Advanced Ozone Oxidation Systems for Organic Pollutant Degradation: Performance Evaluation and Mechanism Insights
Abstract
1. Introduction
2. Results and Discussion
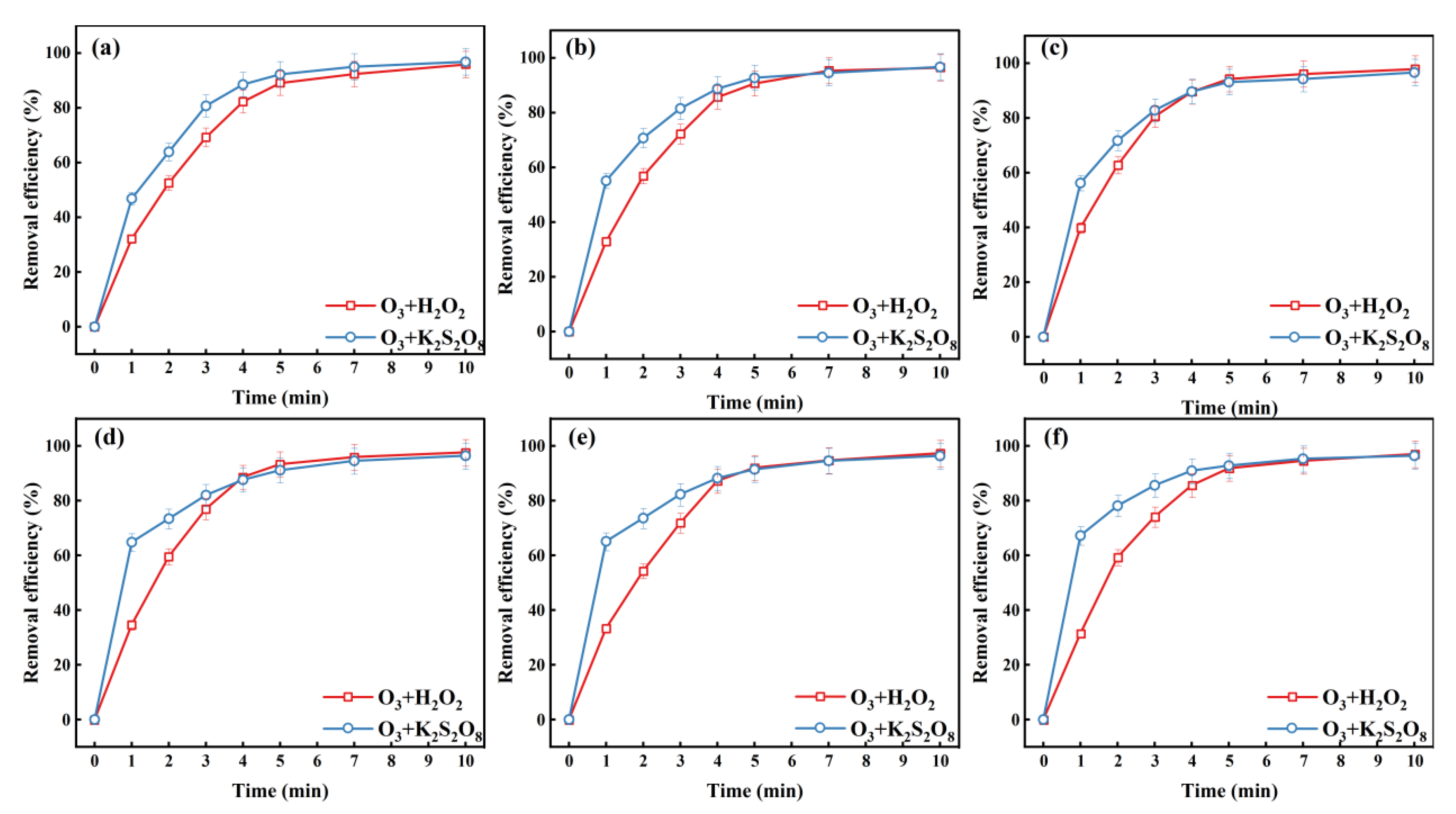
2.1. The Degradation Effectiveness of Pollutants in O3/H2O2 and O3/K2S2O8 Systems
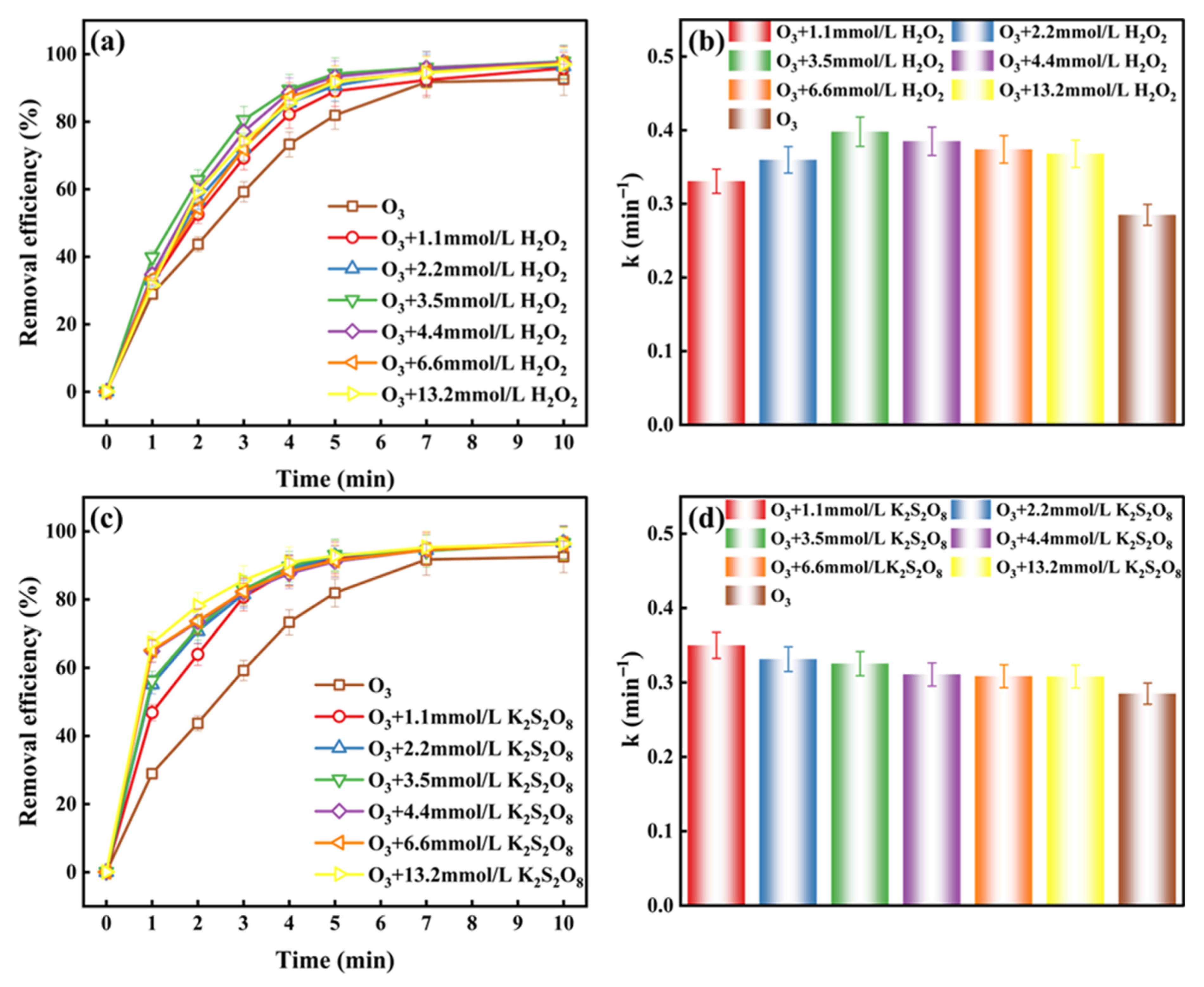
2.1.1. The Initial Concentration of H2O2 and K2S2O8 Affects
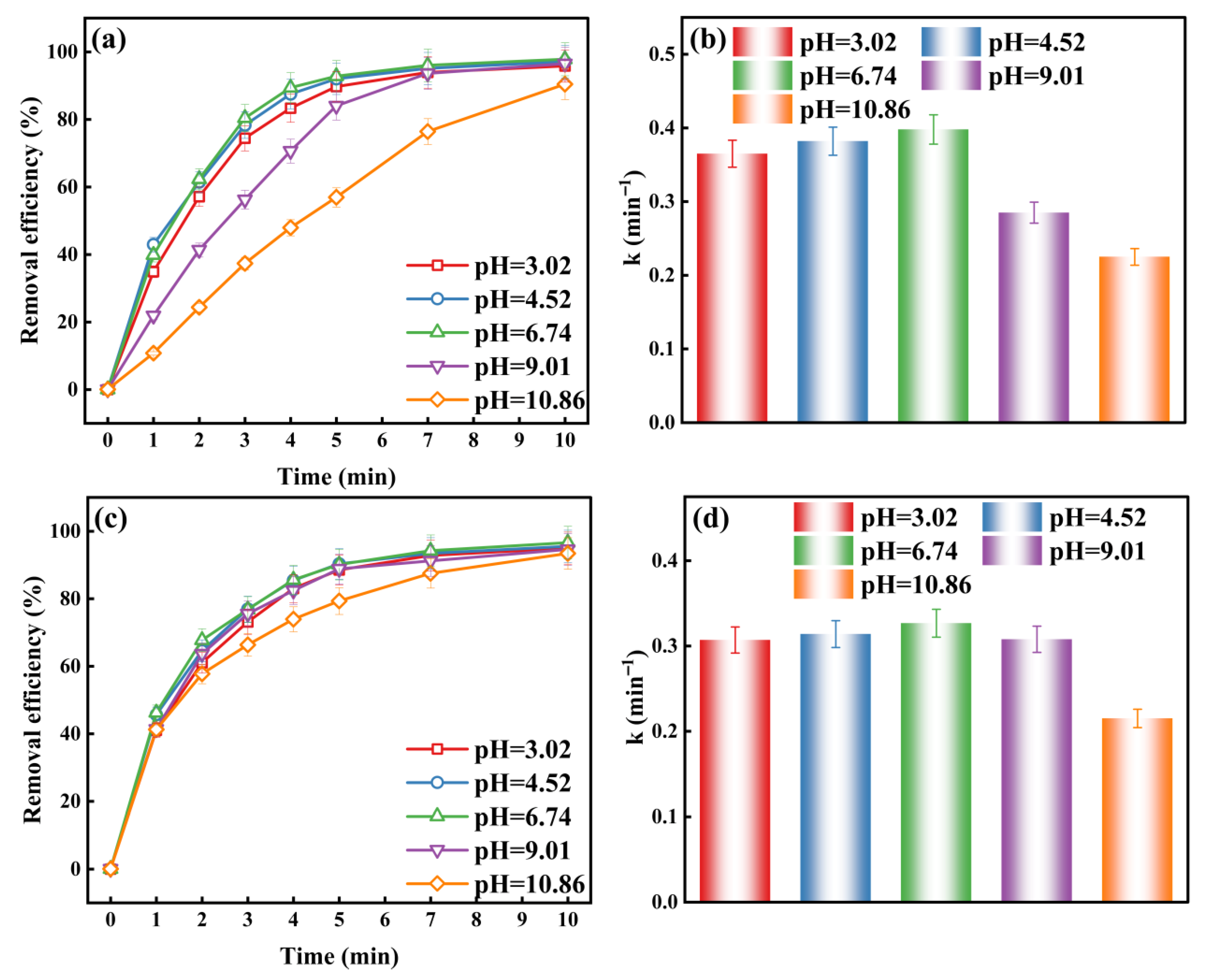
2.1.2. Effect of Initial pH on the O3/H2O2 and O3/K2S2O8 Systems
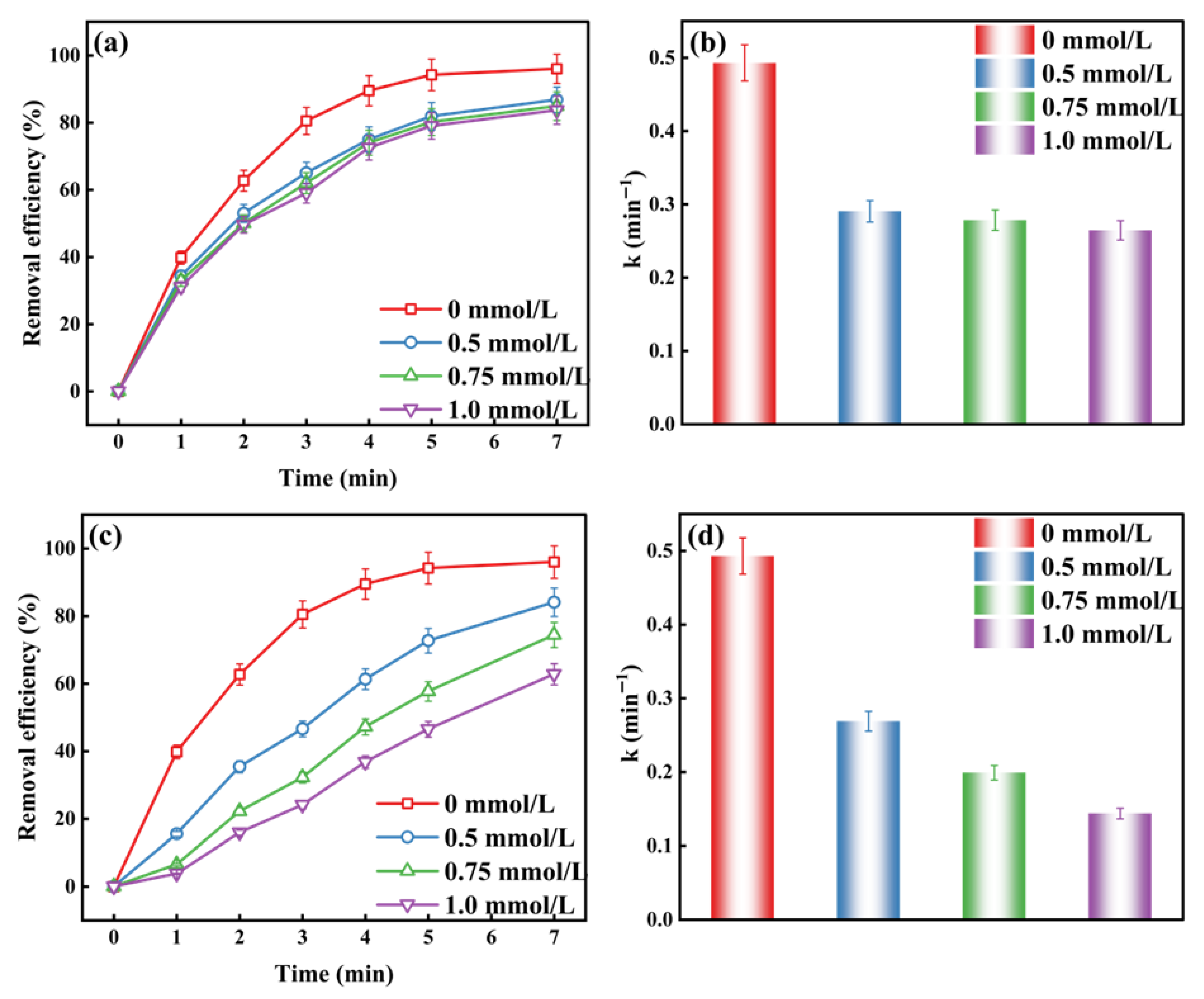
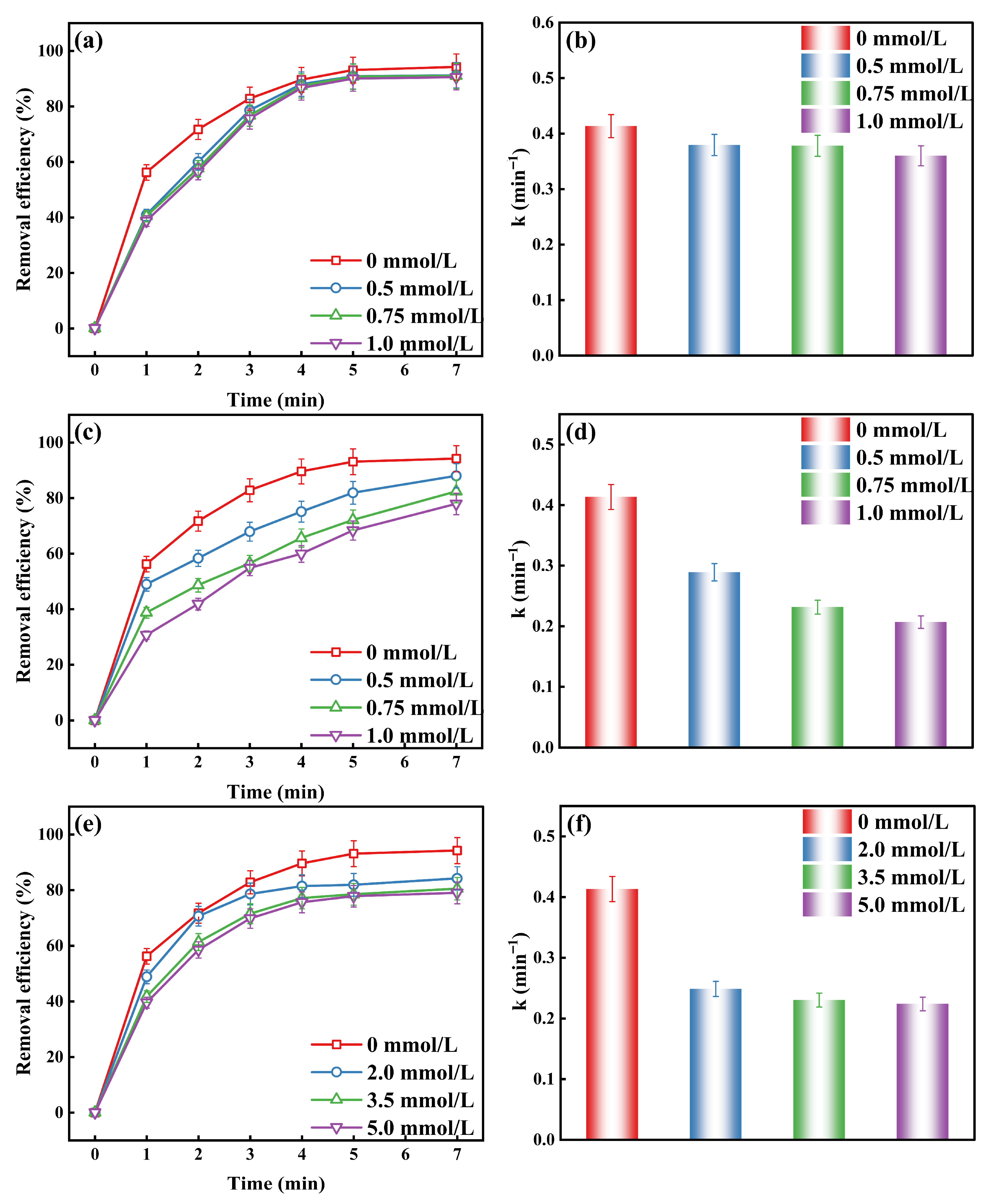
2.1.3. The Effect of Active Substances on the O3/H2O2 and O3/K2S2O8 Systems Degradation of MO
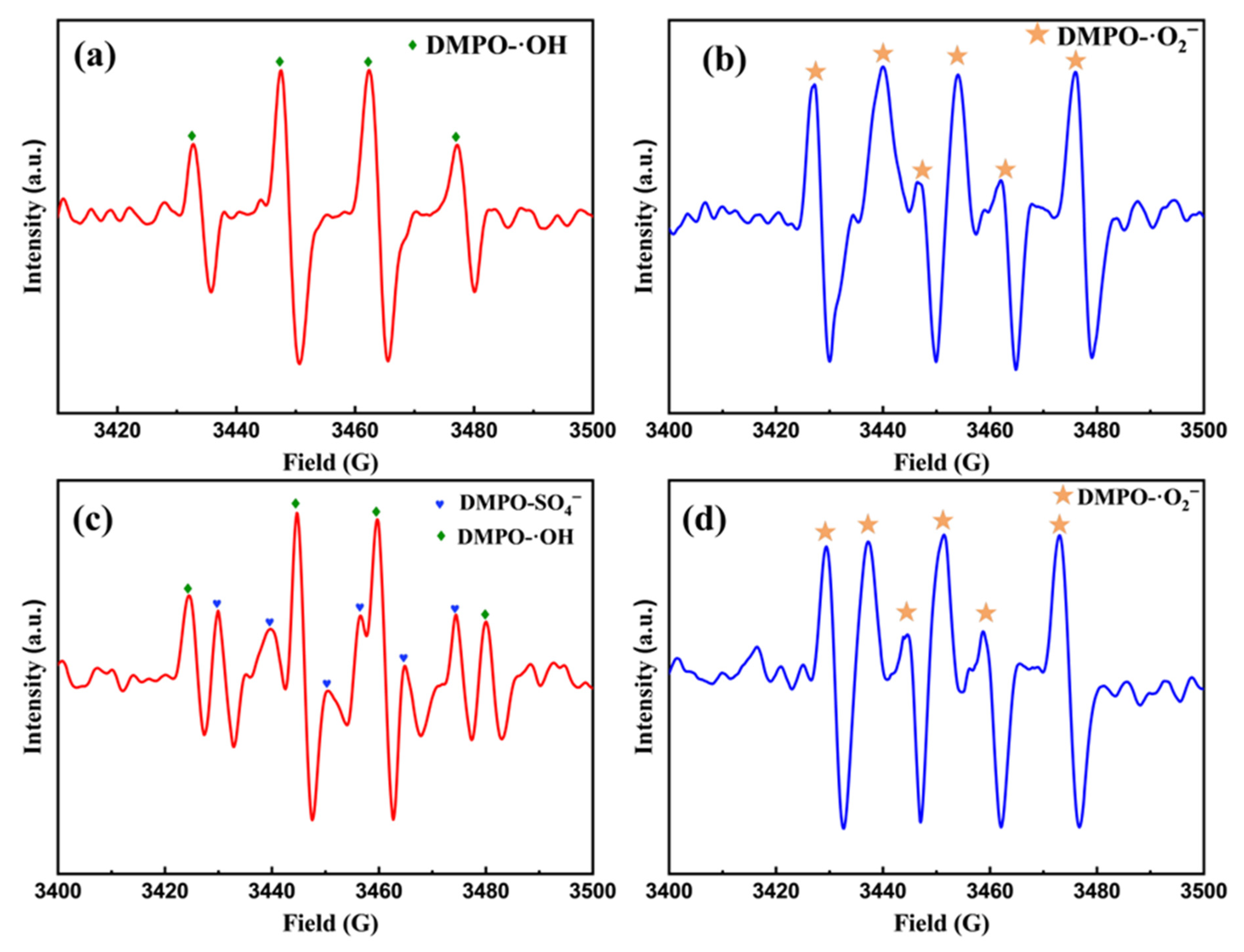
2.1.4. ESR Characterization
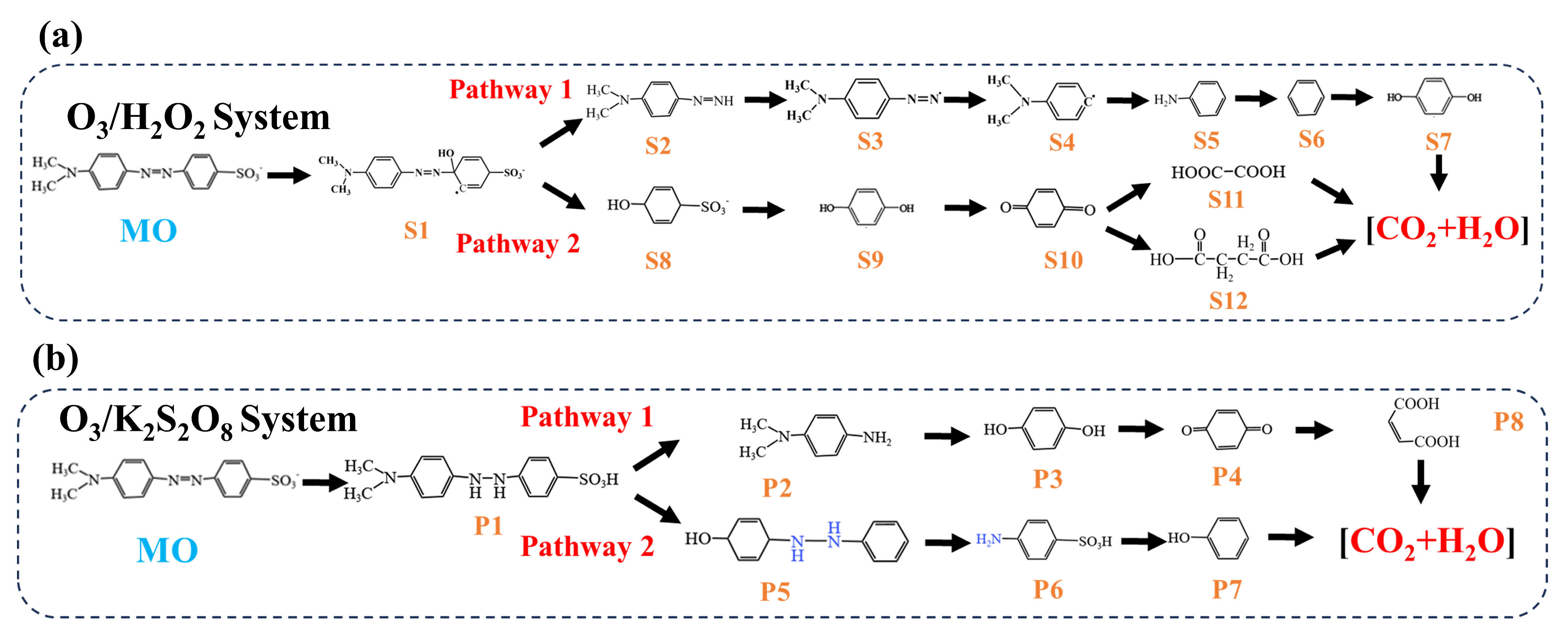
2.1.5. Analysis of Degradation Products in the O3/H2O2 and O3/K2S2O8 Systems
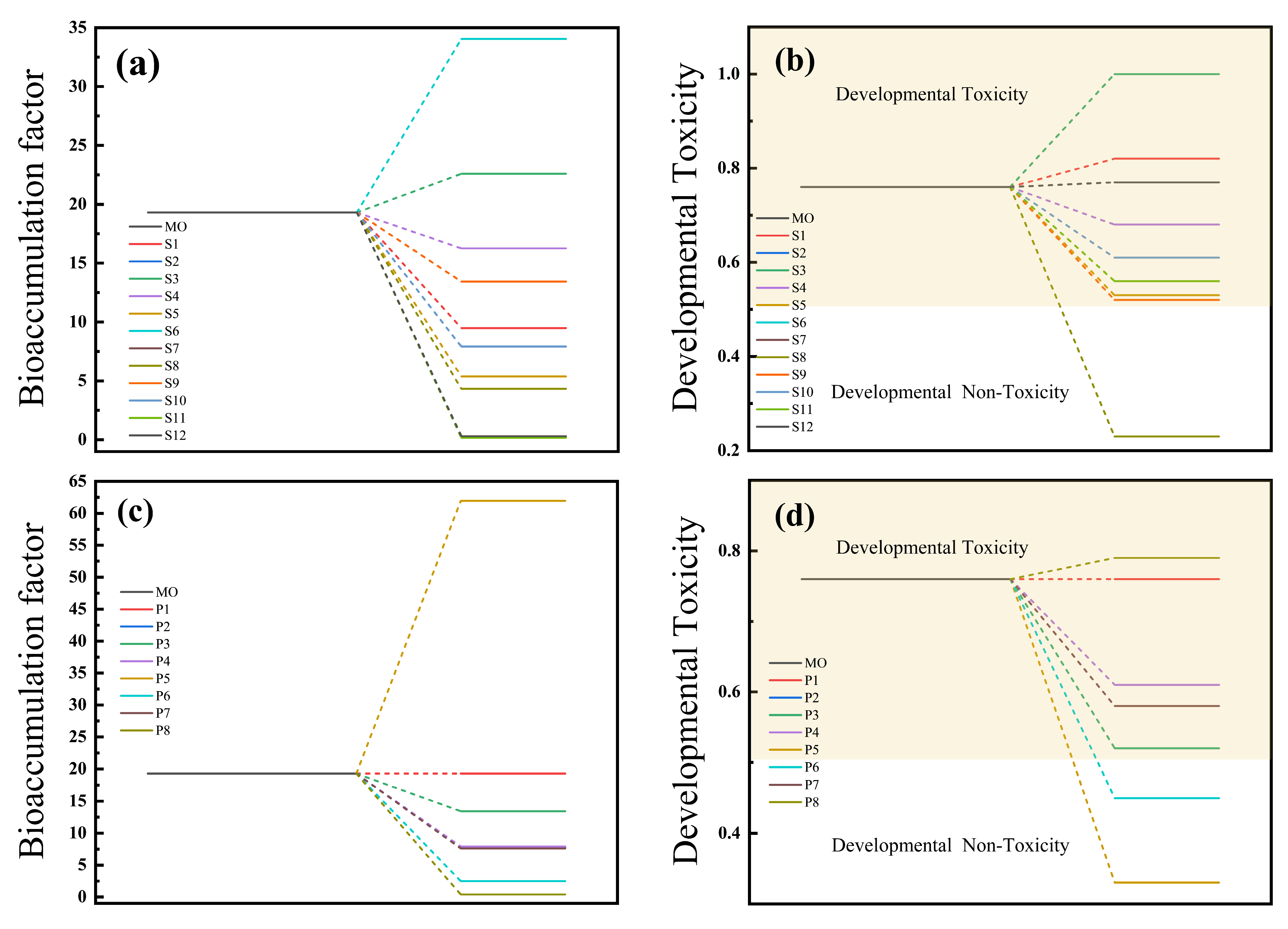
2.1.6. Toxicity Analysis in O3/H2O2 and O3/K2S2O8 Systems
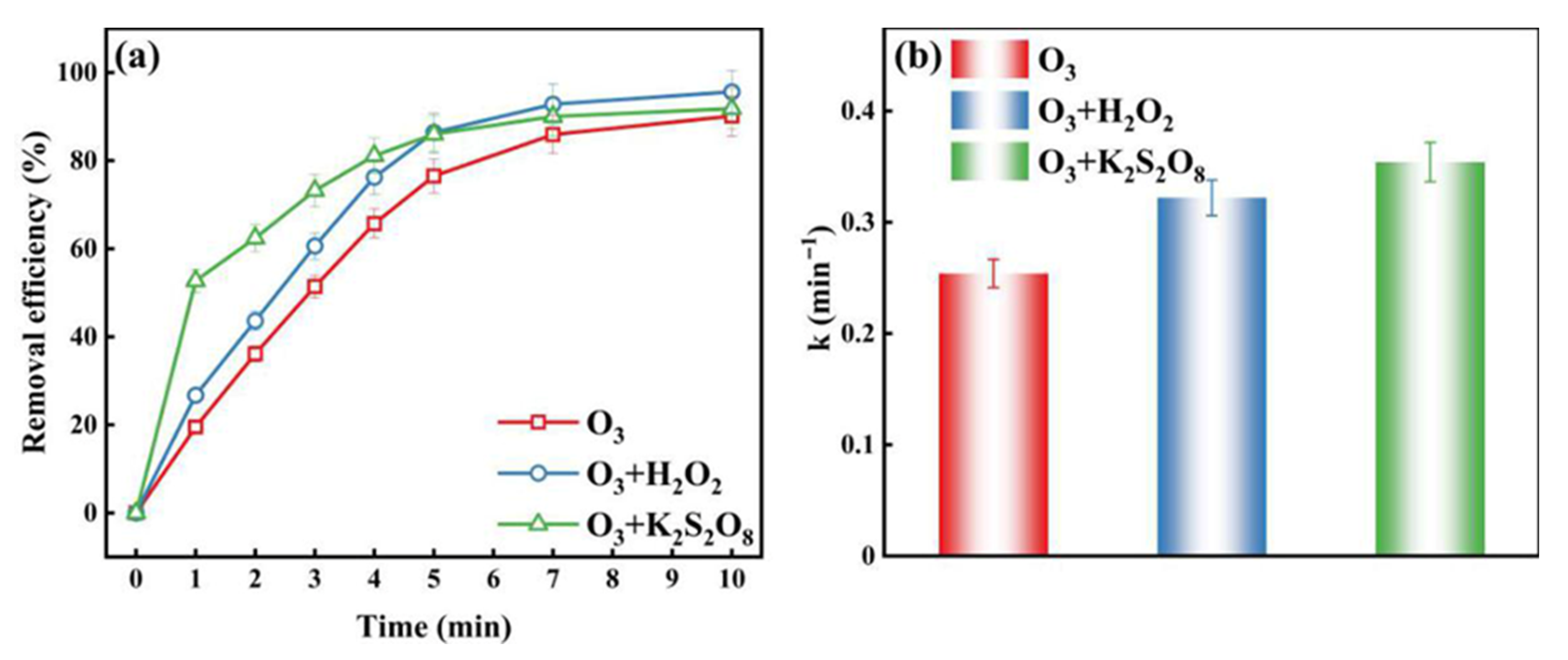
2.2. Comparison of O3 with H2O2 and K2S2O8
2.3. Impact of Different Types of Wastewater Applications
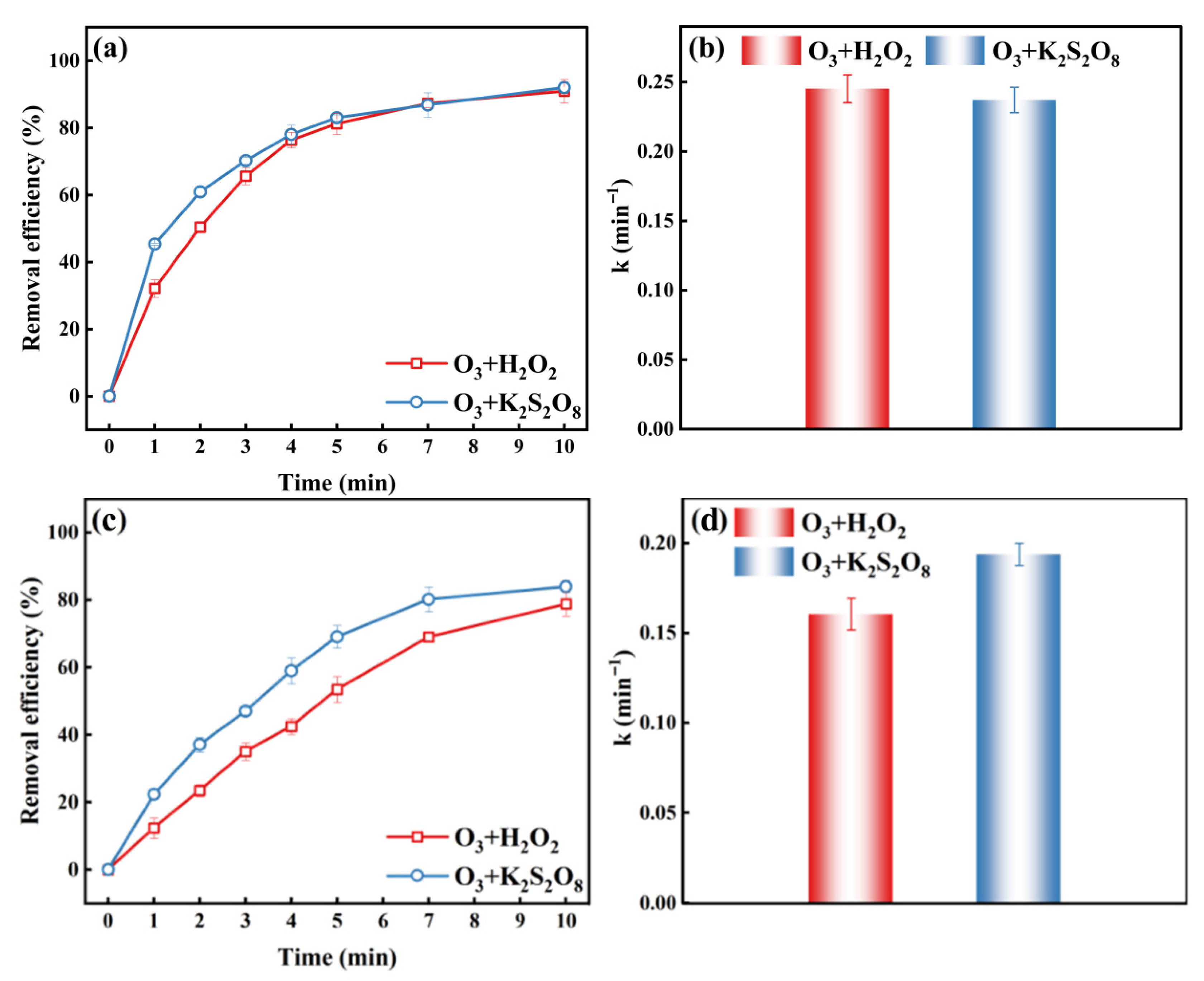
2.3.1. Degradation of Congo Red and Sulfamethoxazole by O3/H2O2 and O3/K2S2O8 Systems
2.3.2. O3/H2O2 and O3/K2S2O8 Systems for Degrading Natural Lake Water
3. Experimental
3.1. Chemicals and Experimental Setup
3.2. Experimental Setup
3.3. Calculation and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amaral-Silva, N.; Martins, R.C.; Catro-Silva, S.; Quinta-Ferreira, R.M. Ozonation and perozonation on the biodegradability improvement of a landfill leachate. J. Environ. Chem. Eng. 2016, 4, 527–533. [Google Scholar] [CrossRef]
- Park, J.; Pineda, M.; Peyot, M.; Yargeau, V. Degradation of oxytetracycline and doxycycline by ozonation: Degradation pathways and toxicity assessment. Sci. Total Environ. 2023, 856, 159076. [Google Scholar] [CrossRef] [PubMed]
- Issaka, E.; Amu-Darko, J.N.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants-A review. Chemosphere 2022, 289, 133208. [Google Scholar] [CrossRef] [PubMed]
- Esplugas, S.; Giménez, J.; Contreras, S.; Pascual, E.; Rodríguez, M. Comparison of different advanced oxidation processes for phenol degradation. Water Res. 2002, 36, 1034–1042. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.L.; Xu, Z.B.; Lee, M.Y.; Wu, Q.Y.; Hu, H.Y. A study of synergistic oxidation between ozone and chlorine on benzalkonium chloride degradation: Reactive species and degradation pathway. Chem. Eng. J. 2020, 382, 122856. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Yang, Z.W.; Du, Y.; Ouyang, W.Y.; Wang, W.L. The promotions on radical formation and micropollutant degradation by the synergies between ozone and chemical reagents (synergistic ozonation): A review. J. Hazard. Mater. 2021, 418, 126327. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Song, W.H.; Puttabyatappa, M. Praegnatio Perturbatio-Impact of Endocrine-Disrupting Chemicals. Endocr. Rev. 2021, 42, 295–353. [Google Scholar] [CrossRef]
- Hong, Y.; Feng, C.L.; Yan, Z.F.; Wang, Y.; Liu, D.Q.; Liao, W.; Bai, Y. Nonylphenol occurrence, distribution, toxicity and analytical methods in freshwater. Environ. Chem. Lett. 2020, 18, 2095–2106. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2022, 334, 1504–1521. [Google Scholar] [CrossRef]
- Droguett, C.; Salazar, R.; Brillas, E.; Sirés, I.; Carlos, C.; Marco, J.F.; Thiam, A. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Sci. Total Environ. 2020, 740, 140154. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wu, C.Y.; Zhou, Y.X.; Zou, J.N.; Song, G.Q.; Tan, Y. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2 and ozone/catalyst. Chemosphere 2019, 233, 34–43. [Google Scholar] [CrossRef]
- Li, C.H.; Jiang, F.; Sun, D.Z.; Qiu, B. Catalytic ozonation for advanced treatment of incineration leachate using (MnO2-Co3O4)/AC as a catalyst. Chem. Eng. J. 2017, 325, 624–631. [Google Scholar] [CrossRef]
- Sani, S.; Dashti, A.F.; Adnan, R. Applications of Fenton oxidation processes for decontamination of palm oil mill effluent: A review. Arab. J. Chem. 2020, 13, 7302–7323. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigné, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676. [Google Scholar] [CrossRef]
- Nöthe, T.; Fahlenkamp, H.; Sonntag, C.V. Ozonation of wastewater: Rate of ozone consumption and hydroxyl radical yield. Environ. Sci. Technol. 2009, 43, 5990. [Google Scholar] [CrossRef] [PubMed]
- Mérenyi, G.; Lind, J.; Naumov, S.; Sonntag, C.V. Reaction of ozone with hydrogen peroxide (peroxone process): A revision of current mechanistic concepts based on thermokinetic and quantum-chemical considerations. Environ. Sci. Technol. 2010, 44, 3505. [Google Scholar] [CrossRef]
- Fischbacher, A.; Sonntag, J.V.; Sonntag, C.V.; Schmidt, T.C. The (•)OH radical yield in the H2O2 + O3 (peroxone) reaction. Environ. Sci. Technol. 2013, 47, 9959. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Effects of factors and interacted factors on the optimal decolorization process of methyl orange by ozone. Water Res. 2000, 34, 974. [Google Scholar] [CrossRef]
- Dong, J.; Yao, J.; Tao, J.; Shi, X.; Wei, F. Degradation of Methyl Orange by ozone microbubble process with packing in the bubble column reactor. Environ. Sci. Technol. 2022, 44, 2512. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, S.; Liu, J.; Zhou, R. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Razali, N.A.; Abidin, C.Z.A.; An, O.S.; Ridwan, F.M.; Ibrahim, A.H.; Sabri, S.N.; Kow, S.H. Preliminary screening: Oxidative degradation of methyl orange using ozonation/persulfate. E3S Web Conf. 2018, 34, 02038. [Google Scholar]
- CCTE; EPA. Toxicity Estimation Software Tool (TEST); The United States Environmental Protection Agency’s Center for Computational Toxicology and Exposure: Triangle Park, NC, USA, 2022. [CrossRef]
- Du, X.D.; Oturan, M.A.; Zhou, M.H.; Belkessa, N.; Su, P.; Cai, J.J.; Trellu, C.; Mousset, E. Nanostructured electrodes for electrocatalytic advanced oxidation processes: From materials preparation to mechanisms understanding and wastewater treatment applications. Appl. Catal. B 2021, 296, 120332. [Google Scholar] [CrossRef]
- Qu, C.; Soomro, G.S.; Ren, N.; Liang, D.W.; Lu, S.F.; Xiang, Y.; Zhang, S.J. Enhanced electro-oxidation/peroxone (in situ) process with a Ti-based nickel-antimony doped tin oxide anode for phenol degradation. J. Hazard. Mater. 2020, 384, 121398. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Ji, J.; Wu, Y.; Chen, S.Q.; Xu, M.Y.; Cao, X.; Liu, H.L.; Wang, Z.; Bi, H.Y.; Guan, G.A.; et al. Nonylphenol and its derivatives: Environmental distribution, treatment strategy, management and future perspectives. Chemosphere 2024, 352, 141377. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.L.; Ren, J.W.; Zhou, Z.W.; Li, X.; Liu, Y.K.; Feng, J.Y. Fate of organic fractions of greywater in combined process of vacuum-ultraviolet (VUV/UV)/ozone pre-oxidation with enhanced coagulation. J. Environ. Chem. Eng. 2022, 10, 107417. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.B.; Ma, D.F.; Wang, Y.; Yao, G.P.; Yue, Q.Y.; Gao, B.Y.; Wang, S.E.; Xu, X. Degradation of organic pollutants by ultraviolet/ozone in high salinity condition: Non-radical pathway dominated by singlet oxygen. Chemosphere 2021, 268, 128796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Yan, T.; Pan, W.G. Study on the sorption mechanism of middle-low temperature sorption thermal storage materials from the microscale simulation: A review. Int. J. Energy Res. 2021, 45, 9719–9752. [Google Scholar] [CrossRef]
- Peng, Y.; Bin, Z.Y.; Wang, F.; Li, S.L.; Xu, S.W.; Wang, H. Electrocatalytic degradation of p-nitrophenol on metal-free cathode: Superoxide radical (O2•−) production via molecular oxygen activation. J. Hazard. Mater. 2023, 462, 132797. [Google Scholar] [CrossRef]
- Rodriguez-Peta, M.; Perez, J.A.B.; Llanos, J.; Saez, C.; Barrera-Díaz, C.E.; Rodrigo, M.A. Electrochemical generation of ozone using a PEM electrolyzer at acidic pHs. Sep. Purif. Technol. 2021, 267, 118672. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation—Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Ge, D.; Zeng, Z.Q.; Arowo, M.; Zou, H.K.; Chen, J.F.; Shao, L. Degradation of methyl orange by ozone in the presence of ferrous and persulfate ions in a rotating packed bed. Chemosphere 2016, 146, 413–418. [Google Scholar] [CrossRef]
- Amado-Pina, D.; Roa-Morales, G.; Molina-Mendieta, M.; Balderas-Hernández, P.; Romero, R.; Barrera-Díaz, C.E.; Natividad, R. E-peroxone process of a chlorinated compound: Oxidant species, degradation pathway and phytotoxicity. J. Environ. Chem. Eng. 2022, 10, 108148. [Google Scholar] [CrossRef]
- Wang, J.J.; Bai, R.B. Formic acid enhanced effective degradation of methyl orange dye in aqueous solutions under UV–Vis irradiation. Water Res. 2016, 101, 103–113. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, L.J.; Zhang, D.B. Decolorization of methyl orange by ozonation in combination with ultrasonic irradiation. J. Hazard. Mater. 2006, 138, 53–59. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, O.; Ji, G.Z.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Chen, S.X.; Huang, Y.F.; Han, X.X.; Wu, Z.L.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.L.; Deng, S.G. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Sun, B.Y.; Deng, S.H.; Wang, Y.J.; Peng, H.; Li, Y.W.; Zhang, X.H. Methyl orange degradation by pulsed discharge in the presence of activated carbon fibers. Chem. Eng. J. 2010, 159, 47–52. [Google Scholar] [CrossRef]
- Ma, Y.H.; Chen, F.; Yang, Q.; Zhong, Y.; Shu, X.Y.; Yao, F.B.; Xie, T.; Li, X.M.; Wang, D.B.; Zeng, G.M. Sulfate radical induced degradation of Methyl Violet azo dye with CuFe layered doubled hydroxide as heterogeneous photoactivator of persulfate. J. Environ. Manag. 2018, 227, 406–414. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.L.; Zhang, H.S.; Zheng, Y.J.; Jing, T.; Ma, J.; Nan, J.; Leong, Y.K.; Chang, J.S. Advanced oxidation process based on hydroxyl and sulfate radicals to degrade refractory organic pollutants in landfill leachate. Chemosphere 2022, 297, 134214. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yuan, P.F.; Jia, S.Y.; Liu, Y.Q.; Zhang, X.J.; Huo, S.; Zhang, H.X.; He, Z.G. Catalytic micro-ozonation by Fe3O4 nanoparticles @cow-dung ash for advanced treatment of biologically pre-treated leachate. Waste Manag. 2019, 83, 23–32. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, L.; Yang, S.; Guo, H. Advanced Ozone Oxidation Systems for Organic Pollutant Degradation: Performance Evaluation and Mechanism Insights. Catalysts 2025, 15, 1057. https://doi.org/10.3390/catal15111057
Xiang L, Yang S, Guo H. Advanced Ozone Oxidation Systems for Organic Pollutant Degradation: Performance Evaluation and Mechanism Insights. Catalysts. 2025; 15(11):1057. https://doi.org/10.3390/catal15111057
Chicago/Turabian StyleXiang, Liangrui, Shuang Yang, and He Guo. 2025. "Advanced Ozone Oxidation Systems for Organic Pollutant Degradation: Performance Evaluation and Mechanism Insights" Catalysts 15, no. 11: 1057. https://doi.org/10.3390/catal15111057
APA StyleXiang, L., Yang, S., & Guo, H. (2025). Advanced Ozone Oxidation Systems for Organic Pollutant Degradation: Performance Evaluation and Mechanism Insights. Catalysts, 15(11), 1057. https://doi.org/10.3390/catal15111057







