Controllable Synthesis of Ultrafine Ag NPs/Functionalized Graphene-Introduced TiO2 Mesoporous Hollow Nanofibers by Coaxial Electrospinning for Photocatalytic Oxidation of CO
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Synthetic Pathway
2.2. Functionalized Graphene–TiO2 Mesoporous Hollow Nanofibers
2.3. Ultrafine Ag NPs Modified SiG-TO MPHNFs
2.4. Discussion on Mechanism
3. Experimental Section
3.1. Materials Synthesis
3.1.1. Reagents and Solvents
3.1.2. Synthesis of the BGR
3.1.3. Synthesis of KH550-BGR
3.1.4. Synthesis of SiG-TO MPHNFs
3.1.5. Synthesis of Ag/SiG-TO MPHNFs
3.2. Characterization of Materials
3.3. Hydroxyl Radical Production Analysis
3.4. Photoelectrochemical Test
3.5. In Situ Fourier Transform Infrared Spectra
3.6. Evaluation of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-Oxide Semiconductors for Carbon Monoxide (CO) Gas Sensing: A Review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Wu, J.; Hu, Q.; Zhou, C. Photothermocatalysis for Efficient Abatement of CO and VOCs. J. Mater. Chem. A 2020, 8, 8171–8194. [Google Scholar] [CrossRef]
- Ma, X.; Albertsma, J.; Gabriels, D.; Horst, R.; Polat, S.; Snoeks, C.; Kapteijn, F.; Eral, H.B.; Vermaas, D.A.; Mei, B.; et al. Carbon Monoxide Separation: Past, Present and Future. Chem. Soc. Rev. 2023, 52, 3741–3777. [Google Scholar] [CrossRef] [PubMed]
- Ovcharov, M.L.; Granchak, V.M. Photocatalytic Activation of Carbon Monoxide on Semiconductors and Derived Nanocomposites: Basic Principles and Mechanisms: A Review. Theor. Exp. Chem. 2019, 55, 173–200. [Google Scholar] [CrossRef]
- Wu, X.; Lang, J.; Sun, Z.; Jin, F.; Hu, Y. Photocatalytic Conversion of Carbon Monoxide: From Pollutant Removal to Fuel Production. Appl. Catal. B Environ. 2021, 295, 120312. [Google Scholar] [CrossRef]
- Eid, K.; Gamal, A.; Abdullah, A.M. Graphitic Carbon Nitride-based Nanostructures as Emergent Catalysts for Carbon Monoxide (CO) Oxidation. Green Chem. 2023, 25, 1276–1310. [Google Scholar] [CrossRef]
- Ruan, X.; Li, S.; Huang, C.; Zheng, W.; Cui, X.; Ravi, S.K. Catalyzing Artificial Photosynthesis with TiO2 Heterostructures and Hybrids: Emerging Trends in a Classical yet Contemporary Photocatalyst. Adv. Mater. 2023, 36, e2305285. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why Five Decades of Massive Research on Heterogeneous Photocatalysis, Especially on TiO2, Has Not yet Driven to Water Disinfection and Detoxification Applications? Critical Review of Drawbacks and Challenges. Chem. Eng. J. 2023, 477, 14687. [Google Scholar] [CrossRef]
- Shoneye, A.; Chang, J.S.; Chong, M.N.; Tang, J.W. Recent Progress in Photocatalytic Degradation of Chlorinated Phenols and Reduction of Heavy Metal Ions in Water by TiO2-Based Catalysts. Int. Mater. Rev. 2021, 67, 47–64. [Google Scholar] [CrossRef]
- Kolobov, N.S.; Svintsitskiy, D.A.; Kozlova, E.A.; Selishchev, D.S.; Kozlov, D.V. UV-LED Photocatalytic Oxidation of Carbon Monoxide over TiO2 Supported with Noble Metal Nanoparticles. Chem. Eng. J. 2017, 314, 600–611. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhang, M.; Jing, X.; Zhang, P.; Zhu, Y.; Zhang, Z. Electrospun Semiconductor-Based Nano-Heterostructures for Photocatalytic Energy Conversion and Environmental Remediation: Opportunities and Challenges. Energy Environ. Mater. 2022, 6, e12338. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Bi, Z.; Ni, W.; Gurzadyan, G.; Zhu, Y.; Zhang, Z. Plasmonic Active “Hot Spots”-Confined Photocatalytic CO2 Reduction with High Selectivity for CH4 Production. Adv. Mater. 2022, 34, 2109330. [Google Scholar] [CrossRef]
- Dou, T.; Zhu, Y.; Chu, Z.; Sun, L.; Li, Z.; Jing, L. Controlled Synthesis of Au@TiO2 Mesoporous Hollow Nanofibers by One-step Polyethylenimine-regulated Electrospinning Method for Efficiently Photocatalytic Oxidation of CO. Appl. Catal. B Environ. Energy 2024, 354, 124112. [Google Scholar] [CrossRef]
- Li, X.; Zhi, L. Graphene Hybridization for Energy Storage Applications. Chem. Soc. Rev. 2018, 47, 3189–3216. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B.H. Graphene Oxide for Photonics, Electronics and Optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, Q.; Mao, D.; Qi, J.; Wei, Y.; Yu, R.; Li, A.; Li, S.; Zhao, H.; Ma, Y.; et al. Dually Ordered Porous TiO2-rGO Composites with Controllable Light Absorption Properties for Efficient Solar Energy Conversion. Adv. Mater. 2017, 29, 1604795. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. rGO-Wrapped Ag-Doped TiO2 Nanofibers for Photocatalytic CO2 Reduction under Visible Light. J. Clean. Prod. 2022, 374, 134022. [Google Scholar] [CrossRef]
- Johns, J.E.; Hersam, M.C. Atomic Covalent Functionalization of Graphene. Acc. Chem. Res. 2013, 46, 77–86. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host-Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Rooyanian, S.; Bagherzadeh, M.; Akrami, Z.; Golikand, A.N. A Simple Route to Surface Functionalization of Graphene Nanosheets by Benzoic Acid and Its Application toward Pb(II) Sensing. New J. Chem. 2018, 42, 17371–17378. [Google Scholar] [CrossRef]
- Sordello, F.; Zeb, G.; Hu, K.; Calza, P.; Minero, C.; Szkopek, T.; Cerruti, M. Tuning TiO2 Nanoparticle Morphology in Graphene–TiO2 Hybrids by Graphene Surface Modification. Nanoscale 2014, 6, 6710–6719. [Google Scholar] [CrossRef]
- Fu, X.; Xie, M.; Luan, P.; Jing, L. Effective Visible-Excited Charge Separation in Silicate-Bridged ZnO/BiVO4 Nanocomposite and Its Contribution to Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2014, 6, 18550–18557. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, B.; Chu, X.; Sun, N.; Qu, Y.; Zhang, X.; Khan, I.; Bai, L.; Jing, L. Synthesis of Si−O-Bridged g-C3N4/WO3 2D-Heterojunctional Nanocomposites as Efficient Photocatalysts for Aerobic Alcohol Oxidation and Mechanism Insight. ACS Sustain. Chem. Eng. 2019, 7, 9916–9927. [Google Scholar] [CrossRef]
- Pan, C.; Wang, C.; Zhao, X.; Xu, P.; Mao, F.; Yang, J.; Zhu, Y.; Yu, R.; Xiao, S.; Fang, Y.; et al. Neighboring sp-Hybridized Carbon Participated Molecular Oxygen Activation on the Interface of Sub-nanocluster CuO/Graphdiyne. J. Am. Chem. Soc. 2022, 144, 4942–4951. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, L.; Li, Z.; Wu, W.; Bian, J.; Jing, L. Interface Modulation of FePc/Porous Ti(HPO4)2 Z-Scheme Heterojunctions with Ultrafine Ag for Efficiently Photocatalytic CO Oxidation. Small Struct. 2022, 3, 2200011. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, L.; Li, Z.; Qu, Y.; Wu, W.; Jing, L. Porous Two-dimension MnO2-C3N4/Titanium Phosphate Nanocomposites as Efficient Photocatalysts for CO Oxidation and Mechanisms. Appl. Catal. B Environ. 2021, 282, 119563. [Google Scholar] [CrossRef]
- Nie, C.; Du, P.; Zhao, H.; Xie, H.; Li, Y.; Yao, L.; Shi, Y.; Hu, L.; Si, S.; Zhang, M.; et al. Ag@TiO2 Nanoprisms with Highly Efficient near-Infrared Photothermal Conversion for Melanoma Therapy. Chem. Asian J. 2019, 15, 148–155. [Google Scholar] [CrossRef]
- Chen, L.; Lei, J.; Tian, L.; Deng, K.; Cheng, G. One-Pot in Situ Fabrication of Cu-Coupled Rugby-Shaped BiVO4 Sosoloid for Enhancing Photocatalytic Activity with SPR Effect via Ultrasonic Hydrothermal Strategy. Ceram. Int. 2021, 47, 23001–23013. [Google Scholar] [CrossRef]
- Zhou, P.; Luo, M.C.; Guo, S.J. Optimizing the Semiconductor-Metal-Single-Atom Interaction for Photocatalytic Reactivity. Nat. Rev. Chem. 2022, 6, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, F.; Zhang, S.; Shen, J.; Zhu, X.; Cui, Y.; Li, P.; Lin, C.; Li, X.; Xiao, Q.; et al. Ice-templated Synthesis of Atomic Cluster co-catalyst with Regulable Coordination Number for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2024, 36, 2400764. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, L.; Bian, J.; Zhang, Z.; Li, Z.; Jing, L. Accelerated Charge Transfer of g-C3N4/BiVO4 Z-scheme 2D Heterojunctions by Controllably Introducing Phosphate Bridges and Ag Nanocluster co-catalysts for Selective CO2 Photoreduction to CO. Appl. Surf. Sci. 2023, 610, 155360. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, D.; Sun, Y.; Qi, D. Controllable Microemulsion Synthesis of Hybrid TiO2-SiO2 Hollow Spheres and Au-Doped Hollow Spheres with Enhanced Photocatalytic Activity. Langmuir 2022, 38, 4001–4013. [Google Scholar] [CrossRef]
- Lee, Y.-F.; Chang, K.-H.; Hu, C.-C.; Lin, K.-M. Synthesis of Activated Carbon-Surrounded and Carbon-Doped Anatase TiO2 Nanocomposites. J. Mater. Chem. 2010, 20, 5682–5688. [Google Scholar] [CrossRef]
- Gul, I.; Sayed, M.; Rehman, F.; Wang, J.; Fu, P.; Zhang, Y.; Nadagouda, M.N. Unlocking the Potential of Multifunctional and Highly Porous Ti3C2/TiO2@Bi2O3-Based MXene: Synergetic Photocatalytic Activation of Peroxymonosulfate, Hydrogen Evolution and Antimicrobial Activity. Appl. Catal. B Environ. Energy 2024, 359, 124493. [Google Scholar] [CrossRef]
- Peng, C.; Yang, X.; Li, Y.; Yu, H.; Wang, H.; Peng, F. Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, G.; Yuan, S.; Wang, L.; Shi, L.; Yu, W.; Zhang, H. Interfacial Encapsulation Stress Management of Micron-Sized Porous SiO2 Anodes for High-Energy Lithium-Ion Batteries. J. Mater. Chem. A 2023, 11, 972–982. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.; Shao, G.; Du, Z.; Yang, W.; Wang, L.; Wang, Z.; Chen, S. Enhanced Piezoresistive Behavior of SiC Nanowire by Coupling with Piezoelectric Effect. ACS Appl. Mater. Interfaces 2020, 12, 21903–21911. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Liu, W.; Xu, Y.; Wang, P.; Fan, J.; Yu, H. Core-Shell Ag@Ni cocatalyst on the TiO2 Photocatalyst: One-Step Photoinduced Deposition and Its Improved H2-Evolution Activity. Appl. Catal. B Environ. 2020, 260, 118190. [Google Scholar] [CrossRef]
- He, L.; Jing, L.; Luan, Y.; Wang, L.; Fu, H. Enhanced Visible Activities of α-Fe2O3 by Coupling N-Doped Graphene and Mechanism Insight. ACS Catal. 2014, 4, 990–998. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Spectroscopic Observation of Dual Catalytic Sites during Oxidation of CO on an Au/TiO2 Catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xiong, J.; Tang, Z.; Wang, Y.; Wang, X.; Xu, H.; Zhao, Z.; Liu, J.; Wei, Y. Localized Surface Plasmon Resonance Effect of Bismuth Nanoparticles in Bi/TiO2 Catalysts for Boosting Visible Light-Driven CO2 Reduction to CH4. Appl. Catal. B Environ. Energy 2024, 344, 123651. [Google Scholar] [CrossRef]
- Xia, X.-C.; Cheng, F.; Sun, P.; Dubale, A.A.; Han, H.; Cui, E.-T.; Song, J.; Yang, X.-L.; Xie, M.-H. Versatile MOF-Based Nanofibrous Heterostructures for Engineering the CO2 Photoreduction Activity and Selectivity: From CO to C2H5OH. Chem. Eng. J. 2024, 491, 152096. [Google Scholar] [CrossRef]
- Gaur, S.; Wu, H.; Stanley, G.G.; More, K.; Kumar, C.S.S.R.; Spivey, J.J. CO Oxidation Studies over Cluster-Derived Au/TiO2 and AUROlite™ Au/TiO2 Catalysts using DRIFTS. Catal. Today 2013, 208, 72–81. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X.; Zhu, T.; Feng, C.; Hu, Y.; Tian, M. New Insights into the Influence Mechanism of H2O and SO2 on Pt-W/Ti Catalysts for CO Oxidation. Catal. Sci. Technol. 2022, 12, 1574–1585. [Google Scholar] [CrossRef]


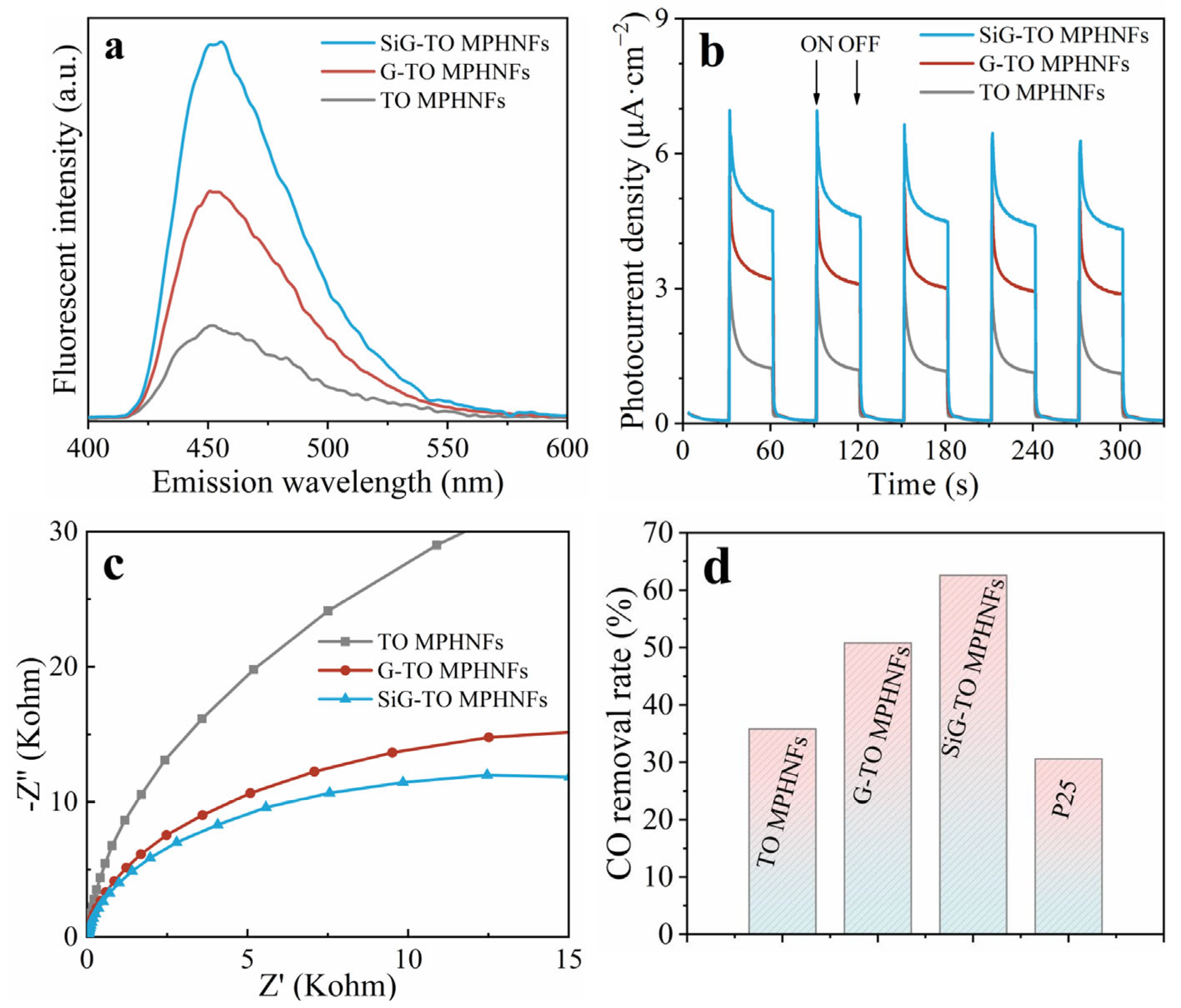

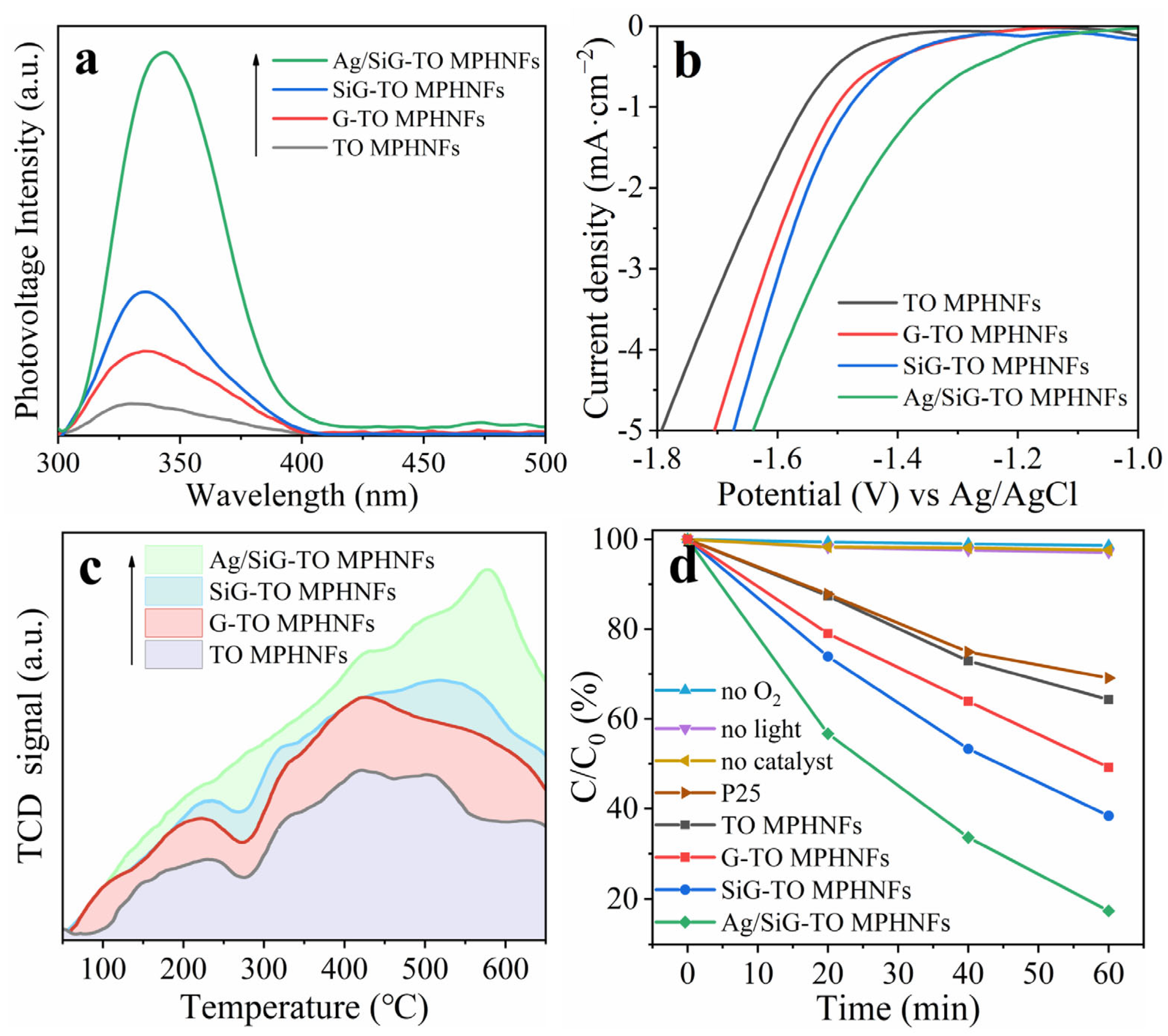

| Catalyst | CO Removal Rate (%) | Reaction Rate Constant k (min−1) |
|---|---|---|
| P25 TiO2 | 30.9 | 0.0063 |
| TO MPHNF | 35.7 | 0.0075 |
| G-TO MPHNF | 50.8 | 0.0117 |
| SiG-TO MPHNF | 61.6 | 0.0160 |
| 0.5Ag/SiG-TO MPHNF | 68.9 | 0.0194 |
| 1Ag/SiG-TO MPHNFs | 78.2 | 0.0253 |
| Ag/SiG-TO MPHNFs | 82.6 | 0.0289 |
| 2Ag/SiG-TO MPHNFs | 73.1 | 0.0216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, T.; Zhu, Y.; Chu, Z.; Li, Z.; Sun, L.; Jing, L. Controllable Synthesis of Ultrafine Ag NPs/Functionalized Graphene-Introduced TiO2 Mesoporous Hollow Nanofibers by Coaxial Electrospinning for Photocatalytic Oxidation of CO. Catalysts 2025, 15, 231. https://doi.org/10.3390/catal15030231
Dou T, Zhu Y, Chu Z, Li Z, Sun L, Jing L. Controllable Synthesis of Ultrafine Ag NPs/Functionalized Graphene-Introduced TiO2 Mesoporous Hollow Nanofibers by Coaxial Electrospinning for Photocatalytic Oxidation of CO. Catalysts. 2025; 15(3):231. https://doi.org/10.3390/catal15030231
Chicago/Turabian StyleDou, Tianwei, Yangyang Zhu, Zhanyu Chu, Zhijun Li, Lei Sun, and Liqiang Jing. 2025. "Controllable Synthesis of Ultrafine Ag NPs/Functionalized Graphene-Introduced TiO2 Mesoporous Hollow Nanofibers by Coaxial Electrospinning for Photocatalytic Oxidation of CO" Catalysts 15, no. 3: 231. https://doi.org/10.3390/catal15030231
APA StyleDou, T., Zhu, Y., Chu, Z., Li, Z., Sun, L., & Jing, L. (2025). Controllable Synthesis of Ultrafine Ag NPs/Functionalized Graphene-Introduced TiO2 Mesoporous Hollow Nanofibers by Coaxial Electrospinning for Photocatalytic Oxidation of CO. Catalysts, 15(3), 231. https://doi.org/10.3390/catal15030231









