Fluorine- and Trifluoromethyl-Substituted Iminopyridinenickel(II) Complexes Immobilized into Fluorotetrasilicic Mica Interlayers as Ethylene Oligomerization Catalysts
Abstract
1. Introduction
2. Results and Discussion
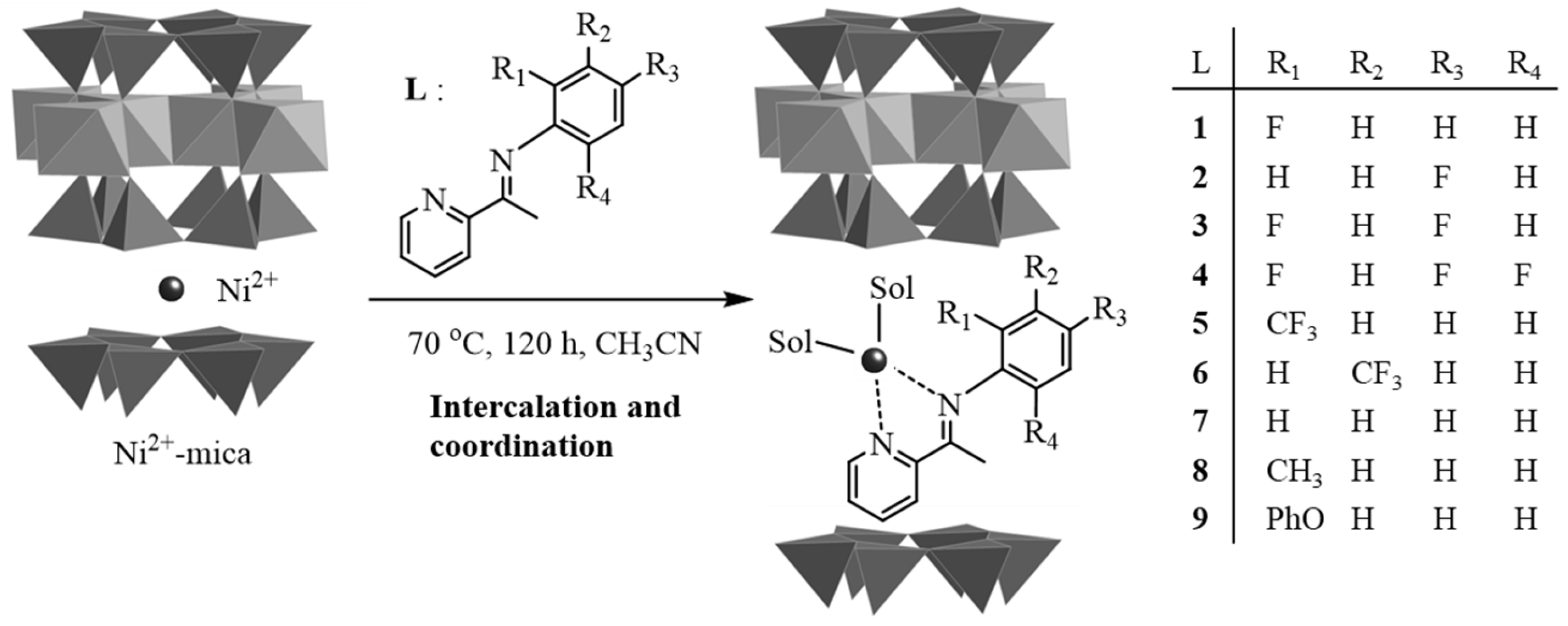
2.1. Characterization of Precatalysts
2.2. Catalyst Performance in Ethylene Oligomerization
2.2.1. Effects of the Ligand Structure on the Catalyst Performance
2.2.2. Detailed Analysis of Alkene Products

2.2.3. Effects of Oligomerization Temperature and Ethylene Pressure on Activity and Product Distribution
2.3. Precatalyst Prepared Using One–Pot Synthesis Method
2.4. Comparison with Homogeneous Catalyst System
3. Materials and Methods
3.1. Precatalyst Preparation
3.2. Procedure of Ethylene Oligomerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Detailed Product Distribution in the Liquid Products
| Entry | Mole Fraction in Liquid Products (C4-C18)/mol% | ||||||
|---|---|---|---|---|---|---|---|
| C4 | C6 | C8 | C10 | C12 | C14 | C16–18 | |
| 1 | 31.4 | 24.4 | 14.0 | 9.6 | 7.1 | 5.2 | 8.4 |
| 2 | 28.4 | 21.4 | 13.7 | 10.4 | 8.0 | 6.6 | 11.6 |
| 3 | 28.9 | 22.8 | 14.0 | 11.6 | 8.2 | 5.7 | 8.8 |
| 4 | 29.0 | 21.0 | 13.7 | 10.3 | 8.1 | 6.3 | 11.6 |
| 5 | 22.1 | 18.9 | 14.6 | 10.3 | 10.3 | 8.8 | 15.1 |
| 6 | 28.9 | 22.5 | 14.9 | 10.1 | 7.7 | 6.1 | 9.8 |
| 7 | 42.8 | 22.5 | 12.2 | 7.8 | 5.4 | 4.0 | 5.5 |
| 8 | 28.5 | 20.0 | 16.1 | 11.6 | 9.0 | 6.2 | 8.6 |
| 9 | 42.4 | 22.6 | 12.1 | 8.1 | 5.4 | 3.7 | 5.6 |
| 10 | 45.9 | 20.3 | 11.2 | 7.9 | 5.4 | 3.9 | 5.6 |
| 11 | 35.6 | 19.3 | 12.4 | 9.1 | 7.2 | 5.6 | 10.8 |
| 12 | 38.1 | 18.4 | 11.8 | 8.9 | 6.8 | 5.7 | 10.2 |
| 13 | 56.4 | 11.4 | 7.0 | 6.0 | 6.0 | 4.5 | 8.8 |
| 14 | 42.4 | 20.3 | 11.6 | 8.3 | 5.8 | 4.5 | 7.0 |
| 16 | 26.3 | 22.1 | 13.5 | 10.7 | 7.8 | 6.6 | 13.0 |
| 17 | 42.2 | 24.4 | 12.6 | 7.3 | 5.2 | 3.9 | 4.4 |
| 18 | 52.8 | 23.0 | 9.5 | 5.0 | 3.9 | 2.8 | 2.9 |
| 19 | 40.6 | 24.0 | 12.4 | 7.9 | 5.7 | 4.0 | 5.3 |
| 20 | 26.9 | 24.8 | 15.4 | 11.1 | 7.9 | 5.4 | 8.6 |
Appendix B
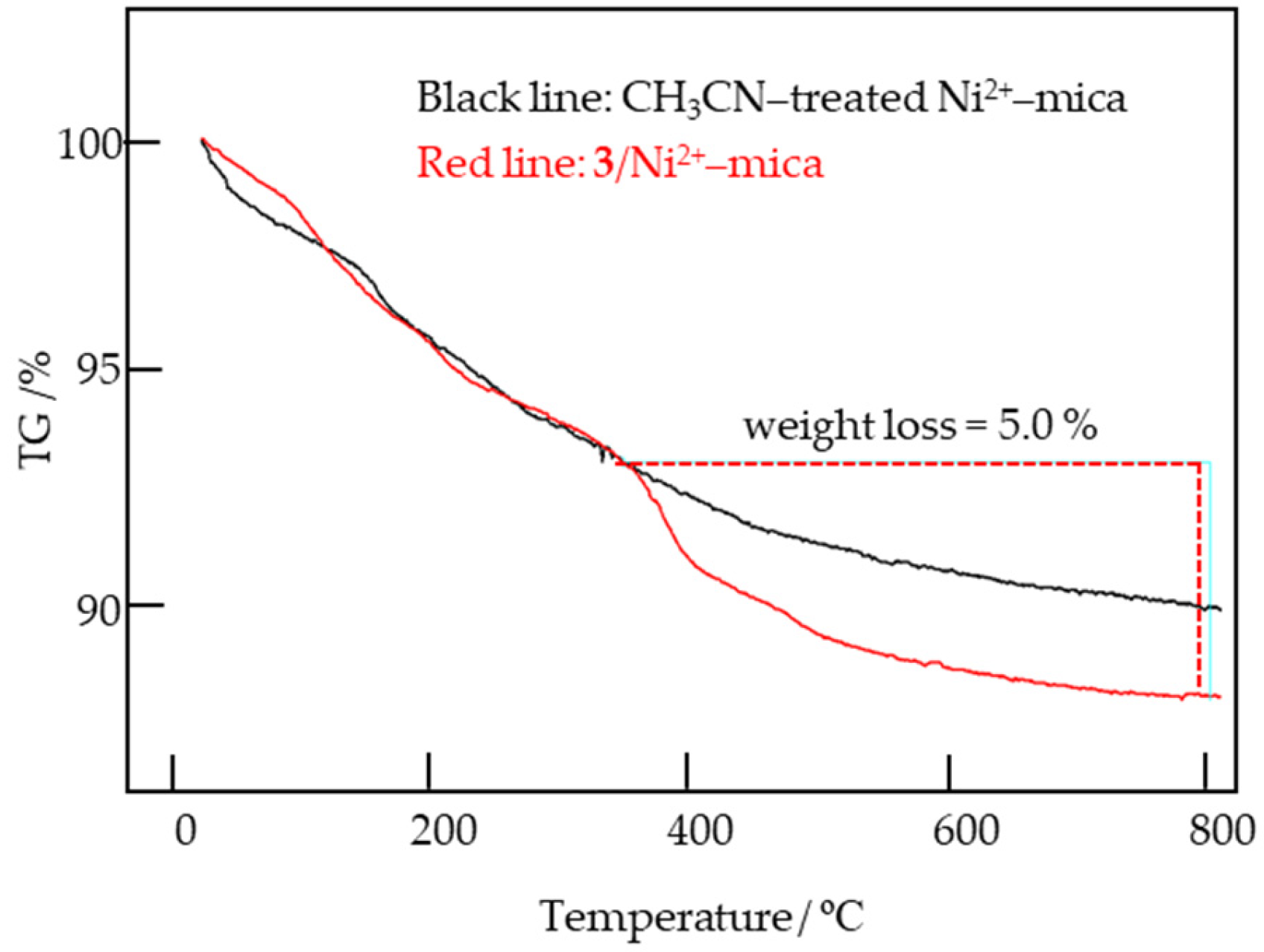
Appendix B.1. TG–DTA Analysis of CH3CN–Treated Ni2+–Mica and 3/Ni2+–Mica (800 μmol–Ligand g–Ni2+–Mica−1)
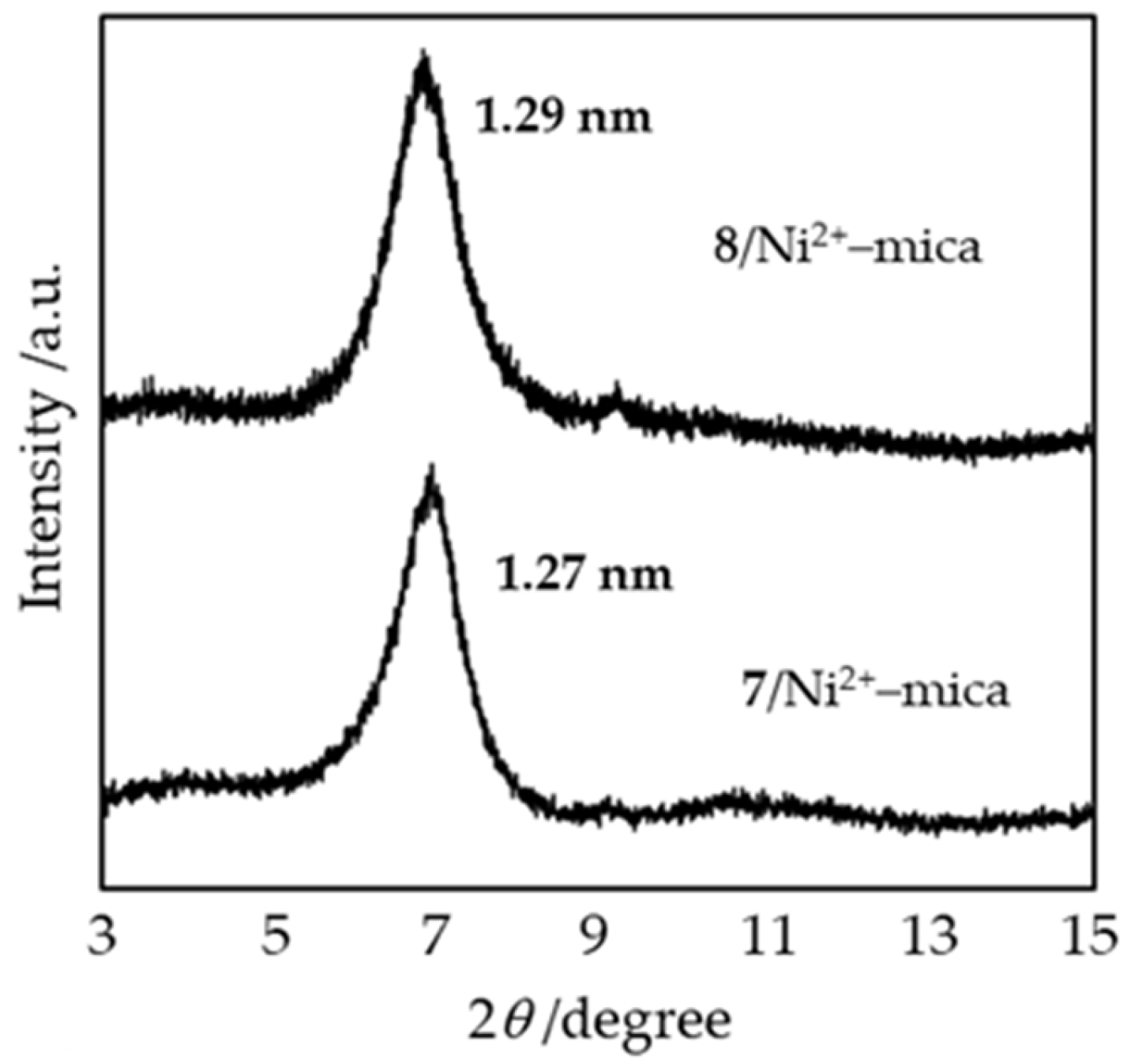
Appendix B.2. Preparation of 7/Ni2+–Mica and 8/Ni2+–Mica Precatalysts Using the One-Pot Synthesis Method
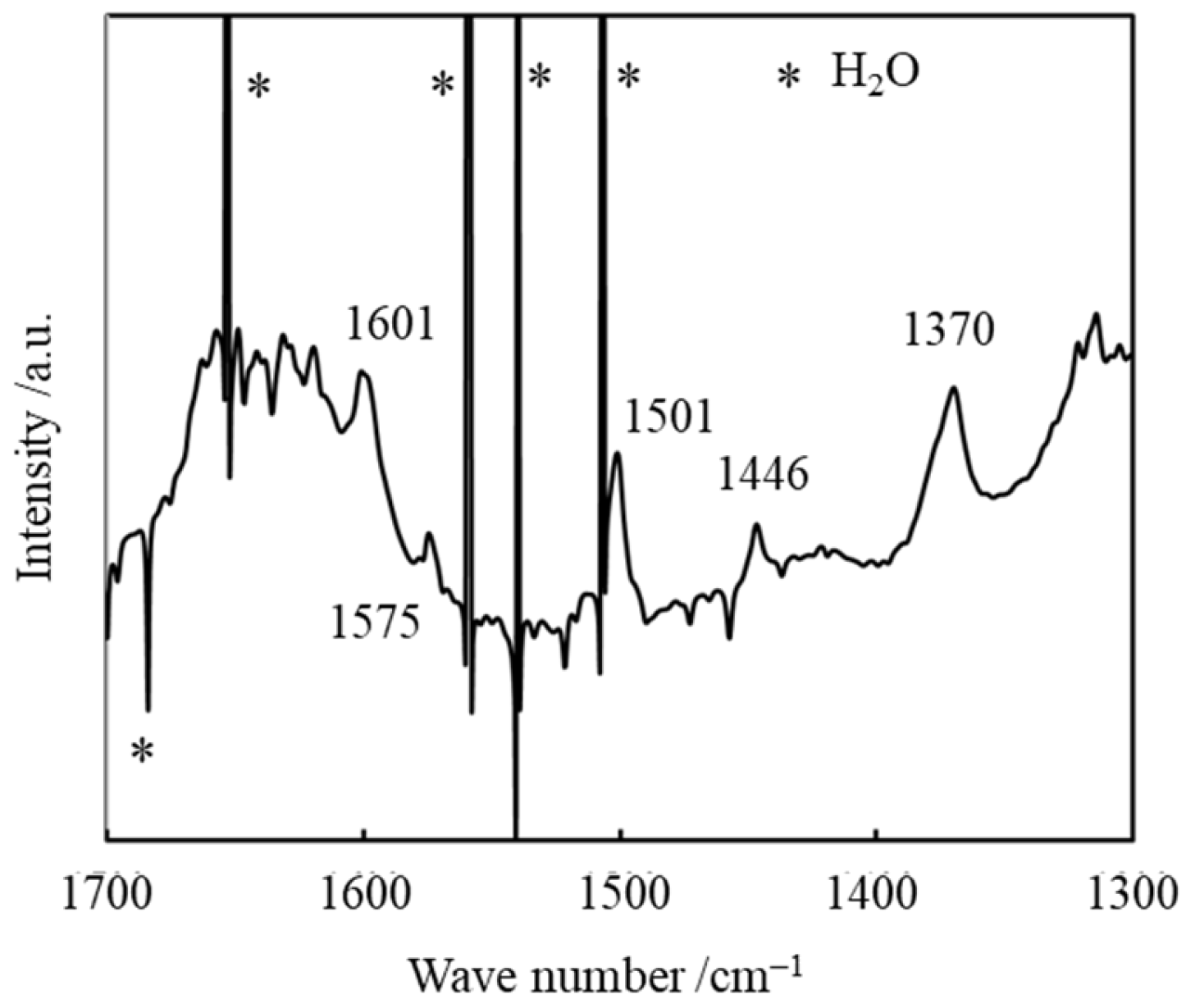
Appendix B.3. FT-IR Spectrum of 3/Ni2+-Mica
References
- Ghosh, R.; Bandyopadhyay, A.R.; Jasra, R.; Gagjibhai, M.M. Mechanistic study of the oligomerization of olefins. Ind. Eng. Chem. Res. 2014, 53, 7622–7628. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Mecking, S. Olefin polymerization by late transition metal complexes—A root of Ziegler catalysts gains new ground. Angew. Chem. Int. Ed. 2001, 40, 534–540. [Google Scholar] [CrossRef]
- Gibson, B.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Suo, H.; Solan, G.A.; Ma, Y.; Sun, W.H. Developments in compartmentalized bimetallic transition metal ethylene polymerization catalysts. Coord. Chem. Rev. 2018, 372, 101–116. [Google Scholar] [CrossRef]
- Champouret, Y.; Hashmi, O.H.; Visseaux, M. Discrete iron-based complexes: Applications in homogeneous coordination-insertion polymerization catalysis. Coord. Chem. Rev. 2019, 390, 127–170. [Google Scholar] [CrossRef]
- Gao, R.; Sun, W.H.; Redshaw, C. Nickel complex precatalysts in ethylene polymerization: New approaches to elastomeric materials. Catal. Sci. Technol. 2013, 3, 1172–1179. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.H.; Redshaw, C. Recent progress on nickel–based systems for ethylene oligo-/polymerization catalysis. J. Organomet. Chem. 2014, 751, 717–741. [Google Scholar] [CrossRef]
- Wu, R.; Wu, W.K.; Stieglitz, L.; Gaan, S.; Rieger, B.; Heuberger, M. Recent advances on α-diimine Ni and Pd complexes for catalyzed ethylene (Co)polymerization: A comprehensive review. Coord. Chem. Rev. 2023, 474, 214844. [Google Scholar] [CrossRef]
- Laine, T.V.; Lappalainen, K.; Liimatta, J.; Aitola, E.; Löfgren, B.; Leskela, M. Polymerization of ethylene with new diimine complexes of late transition metals. Macromol. Rapid Commun. 1999, 20, 487–491. [Google Scholar] [CrossRef]
- Köppl, A.; Alt, H.G. Substituted 1-(2-pyridyl)-2-azaethene-(N,N)-nickel dibromide complexes as catalyst precursors for homogeneous and heterogeneous ethylene polymerization. J. Mol. Catal. A Chem. 2000, 154, 45–53. [Google Scholar] [CrossRef]
- Laine, T.V.; Piironen, U.; Lappalainen, K.; Klinga, M.; Aitola, E.; Leskelä, M. Pyridinylimine-based nickel(II) and palladium(II) complexes: Preparation, structural characterization and use as alkene polymerization catalysts. J. Organomet. Chem. 2000, 606, 112–124. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Baugh, S.P.D.; Hoarau, O.; Gibson, V.C.; Wass, D.F.; White, A.J.P.; Williams, D.J. The role of bulky substituents in the polymerization of ethylene using late transition metal catalysts: A comparative study of nickel and iron catalyst systems. Inorg. Chim. Acta 2003, 345, 279–291. [Google Scholar] [CrossRef]
- Benito, J.M.; de Jesús, E.; de la Mata, F.J.; Flores, J.C.; Gómez, R.; Gómez-Sal, P. Mononuclear and dendritic nickel(II) complexes containing N,N′-iminopyridine chelating ligands: Generation effects on the catalytic oligomerization and polymerization of ethylene. Organometallics 2006, 25, 3876–3887. [Google Scholar] [CrossRef]
- Irrgang, T.; Keller, S.; Maisel, H.; Kretschmer, W.; Kempe, R. Sterically demanding iminopyridine ligands. Eur. J. Inorg. Chem. 2007, 2007, 4221–4228. [Google Scholar] [CrossRef]
- Sun, W.H.; Song, S.; Li, B.; Redshaw, C.; Hao, X.; Li, Y.S.; Wang, F. Ethylene polymerization by 2-iminopyridylnickel halide complexes: Synthesis, characterization and catalytic influence of the benzhydryl group. Dalton Trans. 2012, 41, 11999–12010. [Google Scholar] [CrossRef]
- Yue, E.; Zhang, L.; Xing, Q.; Cao, X.P.; Hao, X.; Redshaw, C.; Sun, W.H. 2-(1-(2-Benzhydrylnaphthylimino)ethyl)pyridylnickel halides: Synthesis, characterization, and ethylene polymerization behavior. Dalton Trans. 2014, 43, 423–431. [Google Scholar] [CrossRef]
- Antonov, A.A.; Semikolenova, N.V.; Talsi, E.P.; Matsko, M.A.; Zakharov, V.A.; Bryliakov, K.P. 2-iminopyridine nickel(II) complexes bearing electron-withdrawing groups in the ligand core: Synthesis, characterization, ethylene oligo and polymerization behavior. J. Organomet. Chem. 2016, 822, 241–249. [Google Scholar] [CrossRef]
- Guo, L.; Li, S.; Ji, M.; Sun, W.; Liu, W.; Li, G.; Zhang, J.; Liu, Z.; Dai, S. Monoligated vs bisligated effect in iminopyridyl nickel catalyzed ethylene polymerization. Organometallics 2019, 38, 2800–2806. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Mu, H.; Jian, Z. Systematic studies on dibenzhydryl and pentiptycenyl substituted pyridine–imine nickel(II) mediated ethylene polymerization. Dalton Trans. 2020, 49, 4824–4833. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sun, W.H.; Gao, T.; Hou, J.; Chen, J.; Chen, W. Nickel (II) complexes bearing 2-ethylcarboxylate-6-iminopyridyl ligands: Synthesis, structures and their catalytic behavior for ethylene oligomerization and polymerization. J. Organomet. Chem. 2005, 690, 1570–1580. [Google Scholar] [CrossRef]
- Champouret, Y.D.M.; Fawcett, J.; Nodes, W.J.; Singh, K.; Solan, G.A. Mono- vs. bi-metallic assembly on a bulky bis(imino)terpyridine framework: A combined experimental and theoretical study. Dalton Trans. 2006, 19, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Chandran, D.; Lee, K.M.; Chang, H.C.; Song, G.Y.; Lee, J.E.; Suh, H.; Kim, I.J. Ni(II) complexes with ligands derived from phenylpyridine, active for selective dimerization and trimerization of ethylene. J. Organomet. Chem. 2012, 718, 8–13. [Google Scholar] [CrossRef]
- Chan, L.; Huo, H.; Wang, L.; Kuang, Q.; Shi, W.; Zhang, N.; Li, Z.; Wang, J. Ethylene oligomerization studies utilizing nickel complexes bearing pyridine-imine ligands. Inorg. Chim. Acta 2019, 491, 67–75. [Google Scholar] [CrossRef]
- Antonov, A.A.; Semikolenova, N.V.; Soshinikov, I.E.; Talsi, E.P.; Bryliakov, K.P. Selective Ethylene Dimerization into 2-Butenes Using Homogeneous and Supported Nickel(II) 2-Iminopyridine Catalysts. Top. Catal. 2020, 63, 222–228. [Google Scholar] [CrossRef]
- Ji, J.; Ma, Y.; Hu, X.; Flisak, Z.; Tongling, L.; Sun, W.H. 2-(N,N-Diethylaminomethyl)-6,7-trihydroquinolinyl-8-ylideneamine–Ni(II) chlorides: Application in ethylene dimerization and trimerization. New J. Chem. 2020, 44, 17047–17052. [Google Scholar]
- Mahmood, Q.; Li, X.; Qin, L.; Wang, L.; Sun, W.H. Structural evolution of iminopyridine support for nickel/palladium catalysts in ethylene (oligo) polymerization. Dalton Trans. 2022, 51, 14375–14407. [Google Scholar] [CrossRef]
- Wang, W.C.; Tao, L.; Markham, J.; Zhang, Y.; Tan, E.; Batan, L.; Warner, E.; Biddy, M. Review of Biojet Fuel Conversion Technologies; Technical Report; NREL/TP-5100-66291; National Renewable Energy Laboratory: Washington, DC, USA, 2016. [CrossRef]
- Attanatho, L.; Lao-ubol, S.; Suemanotham, A.; Prasongthum, N.; Khowattana, P.; Laosombut, T.; Duangwongsa, N.; Larpkiattaworn, S.; Thanmongkhon, Y. Jet fuel range hydrocarbon synthesis through ethylene oligomerization over platelet Ni-AlSBA-15 catalyst. SN Appl. Sci. 2020, 2, 971–982. [Google Scholar] [CrossRef]
- van Dyk, S.; Saddler, J. Progress in Commercialization of Biojet/Sustainable Aviation Fuels (SAF): Technologies, Potential and Challenges; IEA Bioenergy Task 39; IEA: Paris, France, 2021. [Google Scholar]
- Arrozi, U.S.F.; Bon, V.; Kutzscher, C.; Senkovska, I.; Kaskel, S. Towards highly active and stable nickel-based metal–organic frameworks as ethylene oligomerization catalysts. Dalton Trans. 2019, 48, 3415–3421. [Google Scholar] [CrossRef]
- Kurokawa, H.; Matsuda, M.; Fujii, K.; Ishihama, Y.; Sakuragi, T.; Ohshima, M.; Miura, H. Bis(imino)pyridine iron and cobalt complexes immobilized into interlayer space of fluorotetrasilicic mica: Highly active heterogeneous catalysts for polymerization of ethylene. Chem. Lett. 2007, 36, 1004–1005. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Li, D.; Guo, L.; Xiao, Y.; Li, C. Study on the catalytic performance of nickel(II) complexes with distinct triazine support Structures in ethylene oligomerization via different experiment designs. Molecules 2025, 30, 1977. [Google Scholar] [CrossRef]
- Kondo, T.; Yamamoto, K.; Sakuragi, T.; Kurokawa, H.; Miura, H. Acetyliminopyridineiron(III) complexes immobilized in fluorotetrasilicic mica interlayer as efficient catalysts for oligomerization of ethylene. Chem. Lett. 2012, 41, 461–463. [Google Scholar] [CrossRef]
- Fujii, K.; Ishihama, Y.; Sakuragi, T.; Ohshima, M.; Kurokawa, H.; Miura, H. Heterogeneous catalysts immobilizing a-diimine nickel complexes into fluorotetrasilicic mica interlayers to prepare branched polyethylene from only ethylene. Catal. Commun. 2008, 10, 183–186. [Google Scholar] [CrossRef]
- Kurokawa, H.; Nakazato, Y.; Tahara, S.; Katakura, T.; Ishihama, Y.; Sakuragi, T.; Miura, H. Copolymerization of ethylene with vinyl monomers using heterogeneous catalysts consisting of a-diimine Ni(II) complexes immobilized into a fluorotetrasilicic mica interlayer in the presence of an alkylaluminum compound. Macromol. React. Eng. 2013, 7, 125–134. [Google Scholar] [CrossRef]
- Kurokawa, H.; Miura, K.; Yamamoto, K.; Sakuragi, T.; Sugiyama, T.; Ohshima, M.; Miura, H. Oligomerization of ethylene to produce linear a-olefins using heterogeneous catalyst prepared by immobilization of a-diiminenickel(II) complex into fluorotetrasilicic mica interlayer. Catalysts 2013, 3, 125–136. [Google Scholar] [CrossRef]
- Kurokawa, H.; Hayasaka, M.; Yamamoto, K.; Sakuragi, T.; Ohshima, M.; Miura, H. Self-assembled heterogeneous late transition-metal catalysts for ethylene polymerization; New approach to simple preparation of iron and nickel complexes immobilized in clay mineral interlayer. Catal. Commun. 2014, 47, 13–17. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ogawa, R.; Yamamoto, K.; Sakuragi, T.; Ohshima, M.; Miura, H. Nickel (II) complex baring fluorine-substituted a-diimine ligand immobilized in fluorotetrasilicic mica interlayer as heterogeneous catalysts for ethylene oligomerization. J. Jpn. Petrol. Inst. 2014, 57, 146–154. [Google Scholar] [CrossRef]
- Hirahara, M.Y.; Fujiwara, S.; Kurokawa, H. Simple preparation of halogen–substituted a-diimine nickel complexes immobilized into clay interlayer as catalysts for ethylene oligo-/polymerization. Mod. Res. Catal. 2017, 6, 100–120. [Google Scholar] [CrossRef]
- Yamanaka, H.; Yamamoto, K.; Sakuragi, T.; Ohshima, M.; Nagashima, S.; Kurokawa, H.; Miura, H. Ethylene oligomerization using quinoline-iminenickel(II) complex immobilized in fluorotetrasilicic mica interlayer by one-pot preparation method. J. Mol. Catal. A Chem. 2016, 425, 275–282. [Google Scholar] [CrossRef]
- Kurokawa, H.; Morita, S.; Matsuda, M.; Suzuki, H.; Ohshima, M.; Miura, H. Polymerization of ethylene using zirconocenes supported on swellable cation-exchanged fluorotetrasilicic mica. Appl. Catal. A Gen. 2009, 360, 192–198. [Google Scholar] [CrossRef]
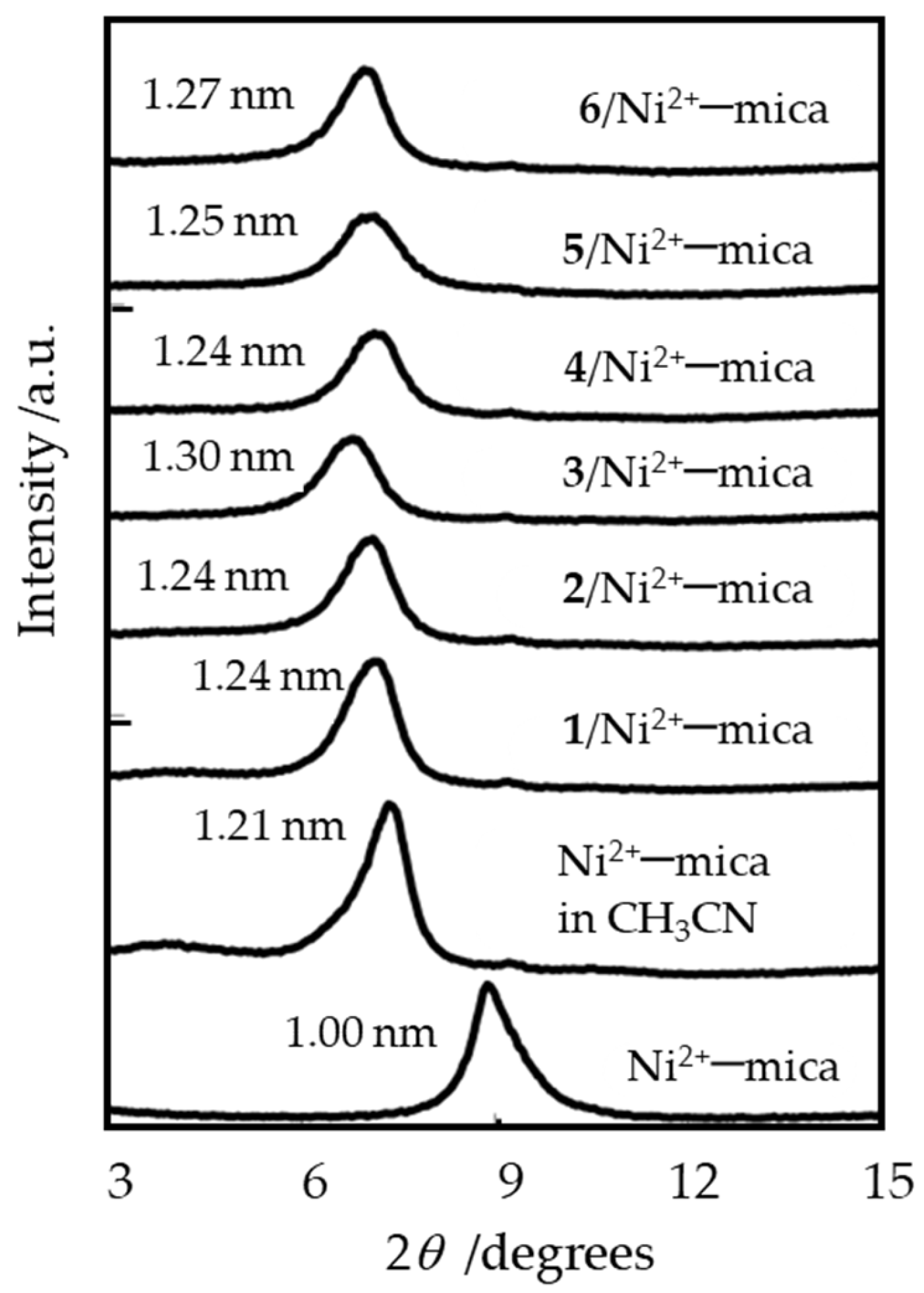
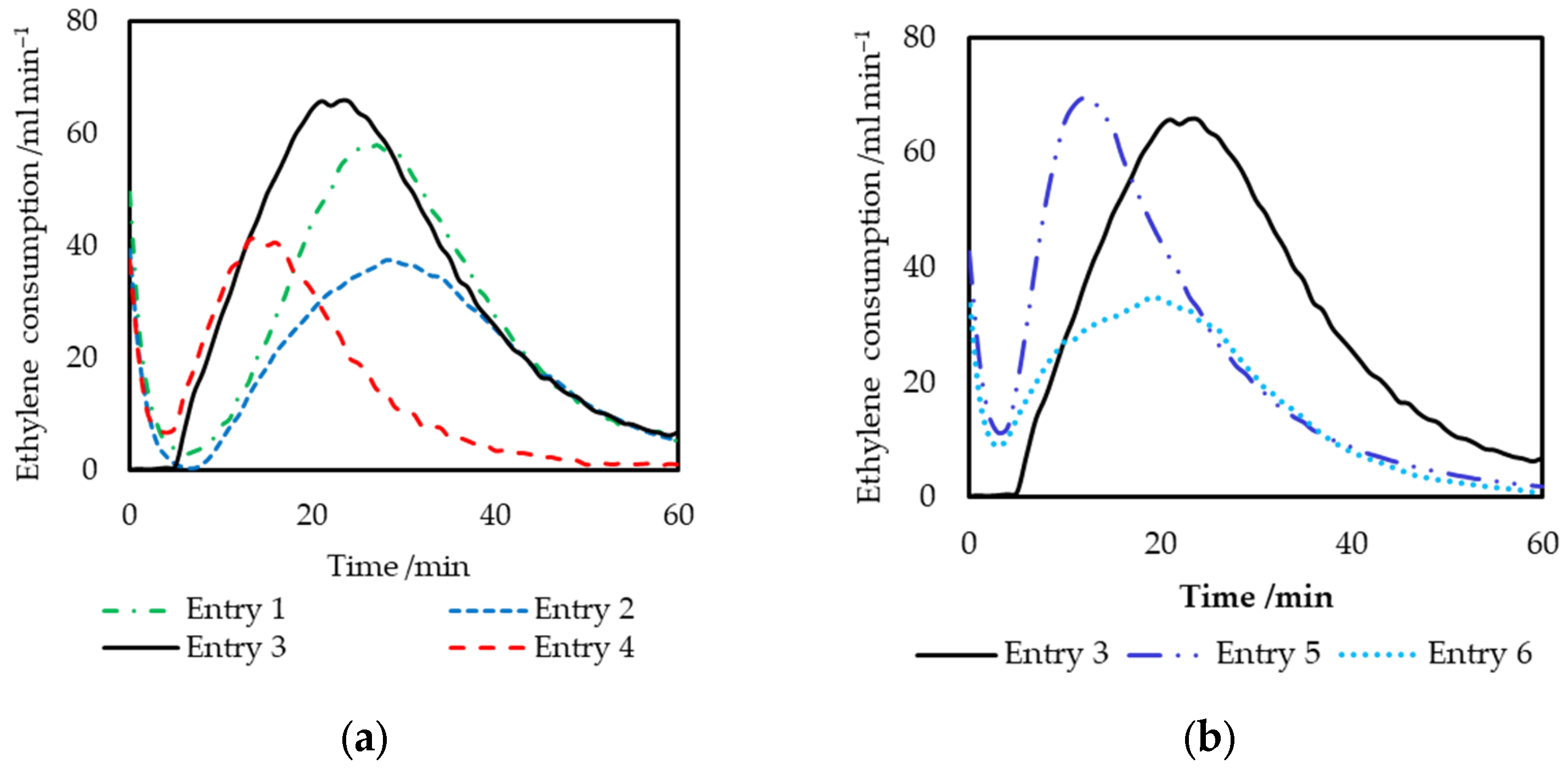

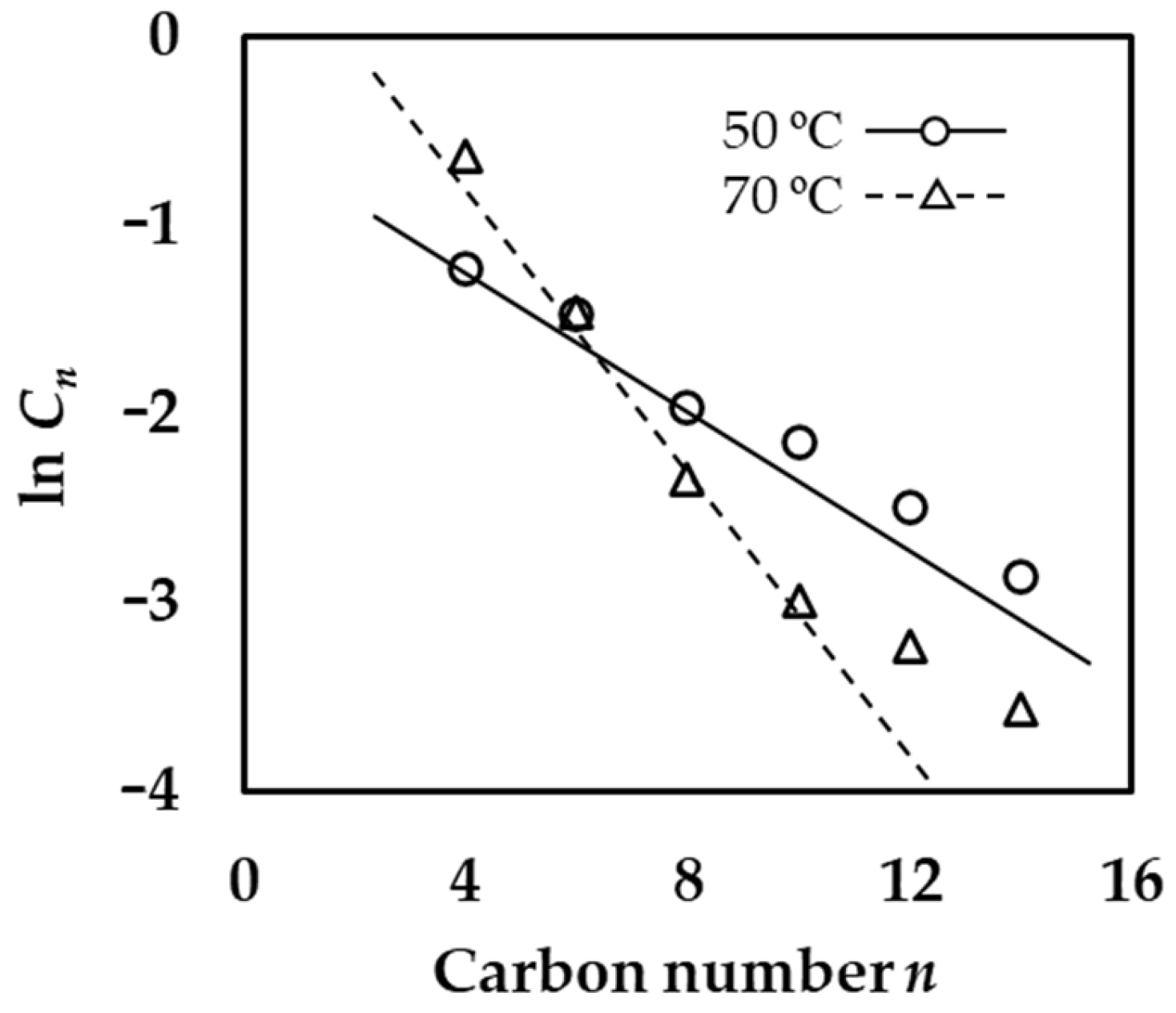
| Entry | L | Time | R3Al | Activity 2 | Selectivity/wt% | Selectivity in Alkenes 4/mol% | MB 5 | α 6 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| /h | Solid | Liquid 3 | SLAO | SIO | SBO | /% | |||||
| 1 | 1 | 1 | TEA | 333 | 9.2 | 55.3 | 43.7 | 46.6 | 9.7 | 64.7 | 0.72 |
| 2 | 2 | 1 | 249 | 16.0 | 46.7 | 42.7 | 48.2 | 9.1 | 62.9 | 0.78 | |
| 3 | 3 | 1 | 408 | 11.5 | 51.0 | 49.6 | 43.5 | 6.9 | 62.9 | 0.73 | |
| 4 | 4 | 1 | 175 | 19.4 | 41.1 | 49.6 | 42.7 | 7.7 | 60.9 | 0.77 | |
| 5 | 5 | 1 | 312 | 40.5 | 10.5 | 34.4 | 44.7 | 20.9 | 50.9 | 0.81 | |
| 6 | 6 | 1 | 210 | 16.2 | 46.8 | 61.3 | 32.0 | 6.7 | 62.9 | 0.73 | |
| 7 7 | 7 | 1.25 | 236 | 14.3 | 50.6 | 43.6 | 47.8 | 8.6 | 64.8 | 0.74 | |
| 8 7 | 8 | 1.25 | 229 | 59.8 | 7.5 | 31.4 | 48.9 | 19.7 | 67.3 | 0.85 | |
| 9 8 | 9 | 1 | 80 | 20.8 | 14.9 | 56.3 | nd 9 | nd 9 | 56.3 | 0.69 | |
| 10 | 1 | 2 | TIBA | 233 | 8.4 | 66.7 | 44.0 | 46.6 | 9.4 | 75.4 | 0.68 |
| 11 | 2 | 2 | 195 | 19.2 | 54.8 | 43.0 | 47.9 | 9.1 | 74.2 | 0.69 | |
| 12 | 3 | 2 | 241 | 15.8 | 52.2 | 49.9 | 43.0 | 7.1 | 68.6 | 0.78 | |
| 13 | 4 | 2 | 168 | 19.1 | 48.1 | 51.4 | 40.7 | 7.9 | 67.6 | 0.78 | |
| 14 | 5 | 1 | 419 | 40.3 | 15.8 | 40.0 | 42.7 | 17.4 | 56.1 | 0.85 | |
| 15 | 6 | 1 | 296 | 20.1 | 57.8 | 44.9 | 45.5 | 9.6 | 78.1 | 0.72 | |
| 16 | 3 | 1 | MAO | ~0 | – | – | – | – | – | – | – |
| Entry | L | Selectivity in C8 Alkenes/mol% | ||||||
|---|---|---|---|---|---|---|---|---|
| 1O | (E)2O | (Z)2O | (E)3O | (Z)3O | 3M1H | 5M1H | ||
| 1 | 1 | 43.1 | 15.1 | 28.1 | 5.4 | 5.8 | 1.6 | 1.0 |
| 2 | 2 | 41.5 | 16.5 | 27.7 | 6.9 | 4.5 | 1.7 | 1.2 |
| 3 | 3 | 48.2 | 14.4 | 26.3 | 4.5 | 4.8 | 1.1 | 0.6 |
| 4 | 4 | 50.3 | 14.3 | 25.4 | 4.5 | 3.6 | 1.1 | 0.9 |
| 5 | 5 | 32.6 | 9.8 | 31.1 | 8.3 | 9.5 | 6.4 | 2.3 |
| 6 | 6 | 60.4 | 11.5 | 21.7 | 2.9 | 2.2 | 0.8 | 0.5 |
| 7 | 7 | 39.0 | 17.3 | 26.7 | 5.7 | 6.0 | 1.5 | 3.8 |
| 8 | 8 | 27.9 | 12.9 | 31.9 | 8.1 | 11.1 | 5.2 | 3.0 |
| 9 | 1 | 43.5 | 15.1 | 28.0 | 5.9 | 4.8 | 1.6 | 1.1 |
| 10 | 2 | 42.2 | 16.0 | 27.8 | 6.6 | 4.6 | 1.8 | 1.1 |
| 11 | 3 | 49.3 | 14.2 | 26.6 | 3.9 | 3.9 | 1.2 | 0.9 |
| 12 | 4 | 51.4 | 14.2 | 24.9 | 4.3 | 3.5 | 1.2 | 0.5 |
| 13 | 5 | 37.7 | 8.8 | 26.4 | 9.4 | 11.3 | 4.8 | 1.7 |
| 14 | 6 | 44.3 | 15.4 | 27.3 | 5.4 | 4.7 | 1.8 | 1.1 |
| Entry | T | PC2 | Time | Activity 2 | Selectivity/wt% | Selectivity in Alkenes 4/mol% | MB 5 | α 6 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| /°C | /MPa | /h | Solid | Liquid 3 | SLAO | SIO | SBO | /% | |||
| 17 | 40 | 0.4 | 1.5 | 388 | 34.9 | 37.5 | 63.5 | 31.2 | 5.4 | 72.4 | 0.79 |
| 3 | 50 | 0.4 | 1.0 | 408 | 11.5 | 51.0 | 49.6 | 43.5 | 6.9 | 62.9 | 0.73 |
| 18 | 60 | 0.4 | 1.0 | 258 | 3.1 | 76.7 | 36.6 | 54.6 | 8.8 | 81.0 | 0.64 |
| 19 | 70 | 0.4 | 1.0 | 171 | 1.1 | 74.0 | 27.3 | 60.2 | 12.6 | 77.0 | 0.61 |
| 20 | 50 | 0.2 | 1.0 | 242 | 5.3 | 67.2 | 34.8 | 55.6 | 9.5 | 72.7 | 0.66 |
| 21 | 50 | 0.7 | 1.0 | 581 | 24.3 | 49.1 | 60.0 | 34.8 | 5.3 | 73.5 | 0.71 |
| 22 7 | 50 | 0.4 | 2.0 | 237 | 17.6 | 58.5 | 51.7 | 41.1 | 7.2 | 76.2 | 0.75 |
| 23 8 | 50 | 0.4 | 1.5 | 283 | 16.3 | 48.9 | 53.8 | 40.0 | 6.3 | 65.2 | 0.72 |
| 24 9 | 50 | 0.4 | 1.5 | 121 | 16.0 | 53.0 | 54.0 | 40.3 | 5.6 | 69.0 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurokawa, H.; Haruta, S.; Sunagawa, R.; Ogihara, H. Fluorine- and Trifluoromethyl-Substituted Iminopyridinenickel(II) Complexes Immobilized into Fluorotetrasilicic Mica Interlayers as Ethylene Oligomerization Catalysts. Catalysts 2025, 15, 1073. https://doi.org/10.3390/catal15111073
Kurokawa H, Haruta S, Sunagawa R, Ogihara H. Fluorine- and Trifluoromethyl-Substituted Iminopyridinenickel(II) Complexes Immobilized into Fluorotetrasilicic Mica Interlayers as Ethylene Oligomerization Catalysts. Catalysts. 2025; 15(11):1073. https://doi.org/10.3390/catal15111073
Chicago/Turabian StyleKurokawa, Hideki, Shingo Haruta, Riku Sunagawa, and Hitoshi Ogihara. 2025. "Fluorine- and Trifluoromethyl-Substituted Iminopyridinenickel(II) Complexes Immobilized into Fluorotetrasilicic Mica Interlayers as Ethylene Oligomerization Catalysts" Catalysts 15, no. 11: 1073. https://doi.org/10.3390/catal15111073
APA StyleKurokawa, H., Haruta, S., Sunagawa, R., & Ogihara, H. (2025). Fluorine- and Trifluoromethyl-Substituted Iminopyridinenickel(II) Complexes Immobilized into Fluorotetrasilicic Mica Interlayers as Ethylene Oligomerization Catalysts. Catalysts, 15(11), 1073. https://doi.org/10.3390/catal15111073







