Abstract
A series of 5 wt.% Cr/SiO2 catalysts were prepared through incipient wet impregnation using different chromium salts as a source of Cr (chromium (III) sulfate, acetylacetonate, nitrate, ammonium dichromate). The obtained catalysts were characterized by SEM-EDX, TEM, DRIFT-CD3CN spectroscopy, UV-VIS diffuse reflectance spectroscopy, and the N2 low-temperature adsorption–desorption technique. The catalysts were tested in propane, and isobutane dehydrogenation assisted with CO2 at 600–750 °C. The highest activity in propane dehydrogenation was observed for the catalyst obtained from chromium acetylacetonate, the yield of propylene was 32% at 750 °C, and in the isobutane dehydrogenation reaction, the catalyst obtained from chromium sulfate was the best one; the yield of isobutene was ~30% at 600 °C. The obtained results show that the type of chromium precursor has a significant effect on the efficiency of the catalyst in the propane and isobutane dehydrogenation with CO2.
1. Introduction
Protection of the environment from the anthropogenic impact is a topic of high importance for modern society. One of the global problems is the increase in carbon dioxide emissions due to the combustion of fossil fuels. The level of carbon dioxide in the atmosphere has reached 417 ppm, which is critical for the climate. One of the ways to solve this problem is to involve carbon dioxide in the chemical processes. The utilization of CO2 by converting it into valuable products is one of the important problems of the modern chemical industry [,]. One promising method of utilizing carbon dioxide is its use as a soft oxidant in the process of dehydrogenation of light hydrocarbons into olefins [,,,,], the large-scale raw material component of the modern petrochemical and organic synthesis industry [,,,,,]. The scheme of the process is in Equations (1) and (2):
CnH2n+2 → CnH2n + H2
CO2 + H2 → CO + H2O
To carry out the oxidative dehydrogenation of lower alkanes to olefins in the presence of carbon dioxide, it is necessary to develop catalysts with high efficiency, controlled selectivity to the target product, and resistance to deactivation. The most promising catalysts are those based on chromium [,] and gallium [,,] and supported on silica [,,]. In the case of chromium oxide-based catalysts, CO2 has a stimulating effect on the yield of olefins due to its mild oxidizing ability. The dehydrogenation of light hydrocarbons assisted with CO2 on chromium-based catalysts occurs through the Mars–Van Krevelen mechanism [,]. CO2 in propane dehydrogenation maintains the oxygen concentration on the catalyst surface and suppresses the formation of molecular H2 (Equations (3) and (4)):
C3H8 + CrOx → C3H6 + CrOx−1 + H2O
CO2 + CrOx−1 → CO + CrOx
The oxidation–reduction cycle involves Cr6+, Cr5+, and Cr3+ and Cr2+ particles [,,]. The proportion of reduced species depends on the loading and dispersion of chromium, the preparation conditions, and the precursor salt used to prepare the active phases of the catalyst, which is then reduced to Cr3+ and Cr2+ particles [].
In the case of chromium oxide catalysts supported on SiO2, Cr6+ particles as chromates or polychromates are dominant at a chromium concentration of 5–6 wt.% [,,], while a high dispersion of chromium particles is assumed. At higher contents, low-dispersed polymeric Cr3+ particles are present, as well as crystalline α-Cr2O3 [], which is not active catalytically; therefore, it leads to a decrease in propylene selectivity and the activity of the catalyst. In addition, the activity of CrOx/SiO2 is significantly affected by the catalyst preparation method []. The high catalytic activity of chromium oxide catalysts due to the good dispersion of CrOx particles can be achieved on the surface of a support with a large surface area.
Agafonov et al. [,] studied the effect of catalyst synthesis conditions on the activity of the CrOx/SiO2 system in ODP-CO2. The yield of propylene depends on the pH of the chromium solution, which reaches a maximum at pH 5; at higher values, chromium hydroxide precipitates. Additionally, the calcination of the support promotes a more uniform distribution of active centers on the surface of the support favoring a high yield of propylene.
One of the important factors affecting the efficiency of the catalyst is the choice of precursor salt. During the process of synthesis of the catalyst, the chosen precursor can have a great impact on the physiochemical and surface properties of the catalysts. More importantly, the precursor could result in very different catalytic activities of the catalyst. The precursor salt is usually transformed into an oxide species without forming byproducts that will contribute to a change in the properties of the media and catalyst.
Currently, there is no work in the literature on the study of the influence of the predecessor of chromium on the activity of catalysts. Chromium (III) nitrate is a commonly used Cr precursor for the preparation of Cr/silica catalysts. Nevertheless, a lot of different possible candidates exist, and it would be quite interesting to compare their effects on the resulting catalysts of ODP-CO2. In the work [], it was shown that the uniformity of the metal distribution on the surface depends on the metal precursor used.
The following precursor salts were chosen to investigate differences in the uniformity of the distribution of chromium oxides on the surface, and their thermal decomposition is as following: nitrate (5), bichromate (6), acetyl acetonate (7), and sulphate (8):
4Cr(NO3)3 → 2Cr2O3 + 12NO2 + 3O2
(NH4)2Cr2O7 → Cr2O3 + N2 + 4H2O
Cr(C5H7O2)3 → Cr2O3 + H2O + gaseous decomposition products
2Cr2(SO4)3 → 2Cr2O3 + 6SO2 + 3O2
This work aimed at revealing the possible effects on the metal distribution and acidity of the catalysts, the effect of the precursor salt on the activity of chromia catalysts in the dehydrogenating reactions of propane, and i-butane in the presence of CO2.
2. Results and Discussion
Silica with a specific surface area of 750 m2/g was used to synthesize the catalytic systems []. Figure 1 shows the nitrogen adsorption–desorption isotherms and the pore size distribution for the support and catalysts. The isotherms of the support and catalysts demonstrate isotherms of type I according to the IUPAC classification, which indicates the microporous structure []. SiO2 demonstrates a pore distribution in the range of 2–5 nm. After impregnation with chromium, the pore size distribution changed insignificantly (Table 1).
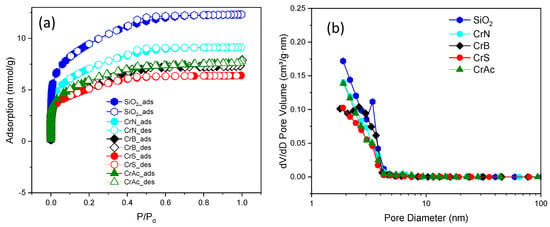
Figure 1.
Nitrogen adsorption–desorption isotherms (a) and pore size distribution (b).

Table 1.
Textural characteristics of supports and catalysts.
A halo in the 2θ range of 20–30° refers to amorphous silica. In the 2θ range of 30–50°, the reflexes corresponding to the crystal phase of α-Cr2O3 were detected (Figure 2). Moreover, it is known that even if Cr3+ salts are used for impregnation, the calcination of the sample in the air atmosphere results in a partial oxidation of the chromium species present on the surface of the support into higher valence states [,,,]. The effectiveness of the Cr3+ oxidation strongly depends on the nature of the support and the Cr loading. On the surface of the calcined, silica-supported chromium oxide catalysts, the Cr6+ species were detected for all the Cr-containing samples. The XPS analysis (Figure 3 and Table 2) of both the ground and unground CrN samples revealed an interesting result. On the surface of the unground CrN sample, which showed the highest activity of propane dehydrogenation reaction in the presence of CO2, the highest concentration of Cr(VI) particles was observed, which may indicate a high dispersion of the active component over the entire surface area and the oxidation–reduction ability of chromium particles. On the surface of the other samples, the surface concentration of Cr(VI) is significantly lower, especially on the CrAc sample, which may indicate the encapsulation of chromium particles in the pores of the support. This fact confirms the XPS results for the ground samples; the highest concentration of chromium (VI) particles among the ground samples was observed in the CrAc sample. It should be noted that for the CrS sample, both ground and unground, the particle concentration was low, which indicates the formation of the α-Cr2O3 phase, which is not subject to the oxidation–reduction cycle.
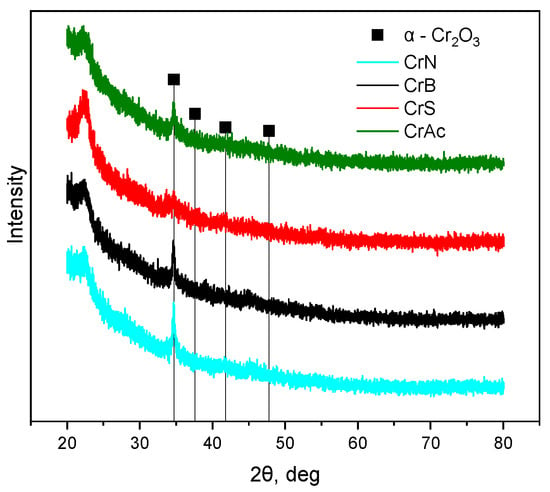
Figure 2.
Diffraction patterns of the freshly prepared catalyst samples.
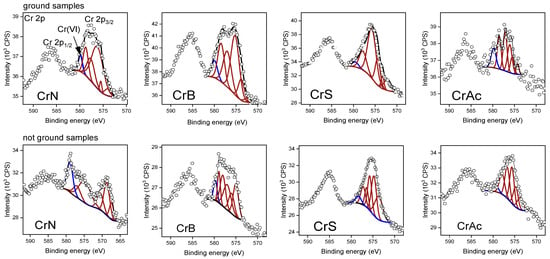
Figure 3.
XPS spectra of the Cr2p of the catalysts.

Table 2.
XPS data for the supported chromium oxide catalysts, including both ground and not ground samples.
The uniformity of the active component distribution on the support’s surface was studied using scanning electron microscopy in combination with energy dispersive spectroscopy (Figure 4). The electron image of the sample CrS showed varying distribution of the active component on the support’s surface. This is in agreement with chromium mapping. The spectra from some areas showed chromium content that was significantly higher than the nominal concentration in the sample. The other samples demonstrated a uniform distribution of chromium on the surface. The standard deviation of the mass fraction of chromium in the samples increased in the following order: CrAc (0.8) < CrB (1.3) < CrN (1.5) << CrS (7.6). Therefore, the choice of precursor affects the uniformity of the chromium distribution on the support’s surface.
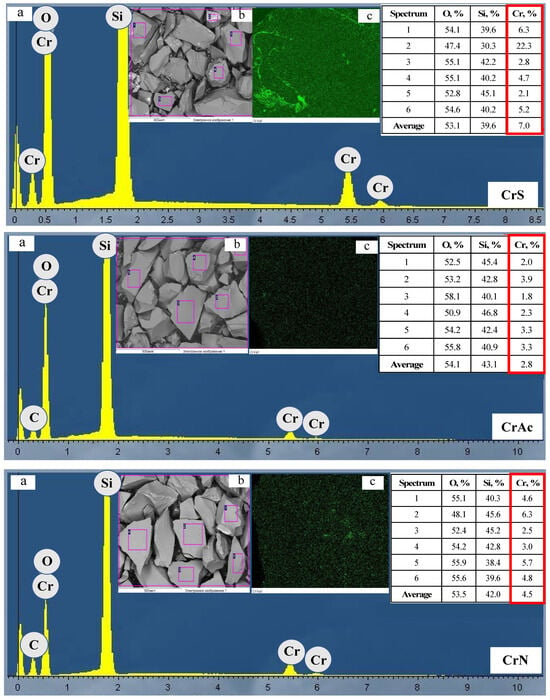
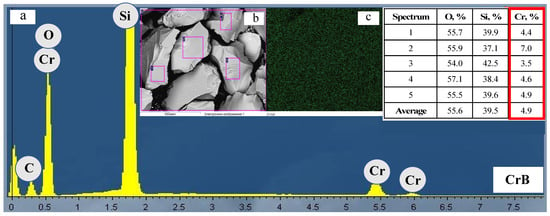
Figure 4.
EDX spectra (a,b) and SEM (c) images.
TEM images of the catalysts are shown in Figure 5. Generally, the separate particles in the images were almost indistinguishable, so the quantitative analysis was not applicable. Nevertheless, some differences in the selected area electron diffraction (SAED) patterns were observed. The sample CrAc showed no distinct pattern, while all the others did. The clearest electron diffraction pattern was observed for CrS. The least crystallinity can be attributed to the smallest particles distributed on the surface of the support. Because the amorphous particles provided the highest surface area, the effectiveness of metal atoms use appeared higher in this case.
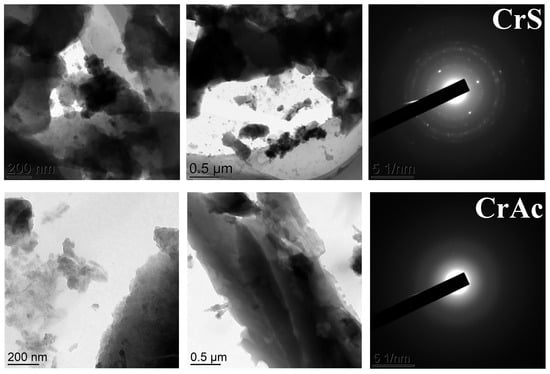
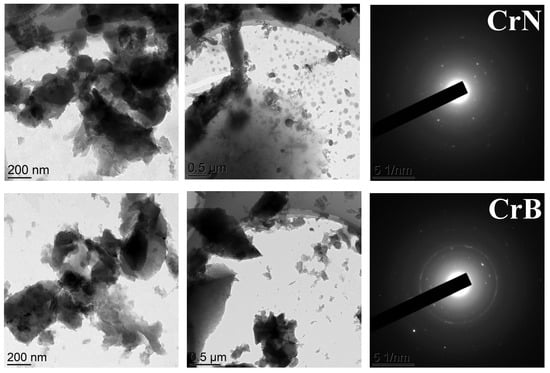
Figure 5.
TEM images of the catalysts and their SAED patterns.
The valence states of chromium on the surface were investigated by diffuse reflectance UV-Vis spectroscopy (Figure 6). The samples exhibited four distinct bands in the range of 200–700 nm. When Cr3+ salts were used for the synthesis of catalysts, the calcination of the sample in the air flow led to the partial oxidation of the chromium present on the support’s surface, causing higher valence states []. All spectra were dominated by the bands at 270 and 360 nm, corresponding to the O2− → Cr6+ charge transfer for chromium ions in tetrahedral coordination [,]. The bands at 455 and 600 nm corresponded to the octahedral coordination of the Cr3+ in Cr2O3 or in the CrOx clusters [,]. The UV-Vis spectra indicated the existence of both forms in the catalysts—Cr(VI) and Cr(III). In the spectrum of the CrS sample, unlike the others, the bands at 455 and 600 nm were more intensive; this indicates either the predominance of Cr3+ particles in Cr2O3 or the CrOx clusters not undergoing the oxidation–reduction cycles.
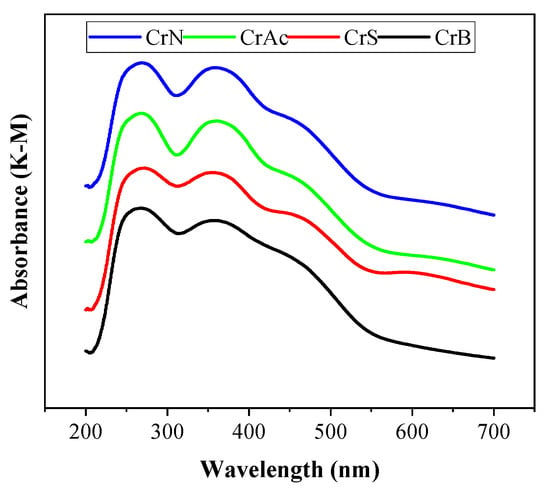
Figure 6.
UV-Vis diffuse reflectance spectra for the fresh catalysts.
An important characteristic of the catalysts for the ODP-CO2 is their acidity, because acid sites can promote the extraction of hydrogen atoms from the hydrocarbon, especially when initiating oxidative dehydrogenation. The acid-base properties of the supports and catalysts were studied by diffuse reflectance FTIR spectroscopy. After thermal vacuum treatment and adsorption of the deuterated acetonitrile, the spectra in the region of OH groups were recorded, and the catalysts prepared using various precursors can be arranged in the following order by the strength of the BAS: CrN > CrB > CrAc > CrS.
Figure 7 compares the spectra of adsorbed CD3CN. CrAc, CrB, and CrS differed little from each other in the amount of adsorbed CD3CN, while the intensity of the spectra of the CrN sample is two times higher. Although all the samples from this series possessed a moderate strength BAS (according to the shift of the OH-group band (429–502 cm−1), the sample CrN showed a greater shift (502 cm−1), and the sample CrS showed the smallest shift (429 cm−1). At the same time, the LAS in all samples exhibited the same strength [,,].
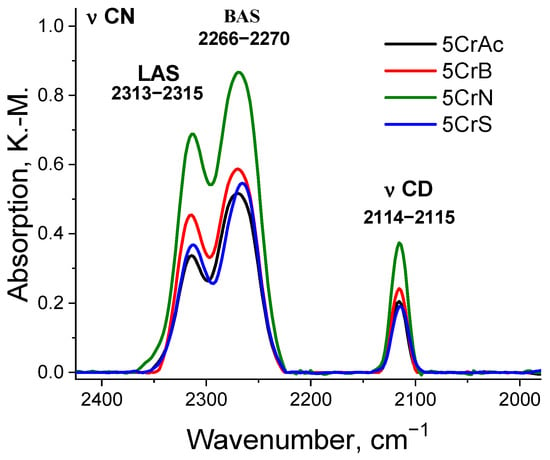
Figure 7.
DRIFT spectra of the adsorbed CD3CN.
2.1. The Catalytic Tests in ODP-CO2
All the catalysts were tested in ODP-CO2. The reaction stoichiometry is as shown in Equation (9). Along with the desired product, propylene, some byproducts were formed (methane, ethane, ethylene).
C3H8 + CO2 → C3H6 + CO + H2O
The results of the catalytic tests show that the activity of the samples varies significantly (Figure 8 and Figure 9). Below 675 °C, the catalyst CrAc was the most active, while the CrN sample obtained from chromium nitrate significantly exceeded all samples in propane and in CO2 conversion at temperatures above 675 °C. The CrS sample appeared to be the least active at all the temperatures, especially moderate ones.
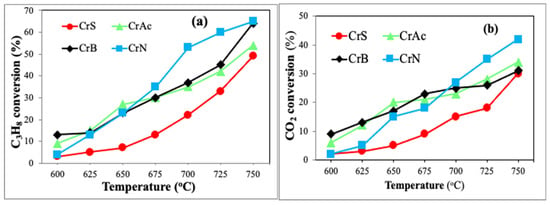
Figure 8.
The catalytic results of ODP-CO2: (a) propane and (b) CO2 conversion at different temperatures.
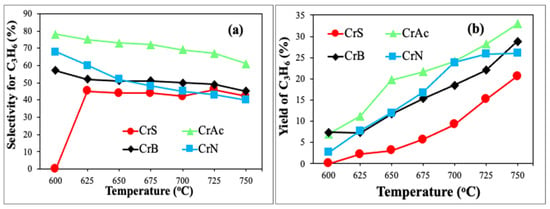
Figure 9.
The catalytic results in ODP-CO2: (a) selectivity to propylene and (b) yield of propylene at different temperatures.
Figure 9 shows the selectivity and propylene yield’s dependence on temperature. The highest selectivity and the propylene yield were demonstrated by the CrAc sample, while the lowest selectivity and propylene yield were observed for the CrS sample over the entire temperature range. The samples CrB and CrAc showed similar behavior at different temperatures, but the curve for CrAc shifted approximately 25% higher. The sample CrN loses its selectivity to propylene in the fastest manner, and above 650 °C, it becomes less active than the CrB sample.
Thus, in terms of propane conversion and propylene selectivity, the CrAc sample showed the highest catalytic activity. A possible reason for such behavior could be that CrB initially possessed a lot of Cr(VI) species but lacked the Cr(III) species that are active in direct dehydrogenation (non-oxidative path). With the increasing temperature, the propylene yield increased, and the propylene selectivity dropped slightly. The highest propylene yield (32%) was observed for the CrAc sample at 750 °C.
2.2. The Catalytic Tests in ODB-CO2
The synthesized catalysts were studied in the reaction of isobutane dehydrogenation assisted with CO2. The scheme of the reaction is given in Equation (10):
i-C4H10 + CO2 = i-C4H8 + CO + H2O
The main reaction product was isobutene, and byproducts such as n-butane, methane, ethane, ethylene, propane, and propylene were also formed. With increasing temperature, the amount of methane increased significantly, indicating the prevalence of the cracking process. Figure 10 shows that the obtained samples differed in catalytic activity. With increasing temperature, the conversion of isobutane increased and reached ~60% for all the samples at 750 °C. For the CrS sample, high conversion (33%) was observed at 600 °C; the yield of isobutene reached ~30%. This can be explained by the low acidity of this sample: the higher the acidity, the more active the cracking processes. The catalytic systems can be arranged in the following order by the reaction activity of isobutane dehydrogenation in the presence of CO2: CrS > CrAc > CrN > CrB.
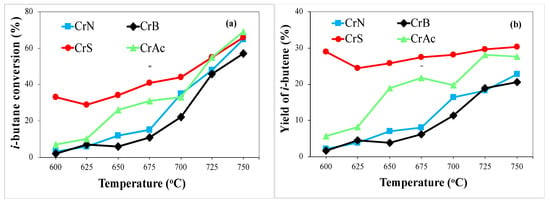
Figure 10.
Isobutane conversion (a) and isobutene yield (b) at different temperatures.
Thus, the actual state of the active surfaces of the catalysts during dehydrogenation is represented by two types of Cr(III) species: the first is Cr(III), found in freshly prepared catalysts (including α-Cr2O3), and the second is Cr(III), formed by the reduction in the Cr(VI) species, so the content of Cr(VI) species on the catalyst surface has a direct effect on the activity of the catalyst []. It is noteworthy that the least active and selective sample in ODP-CO2 was CrS, which appeared to be the most active and selective sample in ODB-CO2. The reason for this may be the formation of any specific ensembles able to activate the molecule of isobutane, which is more sterically complex. Additionally, the sample CrS possessed the highest relative proportion of Cr(III) species according to UV-Vis; therefore, it can be tentatively hypothesized that in the process of isobutane dehydrogenation, the species Cr(III) plays a more significant role than the Cr(VI) also present in the sample. As was shown earlier, Cr(III) species favors the process of the direct dehydrogenation of light alkanes (non-oxidative path), so it seems possible that such a pathway is dominant in this reaction. Propane dehydrogenation proceeds through both oxidative and non-oxidative paths and has different levels of dependence on the Cr valence state on the surface. It is known that the dehydrogenation of light paraffins may be hindered by the internal diffusion of the reagent to the active site and by product elimination from the catalyst pores [,]. The porous structure of the catalyst plays an important role in high-temperature processes []. Wide mesopores provide isobutane diffusion toward active sites on the catalyst’s surface. Therefore, the reaction products (isobutene and hydrogen) should be released from the active surface to prevent hydrogenation reaction and cracking. Wide mesopores provide product transport from the catalyst granules. Blocking of the mesopores by chromia nanoparticles may be a reason for additional diffusion limitation and decreased activity of the catalysts prepared by impregnation. For the dehydrogenation of isobutane, wider mesopores are needed than for the dehydrogenation of propane due to molecule size. In addition, the catalyst must have low acidity to prevent the cracking of the molecule. Therefore, in the reaction of the dehydrogenation of isobutane, CrS is most active due to the presence of wider mesopores and low acidity.
3. Materials and Methods
3.1. Catalyst Preparation
The catalytic systems were synthesized by the wet impregnation method of silica for drying purposes; the silica was provided by Acros Organics. The following salts were used as precursors of Cr: Cr(NO3)3 9H2O, Cr2(SO4)3, (NH4)2Cr2O7, Cr(acac)3. Bidistilled water was used as a solvent, and ethanol was used for Cr(acac)3 because it is practically insoluble in water. The concentration of chromium in all samples was 5 wt.%. The catalyst preparation method is described in []. The catalysts prepared using chromium nitrate, sulphate, bichromate, and acetyl acetonate were denoted as CrN, CrS, CrB, and CrAc, respectively. The impregnated catalysts were dried at 100 °C and calcined at 650 °C for 4 h in a quartz tube reactor in air flow.
3.2. Catalyst Characterization
The texture characteristics of the catalysts were determined based on the nitrogen adsorption isotherms measured at 77 K on an ASAP 2020 Plus unit (Micromeritics, Norcross, GA, USA). The specific surface area was calculated according to BET, and the pore size distribution was found from the desorption branch of isotherm via Barrett–Joyner–Halenda (BJH) analysis. The micropores in the samples were monitored using a T-plot.
The morphology, particle size, and elemental composition on the catalyst’s surface were studied via scanning electron microscopy (SEM) on a LEO EVO 50 XVP electron microscope (Carl Zeiss, Oberkochen, Germany) equipped with an INCA Energy 350 energy dispersive spectrometer (Oxford Instruments, Oxon, UK). The device allows setting the energy of electrons in the range of 200 V–30 kV. The cathode is a heating element made of lanthanum hexaboride LaB6.
Transmission electron microscopy was performed using the JEM 2100 instrument (JEOL, Akishima, Japan) at an accelerating voltage of 200 kV. The catalyst powder was applied to a copper mesh with an amorphous carbon coating, which was loaded into the TEM chamber.
XPS measurements were performed using a PREVAC EA15 spectrometer (PREVAC, Rogów, Poland). In the current work, AlKα radiation (hν = 1486.6 eV, 150 W) was used as a primary radiation source. The pressure in the analytical chamber did not exceed 5 × 10−9 mbar during spectra acquisition. The binding energy scale was pre-calibrated using the positions of Ag 3d5/2 (368.3 eV) and Au 4f7/2 (84.0 eV) from silver and gold foils, respectively. The powdered samples were supported by double-sided conducting scotch tape. To take into account the effect of surface charging, the C1s line at (Eb = 284.8 eV) from carbon contamination was used as an internal standard.
The powder X-Ray diffraction patterns were collected with a STOE STADI P transmission diffractometer (Chicago, IL, USA) using Cu Kα1 radiation monochromatized with a curved germanium (111) monochromator. The samples were examined in the region of 2θ 10–80°, with a step of 0.01° and a 10 s counting time per point. Before the examination, the samples were heated at 600 °C for one hour in a flow of CO2 at 30 mL per minute.
The characteristics of the porous structures of the catalysts were determined on the basis of nitrogen adsorption isotherms measured at −296 °C using an ASAP 2020 Plus unit (Micromeritics Instrument Corporation, Norcross, GA, USA). Prior to measurements, the samples were evacuated at 350 °C for 3 h. The specific surface area was calculated according to the BET method, and the pore size distribution was found from the desorption branch of the isotherm via the Barrett–Joyner–Halenda (BJH) analysis. The volume of micropores was determined as the difference between the total pore volume and the cumulative volume of mesopores at 2 nm in the BJH method.
Diffuse reflectance UV–Vis spectra were obtained via a Shimadzu UV-3600 Plus spectrophotometer (Kyoto, Japan) equipped with an ISR-603 integration sphere. The spectra were recorded at 200–800 nm and room temperature, and BaSO4 was used as the standard and diluent. The weight of the catalysts was 0.1 g, and the weight of BaSO4 was 0.5 mg. The UVProbe software (version 2.3) was used to process the spectra.
Diffuse reflection IR (DRIFT) spectra were recorded at room temperature on a NICOLET “Protege” 460 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a diffuse reflection prefix in the range of 6000–400 cm−1 with a step of 4 cm−1. For a satisfactory signal-to-noise ratio, 500 spectra were accumulated. CaF2 powder was used as a standard. Before measuring the spectra, the samples were subjected to thermal vacuum treatment at a temperature of 400 °C for 2 h (heating rate of 5 °C/min) to remove physically adsorbed gases and water. Deuterated acetonitrile was used as the acid test molecule. Adsorption was carried out at room temperature with a saturated vapor pressure of CD3CN (96 Torr).
The intensity of the bands in the spectra was expressed in Kubelka–Munk units [,]. The data were collected and processed using the OMNIC program (version 9.16). The spectra of adsorbed CO and CD3CN were represented as the difference between those recorded before and after adsorption.
3.3. Catalytic Tests
The dehydrogenation of propane and i-butane in the presence of CO2 was investigated at an atmospheric pressure in the temperature range of 600–750 °C in a flow catalytic setup with a steel reactor with an inner diameter of 4 mm. The gas mixture CnH2n+2 + CO2 was fed into the reactor at a volumetric ratio of 1:2; the total flow rate of the gas mixture was 30 mL/min. The catalyst loading was 1 g (fraction 0.25–0.5 mm). Online analysis of the reaction products was carried out using a Chromatec-Crystal 5000 gas chromatograph (Nizhny Novgorod, Russia) equipped with a thermal conductivity detectors, M ss316 3 m*2 mm Hayesep Q, and CaA molecular sieves with 80/100 mesh columns. The products of the isobutane dehydrogenation reaction in the presence of CO2 were analyzed using a Chromatec-Crystal 5000 gas chromatograph with a flame ionization detector and a capillary column Alumina BOND, 30 м x 0.63 mm. The temperature of the column was ramped according to the following program: 40 °C for 1.5 min, rising to 100 °C at a speed of 15 °C/min. The quantitative analysis was carried out by the absolute calibration method.
The propane and i-butane conversion (X), product selectivity (Si), and yield (Y) were calculated according to the following equations:
4. Conclusions
A series of 5 wt.% Cr/SiO2 catalysts were prepared through incipient wet impregnation using different chromium salts as a source of Cr (chromium (III) sulfate, acetylacetonate, nitrate, ammonium dichromate). The catalysts were tested in propane and isobutane dehydrogenation, assisted with CO2 at 600–750 °C. The highest activity of propane dehydrogenation was observed for the catalyst obtained from chromium acetylacetonate; the yield of propylene was 32% at 750 °C; and in the isobutane dehydrogenation reaction, the catalyst obtained from chromium sulfate was the best one, with an isobutene yield of ~30% at 600 °C. A possible reason for such results could be that the sample obtained through the impregnation of Cr(acac)3 contained the highest fraction of Cr(VI) of all the samples, according to XPS and UV-Vis DRS.
Author Contributions
Conceptualization, M.A.T., M.Y.M. and A.L.K.; methodology, M.Y.M., V.L.B., E.V.M., G.I.K. and A.L.K.; formal analysis, P.V.P., K.B.K., A.A.S. and O.P.T.; data curation, K.B.K., A.A.S., V.L.B., E.V.M., G.I.K. and O.P.T.; writing—original draft, M.A.T., M.Y.M. and A.L.K.; writing—review and editing, L.M.K. and A.L.K.; supervision, L.M.K.; project administration, S.F.D. and L.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Ministry of Science and Higher Education of the Russian Federation (grant no. 075-15-2024-547).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mishanin, I.I.; Bogdan, T.V.; Koklin, A.E.; Bogdan, V.I. Design of highly selective heterogeneous catalyst for CO2-mediated ethane oxidative dehydrogenation based on nonoxidative catalysis in stainless-steel reactor. Chem. Eng. J. 2022, 446, 137184. [Google Scholar] [CrossRef]
- Bugrova, T.A.; Dutov, V.V.; Svetlichnyi, V.A.; Corban, V.C.; Mamontov, G.V. Oxidative dehydrogenation of ethane with CO2 over CrOx catalysts supported on Al2O3, ZrO2, CeO2 and CexZr1−xO2. Catal. Today 2019, 333, 71–80. [Google Scholar] [CrossRef]
- Mashkin, M.; Tedeeva, M.; Fedorova, A.; Vasiliev, A.; Egorov, A.; Pribytkov, P.; Kalmykov, K.; Kapustin, G.; Morozov, I.; Kustov, L.; et al. CrOx/SiO2 mesoporous catalysts prepared using beta-cyclodextrin as a template and their catalytic properties in propane oxidative dehydrogenation in the presence of carbon dioxide. Microporous Mesoporous Mater. 2022, 338, 111967. [Google Scholar] [CrossRef]
- Mashkin, M.Y.; Tedeeva, M.A.; Fedorova, A.A.; Fatula, E.R.; Egorov, A.V.; Dvoryak, S.V.; Maslakov, K.I.; Knotko, A.V.; Baranchikov, A.E.; Kapustin, G.I.; et al. Synthesis of CexZr1−xO2/SiO2 supports for chromium oxide catalysts of oxidative dehydrogenation of propane with carbon dioxide. J. Chem. Technol. Biotechnol. 2023, 98, 1247–1259. [Google Scholar] [CrossRef]
- Salaeva, A.A.; Salaev, M.A.; Mamontov, G.V. Effect of Cu modifier on the performance of CrOx/Al2O3 catalysts for isobutane dehydrogenation. Chem. Eng. Sci. 2020, 215, 115462. [Google Scholar] [CrossRef]
- Bugrova, T.A.; Mamontov, G.V. The Study of CrOx-Containing Catalysts Supported on ZrO2, CeO2, and CexZr(1−x)O2 in Isobutane Dehydrogenation. Kinet. Catal. 2018, 59, 143–149. [Google Scholar] [CrossRef]
- Golubina, E.V.; Kaplin, I.Y.; Gorodnova, A.V.; Lokteva, E.S.; Isaikina, O.Y.; Maslakov, K.I. Non-Oxidative Propane Dehydrogenation on CrOx-ZrO2-SiO2 Catalyst Prepared by One-Pot Template-Assisted Method. Molecules 2022, 27, 6095. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 1995, 24, 307–313. [Google Scholar] [CrossRef]
- Xiong, C.; Chen, S.; Yang, P.; Zha, S.; Zhao, Z.-J.; Gong, J. Structure–Performance Relationships for Propane Dehydrogenation over Aluminum Supported Vanadium Oxide. ACS Catal. 2019, 9, 5816–5827. [Google Scholar] [CrossRef]
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and characterization of SBA-1–supported chromium oxide catalysts for CO2 assisted dehydrogenation of propane. Microporous Mesoporous Mater. 2012, 161, 56–66. [Google Scholar] [CrossRef]
- Melnikov, D.P.; Novikov, A.A.; Glotov, A.P.; Reshetina, M.V.; Smirnova, E.M.; Wang, H.Q.; Vinokurov, V.A. Dehydrogenation of Light Alkanes (A Review). Pet. Chem. 2022, 62, 1027–1046. [Google Scholar] [CrossRef]
- Qi, Q.; Shen, W.; Cai, M.; Cai, J.; Hu, B.; Han, D.; Tang, X.; Zhu, Z.; Huo, P. Construction of Cu-Modified g-C3N4 Nanosheets for Photoinduced CO2 Reduction to CO and Selectivity Mechanism Insight. ACS Appl. Nano Mater. 2024, 7, 24788–24797. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, L.; Xu, J.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Chromium-based catalysts for ethane dehydrogenation: Effect of SBA-15 support. Microporous Mesoporous Mater. 2016, 234, 370–376. [Google Scholar] [CrossRef]
- Wang, S.; Murata, K.; Hayakawa, T.; Hamakawa, S.; Suzuki, K. Dehydrogenation of ethane with carbon dioxide over supported chromium oxide catalysts. Appl. Catal. A Gen. 2000, 196, 1–8. [Google Scholar] [CrossRef]
- Bardool, R.; Dean, D.P.; Pham, H.N.; Datye, A.K.; Raeissi, S.; Rahimpour, M.R.; Miller, J.T. Secondary reactions of propylene on Ga/γ-Al2O3 propane dehydrogenation catalysts. J. Catal. 2023, 428, 115201. [Google Scholar] [CrossRef]
- Schreiber, M.W.; Plaisance, C.P.; Baumgärtl, M.; Reuter, K.; Jentys, A.; Bermejo-Deval, R.; Lercher, J.A. Lewis-Brønsted Acid Pairs in Ga/H-ZSM-5 to Catalyze Dehydrogenation of Light Alkanes. J. Am. Chem. Soc. 2018, 140, 4849–4859. [Google Scholar] [CrossRef] [PubMed]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and Kinetics of Propane Dehydrogenation and Cracking over Ga/H-MFI Prepared via Vapor-Phase Exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2019, 141, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Hammer, N.; Rønning, M.; Holmen, A.; Chen, D.; Walmsley, J.C.; Øye, G. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: New insights by a comprehensive spectroscopic study. J. Catal. 2009, 261, 116–128. [Google Scholar] [CrossRef]
- Baek, J.; Yun, H.J.; Yun, D.; Choi, Y.; Yi, J. Preparation of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2: Insight into the nature of catalytically active chromium sites. ACS Catal. 2012, 2, 1893–1903. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Brito, J.L.F.; Dieguez, L.C. Characterization of chromium species in catalysts for dehydrogenation and polymerization. J. Mol. Catal. A Chem. 2003, 203, 251–266. [Google Scholar] [CrossRef]
- Fridman, V.Z.; Xing, R.; Severance, M. Investigating the CrOx/Al2O3 dehydrogenation catalyst model: I. identification and stability evaluation of the Cr species on the fresh and equilibrated catalysts. Appl. Catal. A: Gen. 2016, 523, 39–53. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Verberckmoes, A.A.; Buttiens, A.L.; Schoonheydt, R.A. Diffuse reflectance spectroscopy study of the thermal genesis and molecular structure of chromium-supported catalysts. J. Phys. Chem. 1994, 98, 579–584. [Google Scholar] [CrossRef]
- Botavina, M.A.; Evangelisti, C.; Agafonov, Y.A.; Gaidai, N.A.; Panziera, N.; Lapidus, A.L.; Martra, G. CrOx/SiO2 catalysts prepared by metal vapour synthesis: Physical-chemical characterisation and functional testing in oxidative dehydrogenation of propane. Chem. Eng. J. 2011, 166, 1132–1138. [Google Scholar] [CrossRef]
- Agafonov, Y.A.; Gaidai, N.A.; Lapidus, A.L. Influence of the preparation conditions for catalysts CrOx/SiO2 on their efficiency in propane dehydrogenation in the presence CO2. Russ. Chem. Bull. 2014, 63, 381–388. [Google Scholar] [CrossRef]
- Botavina, M.A.; Agafonov, Y.A.; Gaidai, N.A.; Groppo, E.; Corberán, V.C.; Lapidus, A.L.; Martra, G. Towards efficient catalysts for the oxidative dehydrogenation of propane in the presence of CO2: Cr/SiO2 systems prepared by direct hydrothermal synthesis. Catal. Sci. Technol. 2016, 6, 840–850. [Google Scholar] [CrossRef]
- Botavina, M.A.; Martra, G.; Agafonov, Y.A.; Gaidai, N.A.; Nekrasov, N.V.; Trushin, D.V.; Coluccia, S.; Lapidus, A.L. Oxidative dehydrogenation of C3-C4 paraffins in the presence of CO2 over CrOx/SiO2 catalysts. Appl. Catal. A Gen. 2008, 347, 126–132. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Kalmykov, K.B.; Mashkin, M.Y.; Shesterkina, A.A.; Dunaev, S.F.; Kustov, L.M. Influence of the Method of Fe Deposition on the Surface of Hydrolytic Lignin on the Activity in the Process of Its Conversion in the Presence of CO2. Int. J. Mol. Sci. 2023, 24, 1279. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Kapustin, G.I.; Leonov, A.V.; Tkachenko, O.P.; Tursunov, O.B.; Evdokimenko, N.D.; Kustov, L.M. Dehydrogenation of propane in the presence of CO2 on GaOx/SiO2 catalyst: Influence of the texture characteristics of the support. Fuel 2022, 313, 122698. [Google Scholar] [CrossRef]
- Igonina, M.; Tedeeva, M.; Kalmykov, K.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Pribytkov, P.; Dunaev, S.; Kustov, L.; Kustov, A. Properties of CrOx/MCM-41 and Its Catalytic Activity in the Reaction of Propane Dehydrogenation in the Presence of CO2. Catalysts 2023, 13, 906. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Evdokimenko, N.D.; Sarkar, B.; Kustov, L.M. Dehydrogenation of propane in the presence of CO2 on Cr (3%)/SiO2 catalyst under supercritical conditions. Mendeleev Commun. 2020, 30, 195–197. [Google Scholar] [CrossRef]
- Haney, M.A.; Franklin, J.L. Mass spectrometric determination of the proton affinities of various molecules. J. Phys. Chem. 1969, 73, 4328–4331. [Google Scholar] [CrossRef]
- Angell, C.L.; Howell, M.V. Infrared spectroscopic investigation of zeolites and adsorbed molecules. IV. Acetonitrile. J. Phys. Chem. 1969, 73, 2551–2554. [Google Scholar] [CrossRef]
- Purcell, K.F.; Drago, R.S. Studies of the Bonding in Acetonitrile Adducts 1. J. Am. Chem. Soc. 1966, 88, 919–924. [Google Scholar] [CrossRef]
- Zolotukhina, A.I.; Romanova, E.V.; Bugrova, T.A.; Knyazev, A.S.; Mamontov, G.V. Influence of impregnation conditions on the activity of CrOx/Al2O3 catalysts in dehydrogenation of isobutane in fixed bed reactor. Arab. J. Chem. 2020, 13, 9130–9138. [Google Scholar] [CrossRef]
- Barghi, B.; Fattahi, M.; Khorasheh, F. Kinetic modeling of propane dehydrogenation over an industrial catalyst in the presence of oxygenated compounds. Reac Kinet. Mech. Cat. 2012, 107, 141–155. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.H. Global approximations of unsteady-state adsorption, diffusion, and reaction in a porous catalyst. AlChe J. 2013, 59, 2540–2548. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).