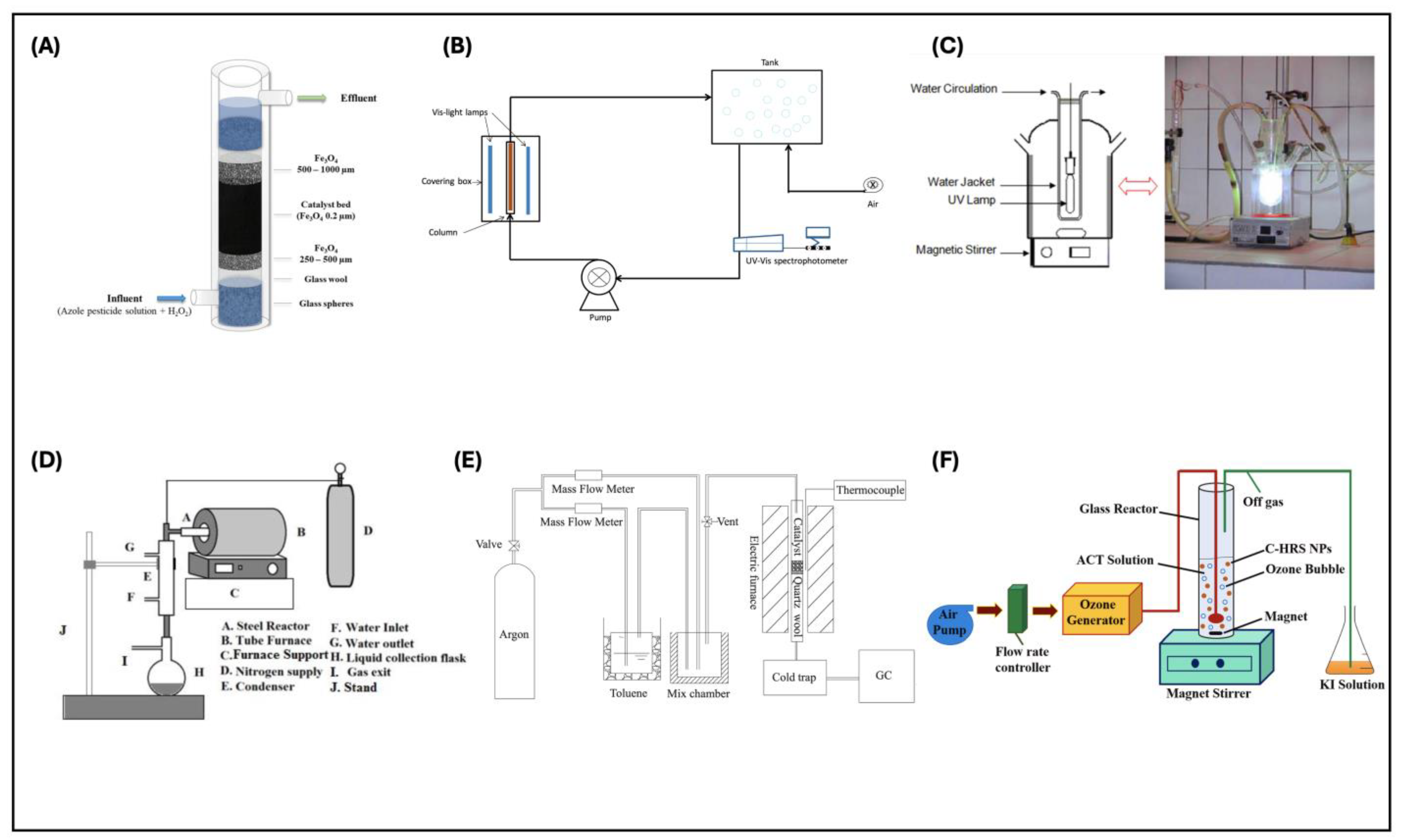
- Lopez-Arago, N.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Natural Magnetite as an Effective and Long-Lasting Catalyst for CWPO of Azole Pesticides in a Continuous up-Flow Fixed-Bed Reactor. Environ. Sci. Pollut. Res. 2024, 31, 29148–29161. [Google Scholar] [CrossRef]
- Mufti, N.; Maryam, S.; Setiyanto, H.; Taufiq, A.; Sunaryono, S. Recyclable Natural Magnetite Nanoparticles for Effective Degradation of Methylene Blue in Water under UV Light Irradiation. Key Eng. Mater. 2020, 855, 315–321. [Google Scholar] [CrossRef]
- Rostamifasih, Z.; Pasalari, H.; Mohammadi, F.; Esrafili, A. Heterogeneous Catalytic Degradation of Methylparaben Using Persulfate Activated by Natural Magnetite; Optimization and Modeling by Response Surface Methodology. J. Chem. Technol. Biotechnol. 2019, 94, 1880–1892. [Google Scholar] [CrossRef]
- Guo, R.; Xi, B.; Guo, C.; Cheng, X.; Lv, N.; Liu, W.; Borthwick, A.G.L.; Xu, J. Persulfate-Based Advanced Oxidation Processes: The New Hope Brought by Nanocatalyst Immobilization. Environ. Funct. Mater. 2022, 1, 67–91. [Google Scholar] [CrossRef]
- Hajiahmadi, M.; Zarei, M.; Khataee, A. Introducing an Effective Iron-Based Catalyst for Heterogeneous Electro-Fenton Removal of Gemcitabine Using Three-Dimensional Graphene as Cathode. J. Ind. Eng. Chem. 2021, 96, 254–268. [Google Scholar] [CrossRef]
- Munoz, M.; Conde, J.; de Pedro, Z.M.; Casas, J.A. Antibiotics Abatement in Synthetic and Real Aqueous Matrices by H2O2/Natural Magnetite. Catal. Today 2018, 313, 142–147. [Google Scholar] [CrossRef]
- Rueda Márquez, J.J.; Levchuk, I.; Sillanpää, M. Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review. Catalysts 2018, 8, 673. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Munoz, M.; Mondejar, V.; de Pedro, Z.M.; Casas, J.A. Boosting the Catalytic Activity of Natural Magnetite for Wet Peroxide Oxidation. Environ. Sci. Pollut. Res. 2020, 27, 1176–1185. [Google Scholar] [CrossRef]
- Lyu, L.; Bagchi, M.; Markoglou, N.; An, C.; Peng, H.; Bi, H.; Yang, X.; Sun, H. Towards Environmentally Sustainable Management: A Review on the Generation, Degradation, and Recycling of Polypropylene Face Mask Waste. J. Hazard. Mater. 2024, 461, 132566. [Google Scholar] [CrossRef]
- He, L.; Hui, H.; Li, S.; Lin, W. Production of Light Aromatic Hydrocarbons by Catalytic Cracking of Coal Pyrolysis Vapors over Natural Iron Ores. Fuel 2018, 216, 227–232. [Google Scholar] [CrossRef]
- Schulz, H. Short History and Present Trends of Fischer–Tropsch Synthesis. Appl. Catal. A Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, J.; Chen, J.; Qiu, S.; Wang, T. Facile Fabrication of Porous Fe@C Nanohybrids from Natural Magnetite as Excellent Fischer–Tropsch Catalysts. Chem. Commun. 2020, 56, 4523–4526. [Google Scholar] [CrossRef]
- Munoz, M.; Domínguez, P.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Naturally-Occurring Iron Minerals as Inexpensive Catalysts for CWPO. Appl. Catal. B 2017, 203, 166–173. [Google Scholar] [CrossRef]
- Widayat; Satriadi, H.; Setyojati, P.W.; Shihab, D.; Buchori, L.; Hadiyanto, H.; Nurushofa, F.A. Preparation CaO/MgO/Fe3O4 Magnetite Catalyst and Catalytic Test for Biodiesel Production. Results Eng. 2024, 22, 102202. [Google Scholar] [CrossRef]
- Roberts, A.P.; Zhao, X.; Heslop, D.; Abrajevitch, A.; Chen, Y.-H.; Hu, P.; Jiang, Z.; Liu, Q.; Pillans, B.J. Hematite (α-Fe2O3) Quantification in Sedimentary Magnetism: Limitations of Existing Proxies and Ways Forward. Geosci. Lett. 2020, 7, 8. [Google Scholar] [CrossRef]
- Manjunatha, M.; Kumar, R.; Anupama, A.V.; Khopkar, V.B.; Damle, R.; Ramesh, K.P.; Sahoo, B. XRD, Internal Field-NMR and Mössbauer Spectroscopy Study of Composition, Structure and Magnetic Properties of Iron Oxide Phases in Iron Ores. J. Mater. Res. Technol. 2019, 8, 2192–2200. [Google Scholar] [CrossRef]
- Amiruddin, E.; Awaluddin, A.; Sinuraya, S.; Hadianto, H.; Noferdi, M.D.; Fitri, A.S. Study of Iron Oxide Nanoparticles Doped with Manganese for Catalytic Degradation of Methylene Blue. J. Phys. Conf. Ser. 2021, 2049, 012021. [Google Scholar] [CrossRef]
- Jalil, Z.; Rahwanto, A.; Mulana, F.; Ismail, I.; Anggreini Akbar, I.; Budi Aulia, T. Effet of Hematite (Fe2O3) from Natural Iron Ore as a Catalyst in MgH2 as Hydrogen Storage Materials. J. Phys. Conf. Ser. 2023, 2498, 012048. [Google Scholar] [CrossRef]
- Mandal, S.; Müller, A.H.E. Facile Route to the Synthesis of Porous α-Fe2O3 Nanorods. Mater. Chem. Phys. 2008, 111, 438–443. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tang, J.Y.; Hu, X.Y. Controllable Synthesis and Magnetic Properties of Pure Hematite and Maghemite Nanocrystals from a Molecular Precursor. J. Alloys Compd. 2008, 462, 24–28. [Google Scholar] [CrossRef]
- Malaidurai, M.; Thangavel, R. Study of Structural and Magnetic Properties of Co-Precipitated α-Fe2O3 Nanocrystals. Superlattices Microstruct. 2018, 120, 553–560. [Google Scholar] [CrossRef]
- Lohaus, C.; Klein, A.; Jaegermann, W. Limitation of Fermi Level Shifts by Polaron Defect States in Hematite Photoelectrodes. Nat. Commun. 2018, 9, 4309. [Google Scholar] [CrossRef]
- Omri, A.; Hamza, W.; Benzina, M. Photo-Fenton Oxidation and Mineralization of Methyl Orange Using Fe-Sand as Effective Heterogeneous Catalyst. J. Photochem. Photobiol. A Chem. 2020, 393, 112444. [Google Scholar] [CrossRef]
- Kermani, M.; Mohammadi, F.; Kakavandi, B.; Esrafili, A.; Rostamifasih, Z. Simultaneous Catalytic Degradation of 2,4-D and MCPA Herbicides Using Sulfate Radical-Based Heterogeneous Oxidation over Persulfate Activated by Natural Hematite (α-Fe2O3/PS). J. Phys. Chem. Solids 2018, 117, 49–59. [Google Scholar] [CrossRef]
- Hollanda, L.R.; de Souza, J.A.B.; Dotto, G.L.; Foletto, E.L.; Chiavone-Filho, O. Iron-Bearing Mining Reject as an Alternative and Effective Catalyst for Photo-Fenton Oxidation of Phenol in Water. Environ. Sci. Pollut. Res. 2024, 31, 21291–21301. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Moussavi, G.; Shekoohiyan, S.; Marín, M.L.; Boscá, F.; Giannakis, S. A Continuous-Flow Catalytic Process with Natural Hematite-Alginate Beads for Effective Water Decontamination and Disinfection: Peroxymonosulfate Activation Leading to Dominant Sulfate Radical and Minor Non-Radical Pathways. Chem. Eng. J. 2021, 411, 127738. [Google Scholar] [CrossRef]
- Lubis, S.; Sheilatina; Murisna. Synthesis, Characterization and Photocatalytic Activity of α-Fe2O3/Bentonite Composite Prepared by Mechanical Milling. J. Phys. Conf. Ser. 2018, 1116, 042016. [Google Scholar] [CrossRef]
- Elfiad, A.; Galli, F.; Boukhobza, L.M.; Djadoun, A.; Boffito, D.C. Low-Cost Synthesis of Cu/α-Fe2O3 from Natural HFeO2: Application in 4-Nitrophenol Reduction. J. Environ. Chem. Eng. 2020, 8, 104214. [Google Scholar] [CrossRef]
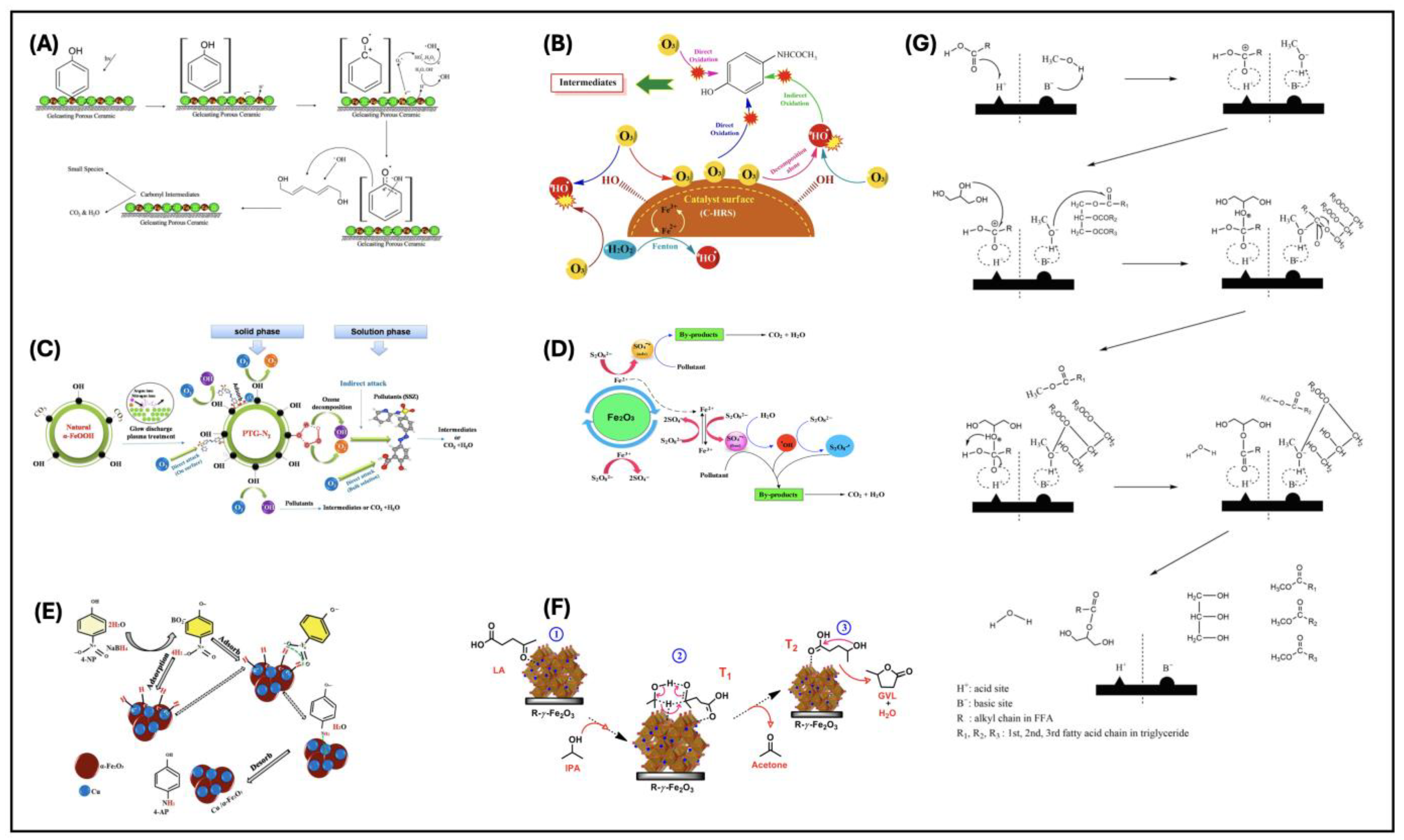
- Putri, S.E.; Pratiwi, D.E.; Tjahjanto, R.T.; Hasri; Andi, I.; Rahman, A.; Ramadani, A.I.W.S.; Ramadhani, A.N.; Subaer; Fudholi, A. The Renewable of Low Toxicity Gelcasting Porous Ceramic as Fe2O3 Catalyst Support on Phenol Photodegradation. Int. J. Des. Nat. Ecodynamics 2022, 17, 503–511. [Google Scholar] [CrossRef]
- Elfiad, A.; Galli, F.; Djadoun, A.; Sennour, M.; Chegrouche, S.; Meddour-Boukhobza, L.; Boffito, D.C. Natural α-Fe2O3 as an Efficient Catalyst for the p-Nitrophenol Reduction. Mater. Sci. Eng. B 2018, 229, 126–134. [Google Scholar] [CrossRef]
- Jaimes-López, R.; Jiménez-Vázquez, A.; Pérez-Rodríguez, S.; Estudillo-Wong, L.A.; Alonso-Vante, N. Catalyst for the Generation of OH Radicals in Advanced Electrochemical Oxidation Processes: Present and Future Perspectives. Catalysts 2024, 14, 703. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Oulego, P.; Giannakis, S. Heterogeneous Catalytic Ozonation and Peroxone-Mediated Removal of Acetaminophen Using Natural and Modified Hematite-Rich Soil, as Efficient and Environmentally Friendly Catalysts. Appl. Catal. B 2022, 301, 120786. [Google Scholar] [CrossRef]
- Yue, Y.; Niu, P.; Jiang, L.; Cao, Y.; Bao, X. Acid-Modified Natural Bauxite Mineral as a Cost-Effective and High-Efficient Catalyst Support for Slurry-Phase Hydrocracking of High-Temperature Coal Tar. Energy Fuels 2016, 30, 9203–9209. [Google Scholar] [CrossRef]
- Robinson, P.R. Hydroconversion Processes and Technology for Clean Fuel and Chemical Production. In Advances in Clean Hydrocarbon Fuel Processing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 287–325. [Google Scholar]
- Kairbekov, Z.K.; Maloletnev, A.S.; Dzheldybaeva, I.M.; Sabitova, A.N.; Ermoldina, E.T. Application of Modified Iron-Containing Catalysts and Preliminary Ozonization of Coal from the Shubarkol Deposit to the Hydrogenation of This Coal. Solid. Fuel Chem. 2017, 51, 365–369. [Google Scholar] [CrossRef]
- Kretzschmar, N.; Busse, O.; Seifert, M. Impact of Geometric and Electronic Factors on Selective Hydro-Deoxygenation of Guaiacol by Surface-Rich Metal/Silica Catalysts. Catalysts 2023, 13, 425. [Google Scholar] [CrossRef]
- Widayat; Putra, D.A.; Nursafitri, I. Synthesis and Catalytic Evaluation of Hematite (α-Fe2O3) Magnetic Nanoparticles from Iron Sand for Waste Cooking Oil Conversion to Produce Biodiesel through Esterification-Transesterification Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012035. [Google Scholar] [CrossRef]
- Prameswari, J.; Widayat, W.; Buchori, L.; Hadiyanto, H. Novel Iron Sand-Derived α-Fe2O3/CaO2 Bifunctional Catalyst for Waste Cooking Oil-Based Biodiesel Production. Environ. Sci. Pollut. Res. 2022, 30, 98832–98847. [Google Scholar] [CrossRef]
- Abdrafikova, I.M.; Kayukova, G.P.; Petrov, S.M.; Ramazanova, A.I.; Musin, R.Z.; Morozov, V.I. Conversion of Extra-Heavy Ashal’chinskoe Oil in Hydrothermal Catalytic System. Pet. Chem. 2015, 55, 104–111. [Google Scholar] [CrossRef]
- Zou, X.; Chen, T.; Liu, H.; Zhang, P.; Chen, D.; Zhu, C. Catalytic Cracking of Toluene over Hematite Derived from Thermally Treated Natural Limonite. Fuel 2016, 177, 180–189. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Huang, F.; Bai, H.; Wang, F.; Wang, C. Carbon Deposition and Sintering Characteristics on Iron-Based Oxygen Carriers in the Catalytic Cracking Process of Coal Tar. Energy Fuels 2017, 31, 6501–6506. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Fernando, J.F.S.; O’Mullane, A.P. Conversion of Iron Ore into an Active Catalyst for the Oxygen Evolution Reaction. Adv. Sustain. Syst. 2018, 2, 1800019. [Google Scholar] [CrossRef]
- Fabbri, E.; Schmidt, T.J. Oxygen Evolution Reaction—The Enigma in Water Electrolysis. ACS Catal. 2018, 8, 9765–9774. [Google Scholar] [CrossRef]
- Alduhaish, O.; Ubaidullah, M.; Al-Enizi, A.M.; Alhokbany, N.; Alshehri, S.M.; Ahmed, J. Facile Synthesis of Mesoporous α-Fe2O3@g-C3N4-NCs for Efficient Bifunctional Electro-Catalytic Activity (OER/ORR). Sci. Rep. 2019, 9, 14139. [Google Scholar] [CrossRef]
- Farooq, A.; Khalil, S.; Basha, B.; Habib, A.; Al-Buriahi, M.S.; Warsi, M.F.; Yousaf, S.; Shahid, M. Electrochemical Investigation of C-Doped CoFe2O4/Fe2O3Nanostructures for Efficient Electrochemical Water Splitting. Int. J. Hydrogen Energy 2024, 51, 1318–1332. [Google Scholar] [CrossRef]
- Ng, K.-L.; Kok, K.-Y.; Ong, B.-H. Facile Synthesis of Self-Assembled Cobalt Oxide Supported on Iron Oxide as the Novel Electrocatalyst for Enhanced Electrochemical Water Electrolysis. ACS Appl. Nano Mater. 2018, 1, 401–409. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, H.; Zhang, X.; Liu, R.; Liu, S.; Wang, G.; Zhang, Y.; Zhao, H. Fe/Fe2O3 Nanoparticles Anchored on Fe-N-Doped Carbon Nanosheets as Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc-Air Batteries. Nano Res. 2016, 9, 2123–2137. [Google Scholar] [CrossRef]
- Guo, W.H.; Zhang, Q.; Fu, H.C.; Yang, Y.X.; Chen, X.H.; Luo, H.Q.; Li, N.B. Semi-Sacrificial Template Growth of FeS/Fe2O3 Heterogeneous Nanosheets on Iron Foam for Efficient Oxygen Evolution Reaction. J. Alloys Compd. 2022, 909, 164670. [Google Scholar] [CrossRef]
- Khan, N.A.; Rashid, N.; Ahmad, I.; Zahidullah; Zairov, R.; Rehman, H.U.; Nazar, M.F.; Jabeen, U. An Efficient Fe2O3/FeS Heterostructures Water Oxidation Catalyst. Int. J. Hydrogen Energy 2022, 47, 22340–22347. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Chaugule, A.A.; Chung, W.-J.; Kim, H. Fe2O3 Hollow Nanorods/CNT Composites as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Electrochim. Acta 2016, 222, 1316–1325. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, J.; Batool, S.; Zafar, M.N.; Hanif, A.; Zahidullah; Nazar, M.F.; Ul-Hamid, A.; Jabeen, U.; Dahshan, A.; et al. Design and Fabrication of Fe2O3/FeP Heterostructure for Oxygen Evolution Reaction Electrocatalysis. J. Alloys Compd. 2022, 894, 162409. [Google Scholar] [CrossRef]
- Cui, T.; Zhai, X.; Guo, L.; Chi, J.-Q.; Zhang, Y.; Zhu, J.; Sun, X.; Wang, L. Controllable Synthesis of a Self-Assembled Ultralow Ru, Ni-Doped Fe2O3 Lily as a Bifunctional Electrocatalyst for Large-Current-Density Alkaline Seawater Electrolysis. Chin. J. Catal. 2022, 43, 2202–2211. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, R.; Li, D.; Yang, M.; Tong, Y.; Qiu, Z.; Jing, B.; Yang, T.; Hu, R.; Yang, Y.; et al. Tungsten Oxide Decorating-Regulated Iron-Nickel Oxide Heterostructure as Electrocatalyst for Oxygen Evolution Reaction. J. Alloys Compd. 2023, 968, 171910. [Google Scholar] [CrossRef]
- Tong, H.; Zheng, X.; Qi, M.; Li, D.; Zhu, J.; Jiang, D. Synergistically Coupled CoMo/Fe2O3 Electrocatalyst for Highly Efficient and Stable Overall Water Splitting. J. Colloid. Interface Sci. 2024, 676, 837–846. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Deng, L.; Li, W.; Ren, Z.; Yang, M.; Yang, X.; Zhu, Y. Synthesis and Characterization of an IrO2 –Fe2O3 Electrocatalyst for the Hydrogen Evolution Reaction in Acidic Water Electrolysis. RSC Adv. 2017, 7, 20252–20258. [Google Scholar] [CrossRef]
- Kim, J.; Heo, J.N.; Do, J.Y.; Chava, R.K.; Kang, M. Electrochemical Synergies of Heterostructured Fe2O3-MnO Catalyst for Oxygen Evolution Reaction in Alkaline Water Splitting. Nanomaterials 2019, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cao, X.; Zhou, W.; Li, Y.; Johnson, D.M.; Huang, Y. Catalytic Hydrolysis of Microcystin-LR Peptides on the Surface of Naturally Occurring Minerals. Res. Chem. Intermed. 2020, 46, 1141–1152. [Google Scholar] [CrossRef]
- Ramutsindela, F.K.; Okoye-Chine, C.G.; Mbuya, C.O.L.; Mubenesha, S.; Gorimbo, J.; Okonye, L.U.; Liu, X.; Hildebrandt, D. The Effect of Reducing Gases on Raw Iron Ore Catalyst for Fischer-Tropsch Synthesis. J. Taiwan. Inst. Chem. Eng. 2022, 131, 104163. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Xie, Q.; Zou, X.; Qing, C.; Frost, R.L. Kinetic Study of Goethite Dehydration and the Effect of Aluminium Substitution on the Dehydrate. Thermochim. Acta 2012, 545, 20–25. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Frost, R.L. An Overview of the Role of Goethite Surfaces in the Environment. Chemosphere 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Strangway, D.W.; Honea, R.M.; McMahon, B.E.; Larson, E.E. The Magnetic Properties of Naturally Occurring Goethite. Geophys. J. Int. 1968, 15, 345–359. [Google Scholar] [CrossRef]
- Valezi, D.F.; Carneiro, C.E.A.; Costa, A.C.S.; Paesano, A.; Spadotto, J.C.; Solórzano, I.G.; Londoño, O.M.; Di Mauro, E. Weak Ferromagnetic Component in Goethite (α-FeOOH) and Its Relation with Microstructural Characteristics. Mater. Chem. Phys. 2020, 246, 122851. [Google Scholar] [CrossRef]
- Han, S.K.; Hwang, T.-M.; Yoon, Y.; Kang, J.-W. Evidence of Singlet Oxygen and Hydroxyl Radical Formation in Aqueous Goethite Suspension Using Spin-Trapping Electron Paramagnetic Resonance (EPR). Chemosphere 2011, 84, 1095–1101. [Google Scholar] [CrossRef]
- Shindo, H.; Huang, P.M. Catalytic Effects of Manganese (IV), Iron(III), Aluminum, and Silicon Oxides on the Formation of Phenolic Polymers. Soil. Sci. Soc. Am. J. 1984, 48, 927–934. [Google Scholar] [CrossRef]
- Cunningham, K.M.; Goldberg, M.C.; Weiner, E.R. Mechanisms for Aqueous Photolysis of Adsorbed Benzoate, Oxalate, and Succinate on Iron Oxyhydroxide (Goethite) Surfaces. Environ. Sci. Technol. 1988, 22, 1090–1097. [Google Scholar] [CrossRef]
- Lin, K.; Ding, J.; Wang, H.; Huang, X.; Gan, J. Goethite-Mediated Transformation of Bisphenol A. Chemosphere 2012, 89, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C. Oxidation of Chlorophenols with Hydrogen Peroxide in the Presence of Goethite. Chemosphere 2000, 40, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de la Plata, G.B.; Alfano, O.M.; Cassano, A.E. Optical Properties of Goethite Catalyst for Heterogeneous Photo-Fenton ReactionsComparison with a Titanium Dioxide Catalyst. Chem. Eng. J. 2008, 137, 396–410. [Google Scholar] [CrossRef]
- He, J.; Ma, W.; Song, W.; Zhao, J.; Qian, X.; Zhang, S.; Yu, J.C. Photoreaction of Aromatic Compounds at α-FeOOH/H2O Interface in the Presence of H2O2: Evidence for Organic-Goethite Surface Complex Formation. Water Res. 2005, 39, 119–128. [Google Scholar] [CrossRef]
- Yeh, C.K.-J.; Hsu, C.-Y.; Chiu, C.-H.; Huang, K.-L. Reaction Efficiencies and Rate Constants for the Goethite-Catalyzed Fenton-like Reaction of NAPL-Form Aromatic Hydrocarbons and Chloroethylenes. J. Hazard. Mater. 2008, 151, 562–569. [Google Scholar] [CrossRef]
- Lin, Z.-R.; Zhao, L.; Dong, Y.-H. Quantitative Characterization of Hydroxyl Radical Generation in a Goethite-Catalyzed Fenton-like Reaction. Chemosphere 2015, 141, 7–12. [Google Scholar] [CrossRef]
- Andersen, S.L.F.; Flores, R.G.; Madeira, V.S.; José, H.J.; Moreira, R.F.P.M. Synthesis and Characterization of Acicular Iron Oxide Particles Obtained from Acid Mine Drainage and Their Catalytic Properties in Toluene Oxidation. Ind. Eng. Chem. Res. 2012, 51, 767–774. [Google Scholar] [CrossRef]
- Aziz, F.; El Achaby, M.; Aziz, K.; Ouazzani, N.; Mandi, L.; Ghazzal, M.N. Nanocomposite Fiber Based on Natural Material for Water Disinfection under Visible Light Irradiation. Nanomaterials 2020, 10, 1192. [Google Scholar] [CrossRef]
- Kuang, J.; Guo, H.; Si, Q.; Guo, W.; Ma, F. Highly Dispersed Natural Goethite/Fe3O4 Heterogeneous Fenton-like Catalyst for Efficient Tetracycline Degradation: Rapid Accumulation of Hydroxyl Radicals by Fe–O–Si Bonds. J. Clean. Prod. 2023, 420, 138262. [Google Scholar] [CrossRef]
- Muruganandham, M.; Wu, J.J. Granular α-FeOOH—A Stable and Efficient Catalyst for the Decomposition of Dissolved Ozone in Water. Catal. Commun. 2007, 8, 668–672. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Ma, J.; Tian, H.; Qiang, Z. Surface Hydroxyl Groups of Synthetic α-FeOOH in Promoting OH Generation from Aqueous Ozone: Property and Activity Relationship. Appl. Catal. B 2008, 82, 131–137. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Du, J.; Xing, B. Goethite Catalyzed Cr(VI) Reduction by Tartaric Acid via Surface Adsorption. Ecotoxicol. Environ. Saf. 2019, 171, 594–599. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, T.; Wang, W.; Liu, B.; Li, W.; Liu, Y. Reduction and Stabilization of Cr(VI) in Soil by Using Calcium Polysulfide: Catalysis of Natural Iron Oxides. Environ. Res. 2020, 190, 109992. [Google Scholar] [CrossRef]
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E. Enhanced Heterogeneous Catalytic Ozonation of Pharmaceutical Pollutants Using a Novel Nanostructure of Iron-Based Mineral Prepared via Plasma Technology: A Comparative Study. J. Hazard. Mater. 2020, 392, 122269. [Google Scholar] [CrossRef]
- Fernández-Marchante, C.M.; Raschitor, A.; Mena, I.F.; Rodrigo, M.A.; Lobato, J. Evaluation of Goethite as a Catalyst for the Thermal Stage of the Westinghouse Process for Hydrogen Production. Catalysts 2021, 11, 1145. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Yasmin, A.; Ge, X.; Ge, Z.; Zhang, K.; Liang, X.; Zhang, J.; Li, G.; Xiao, X.; Jiang, B.; et al. Shielded Goethite Catalyst That Enables Fast Water Dissociation in Bipolar Membranes. Nat. Commun. 2021, 12, 9. [Google Scholar] [CrossRef]
- Hu, Z.; Ai, Y.; Liu, L.; Zhou, J.; Zhang, G.; Liu, H.; Liu, X.; Liu, Z.; Hu, J.; Sun, H.; et al. Hydroxyl Assisted Rhodium Catalyst Supported on Goethite Nanoflower for Chemoselective Catalytic Transfer Hydrogenation of Fully Converted Nitrostyrenes. Adv. Synth. Catal. 2019, 361, 3146–3154. [Google Scholar] [CrossRef]
- Kuncser, A.C.; Vlaicu, I.D.; Pavel, O.D.; Zavoianu, R.; Badea, M.; Radu, D.; Culita, D.C.; Rostas, A.M.; Olar, R. Soft Synthesis and Characterization of Goethite-Based Nanocomposites as Promising Cyclooctene Oxidation Catalysts. RSC Adv. 2021, 11, 27589–27602. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghadge, A.N.; Ghangrekar, M.M. Enhancing the Power Generation in Microbial Fuel Cells with Effective Utilization of Goethite Recovered from Mining Mud as Anodic Catalyst. Bioresour. Technol. 2015, 191, 110–116. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Song, Q.; Liu, S.; Ma, Q.; Ma, L.; Shu, X. Catalytic Reforming of Volatiles from Co-Pyrolysis of Lignite Blended with Corn Straw over Three Different Structures of Iron Ores. J. Anal. Appl. Pyrolysis 2019, 144, 104714. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Oxidative C-H Activation of Amines Using Protuberant Lychee-like Goethite. Sci. Rep. 2018, 8, 2024. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, B.; Zhou, C.; Liu, T.; Wang, Y.; Zhao, G.; Chen, B.; Chu, C. Sunlight-Driven Production of Reactive Oxygen Species from Natural Iron Minerals: Quantum Yield and Wavelength Dependence. Environ. Sci. Technol. 2023, 57, 1177–1185. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, J.; Jiang, J.; Bloemendal, J.; Hesse, P.; Rolph, T.; Mao, X. Analysis on Variety and Characteristics of Maghemite. Sci. China Earth Sci. 2010, 53, 1153–1162. [Google Scholar] [CrossRef]
- Chen, Y.H. Thermal Properties of Nanocrystalline Goethite, Magnetite, and Maghemite. J. Alloys Compd. 2013, 553, 194–198. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A New Method for the Identification and Quantification of Magnetite–Maghemite Mixture Using Conventional X-Ray Diffraction Technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of Initial PH and Temperature of Iron Salt Solutions on Formation of Magnetite Nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Murad, E. Properties and Behavior of Iron Oxides as Determined by Mössbauer Spectroscopy. In Iron in Soils and Clay Minerals; Springer: Dordrecht, The Netherlands, 1988; pp. 309–350. [Google Scholar]
- Dunlop, D.J.; Özdemir, Ö. Magnetizations in Rocks and Minerals. In Treatise on Geophysics; Elsevier: Amsterdam, The Netherlands, 2007; pp. 277–336. [Google Scholar]
- Nikiforov, V.N.; Goldt, A.E.; Gudilin, E.A.; Sredin, V.G.; Irhin, V.Y. Magnetic Properties of Maghemite Nanoparticles. Bull. Russ. Acad. Sci. Phys. 2014, 78, 1075–1080. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Al-Baitai, A.Y.; Saadoune, I.; De Leeuw, N.H. Vacancy Ordering and Electronic Structure of γ-Fe2O3(Maghemite): A Theoretical Investigation. J. Phys. Condens. Matter 2010, 22, 255401–255408. [Google Scholar] [CrossRef]
- Ramirez, A.; Ould-Chikh, S.; Gevers, L.; Chowdhury, A.D.; Abou-Hamad, E.; Aguilar-Tapia, A.; Hazemann, J.; Wehbe, N.; Al Abdulghani, A.J.; Kozlov, S.M.; et al. Tandem Conversion of CO2 to Valuable Hydrocarbons in Highly Concentrated Potassium Iron Catalysts. ChemCatChem 2019, 11, 2879–2886. [Google Scholar] [CrossRef]
- Xu, D.; Lai, X.; Guo, W.; Dai, P. Microwave-Assisted Catalytic Degradation of Methyl Orange in Aqueous Solution by Ferrihydrite/Maghemite Nanoparticles. J. Water Process Eng. 2017, 16, 270–276. [Google Scholar] [CrossRef]
- Vasanthakumar, P.; Karvembu, R. Unmodified Maghemite from River Sand as a Selective Catalyst for Base-Free Transfer Hydrogenation of Furfural, Levulinic Acid, and o-Vanillin: A Pathway for Sustainable Biomass Conversions. ACS Sustain. Chem. Eng. 2020, 8, 17069–17078. [Google Scholar] [CrossRef]
- Filip, J.; Zboril, R.; Schneeweiss, O.; Zeman, J.; Cernik, M.; Kvapil, P.; Otyepka, M. Environmental Applications of Chemically Pure Natural Ferrihydrite. Environ. Sci. Technol. 2007, 41, 4367–4374. [Google Scholar] [CrossRef]
- Jojoa-Sierra, S.D.; Herrero-Albillos, J.; Ormad, M.P.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Mosteo, R. Wüstite as a Catalyst Source for Water Remediation: Differentiated Antimicrobial Activity of by-Products, Action Routes of the Process, and Transformation of Fluoroquinolones. Chem. Eng. J. 2022, 435, 134850. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.; Fareed, S. Low-Temperature Selective Catalytic Reduction of NO with NH3 over Natural Iron Ore Catalyst. Catalysts 2019, 9, 956. [Google Scholar] [CrossRef]
- Cabrera, A.F.; Rodríguez Torres, C.E.; Stewart, S.J. Nanostructured Al-doped maghemite: A low-cost and eco-friendly material tested for methylene blue removal from water and as an accelerator in ammonium nitrate decomposition. J. Phys. Chem. Solids 2024, 185, 111784. [Google Scholar] [CrossRef]
- Shopska, M.; Paneva, D.; Kolev, H.; Kadinov, G.; Briančin, J.; Fabián, M.; Cherkezova-Zheleva, Z.; Mitov, I. Characterization and Catalytic Activity in CO Oxidation of Biogenic Lepidocrocite Layered on Anodic Alumina. Catal. Today 2020, 357, 436–441. [Google Scholar] [CrossRef]
- Guyodo, Y.; Bonville, P.; Till, J.L.; Ona-Nguema, G.; Lagroix, F.; Menguy, N. Constraining the Origins of the Magnetism of Lepidocrocite (γ-FeOOH): A Mössbauer and Magnetization Study. Front. Earth Sci. 2016, 4, 28. [Google Scholar] [CrossRef]
- Zhukhlistov, A.P. Crystal Structure of Lepidocrocite FeO(OH) from the Electron-Diffractometry Data. Crystallogr. Rep. 2001, 46, 730–733. [Google Scholar] [CrossRef]
- Hirt, A.M.; Lanci, L.; Dobson, J.; Weidler, P.; Gehring, A.U. Low-temperature Magnetic Properties of Lepidocrocite. J. Geophys. Res. Solid. Earth 2002, 107, EPM-5. [Google Scholar] [CrossRef]
- Zhong, D.; Feng, W.; Ma, W.; Liu, X.; Ma, J.; Zhou, Z.; Du, X.; He, F. Goethite and Lepidocrocite Catalyzing Different Double-Oxidant Systems to Degrade Chlorophenol. Environ. Sci. Pollut. Res. 2022, 29, 72764–72776. [Google Scholar] [CrossRef]
- Matta, R.; Hanna, K.; Chiron, S. Fenton-like Oxidation of 2,4,6-Trinitrotoluene Using Different Iron Minerals. Sci. Total Environ. 2007, 385, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Huang, C. Application of a Supported Iron Oxyhydroxide Catalyst in Oxidation of Benzoic Acid by Hydrogen Peroxide. Chemosphere 1999, 38, 2719–2731. [Google Scholar] [CrossRef]
- Qin, M.; Lu, B.; Feng, S.; Zhen, Z.; Chen, R.; Liu, H. Role of Exposed Facets and Surface OH Groups in the Fenton-like Reactivity of Lepidocrocite Catalyst. Chemosphere 2019, 230, 286–293. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, S.; Liu, H.; Song, X.; Wei, Y. Preparation and Photocatalytic Activity of Lepidocrocites Obtained Byphotocatalytic Oxidation of Fe(II) in the Presence of Citric Acid. J. Photochem. Photobiol. A Chem. 2015, 312, 73–80. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, Y.; Sun, Y. Room-Temperature Synthesis and Photocatalytic Properties of Lepidocrocite by Monowavelength Visible Light Irradiation. J. Mol. Catal. A Chem. 2012, 353–354, 67–73. [Google Scholar] [CrossRef]
- Shi, H.; Liang, H.; Ming, F.; Wang, Z. Efficient Overall Water-Splitting Electrocatalysis Using Lepidocrocite VOOH Hollow Nanospheres. Angew. Chem. 2017, 129, 588–592. [Google Scholar] [CrossRef]
- Wang, L.; Giammar, D.E. Effects of PH, Dissolved Oxygen, and Aqueous Ferrous Iron on the Adsorption of Arsenic to Lepidocrocite. J. Colloid. Interface Sci. 2015, 448, 331–338. [Google Scholar] [CrossRef]
- He, F.; Zhong, D.; Ma, W.; Yuan, Y.; Li, K.; Dai, C. Activation of the Combined Hydrogen Peroxide and Peroxymonosulphate by Lepidocrocite for Chloramphenicol Removal: Kinetics and Mechanisms. Environ. Technol. 2023, 44, 1936–1946. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Zhang, X.; Coulon, F.; Wang, C. Harnessing the Power of Natural Minerals: A Comprehensive Review of Their Application as Heterogeneous Catalysts in Advanced Oxidation Processes for Organic Pollutant Degradation. Chemosphere 2023, 337, 139404. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yuan, Y.; Zhong, D.; Zhang, P.; Liangdy, A.; Lim, T.-T.; Ma, W.; Yuan, Y. Catalytic Activity of H2O2 by Goethite and Lepidocrocite: Insight from 5-Bromosalicylic Acid Removal Mechanism and Density Functional Theory Calculation (ID:CHEM114760). Chemosphere 2023, 329, 138551. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.W. Ferrihydrite: A Review of Structure, Properties and Occurrence in Relation to Soils. Z. Pflanzenernährung Bodenkd. 1992, 155, 441–448. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Tyliszczak, T.; Brown, J.G.E. Composition and Structural Aspects of Naturally Occurring Ferrihydrite. Comptes Rendus. Géoscience 2011, 343, 210–218. [Google Scholar] [CrossRef]
- Fleischer, M.; Chao, G.Y.; Cabri, L.J. New Minerals Names. Am. Mineral. J. Earth Planet. Mater. 1975, 60, 736–739. [Google Scholar]
- Eggleton, R.A.; Fitzpatrick, R.W. New Data and a Revised Structural Model for Ferrihydrite. Clays Clay Min. 1988, 36, 111–124. [Google Scholar] [CrossRef]
- Cardile, C.M. Tetrahedral Fe3+ in Ferrihydrite: 57 Fe Mössbauer Spectroscopic Evidence. Clays Clay Min. 1988, 36, 537–539. [Google Scholar] [CrossRef]
- Towe, K.M.; Bradley, W.F. Mineralogical Constitution of Colloidal “Hydrous Ferric Oxides”. J. Colloid. Interface Sci. 1967, 24, 384–392. [Google Scholar] [CrossRef]
- Russell, J.D. Infrared Spectroscopy of Ferrihydrite: Evidence for the Presence of Structural Hydroxyl Groups. Clay Min. 1979, 14, 109–114. [Google Scholar] [CrossRef]
- Barrón, V.; Torrent, J. Diffuse Reflectance Spectroscopy of Iron Oxides. Encycl. Surf. Colloid. Sci. 2002, 1, 1438–1446. [Google Scholar]
- Hiemstra, T. Surface and Mineral Structure of Ferrihydrite. Geochim. Cosmochim. Acta 2013, 105, 316–325. [Google Scholar] [CrossRef]
- Funnell, N.P.; Fulford, M.F.; Inoué, S.; Kletetschka, K.; Michel, F.M.; Goodwin, A.L. Nanocomposite Structure of Two-Line Ferrihydrite Powder from Total Scattering. Commun. Chem. 2020, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Boily, J.-F.; Song, X. Direct Identification of Reaction Sites on Ferrihydrite. Commun. Chem. 2020, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Koopal, L.K.; Li, W.; Xu, W.; Liu, F.; Zhang, J.; Liu, Q.; Feng, X.; Sparks, D.L. Effects of Crystallite Size on the Structure and Magnetism of Ferrihydrite. Environ. Sci. Nano 2016, 3, 190–202. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, Q.; Deng, F.; Ni, Z.; Lin, Q.; Cheng, L.; Chen, X.; Qiu, R.; Zhu, R. The Differences in Heterogeneous Fenton Catalytic Performance and Mechanism of Various Iron Minerals and Their Influencing Factors: A Review. Sep. Purif. Technol. 2023, 325, 124702. [Google Scholar] [CrossRef]
- Seomoon, K. On-Line GC and GC–MS Analyses of the Fischer–Tropsch Products Synthesized Using Ferrihydrite Catalyst. J. Ind. Eng. Chem. 2013, 19, 2108–2114. [Google Scholar] [CrossRef]
- Bali, S.; Bali, G.; Huggins, F.E.; Seehra, M.S.; Singh, V.; Hancock, J.M.; Harrison, R.; Huffman, G.P.; Pugmire, R.J.; Ernst, R.D.; et al. Synthetic Doped Amorphous Ferrihydrite for the Fischer–Tropsch Synthesis of Alternative Fuels. Ind. Eng. Chem. Res. 2012, 51, 4515–4522. [Google Scholar] [CrossRef]
- Trivedi, P.; Dyer, J.A.; Sparks, D.L. Lead Sorption onto Ferrihydrite. 1. A Macroscopic and Spectroscopic Assessment. Environ. Sci. Technol. 2003, 37, 908–914. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Abend, S.; Pandya, K.I.; Sparks, D.L. Kinetic Controls on Cu and Pb Sorption by Ferrihydrite. Environ. Sci. Technol. 2001, 35, 1090–1096. [Google Scholar] [CrossRef]
- Brinza, L.; Vu, H.P.; Neamtu, M.; Benning, L.G. Experimental and Simulation Results of the Adsorption of Mo and V onto Ferrihydrite. Sci. Rep. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Johnston, C.P.; Chrysochoou, M. Mechanisms of Chromate, Selenate, and Sulfate Adsorption on Al-Substituted Ferrihydrite: Implications for Ferrihydrite Surface Structure and Reactivity. Environ. Sci. Technol. 2016, 50, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Morita, M.; Matsuoka, M.; Tokoro, C. Sorption Mechanisms of Chromate with Coprecipitated Ferrihydrite in Aqueous Solution. J. Hazard. Mater. 2017, 334, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Dyer, J.A.; Sparks, D.L.; Pandya, K. Mechanistic and Thermodynamic Interpretations of Zinc Sorption onto Ferrihydrite. J. Colloid. Interface Sci. 2004, 270, 77–85. [Google Scholar] [CrossRef]
- Sajih, M.; Bryan, N.D.; Livens, F.R.; Vaughan, D.J.; Descostes, M.; Phrommavanh, V.; Nos, J.; Morris, K. Adsorption of Radium and Barium on Goethite and Ferrihydrite: A Kinetic and Surface Complexation Modelling Study. Geochim. Cosmochim. Acta 2014, 146, 150–163. [Google Scholar] [CrossRef]
- Rojo, I.; Seco, F.; Rovira, M.; Giménez, J.; Cervantes, G.; Martí, V.; de Pablo, J. Thorium Sorption onto Magnetite and Ferrihydrite in Acidic Conditions. J. Nucl. Mater. 2009, 385, 474–478. [Google Scholar] [CrossRef]
- Smith, K.F.; Morris, K.; Law, G.T.W.; Winstanley, E.H.; Livens, F.R.; Weatherill, J.S.; Abrahamsen-Mills, L.G.; Bryan, N.D.; Mosselmans, J.F.W.; Cibin, G.; et al. Plutonium(IV) Sorption during Ferrihydrite Nanoparticle Formation. ACS Earth Space Chem. 2019, 3, 2437–2442. [Google Scholar] [CrossRef]
- Winstanley, E.H.; Morris, K.; Abrahamsen-Mills, L.G.; Blackham, R.; Shaw, S. U(VI) Sorption during Ferrihydrite Formation: Underpinning Radioactive Effluent Treatment. J. Hazard. Mater. 2019, 366, 98–104. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Carbonate Adsorption to Ferrihydrite: Competitive Interaction with Phosphate for Use in Soil Systems. ACS Earth Space Chem. 2019, 3, 129–141. [Google Scholar] [CrossRef]
- Tiberg, C.; Gustafsson, J.P. Phosphate Effects on Cadmium(II) Sorption to Ferrihydrite. J. Colloid. Interface Sci. 2016, 471, 103–111. [Google Scholar] [CrossRef]
- Zhu, J.; Pigna, M.; Cozzolino, V.; Caporale, A.G.; Violante, A. Sorption of Arsenite and Arsenate on Ferrihydrite: Effect of Organic and Inorganic Ligands. J. Hazard. Mater. 2011, 189, 564–571. [Google Scholar] [CrossRef]
- Mao, H.; Shu, J.; Fei, Y.; Hu, J.; Hemley, R.J. The Wüstite Enigma. Phys. Earth Planet. Inter. 1996, 96, 135–145. [Google Scholar] [CrossRef]
- Schrettle, F.; Kant, C.; Lunkenheimer, P.; Mayr, F.; Deisenhofer, J.; Loidl, A. Wüstite: Electric, Thermodynamic and Optical Properties of FeO. Eur. Phys. J. B 2012, 85, 164. [Google Scholar] [CrossRef]
- Yin, M.; Chen, Z.; Deegan, B.; O’Brien, S. Wüstite Nanocrystals: Synthesis, Structure and Superlattice Formation. J. Mater. Res. 2007, 22, 1987–1995. [Google Scholar] [CrossRef]
- Fjellvag, H.; Hauback, B.; Vogt, T.; Stolen, S. Monoclinic Nearly Stoichiometric Wüstite at Low Temperatures. Am. Mineral. 2002, 87, 347–349. [Google Scholar] [CrossRef]
- Liu, H.; Han, W.; Huo, C.; Cen, Y. Development and Application of Wüstite-Based Ammonia Synthesis Catalysts. Catal. Today 2020, 355, 110–127. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Pernicone, N. Wustite as a New Precursor of Industrial Ammonia Synthesis Catalysts. Appl. Catal. A Gen. 2003, 251, 121–129. [Google Scholar] [CrossRef]
- Rossetti, I.; Pernicone, N.; Forni, L. Promoters Effect in Ru/C Ammonia Synthesis Catalyst. Appl. Catal. A Gen. 2001, 208, 271–278. [Google Scholar] [CrossRef]
- Rakshit, S.; Matocha, C.J.; Haszler, G.R. Nitrate Reduction in the Presence of Wüstite. J. Environ. Qual. 2005, 34, 1286–1292. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current Advances and Trends in Electro-Fenton Process Using Heterogeneous Catalysts—A Review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef]
- Expoósito, E.; Saánchez-Saánchez, C.M.; Montiel, V. Mineral Iron Oxides as Iron Source in Electro-Fenton and Photoelectro-Fenton Mineralization Processes. J. Electrochem. Soc. 2007, 154, E116. [Google Scholar] [CrossRef]
- Ren, J.; Yao, Z.; Wei, Q.; Wang, R.; Wang, L.; Liu, Y.; Ren, Z.; Guo, H.; Niu, Z.; Wang, J.; et al. Catalytic Degradation of Chloramphenicol by Water Falling Film Dielectric Barrier Discharge and FeO Catalyst. Sep. Purif. Technol. 2022, 290, 120826. [Google Scholar] [CrossRef]
- Mackay, A.L. β-Ferric Oxyhydroxide—Akaganéite. Mineral. Mag. J. Mineral. Soc. 1962, 33, 270–280. [Google Scholar] [CrossRef]
- Jegdić, B.V.; Ristić, S.S.; Polić-Radovanović, S.R.; Alil, A.B. Corrosion and Conservation of Weapons and Military Equipment. Vojnoteh. Glas. 2012, 60, 169–182. [Google Scholar] [CrossRef]
- Holm, N.G.; Dowler, M.J.; Wadsten, T.; Arrhenius, G. β-FeOOH · Cln (Akaganéite) and Fe1-XO (Wüstite) in Hot Brine from the Atlantis II Deep (Red Sea) and the Uptake of Amino Acids by Synthetic β-FeOOH · Cln. Geochim. Cosmochim. Acta 1983, 47, 1465–1470. [Google Scholar] [CrossRef]
- Post, J.F.; Buchwald, V.F. Crystal Structure Refinement of Akaganeite. Am. Mineral. 1991, 76, 272–277. [Google Scholar]
- Tadic, M.; Milosevic, I.; Kralj, S.; Saboungi, M.-L.; Motte, L. Ferromagnetic Behavior and Exchange Bias Effect in Akaganeite Nanorods. Appl. Phys. Lett. 2015, 106, 183706. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, W.; Chang, Q.; Li, W.; Lai, Y. Adsorptive Characteristics of Akaganeite and Its Environmental Applications: A Review. Environ. Technol. Rev. 2012, 1, 114–126. [Google Scholar] [CrossRef]
- Marsac, R.; Martin, S.; Boily, J.-F.; Hanna, K. Oxolinic Acid Binding at Goethite and Akaganéite Surfaces: Experimental Study and Modeling. Environ. Sci. Technol. 2016, 50, 660–668. [Google Scholar] [CrossRef]
- Xiong, H.; Shen, X.; Cui, C.; Cheng, L.; Xu, Y. Synthesis of Nanospindle-Akaganéite and Its Photocatalytic Degradation for Methyl Orange. Water Sci. Technol. 2020, 82, 481–491. [Google Scholar] [CrossRef]
- Xiong, H.; Shi, K.; Han, J.; Cui, C.; Liu, Y.; Zhang, B. Synthesis of β-FeOOH/Polyaniline Heterogeneous Catalyst for Efficient Photo-Fenton Degradation of AOII Dye. Environ. Sci. Pollut. Res. 2023, 30, 59366–59381. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Liu, Y.; Liu, X.; Jin, W.; Zhao, Y. Transformation Pathway and Degradation Mechanism of Methylene Blue through β-FeOOH@GO Catalyzed Photo-Fenton-like System. Chemosphere 2019, 218, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Majzlan, J.; Koch, C.B.; Navrotsky, A. Thermodynamic Properties of Feroxyhyte (Δ′-FeOOH). Clays Clay Min. 2008, 56, 526–530. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Iron Hydroxides in Soils: A Review of Publications. Eurasian Soil. Sci. 2010, 43, 1244–1254. [Google Scholar] [CrossRef]
- Li, Y.; Fu, F.; Cai, W.; Tang, B. Synergistic Effect of Mesoporous Feroxyhyte Nanoparticles and Fe(II) on Phosphate Immobilization: Adsorption and Chemical Precipitation. Powder Technol. 2019, 345, 786–795. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Fang, D.; Xu, J.; Liang, J.; Zhou, L. Microwave–ultrasound assisted synthesis of β-FeOOH and its catalytic property in a photo-Fenton-like process. Ultrason. Sonochem. 2015, 27, 287–295. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Peng, H.Q.; Yang, R.; Jiang, Z.; Zhou, X.; Lee, C.-S.; Zhao, H.; Zhang, W. Iron vacancies induced bifunctionality in ultrathin feroxyhyte nanosheets for overall water splitting. Adv. Mater. 2018, 30, 1803144. [Google Scholar] [CrossRef]
- Kokkinos, E.; Soukakos, K.; Kostoglou, M.; Mitrakas, M. Cadmium, mercury, and nickel adsorption by tetravalent manganese feroxyhyte: Selectivity, kinetic modeling, and thermodynamic study. Environ. Sci. Pollut. Res. 2018, 25, 12263–12273. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Wang, Y. Photoinduced Degradation of Orange II on Different Iron (Hydr)Oxides in Aqueous Suspension: Rate Enhancement on Addition of Hydrogen Peroxide, Silver Nitrate, and Sodium Fluoride. Langmuir 2008, 24, 175–181. [Google Scholar] [CrossRef]
- Pinto, I.S.X.; Pacheco, P.H.V.V.; Coelho, J.V.; Lorençon, E.; Ardisson, J.D.; Fabris, J.D.; de Souza, P.P.; Krambrock, K.W.H.; Oliveira, L.C.A.; Pereira, M.C. Nanostructured δ-FeOOH: An Efficient Fenton-like Catalyst for the Oxidation of Organics in Water. Appl. Catal. B 2012, 119–120, 175–182. [Google Scholar] [CrossRef]
- Li, J.; Ding, Y.; Chen, K.; Li, Z.; Yang, H.; Yue, S.; Tang, Y.; Wang, Q. δ-FeOOH Coupled BiOBr0.5I0.5 for Efficient Photocatalysis-Fenton Synergistic Degradation of Organic Pollutants. J. Alloys Compd. 2022, 903, 163795. [Google Scholar] [CrossRef]
- Wang, C.; Shi, P.; Wang, Z.; Guo, R.; You, J.; Zhang, H. Efficient Wastewater Disinfection through FeOOH-Mediated Photo-Fenton Reaction: A Review. J. Environ. Chem. Eng. 2023, 11, 111269. [Google Scholar] [CrossRef]
- Pereira, M.C.; Garcia, E.M.; Cândido Da Silva, A.; Lorenon, E.; Ardisson, J.D.; Murad, E.; Fabris, J.D.; Matencio, T.; De Castro Ramalho, T.; Rocha, M.V.J. Nanostructured δ-FeOOH: A Novel Photocatalyst for Water Splitting. J. Mater. Chem. 2011, 21, 10280–10282. [Google Scholar] [CrossRef]
- da Silva Rocha, T.; Nascimento, E.S.; da Silva, A.C.; dos Santos Oliveira, H.; Garcia, E.M.; de Oliveira, L.C.A.; Monteiro, D.S.; Rodriguez, M.; Pereira, M.C. Enhanced Photocatalytic Hydrogen Generation from Water by Ni(OH)2 Loaded on Ni-Doped δ-FeOOH Nanoparticles Obtained by One-Step Synthesis. RSC Adv. 2013, 3, 20308. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.; Chen, G. Performance and Mechanism of Chromate (VI) Adsorption by δ-FeOOH-Coated Maghemite (γ-Fe2O3) Nanoparticles. Sep. Purif. Technol. 2007, 58, 76–82. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Estradé, S.; Martinez-Boubeta, C.; Vourlias, G.; Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Stavropoulos, G.; Mitrakas, M. Tetravalent Manganese Feroxyhyte: A Novel Nanoadsorbent Equally Selective for As(III) and As(V) Removal from Drinking Water. Environ. Sci. Technol. 2013, 47, 9699–9705. [Google Scholar] [CrossRef]
- Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Simeonidis, K.; Mitraka, E.; Mitrakas, M. Monitoring the Role of Mn and Fe in the As-Removal Efficiency of Tetravalent Manganese Feroxyhyte Nanoparticles from Drinking Water: An X-Ray Absorption Spectroscopy Study. J. Colloid. Interface Sci. 2016, 477, 148–155. [Google Scholar] [CrossRef]
- Kokkinos, E.; Kellartzis, I.; Diamantopoulou, I.; Stavropoulos, G.; Vourlias, G.; Mitrakas, M. Study of Elemental Mercury Removal from Flue Gases Using Tetravalent Manganese Feroxyhyte. Chem. Eng. J. 2017, 315, 152–158. [Google Scholar] [CrossRef]
- Chagas, P.; Da Silva, A.C.; Passamani, E.C.; Ardisson, J.D.; De Oliveira, L.C.A.; Fabris, J.D.; Paniago, R.M.; Monteiro, D.S.; Pereira, M.C. δ-FeOOH: A Superparamagnetic Material for Controlled Heat Release under AC Magnetic Field. J. Nanoparticle Res. 2013, 15, 1544. [Google Scholar] [CrossRef]
- Lacerda, L.C.T.; Pires, M.S.; Oliveira, I.S.S.; Silva, T.C.; de Castro, A.A.; Corrêa, S.; Vaiss, V.S.; Ramalho, T.C. Bulk and Surface Theoretical Investigation of Nb-Doped δ-FeOOH as a Promising Bifunctional Catalyst. J. Mol. Model. 2021, 27, 249. [Google Scholar] [CrossRef]
- Kolitsch, U. Bernalite from the Clara Mine, Germany, and the Incorporation of Tungsten in Minerals Containing Ferric Iron. Can. Mineral. 1998, 36, 1211–1216. [Google Scholar]
- McCammon, C.A.; Pring, A.; Keppler, H.; Sharp, T. A Study of Bernalite, Fe(OH)3, Using Mössbauer Spectroscopy, Optical Spectroscopy and Transmission Electron Microscopy. Phys. Chem. Min. 1995, 22, 11–20. [Google Scholar] [CrossRef]
- Welch, M.D.; Crichton, W.A.; Ross, N.L. Compression of the Perovskite-Related Mineral Bernalite Fe(OH) 3 to 9 GPa and a Reappraisal of Its Structure. Min. Mag. 2005, 69, 309–315. [Google Scholar] [CrossRef]
- McCammon, C.A.; De Grave, E.; Pring, A. The Magnetic Structure of Bernalite, Fe(OH)3. J. Magn. Magn. Mater. 1996, 152, 33–39. [Google Scholar] [CrossRef]
- Han, J.; Ro, H.-M. Identification of Bernalite Transformation and Tridentate Arsenate Complex at Nano-Goethite under Effects of Drying, PH and Surface Loading. Sci. Rep. 2018, 8, 8369. [Google Scholar] [CrossRef]
- Han, J.; Ro, H.-M. Interpreting competitive adsorption of arsenate and phosphate on nanosized iron (hydr)oxides: Effects of pH and surface loading. Environ. Sci. Pollut. Res. 2018, 25, 28572–28582. [Google Scholar] [CrossRef]
- Su-Gallegos, J.; Magallón-Cacho, L.; Borja-Arco, E.; García-Valdés, J.; Sebastian, P.J. Oxygen Reduction and Hydrogen Oxidation Reactions on Ru-Fe Electrocatalyst Synthesized by a Microwave-Assisted Synthesis. Mater. Res. Express 2020, 7, 035505. [Google Scholar] [CrossRef]
- Nur’aeni; Chae, A.; Jo, S.; Choi, Y.; Park, B.; Park, S.Y.; In, I. Synthesis of β-FeOOH/Fe3O4 Hybrid Photocatalyst Using Catechol-Quaternized Poly(N-Vinyl Pyrrolidone) as a Double-Sided Molecular Tape. J. Mater. Sci. 2017, 52, 8493–8501. [Google Scholar] [CrossRef]
- Sciscenko, I.; Mestre, S.; Climent, J.; Valero, F.; Escudero-Oñate, C.; Oller, I.; Arques, A. Magnetic Photocatalyst for Wastewater Tertiary Treatment at Pilot Plant Scale: Disinfection and Enrofloxacin Abatement. Water 2021, 13, 329. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Vreuls, C.; Drot, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Hermans, S.; Lambert, S.D. Advanced Oxidation Processes for Waste Water Treatment: From Laboratory-Scale Model Water to on-Site Real Waste Water. Environ. Technol. 2021, 42, 3974–3986. [Google Scholar] [CrossRef]



| Name | Formula | Crystal Symmetry |
|---|---|---|
| Magnetite (Mt) | Fe3O4 | Cubic spinel |
| Hematite (Ht) | α-Fe2O3 | Rhombohedral |
| Goethite (Gt) | α-FeOOH | Orthorhombic |
| Lepidocrocite | γ-FeOOH | Orthorhombic |
| Ferrihydrite (Fh) | 5Fe2O3⋅9H2O | Hexagonal |
| Maghemite (Mh) | γ-Fe2O3 | Cubic spinel |
| Wüstite | FeO | Isometric-hexoctahedral |
| Akaganéite | β-FeOOH | Monoclinic |
| Feroxyhyte | δ′-FeOOH | Hexagonal |
| Bernalite | Fe(OH)3 | Orthorhombic |
| Characteristics | Bulk Fe3O4 | Fe3O4 NPs |
|---|---|---|
| Magnetism | Ferromagnetism | Superparamagnetism or ferrimagnetism |
| Saturation magnetization (Ms, 300 K, emu/g) | ~(84–100) | Depend on size, shape, and coating: ~(0.5–92) |
| Size controllability | Unachievable | Precisely controllable: ~(2–100) nm |
| Shape controllability (Spherical, cubic, rod, hollow, and 2D nanoplate) | Unachievable | Precisely controllable |
| Specific surface area (m2/g) | 0.34 * | Depend on size, shape, and coating: ~(20–300) |
| Magnetothermal conversion (W/g) | Absent | ~(100–2500) |
| Electrocatalytic activity | Achievable | Achievable |
| Catalytic Reaction | Natural Source | Pretreatment Method | SBET (m2·g−1) | Reaction Conditions | Results of Catalytic Evaluation | Rate Constant | Ref. |
|---|---|---|---|---|---|---|---|
| Fenton p-NP degradation | Ores | Crushing, grinding, and magnetic and gravity separation | 0.54–2.46 | Cat = 1.0 g·L−1; p-nitrophenol = 10 mg·L−1; pH = 7; H2O2 = 0.05 mol·L−1; NaOH = 0.1 mol·L−1 | 17.8–95.0% degradation | 0.24, 0.19, and 0.12 μg·L−1·min−1 | [1] |
| Photo-Fenton degradation of MB | Natural iron sands | Magnetic separation and coprecipitation | 147.12 | Cat = 2 g·L−1; MB = 10 mL of 20 ppm; UV irradiation | 68.52–76.32% degradation | 0.000194, 0.000262, and 0.000336 g·mg−1·min−1 | [51] |
| Fenton degradation of MB | Beach sand | Drying, magnetic separation, and ball milling | ND | Cat = 1 g·L−1; MB = 15 mg·L−1; pH = 4.8; H2O2 = 150 mL L−1 | 86.79% degradation | ND | [44] |
| Photo-Fenton degradation of cefotaxime * | Natural magnetite | Ball milling method | 2.92–5.64 | Cat = 0.2 g·L−1; cefotaxime = 0.4 mmol·L−1; pH = 5.6; H2O2 = 10 mmol·L−1 | 100% degradation after 60 min | 0.09 min−1 | [46] |
| Fenton-like degradation of AO7 | Natural magnetite | ND | ND | Cat = 0.5 mg·L−1; AO7 = 15 mg·L−1; pH = 5; S2 = 0.2 mM | 75% degradation after 120 min | 0.0019 min−1 | [49] |
| Fenton-like degradation of MeP | Natural magnetite | As received | 62.5 | Cat = 0.3 g·L−1; MeP = 10 µmol·L−1; pH = 6.5; SPS = 5 mmol·L−1 | 90.2–99.5% degradation | 0.0066 min−1 | [52] |
| Electro-Fenton removal of Gemcitabine | Natural mineral | Crushing, sieving, and milling | ND | Pt anode; 140 mL·min−1 of air; 1 cm separation between electrodes; 0.05 mol·L−1 of Na2SO4 | 25–35% degradation | 63 M−1s−1 min−1 | [54] |
| CWPO of phenol | Natural iron minerals | Sieving | 8.0 | Cat = 1 g·L−1; phenol = 100 mg·L−1; pH = 3; H2O2 = 500 mg·L−1; T = 75 °C; t = 4 h | 70–80% mineralization and 100% of conversion | 11 L2 mg−1 gcat−1 min−1 | [62] |
| CWPO of azole pesticide | Pristine magnetite | As received | 7.5 | Cat = 8 g; H2O2 = 6.7 mg·L−1; T = 25 °C; pH = 5; flow rate = 0.5 mL·min−1 | Conversion of the pollutants > 95% | 0.22 mL·gcat−1·min−1 | [50] |
| CWPO of SMX | Pristine magnetite | As received | 7.5 | Cat = 1 g·L−1; H2O2 = 25 mg·L−1; T = 25 °C; pH = 5 | 50% mineralization and 100% removal | 0.0263 mg·L−1·min−1 | [55] |
| Fischer–Tropsch synthesis ** | Natural magnetite | Sol–gel and high-temperature pyrolysis | 73.4–157.7 | Cat = 1 g; 2 g of quartz sand; T = 300 °C; P = 2 MPa; GHSV of 3000 mL·g−1·h−1; t = 180 h | 93–98.5% conversion of CO | ND | [61] |
| Production of diesel by catalytic pyrolysis | Magnetite ore | Magnetic separation, crushing, grinding, and ball milling | ND | Cat = 3 wt%; T = 500 °C; t = 90 min | Selectivity towards the formation of hydrocarbons having fuel value in conformity with diesel fuel | ND | [45] |
| Cracking of coal | Mineral magnetite | As received | 3.32 | Cat = 1 mg; coal; 1 mg; T = 700 °C; Ar atmosphere | Selectivity to aliphatics | ND | [59] |
| Biodiesel production from waste cooking oil *** | Iron sand | Coprecipitation and wet impregnation | ND | Ratio of methanol to oil = 9:1; Cat = 1 wt%; T = 60 °C; t = 6 h; 1100 rpm | 95.64% yield | ND | [63] |
| Catalytic Reaction | Catalyst | Natural Source | Pretreatment Method | SBET (m2·g−1) | Reaction Conditions | Results of Catalytic Evaluation | Rate Constant | Ref. |
|---|---|---|---|---|---|---|---|---|
| Photocatalytic degradation of MB | Mg/α-Fe2O3 | Natural sand | Magnetic separation, drying, and ball milling | ND | UV irradiation; t = 300 min | Removal of 88.8% of MB | ND | [66] |
| Photocatalytic degradation of phenol | α-Fe2O3/gelcasting porous ceramic | Natural clay | Grinding, sieving, and extraction by 3 M of H2SO4 at 80 °C | 31.92 | UV irradiation; T = 23 °C; pH = 8; t = 3 h; phenol = 10 mg·L−1 | Removal of 57% of phenol; catalytic activity remains without changes after 8 cycles | ND | [78] |
| Photo-Fenton degradation of MO | α-Fe2O3/SiO2 | Iron sand | Sieving, immersion in HCl solution, washing, and drying | 11.16 | Cat = 1.5 g·L−1; MO = 100 mg·L−1; H2O2 = 200 mg·L−1; pH = 3; UV irradiation | 93% of degradation of MO. 89% after 4 cycles of reaction | 0.048 min−1 | [72] |
| Fenton degradation of (2,4-D) and MCPA | α-Fe2O3 | Natural mineral | Crushing, sieving, washing, sonication, and calcination | 60.4 | Cat = 0.5 g·L−1; 2,4-D and MCPA = 200 mg·L−1; PS = 0.025 M; pH = 3; T = 50 °C; t = 120 min | 36% of mineralization | MCPA = 0.0064; 2,4-D = 0.0059 min−1 | [73] |
| Fenton and photo-Fenton oxidation of phenol in water | Mining reject | Mining reject | Ball milling and sieving | 16.35 | Cat = 0.75 g·L−1; phenol = 50 mg·L−1; H2O2 = 0.75 g·L−1; pH = 3 | 96.5% degradation at 180 min | 0.0411 min−1 | [74] |
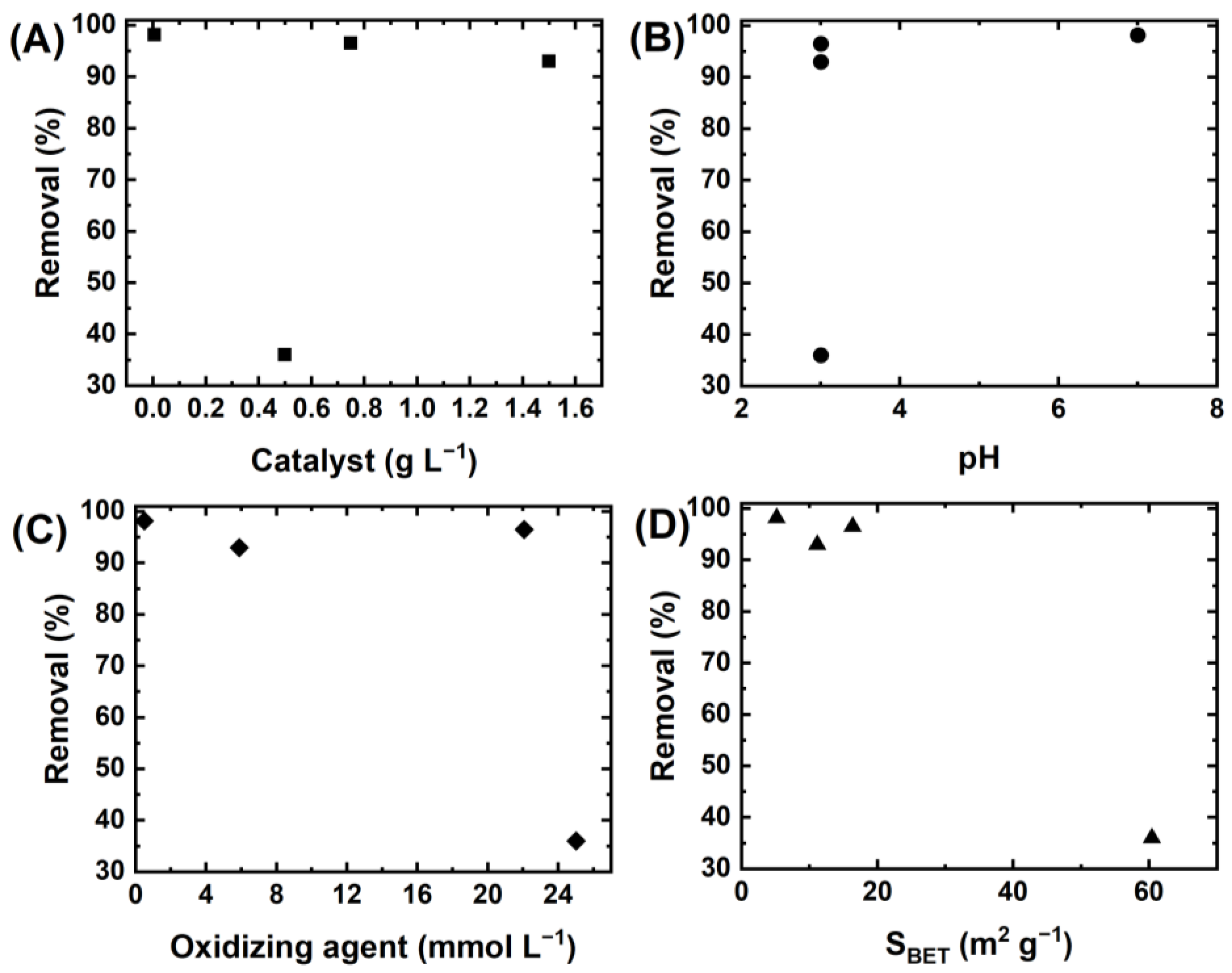
| Degradation of DCF via PMS activation | α-Fe2O3 | HRS | As received | 5.17 | Cat = 5 mg·L−1; DCF = 50 mg·L−1; PMS = 75 mg·L−1; t = 10 min; neutral pH | 98.2% of degradation in 10 min | 0.334 min−1 | [75] |
| Photocatalytic degradation of IC | α-Fe2O3/bentonite | Iron ore | Magnetic separation, grinding, ball milling, and coprecipitation | ND | Cat = 250 mg; IC = 5 mg·L−1; pH = 1; solar light | 100% of degradation after 2 h | ND | [76] |
| Photocatalytic reduction of 4 NP to 4 AP | Cu/Fe2O3 | Natural iron ore rock | Crushing, sieving, calcination, and impregnation | 10–42 | Cat = 33.3 mg·L−1; 4 NP = 5 × 10−5 mol·L−1; NaBH4 = 12.5 mL (0.5 M); pH = 11.5; λ = 200–500 nm | >99% conversion in less than 1 min | 2.34, 3.36, and 5.4 min−1 | [77] |
| Photocatalytic reduction of 4 NP to 4 AP | α-Fe2O3 | Natural iron ore rock | Crushing, sieving, heat treatment, and calcination | 18–85 | Cat = 33.3 mg·L−1; 4 NP = 100 mL (0.1 mM); λ = 220–550 nm; NaBH4 = 12.5 mL (0.5 M) | >99% conversion in less than 3 min | 1.38 min−1 | [79] |
| Catalytic ozonation and peroxone-mediated removal of ACT | α-Fe2O3 | HRS | Calcination in air atmosphere | 3.63 | Cat = 1 g·L−1; ACT = 50 mg·L−1; O3 = 1.2 mg/min; t= 10 min; pH = 7 | 100% degradation | 0.40 min−1 | [81] |
| Hydrocracking of high-temperature coal tar | Mo/Al2O3-Fe2O3 | Natural bauxite | Crushing, calcination, purification, washing, and wet impregnation | 126.9–237.9 | Cat = 1.3 g; sulfur powder = 0.4 g; HTCT = 42 g; T = 430 °C; P = 12.5 MPa; t = 90 min | The presence of Fe2O3 is not favorable to the hydrocracking of high-temperature coal tar | ND | [82] |
| Hydrogenation of coal | Fe2O3–SiO2–Al2O3–TiO2–MnO2 | Natural bauxite | ND | ND | T = 380–440 °C; P = 3–5 MPa; sulfur additive = 0–2%; t = 90 min | Selectivity to liquid products | ND | [84] |
| Biodiesel production from waste cooking oil | α-Fe2O3 | Iron sand | Magnetic separation, coprecipitation, and calcination | 10.5–22.9 | Esterification: methanol: waste cooking oil molar ratio of 5:1; H2SO4 = 1 wt%; T = 70 °C; t = 300 min. Transesterification: waste cooking oil: methanol molar ratio of 15:1; Cat = 1 wt%; T = 65 °C; t = 3 h | 87.88% biodiesel yield | ND | [86] |
| Biodiesel production from waste cooking oil | Fe2O3/CaO2 | Iron sand | Magnetic separation, coprecipitation, and calcination | ND | Waste cooking oil: methanol molar ratio of 1:15; Cat = 1 wt%; T = 65 °C; t = 3 h | 97.04% biodiesel yield with a Fe/Ca ratio of 1:4 | ND | [87] |
| Hydrothermal catalytic conversion of extra-heavy Ashal’chinskoe oil | α-Fe2O3 | Natural hematite | As received | ND | T = 210, 230, and 300 °C; P = 2–18 MPa; t = 2 h | The possibility of increasing the number of lighter hydrocarbons in heavy oil and reducing its density by a regular decrease in the amount of resin–asphaltene components | ND | [88] |
| Catalytic cracking of toluene | α-Fe2O3 | Natural limonite | Crushing, sieving, and calcination | 12.4 | Cat = 0.5–1.5 g; toluene = 1000 ppm; T = 500–800 °C; t = 60 min | 95% of toluene conversion | ND | [89] |
| Catalytic cracking of coal tar | α-Fe2O3/γ-Al2O3 α-Fe2O3/NiO | Mineral hematite | Crushing, drying, and mechanical mixing | ND | T = 700–900 °C; consumption of coal tar = 0.3 kg·h−1; mass flow rate of coal tar = 5 g·min−1 | Poor performance of hematite/NiO; the addition of γ-Al2O3 could effectively inhibit carbon deposition | ND | [90] |
| OER | Ni/iron ore | Iron ore | Ball milling and solution-assisted electrode preparation | ND | Cat = 150 mg; NaOH = 1 M | Achieves a current density of 10 mA cm−2 at a low overpotential of 280 mV; potentially scalable to industrial applications | ND | [91] |
| Catalytic hydrolysis of microcystin-LR peptides | α-Fe2O3 | Mineral hematite | Washing, drying, milling, and sieving | ND | Cat = 20 mg; MC-LR = 1 mL (10 ppm); T = 60 °C | 20.7% hydrolysis yield | ND | [106] |
| Fischer–Tropsch synthesis | α-Fe2O3 | Raw iron ore | Grinding and sieving | 59.0 | Syngas at space velocity of 60 Nml·gcat−1; T = 270 °C; P = 20 bar | The CO-reduced catalyst exhibited the highest CO conversion of 94.1%, followed by the H2-reduced catalyst with a CO conversion of 80.1%, while the syngas-reduced catalyst showed the least CO conversion of 54.1% | ND | [107] |
| Catalytic Reaction | Natural Source | Pretreatment Method | SBET (m2·g−1) | Reaction Conditions | Results of Catalytic Evaluation | Rate Constant | Ref. |
|---|---|---|---|---|---|---|---|
| Oxidation of toluene * | Acid mine drainage | Sequential precipitation method and wetness impregnation | 115.70 | Cat = 1 g; T = 250–450 °C; toluene = 0.9 vol%; flow rate = 500 L·min−1; GHSV = 18,000 h−1 | 30% of toluene conversion; selectivity to CO2 | ND | [121] |
| Photocatalytic degradation of MB ** | Goethite rocks | Ball milling, stirring, sonication, and electro-spinning process | ND | Cat = 0.5 g; MB = 100 mL (10 ppm); t = 5 h; visible light and UV | 90% of bleaching after 5 h of illumination | 0.0141 min−1; similar in UV and visible light | [122] |
| Fenton-like TC degradation *** | Mineral goethite | Hydrothermal method | 20.4 | Cat = 0.3 g·L−1; pH = 3; TC = 100 mg·L−1; H2O2 = 6.0 mM | 90.1% removal after 30 min of treatment | ND | [123] |
| Reduction and stabilization of Cr(VI) in soil | Natural goethite | As received | ND | Cat = 3, 6, 9, and 12 g; pH = 8.6 to 9 | Goethite increased the apparent rate constant | 3.33 × 10−6 kg·mg−1·min−1 to 8.33 × 10−6 kg·mg−·min−1 | [128] |
| Ozonation of SSZ **** | Natural goethite | Crushing, milling, washing, and plasma process | 29.65, 73.24 and 77.31, respectively | Cat = 1–9 mg·L−1; T = 25 °C; P = 1 atm; inlet flow = 1 L·h−1; Na2SO3 = 1 mL (0.01 M) | 96.5% degradation efficiency reached by N2-goethite after 40 min of reaction | 0.076 min−1 | [129] |
| Catalytic reforming of volatiles from co-pyrolysis | Mineral goethite | Dehydration | 18.05–140.28 | 15–20 mg of a mixture of raw lignite and corn straw; T = room temperature to 800 °C; Ar flow = 100 mL·min−1 | Goethite significantly promotes the production of light aromatic hydrocarbons | ND | [135] |
| Production of ROS | Natural goethite | Mechanical crushing, milling, and sieving | ND | Cat = 0.1 g·L−1; LI = 10 mW cm−2; pH = 7; T = 25 °C | Natural goethite ROS production was 4.9-fold higher than the standard | 0.648 nM min−1 | [137] |
| Catalytic Reaction | Catalyst | Mineral Source | Pretreatment Method | SBET (m2·g−1) | Reaction Conditions | Results of Catalytic Evaluation | Ref. |
|---|---|---|---|---|---|---|---|
| Transfer hydrogenation of furfural, levulinic acid, and o-vanillin | γ-Fe2O3 | River sand | Magnetic separation, grinding, washing, and drying at 80 °C | 22.3 | Cat = 20 mg (7 mol %); T = 150 °C; 1 mmol of substrate in 2-propanol (3 mL); t = 30–90 min | γ-Fe2O3 recovered from the natural source exhibited superior activity compared to the synthetic counterpart under base-free conditions | [149] |
| Catalytic reduction of NO with NH3 | α-Fe2O3/γ-Fe2O3 | Iron ores | Drying, grinding, and calcination | 22.8–42.5 | NO = 500 ppm; NH3 = 500 ppm; O2 = 3 vol %; SO2 (when used) = 150 ppm; H2O (when used) = 5 vol%; N2 = 145 mL·min−1; GHSV = 20,000 h−1 | Selectivity catalytic reduction activity above 80% at 170–350 °C and N2 selectivity (above 90% up to 250 °C) at low temperatures | [152] |
| Decomposition of hydrogen peroxide | Ferrihydrite | Acid mine drainage | Vacuum drying | 270 | Cat = 1 g·L−1; H2O2 = 0.02 M | Waste ferrihydrite shows the same catalytic activity for H2O2 decomposition as the commercial goethite-based catalyst | [150] |
| Photo-Fenton degradation of ENR and LEV | Wüstite | ND | ND | 108.7 | Cat = 10 mg·L−1; ENR = LEV = 0.05 mmol·L−1; H2O2 = 1.0 mmol·L−1; pH = 6.5; T = 35 °C; t = 180 min | 100% and 55 % of antibiotic activity elimination in 180 min for ENR and LEV, respectively; complete antibiotic activity elimination for ENR in the next four recycling cycles | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Vázquez, A.; Jaimes-López, R.; Morales-Bautista, C.M.; Pérez-Rodríguez, S.; Gochi-Ponce, Y.; Estudillo-Wong, L.A. Catalytic Applications of Natural Iron Oxides and Hydroxides: A Review. Catalysts 2025, 15, 236. https://doi.org/10.3390/catal15030236
Jiménez-Vázquez A, Jaimes-López R, Morales-Bautista CM, Pérez-Rodríguez S, Gochi-Ponce Y, Estudillo-Wong LA. Catalytic Applications of Natural Iron Oxides and Hydroxides: A Review. Catalysts. 2025; 15(3):236. https://doi.org/10.3390/catal15030236
Chicago/Turabian StyleJiménez-Vázquez, Adriana, Raciel Jaimes-López, Carlos Mario Morales-Bautista, Samuel Pérez-Rodríguez, Yadira Gochi-Ponce, and Luis Alberto Estudillo-Wong. 2025. "Catalytic Applications of Natural Iron Oxides and Hydroxides: A Review" Catalysts 15, no. 3: 236. https://doi.org/10.3390/catal15030236
APA StyleJiménez-Vázquez, A., Jaimes-López, R., Morales-Bautista, C. M., Pérez-Rodríguez, S., Gochi-Ponce, Y., & Estudillo-Wong, L. A. (2025). Catalytic Applications of Natural Iron Oxides and Hydroxides: A Review. Catalysts, 15(3), 236. https://doi.org/10.3390/catal15030236








