Effect of Mn/Cu Ratio on the Structure–Performance Relationship of Spinel-Type Mn–Cu/Al2Ox Catalysts for Methanol Steam Reforming
Abstract
1. Introduction
2. Results and Discussion
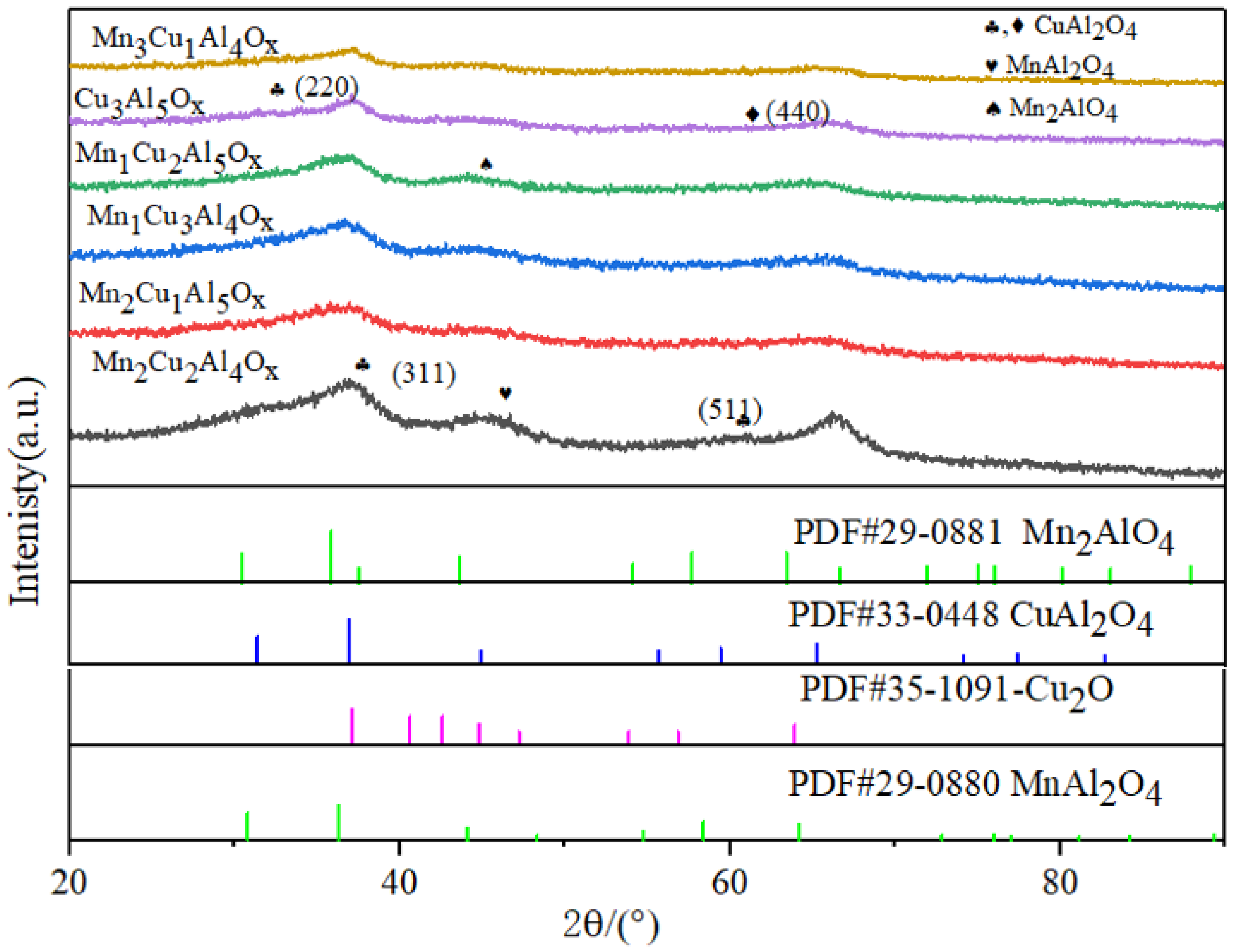
2.1. XRD Analysis
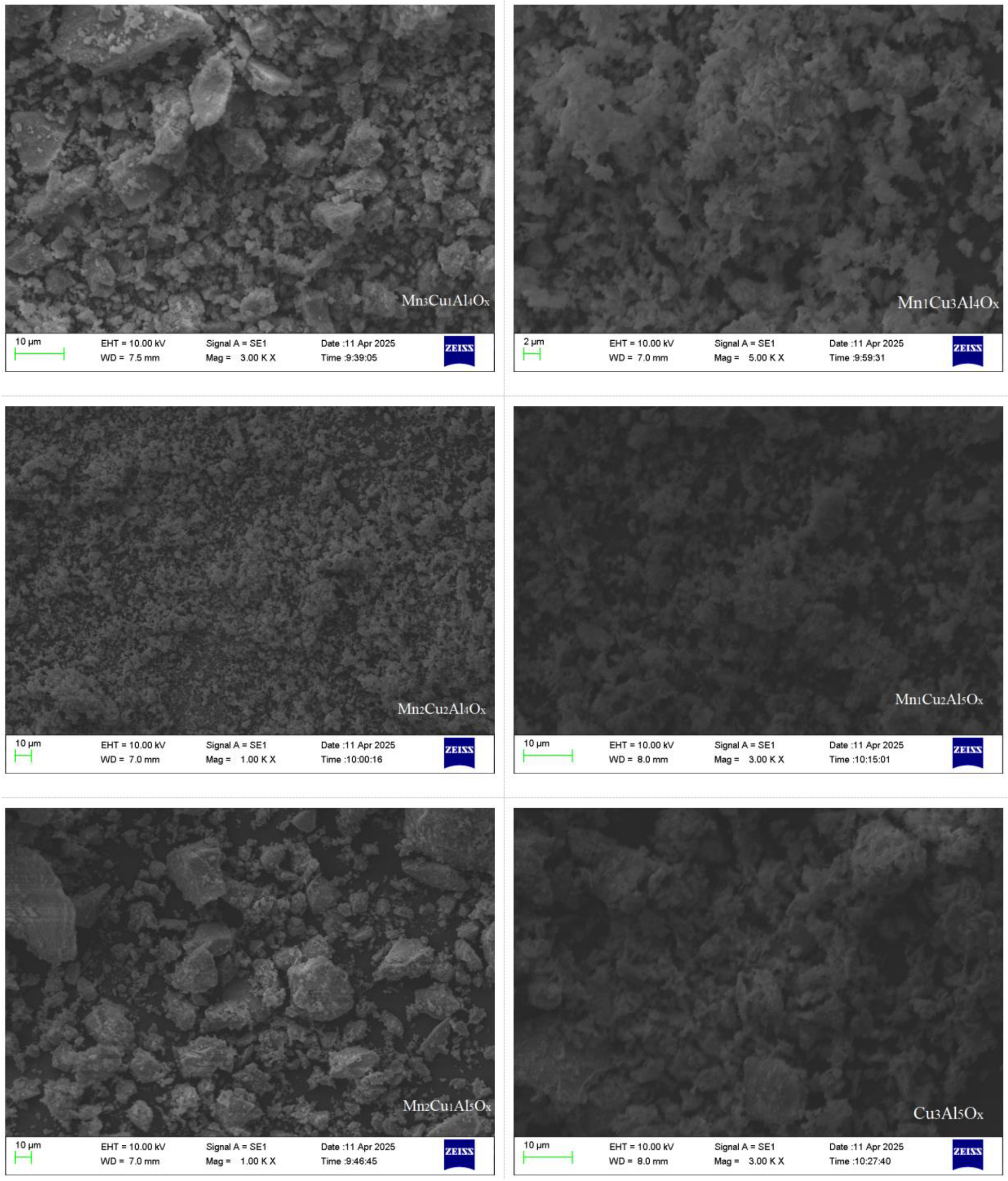
2.2. SEM Analysis
2.3. H2-TPR Analysis
2.4. BET Analysis
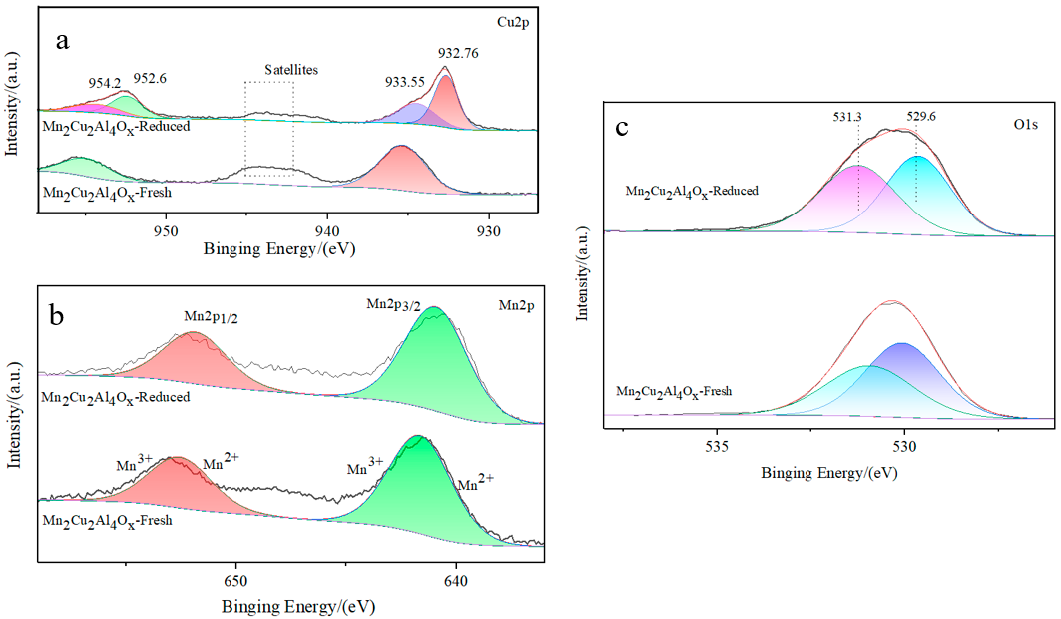
2.5. XPS Analysis
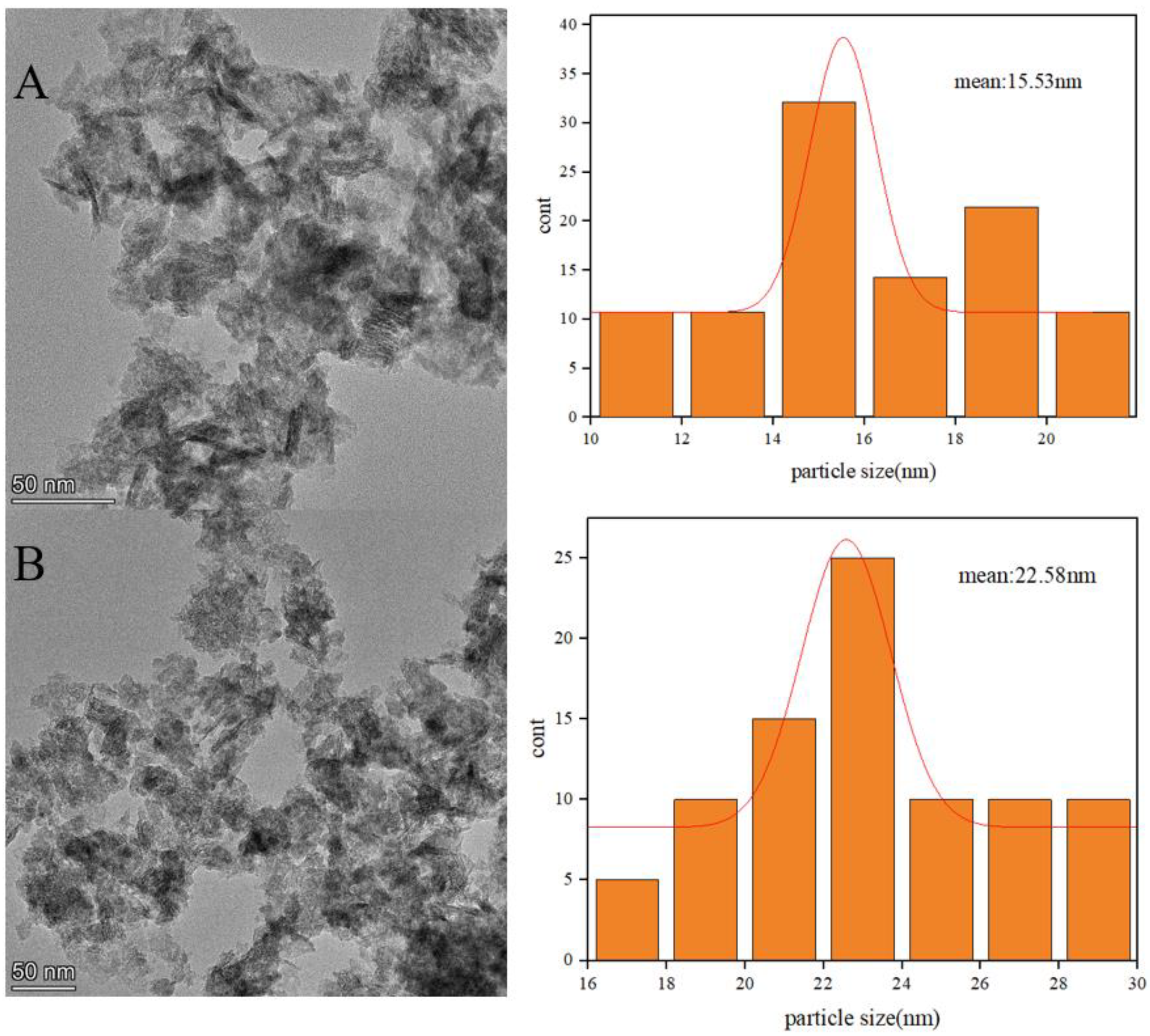
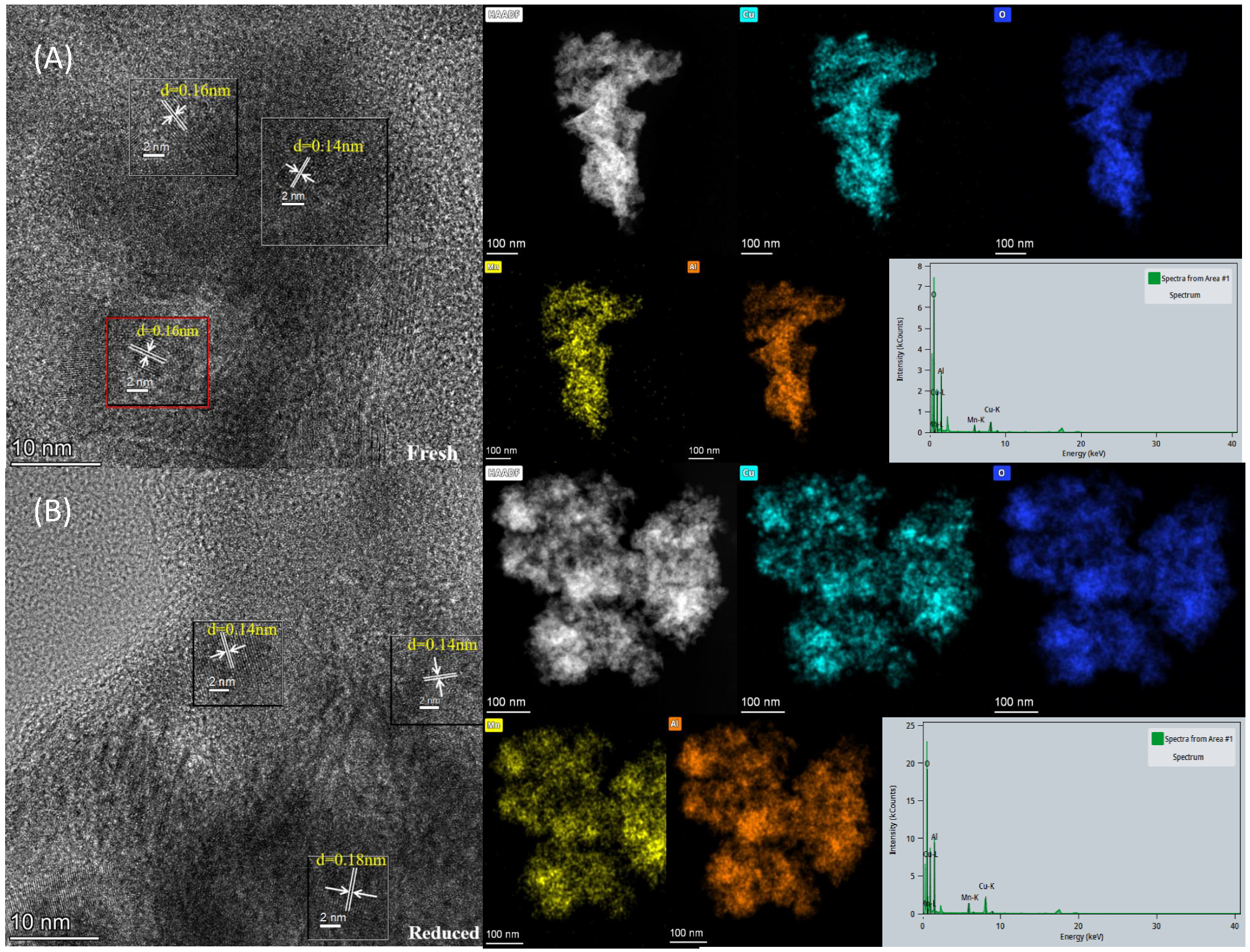
2.6. TEM Characterization
2.7. Assessment of Catalytic Activity and Selectivity
3. Experimental Section
3.1. Materials
3.2. Instruments
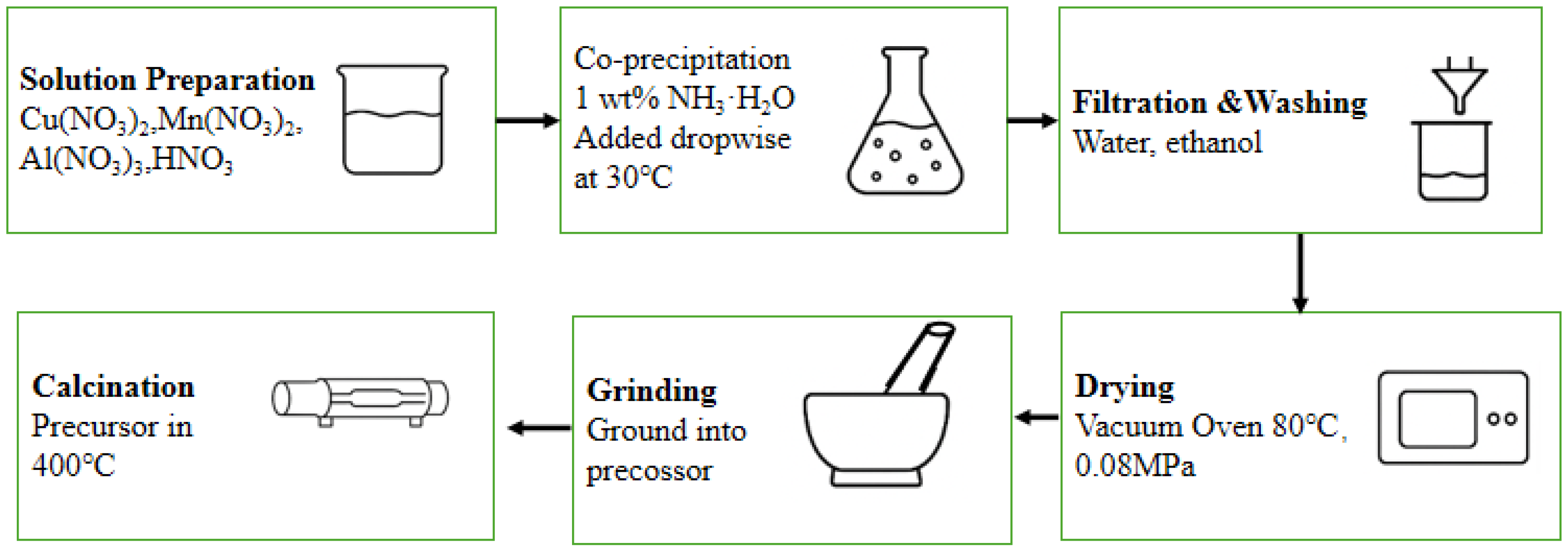
3.3. Catalyst Preparation
3.4. Characterization of Catalysts
3.4.1. X-Ray Diffraction (XRD)
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. H2-TPR Characterization
3.4.4. Determination of Elemental Content by ICP
3.4.5. N2 Physisorption Analysis
3.4.6. XPS and TEM Analysis
3.5. Catalyst Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiba, S.; Omri, A. Literature survey on the relationships between energy, environment and economic growth. Renew. Sustain. Energy Rev. 2017, 69, 1129–1146. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Rao, R.P.; Reddy, M.; Chellappan, V.; Ramakrishna, S. Novel low-carbon energy solutions for powering emerging wearables, smart textiles, and medical devices. Energy Environ. Sci. 2022, 15, 4928–4981. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Rostami, M.; Farajollahi, A.H.; Amirkhani, R.; Farshchi, M.E. A review study on methanol steam reforming catalysts: Evaluation of the catalytic performance, characterizations, and operational parameters. AIP Adv. 2023, 13, 030701. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, S.; Ma, J.; Deng, S.; Fang, X.; Xu, X.; Meng, H.; Wang, X. Enhancing the Reactivity of Cu/Al2O3 for Methanol Steam Reforming through adding CrOx: Unraveling Reaction Pathways and the Mechanism for Improvement. ACS Catal. 2025, 15, 7138–7152. [Google Scholar] [CrossRef]
- Shahmohammadi, N.; Rezaei, M.; Alavi, S.M.; Akbari, E. Methanol steam reforming over highly efficient CuO–Al2O3 nanocatalysts synthesized by the solid-state mechanochemical method. Int. J. Hydrogen Energy 2023, 48, 13139–13150. [Google Scholar] [CrossRef]
- Pang, S.; Dou, X.; Zhao, W.; Bai, S.; Wan, B.; Wang, T.; Yang, J.H. A Review on the Design Strategies of Copper-Based Catalysts for Enhanced Activity and Stability in Methanol Reforming to Hydrogen. Nanomaterials 2025, 15, 1118. [Google Scholar] [CrossRef]
- Kamsuwan, T.; Guntida, A.; Praserthdam, P.; Jongsomjit, B. Differences in Deterioration Behaviors of Cu/ZnO/Al2O3 Catalysts with Different Cu Contents toward Hydrogenation of CO and CO2. ACS Omega 2022, 7, 25783–25797. [Google Scholar] [CrossRef]
- Zamel, N.; Groos, U. Hydrogen Energy Recovery–H2-Based Fuel Cells. Adv. Energy Storage Latest Dev. RD Mark. 2022, 559–588. [Google Scholar]
- Jambur, S.R. Techno-Economic Assessment of High-Temperature H2O/CO2: Co-Electrolysis in Solid Oxide Electrolysers for Syngas Production; Technical University of Denmark/DTU: Kongens Lyngby, Denmark, 2022. [Google Scholar]
- Hammoud, D.; Gennequin, C.; Aboukaïs, A.; Aad, E.A. Steam reforming of methanol over x% Cu/Zn–Al 400 500 based catalysts for production of hydrogen: Preparation by adopting memory effect of hydrotalcite and behavior evaluation. Int. J. Hydrogen Energy 2015, 40, 1283–1297. [Google Scholar] [CrossRef]
- Shan, J.; Zheng, Y.; Shi, B.; Davey, K.; Qiao, S.-Z. Regulating Electrocatalysts via Surface and Interface Engineering for Acidic Water Electrooxidation. ACS Energy Lett. 2019, 4, 2719–2730. [Google Scholar] [CrossRef]
- Liao, M.; Duan, Q.; Xiang, R.; Tan, X.; Dai, Z.; Qin, H.; Xiao, H. Compounds: Rare earth-doped CuAl2O4 catalysts with superior catalytic performance for methanol steam reforming. J. Alloys Compd. 2025, 1024, 180229. [Google Scholar] [CrossRef]
- Frank, B. Steam Reforming of Methanol over Cu/ZnO/Al2O3 Modified with Hydrotalcites. Catal. Commun. 2007, 8, 1684–1690. [Google Scholar] [CrossRef]
- Tan, Z.; Fan, G.; Zheng, L.; Li, F. Promoted Surface-Interface Catalysis over Mn–Cr Incorporated Cu-Based Catalysts for Efficient Hydrogen Production from Methanol Decomposition. ACS Catal. 2024, 14, 11218–11230. [Google Scholar] [CrossRef]
- Ye, R.; Xiao, S.; Lai, Q.; Wang, D.; Huang, Y.; Feng, G.; Zhang, R.; Wang, T. Advances in enhancing the stability of Cu-based catalysts for methanol reforming. Catalysts 2022, 12, 747. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, D.; Wang, Y.; Zhao, L.; Xu, G.; Yu, Y.; He, H. Recent advances in methanol steam reforming catalysts for hydrogen production. Catalysts 2025, 15, 36. [Google Scholar] [CrossRef]
- Mao, Q.; Gao, Z.; Liu, X.; Guo, Y.; Wang, Y.; Ma, D. The Cu–Al2O3 interface: An unignorable active site for methanol steam reforming hydrogen production. Catal. Sci. Technol. 2024, 14, 3448–3458. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Likozar, B. Cu–Mn–O nano-particle/nano-sheet spinel-type materials as catalysts in methanol steam reforming (MSR) and preferential oxidation (PROX) reaction for purified hydrogen production. Renew. Energy 2022, 182, 713–724. [Google Scholar] [CrossRef]
- Kang, N.; Zhao, Z.; Yu, X.; Lin, J.; Lu, S. Tuning surface properties of CuCeOx catalysts with manganese and cobalt co-doping for CO oxidation. J. Rare Earths 2025, 43, 1643–1651. [Google Scholar] [CrossRef]
- Rani, B.J.; Sivanantham, A.; Cho, I.S. Nanostructured spinel manganates and their composites for electrochemical energy conversion and storage. Adv. Funct. Mater. 2023, 33, 2303002. [Google Scholar] [CrossRef]
- Liao, M.; Xiang, R.; Zhou, X.; Dai, Z.; Wang, L.; Qin, H.; Xiao, H. Enhancing effect of Mn2+ substitution in CuAl2O4 spinel for methanol steam reforming in a microreactor. Renew. Energy 2024, 230, 120815. [Google Scholar] [CrossRef]
- Meng, H.; Yang, Y.; Shen, T.; Yin, Z.; Wang, L.; Liu, W.; Yin, P.; Ren, Z.; Zheng, L.; Zhang, J.; et al. Designing Cu0−Cu+ dual sites for improved C−H bond fracture towards methanol steam reforming. Nat. Commun. 2023, 14, 7980. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Qiu, L.; Gao, Y.; O’Hare, D.; Wang, Q. The synthesis of CuyMnzAl1−zOx mixed oxide as a low-temperature NH3-SCR catalyst with enhanced catalytic performance. Dalton Trans. 2018, 47, 2992–3004. [Google Scholar] [CrossRef]
- Herdem, M.S.; Sinaki, M.Y.; Farhad, S.; Hamdullahpur, F. An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qian, K.; Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 2022, 12, 1268–1287. [Google Scholar] [CrossRef]
- Pang, J.; Li, Q.; Su, G.; Sun, B.; Song, M.; Hua, Y.; Zhang, W.; Meng, J.; Shi, B. Tailoring Dual High-Valence Cu–O–Mn Active Sites to Enhance VOC Catalytic Oxidation. Environ. Sci. Technol. 2025, 59, 9812–9826. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhao, L.; Yang, Y.; Liu, J. Unraveling the role of a Cu dopant in formaldehyde catalytic oxidation over a La0.8Sr0.2Mn1−xCuxO3 perovskite: An experimental and theoretical study. J. Mater. Chem. A 2025, 13, 7371–7380. [Google Scholar] [CrossRef]
- Lu, T.; Su, F.; Zhao, Q.; Li, J.; Zhang, C.; Zhang, R.; Liu, P. Catalytic oxidation of volatile organic compounds over manganese-based oxide catalysts: Performance, deactivation and future opportunities. Sep. Purif. Technol. 2022, 296, 121436. [Google Scholar] [CrossRef]
- Saw, S.; Datta, S.; Chavan, P.; Gupta, P.; Kumari, S.; Sahu, G.; Chauhan, V. Significance and influence of various promoters on Cu-based catalyst for synthesizing methanol from syngas: A critical review. J. Chem. Technol. Biotechnol. 2023, 98, 1083–1102. [Google Scholar] [CrossRef]
- Fajin, J.L.; Cordeiro, M.N.D. Insights into the mechanism of methanol steam reforming for hydrogen production over Ni–Cu-based catalysts. ACS Catal. 2021, 12, 512–526. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Y.; Chen, X.; Li, C.; Lu, S.; Liew, K.M. CO Oxidation over Nanostructured Ceria Supported Bimetallic Cu–Mn Oxides Catalysts: Effect of Cu/Mn Ratio and Calcination Temperature. Catal. Lett. 2018, 148, 181–193. [Google Scholar] [CrossRef]
- Kang, R.; Wei, X.; Bin, F.; Wang, Z.; Hao, Q.; Dou, B. Reaction mechanism and kinetics of CO oxidation over a CuO/Ce0.75Zr0.25O2-δ catalyst. Appl. Catal. A Gen. 2018, 565, 46–58. [Google Scholar] [CrossRef]
- Zedan, A.F.; Polychronopoulou, K.; Asif, A.; AlQaradawi, S.Y.; AlJaber, A.S. Cu-Ce-O catalyst revisited for exceptional activity at low temperature CO oxidation reaction. Surf. Coat. Technol. 2018, 354, 313–323. [Google Scholar] [CrossRef]
- Abed, S.H.; Reshak, A.H. Optimization of CuAl2O4 Nanoparticles for Enhanced Photocatalytic Degradation of Organic Dyes Under UV and Visible Light. J. Fluoresc. 2025, 35, 6797–6812. [Google Scholar] [CrossRef]
- Torres-Ochoa, J.A.; Cabrera-German, D.; Cortazar-Martinez, O.; Bravo-Sanchez, M.; Gomez-Sosa, G.; Herrera-Gomez, A. Peak-fitting of Cu 2p photoemission spectra in Cu0, Cu1+, and Cu2+ oxides: A method for discriminating Cu0 from Cu1+. Appl. Surf. Sci. 2023, 622, 156960. [Google Scholar] [CrossRef]
- Napruszewska, B.; Walczyk, A.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Michalik, A.; Karcz, R.; Śliwa, M.; Serwicka, E. The Synthesis of Cu–Mn–Al Mixed-Oxide Combustion Catalysts by Co-Precipitation in the Presence of Starch: A Comparison of NaOH with Organic Precipitants. Catalysts 2022, 12, 1159. [Google Scholar] [CrossRef]
- Liu, P.; Wei, G.; Liang, X.; Chen, D.; He, H.; Chen, T.; Xi, Y.; Chen, H.; Han, D. Synergetic effect of Cu and Mn oxides supported on palygorskite for the catalytic oxidation of formaldehyde: Dispersion, microstructure, and catalytic performance. Appl. Clay Sci. 2018, 161, 265–273. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.; Wei, L.; Shi, Z.; Han, L.; Yuan, H.; Li, B.; Dong, L.; Wang, F.; Sun, C.-Z. Preparation and Evaluation of Cu-Mn-Oxide as High Efficiency Catalyst for CO Oxidation and NO Reduction by CO. J. Phys. Chem. C 2017, 121, 12757–12770. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Ma, L.; Liang, N.; Yang, S.; Liu, H.; Sun, J.; Huang, F.; Sun, C.; Dong, L. Construction of Surface Synergetic Oxygen Vacancies on CuMn2O4 Spinel for Enhancing NO Reduction with CO. ACS Catal. 2024, 14, 3028–3040. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures. Appl. Catal. B Environ. 2015, 162, 122–132. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Castaño, M.H.; Molina, R.; Moreno, S. Cooperative effect of the Co–Mn mixed oxides for the catalytic oxidation of VOCs: Influence of the synthesis method. Appl. Catal. A Gen. 2015, 492, 48–59. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Nishimoto, M.; Tokura, R.; Nguyen, M.T.; Yonezawa, T. Copper materials for low temperature sintering. Mater. Trans. 2022, 63, 663–675. [Google Scholar] [CrossRef]
- Sanches, S.; Flores, J.H.; De Avillez, R.; Da Silva, M.P. Influence of preparation methods and Zr and Y promoters on Cu/ZnO catalysts used for methanol steam reforming. Int. J. Hydrogen Energy 2012, 37, 6572–6579. [Google Scholar] [CrossRef]




| Sample | S_BET (m2/g) | C_Cu (%) | D_Cu (%) | S_Cu (m2/g) | TPR1 (°C) | TPR2 (°C) | H2 Consumption of TPR1 (mmol/g) | H2 Consumption of TPR2 (mmol/g) |
|---|---|---|---|---|---|---|---|---|
| Cu3Al5Ox | 51.43 | 0.34 | 11.71 | 15.91 | 287.17 | 171.22 | 12.37 | 0.99 |
| Mn1Cu3Al4Ox | 55.87 | 0.31 | 18.40 | 21.65 | 244.01 | 171.63 | 11.35 | 1.05 |
| Mn1Cu2Al5Ox | 57.45 | 0.26 | 13.47 | 17.56 | 251.51 | 162.45 | 8.57 | 1.26 |
| Mn2Cu2Al4Ox | 76.44 | 0.25 | 24.60 | 26.11 | 257.86 | 144.90 | 6.34 | 0.88 |
| Mn2Cu1Al5Ox | 71.62 | 0.16 | 27.42 | 19.88 | 215.23 | 170.83 | 14.45 | 1.97 |
| Mn3Cu1Al4Ox | 65.32 | 0.15 | 31.25 | 21.65 | 277.38 | 151.66 | 11.29 | 1.77 |
| Element | Composition Before Reduction (%) | Composition After Reduction (%) | Numerical Change (%) |
|---|---|---|---|
| Mn | 10.21 | 14.32 | +40.3 |
| Cu | 11.86 | 15.56 | +31.2 |
| Al | 18.20 | 19.37 | +6.4 |
| O | 59.73 | 50.75 | −15.0 |
| Catalyst Composition | Temperature (°C) | MeOH Conv. (%) | CO Sel. (%) | Stability/Notes | Reference |
|---|---|---|---|---|---|
| Mn2Cu2Al4Ox | 300 | >95 | 6 | Excellent stability (>88% conv. after 24h) | This work |
| Mn2Cu2Al4Ox | 260 | 87 | 3 | This work | |
| 10% Cu/Al-400-500 | 250 | 51.9 | N/A | [11] | |
| Cu/ZnO/Al2O3 | 250 | >90 | ~3–5 | Prone to sintering and deactivation | [5,6] |
| 5%Mn-15%Cu/Al2O3 | 300 | ~90 | 12.0 | [18] | |
| 4.25Cu/Cu(Al)Ox | 270 | ~92.3 | N/A | Good performance, complex structure | [23] |
| Cu/ZnO/Al2O3 | 280 | ~75 | ~15 | Lower activity, higher CO selectivity | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Qiu, S.; Zheng, Y.; Huang, Y. Effect of Mn/Cu Ratio on the Structure–Performance Relationship of Spinel-Type Mn–Cu/Al2Ox Catalysts for Methanol Steam Reforming. Catalysts 2025, 15, 1091. https://doi.org/10.3390/catal15111091
Zhang Q, Qiu S, Zheng Y, Huang Y. Effect of Mn/Cu Ratio on the Structure–Performance Relationship of Spinel-Type Mn–Cu/Al2Ox Catalysts for Methanol Steam Reforming. Catalysts. 2025; 15(11):1091. https://doi.org/10.3390/catal15111091
Chicago/Turabian StyleZhang, Qiang, Shiming Qiu, Yanfei Zheng, and Yingying Huang. 2025. "Effect of Mn/Cu Ratio on the Structure–Performance Relationship of Spinel-Type Mn–Cu/Al2Ox Catalysts for Methanol Steam Reforming" Catalysts 15, no. 11: 1091. https://doi.org/10.3390/catal15111091
APA StyleZhang, Q., Qiu, S., Zheng, Y., & Huang, Y. (2025). Effect of Mn/Cu Ratio on the Structure–Performance Relationship of Spinel-Type Mn–Cu/Al2Ox Catalysts for Methanol Steam Reforming. Catalysts, 15(11), 1091. https://doi.org/10.3390/catal15111091








