Enhancement of Photocatalytic and Anticancer Properties in Y2O3 Nanocomposites Embedded in Reduced Graphene Oxide and Carbon Nanotubes
Abstract
1. Introduction
2. Results and Discussions
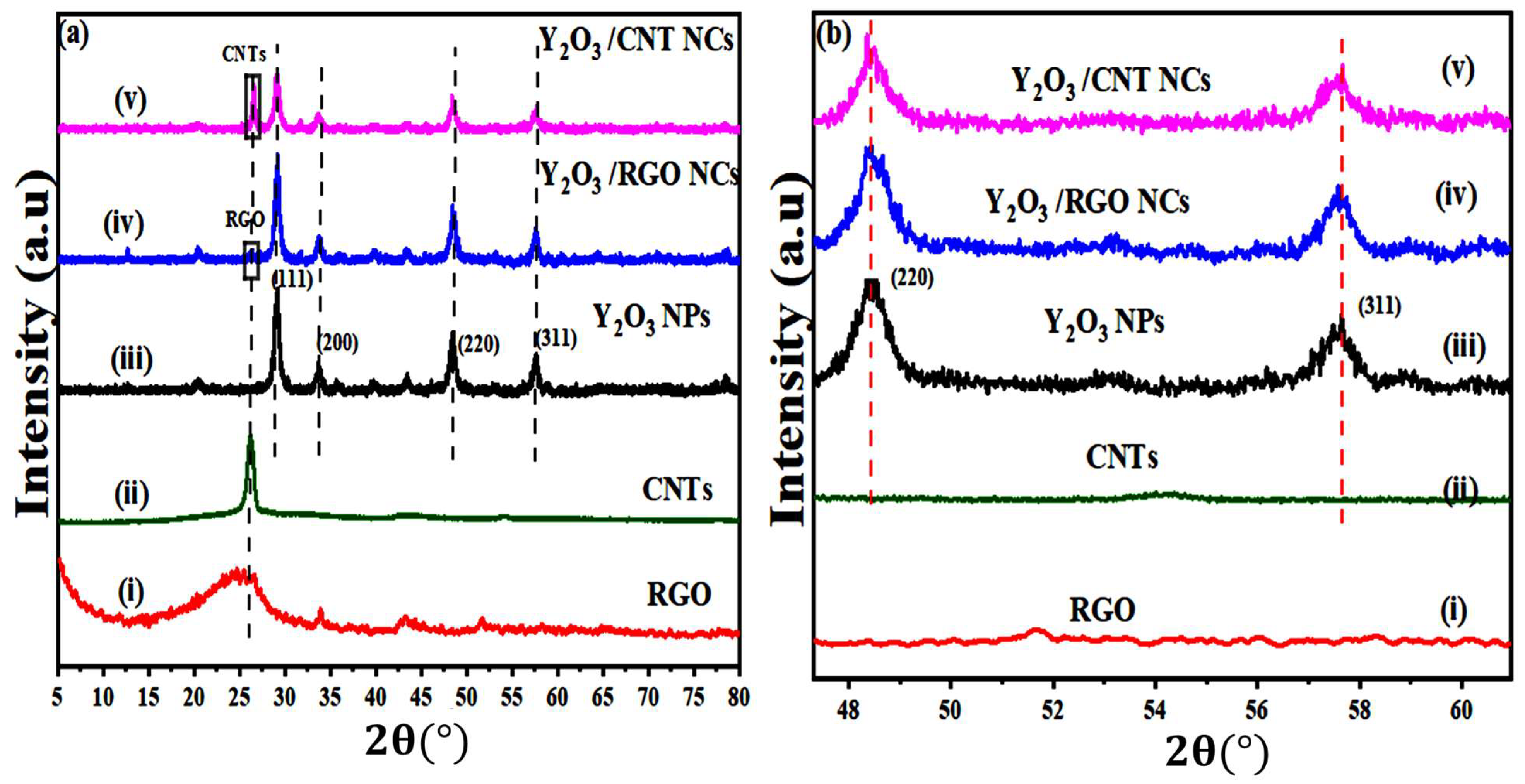
2.1. X-Ray Diffraction (XRD)
2.2. SEM Characterization
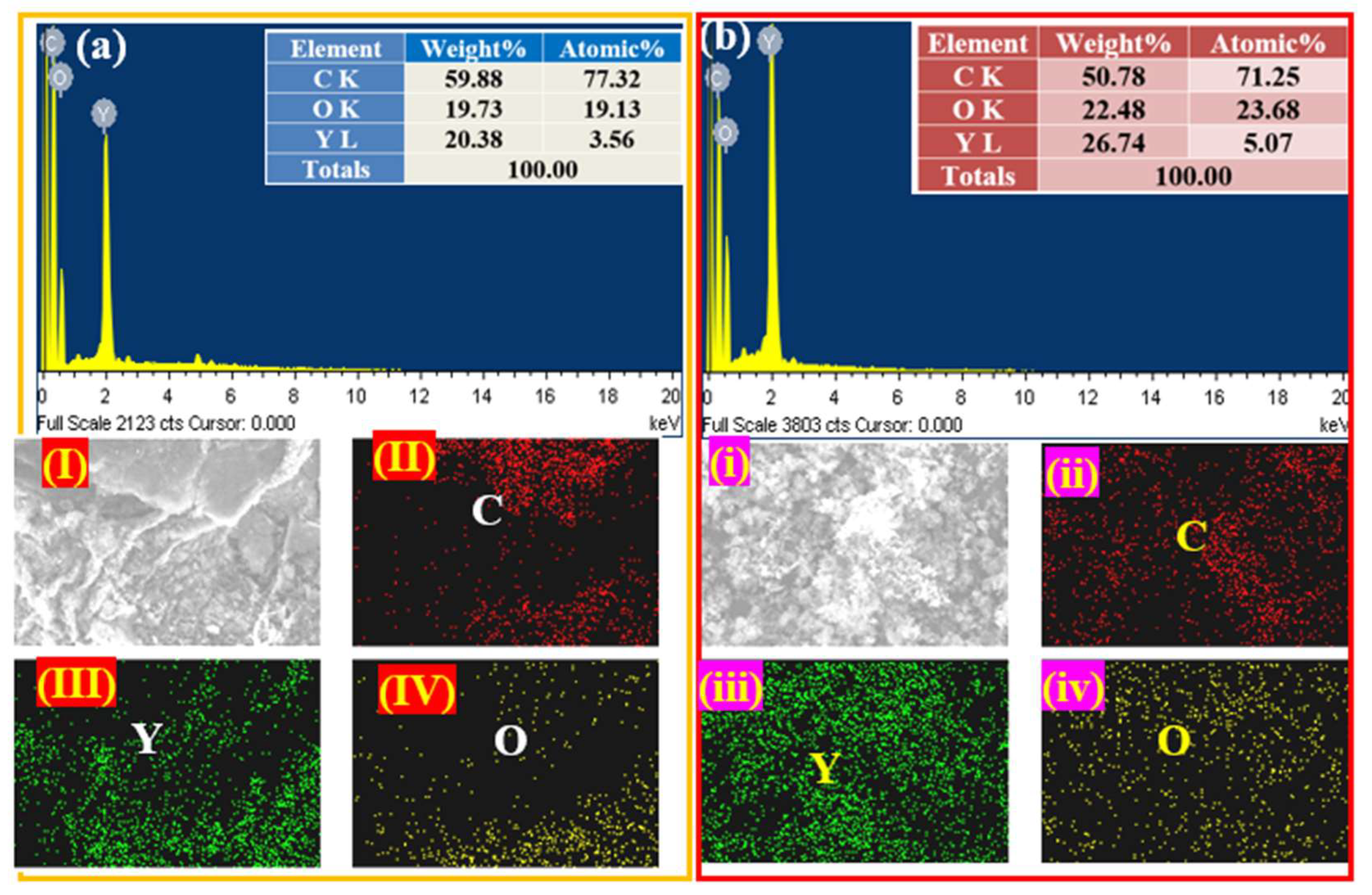
2.3. EDX and Elemental Mapping Analysis
2.4. X-Ray Photoelectron Spectroscopy (XPS)
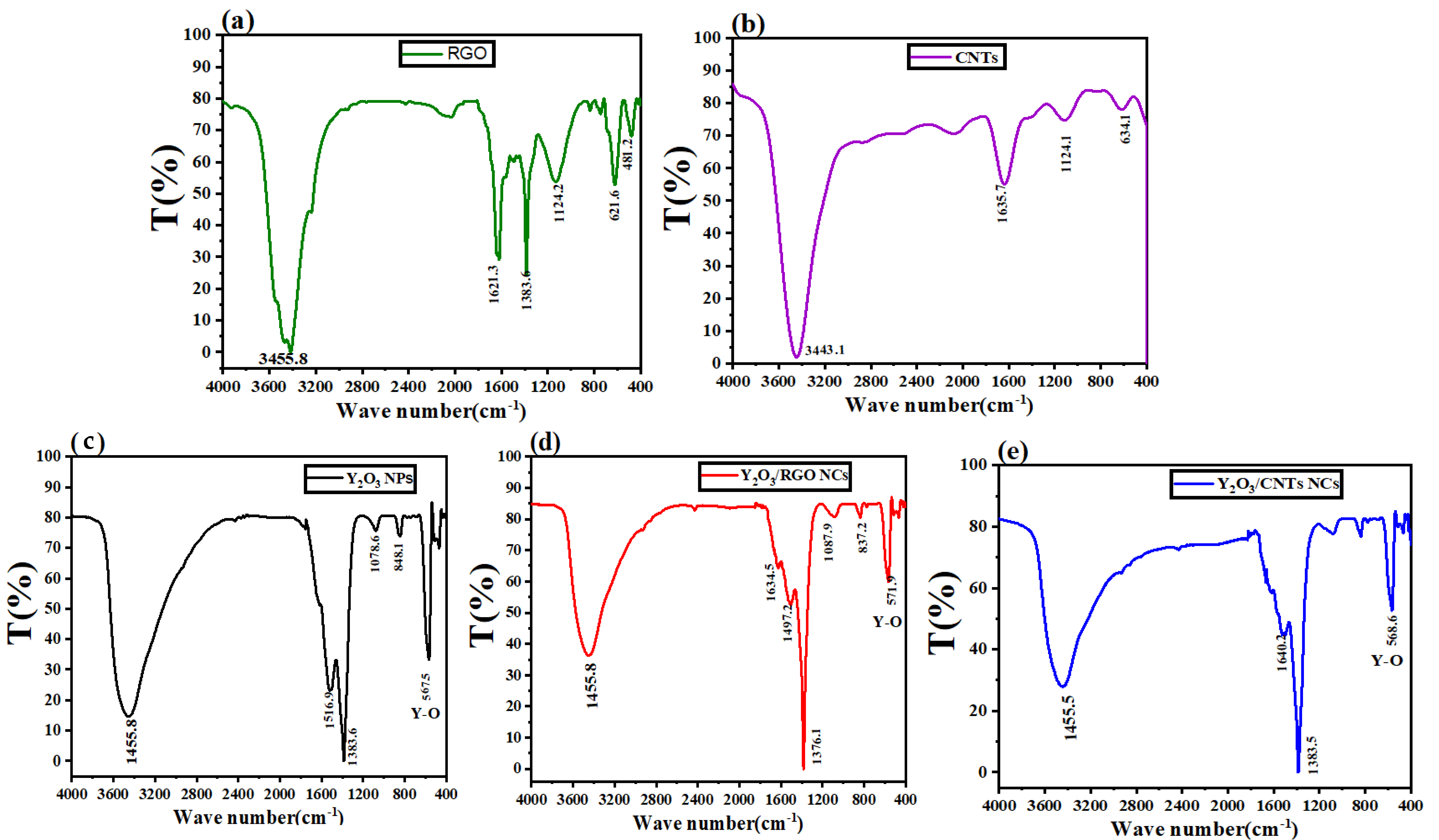
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. Optical Properties Analysis
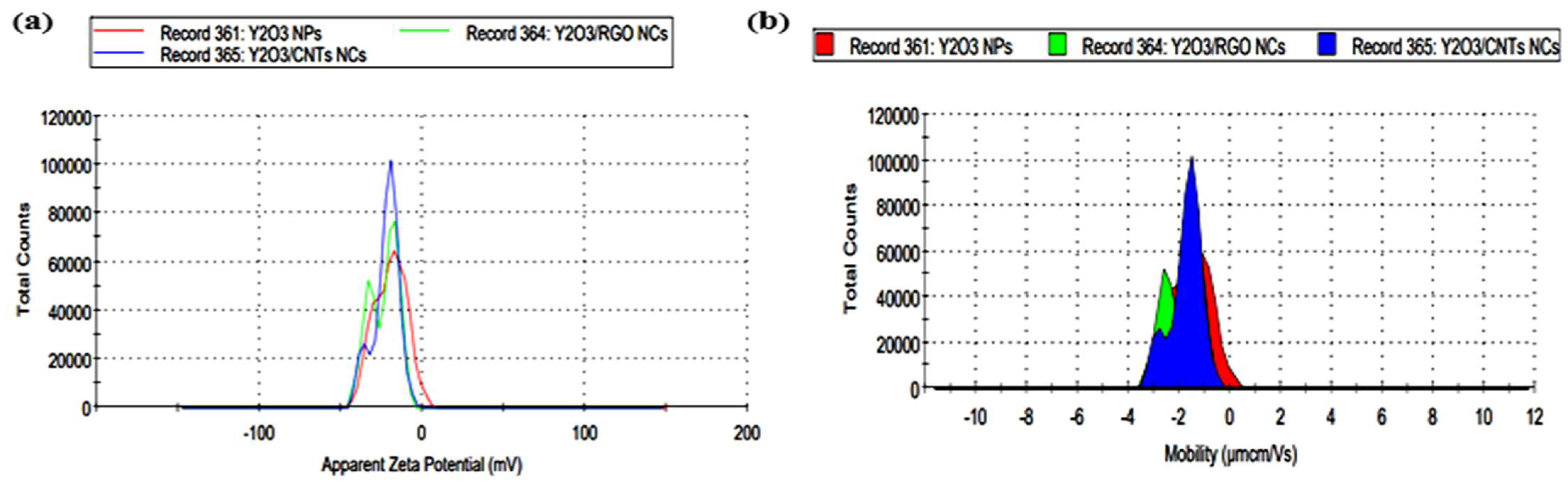
2.7. DLS Analysis
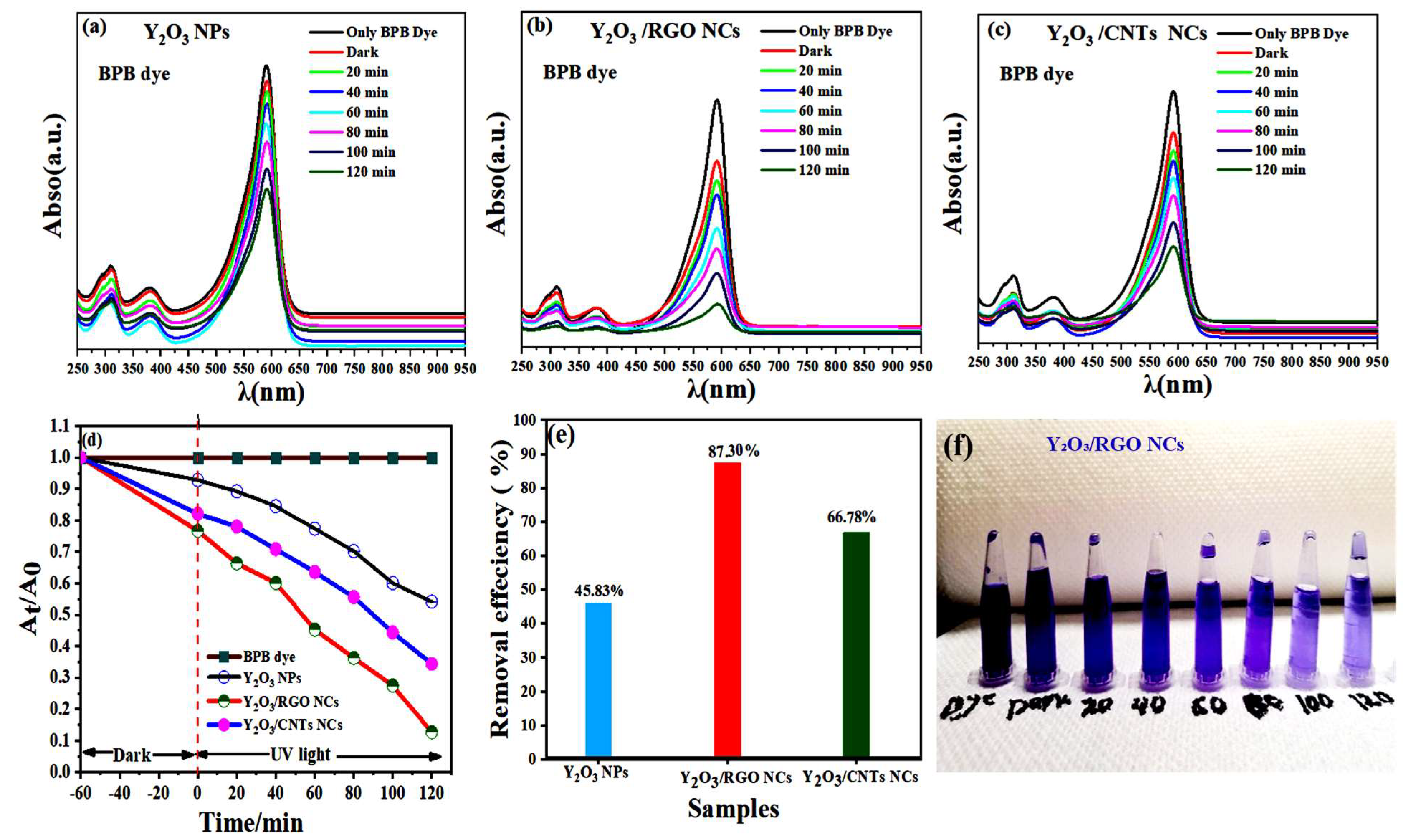
2.8. Photocatalytic Activity
2.8.1. Stability
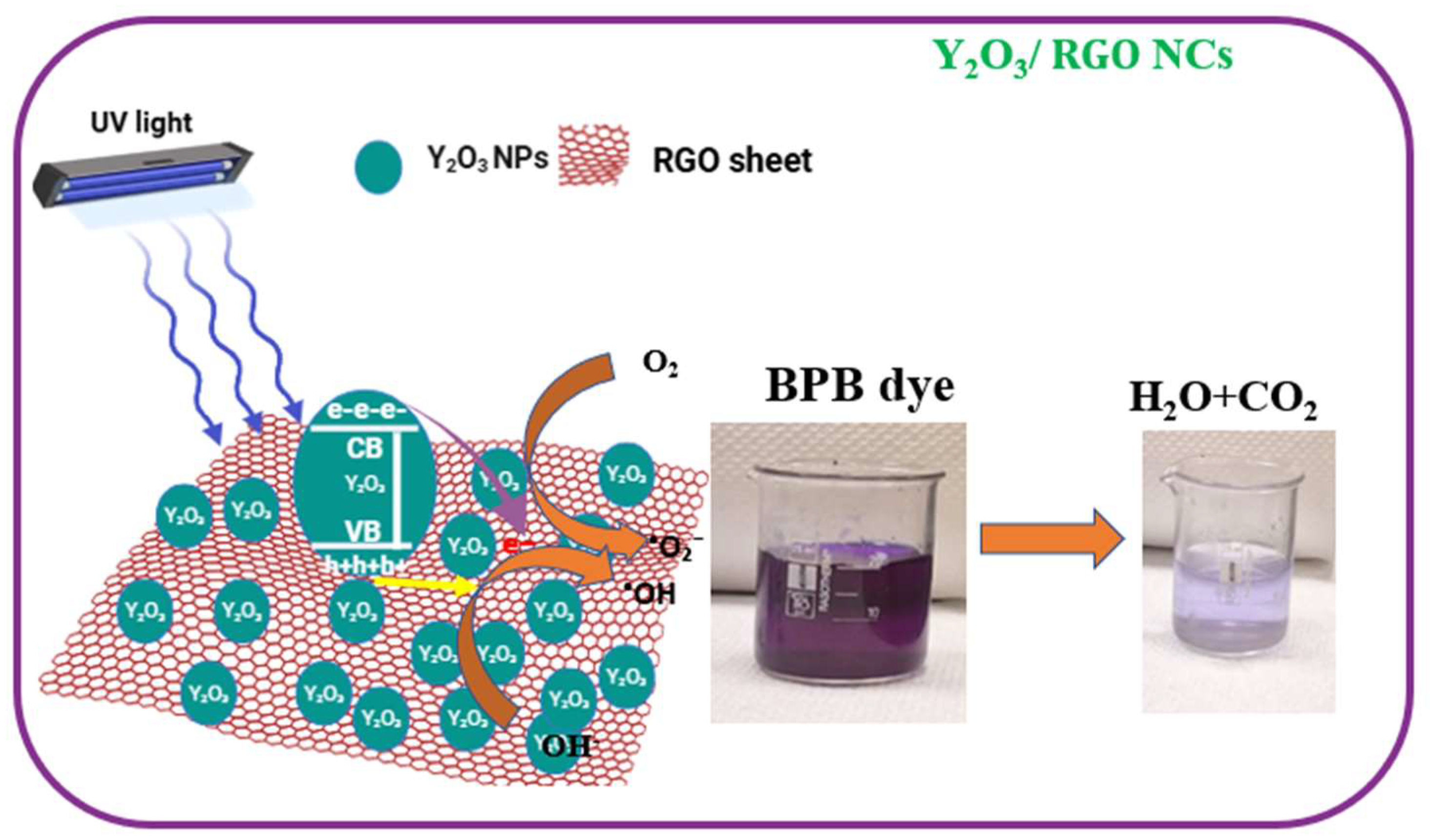
2.8.2. Reaction Mechanism
2.9. Anticancer Study
3. Methodology
3.1. Materials and Chemicals Used
3.2. Preparation of Reduced Graphene Oxide (RGO) and Carbon Nanotubes (CNTs)
3.3. Preparation of Y2O3/RGO NCs, and Y2O3/CNTs NCs and Characterization
3.4. Photocatalytic Degradation Experiment
3.5. MTT Experiment with Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dabhi, S.D.; Gahlot, S.; Mirza, S.; Pandya, M.; Rawal, R.; Kulshrestha, V.; Jha, P.K. Study of Anticancer Properties of Graphene Oxide–Metal Oxide Composite. Macromol. Symp. 2024, 413, 2300086. [Google Scholar] [CrossRef]
- Loh, T.A.J.; Hu, Y.; Pham, K.-C.; Tan, Z.; Chua, D.H.C. Multifunctional Metal Oxides and 2D Materials Utilizing Carbon Nanotubes as a Base Template for Clean Energy and Other Applications. In Proceedings of the 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), Sendai, Japan, 22–25 August 2016; pp. 899–900. [Google Scholar] [CrossRef]
- Bijesh, P.; Selvaraj, V.; Andal, V. A Review on Synthesis and Applications of Nano Metal Oxide/Porous Carbon Composite. Mater. Today Proc. 2021, 55, 212–219. [Google Scholar] [CrossRef]
- Ansari, A.S.; Azzahra, G.; Nugroho, F.G.; Mujtaba, M.M.; Ahmed, A.T.A. Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal. Catalysts 2025, 15, 134. [Google Scholar] [CrossRef]
- Silva, C.; Pastrana Martinez, L.; Morales-Torres, S. When Carbon Meets Light: Synergistic Effect between Carbon Nanomaterials and Metal Oxide Semiconductors for Photocatalytic Applications. Boletín Grupo Español Carbón 2016, 40, 28–35. [Google Scholar]
- Parangusan, K.; Subramanium, V.; Sundarabharathi, L.; Kannan, K.; Radhika, D. Influence of PH on Structural, Morphological, Optical, Photocatalytic, and Antibacterial Properties of Yttrium Oxide Nanoparticles via Co-Precipitation Method. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Myvizhi, G.; Krishna, S.K. Structural and Optical Studies of Hydrothermal Synthesis of Yttrium Oxide Nanoparticles: In Vitro Antioxidant Activity. Asian J. Chem. 2022, 34, 651–656. [Google Scholar] [CrossRef]
- Lagos, K.J.; Buzzá, H.H.; Bagnato, V.S.; Romero, M.P. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int. J. Mol. Sci. 2022, 23, 22. [Google Scholar] [CrossRef]
- Soren, S.; Chakroborty, S.; Pal, K. Enhanced in Tunning of Photochemical and Electrochemical Responses of Inorganic Metal Oxide Nanoparticles via RGO Frameworks (MO/RGO): A Comprehensive Review. Mater. Sci. Eng. B 2022, 278, 115632. [Google Scholar] [CrossRef]
- Rasheed, T.; Ahmad, N.; Nawaz, S.; Sher, F. Photocatalytic and Adsorptive Remediation of Hazardous Environmental Pollutants by Hybrid Nanocomposites. Case Stud. Chem. Environ. Eng. 2020, 2, 100037. [Google Scholar] [CrossRef]
- Gang, R.; Xu, L.; Xia, Y.; Zhang, L.; Wang, S.; Li, R. Facile One-Step Production of 2D/2D ZnO/RGO Nanocomposites under Microwave Irradiation for Photocatalytic Removal of Tetracycline. ACS Omega 2021, 6, 3831–3839. [Google Scholar] [CrossRef]
- Zhu, H.; Pan, Y.; Wang, Y.; Xiang, Y.; Han, R.; Huang, R. Photocatalytic Activity and Antibacterial Properties of ZnO/CNTs Composites. J. Nano Res. 2024, 82, 55–75. [Google Scholar] [CrossRef]
- Gangadhar, A.; Ramesh, A.M.; Krishnegowda, J.; Shivanna, S. Photo-Catalytic Dye Degradation of Methylene Blue by Using ZrO2/MWCNT Nanocomposites. Water Pract. Technol. 2021, 16, 1265–1276. [Google Scholar] [CrossRef]
- Mohamed, H.R.; Hemdan, S.H.A.; El-Sherif, A.A. Y2O3 NPs Induce Selective Cytotoxicity, Genomic Instability, Oxidative Stress and ROS Mediated Mitochondrial Apoptosis in Human Epidermoid Skin A-431 Cancer Cells. Sci. Rep. 2025, 15, 1543. [Google Scholar] [CrossRef]
- Mohamed, M.; Sedky, A.; Alshammari, A.S.; Khan, Z.R.; Bouzidi, M.; Gandouzi, M. Structural, Morphological, Optical, Photocatalytic Activity Investigations of Bi Doped ZnO Nanoparticles. Opt. Mater. 2023, 136, 113347. [Google Scholar] [CrossRef]
- Vats, R.; Bhukkal, C.; Goswami, B.; Rani, N.; Ahlawat, R. Structural and Dye Degradation Study of Cubic Nanocrystalline Yttria. AIP Conf. Proc. 2021, 2352, 040035. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Magdalane, C.M.; Kaviyarasu, K.; Kaviyarasu, K.; Vijaya, J.J.; Siddhardha, B.; Jeyaraj, B. Facile Synthesis of Heterostructured Cerium Oxide/Yttrium Oxide Nanocomposite in UV Light Induced Photocatalytic Degradation and Catalytic Reduction: Synergistic Effect of Antimicrobial Studies. J. Photochem. Photobiol. B 2017, 173, 23–34. [Google Scholar] [CrossRef]
- El-Sayed, F.; Ganesh, V.; Hussien, M.S.A.; AlAbdulaal, T.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S.; Shakir, M.; Bitla, Y. Facile Synthesis of Y2O3/CuO Nanocomposites for Photodegradation of Dyes/Mixed Dyes under UV and Visible Light Irradiation. J. Mater. Res. Technol. 2022, 19, 4867–4880. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Enhanced Anticancer Performance of Eco-Friendly-Prepared Mo-ZnO/RGO Nanocomposites: Role of Oxidative Stress and Apoptosis. ACS Omega 2022, 7, 7103–7115. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Ramadan, M.S.; Amin, M.A.; El-Mehasseb, I.M. Graphene Oxide/Cellulose Derivative Nanohybrid Membrane with Yttrium Oxide: Upgrading the Optical and Electrochemical Properties for Removing Organic Pollutants and Supercapacitors Implementations. J. Energy Storage 2021, 44, 103344. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Zhao, Y.; Wang, H.; He, C. Synthesis of Novel Yttrium-Doped Graphene Oxide Nanocomposite for Dye Removal. J. Mater. Chem. 2014, 2, 7897–7903. [Google Scholar] [CrossRef]
- Alaizeri, Z.A.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Aziz, A.A.; Ahamed, M. Photocatalytic Degradation of Methylene Blue and Anticancer Response of In2O3/RGO Nanocomposites Prepared by a Microwave-Assisted Hydrothermal Synthesis Process. Molecules 2023, 28, 5153. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, M.; Zhang, K.; Oh, W.-C. Visible Light Photoelectrocatalytic Properties of Novel Yttrium Treated Carbon Nanotube/Titania Composite Electrodes. Bull. Korean Chem. Soc. 2010, 31, 133–139. [Google Scholar] [CrossRef][Green Version]
- Bhuvaneswari, K.; Sreeja, B.S.; Radha, S.; Saranya, J.; Palanisamy, G.; Srinivasan, M.; Pazhanivel, T. Facile Assembly of Effective Carbon Quantum Dots and Multiwall Carbon Nanotubes Supported MnO2 Hybrid Nanoparticles for Enhanced Photocatalytic and Anticancer Activity. Inorg. Chem. Commun. 2022, 148, 110250. [Google Scholar] [CrossRef]
- Wagh, S.S.; Chougale, A.S.; Survase, A.A.; Patil, R.S.; Naik, N.; Naushad, M.; Pathan, H.M. Rapid Photocatalytic Dye Degradation, Enhanced Antibacterial and Antifungal Activities of Silver Stacked Zinc Oxide Garnished on Carbon Nanotubes. Sci. Rep. 2024, 14, 14045. [Google Scholar] [CrossRef]
- Phophayu, S.; Pimpang, P.; Wongrerkdee, S.; Sujinnapram, S.; Wongrerkdee, S. Modified Graphene Quantum Dots-Zinc Oxide Nanocomposites for Photocatalytic Degradation of Organic Dyes and Commercial Herbicide. J. Reinf. Plast. Compos. 2020, 39, 81–94. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Ahamed, M. One-Pot Synthesis of SnO2-RGO Nanocomposite for Enhanced Photocatalytic and Anticancer Activity. Polymers 2022, 14, 2036. [Google Scholar] [CrossRef]
- Alaizeri, Z.A.M.; Alahmari, A.S.; Alhadlaq, H.A.; Aziz, I.M.; Mohammed, O.B.; Ali, S.M. Enhancement of Physicochemical Properties and Biological Impact of Fe2O3/ZnO/ZrO2 Nanocomposites in Cancer Applications. J. Cryst. Growth 2025, 667, 128250. [Google Scholar] [CrossRef]
- Alaizeri, Z.A.M.; Alhadlaq, H.A.; Aldawood, S.; Ahamed, M. Chemical Synthesis, Characterization, and Anticancer Potential of CuO/ZrO2/TiO2/RGO Nanocomposites against Human Breast (MCF-7) Cancer Cells. RSC Adv. 2024, 14, 37697–37708. [Google Scholar] [CrossRef] [PubMed]
- Alaizeri, Z.A.M.; Alhadlaq, H.A.; Aldawood, S.; Javed Akhtar, M.; Ahamed, M. One-Step Preparation, Characterization, and Anticancer Potential of ZnFe2O4/RGO Nanocomposites. Saudi Pharm. J. 2023, 31, 101735. [Google Scholar] [CrossRef] [PubMed]
- Amir Faiz, M.S.; Che Azurahanim, C.A.; Raba’ah, S.A.; Ruzniza, M.Z. Low Cost and Green Approach in the Reduction of Graphene Oxide (GO) Using Palm Oil Leaves Extract for Potential in Industrial Applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Ngoma, M.M.; Mathaba, M.; Moothi, K. Effect of Carbon Nanotubes Loading and Pressure on the Performance of a Polyethersulfone (PES)/Carbon Nanotubes (CNT) Membrane. Sci. Rep. 2021, 11, 23805. [Google Scholar] [CrossRef] [PubMed]
- Mandal, G.; Choudhary, R.B. RGO–Y2O3 Intercalated PANI Matrix (PANI–RGO–Y2O3) Based Polymeric Nanohybrid Material as Electron Transport Layer for OLED Application. Res. Chem. Intermed. 2019, 45, 3755–3775. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathi, H.; Bhardwaj, S.; Garbiec, D.; Gupta, A.; Batra, U.; Sharma, J.D. Structural and Opto-Electrical Properties of Y2O3 Nanopowders Synthesized by Co-Precipitation Method. J. Mol. Struct. 2024, 1302, 137463. [Google Scholar] [CrossRef]
- Porosnicu, I.; Butnaru, C.M.; Tiseanu, I.; Stancu, E.; Munteanu, C.V.A.; Bita, B.I.; Duliu, O.G.; Sima, F. Y2O3 Nanoparticles and X-Ray Radiation-Induced Effects in Melanoma Cells. Molecules 2021, 26, 3403. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/RGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route. Nanomaterials 2019, 9, 811. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A Concise Review on GO, RGO and Metal Oxide/RGO Composites: Fabrication and Their Supercapacitor and Catalytic Applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Alaizeri, Z.A.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Ahamed, M. Bi2O3-Doped WO3 Nanoparticles Decorated on RGO Sheets: Simple Synthesis, Characterization, Photocatalytic Performance, and Selective Cytotoxicity toward Human Cancer Cells. ACS Omega 2023, 8, 25020–25033. [Google Scholar] [CrossRef] [PubMed]
- Pirsaheb, M.; Hossaini, H.; Fatahi, N.; Jafari, Z.; Jafari, F.; Jafari Motlagh, R. Photocatalytic Removal of Organophosphorus Pesticide by the WO3-Fe3O4/RGO Photocatalyst under Visible Light. Environ. Sci. Pollut. Res. Int. 2024, 31, 2555–2568. [Google Scholar] [CrossRef]
- Song, J.B.; Kim, J.T.; Oh, S.G.; Yun, J.Y. Contamination Particles and Plasma Etching Behavior of Atmospheric Plasma Sprayed Y2O3 and YF3 Coatings under NF3 Plasma. Coatings 2019, 9, 102. [Google Scholar] [CrossRef]
- Yao, Q.-L.; He, M.; Kong, Y.-R.; Gui, T.; Lu, Z.-H. Y2O3-Functionalized Graphene-Immobilized Ni–Pt Nanoparticles for Enhanced Hydrous Hydrazine and Hydrazine Borane Dehydrogenation. Rare Met. 2023, 42, 3410–3419. [Google Scholar] [CrossRef]
- Mariscal-Becerra, L.; Vázquez-Arreguín, R.; Balderas, U.; Carmona-Téllez, S.; Murrieta Sánchez, H.; Falcony, C. Luminescent Characteristics of Layered Yttrium Oxide Nano-Phosphors Doped with Europium. J. Appl. Phys. 2017, 121, 125111. [Google Scholar] [CrossRef]
- Saeed, M.; Marwani, H.M.; Shalauddin, M.; Alfaifi, S.Y.; Akhter, S.; Alzahrani, K.A.; Jefrey Basirun, W.; Rahman, M.M. Sensitive Detection of Unsafe Nitrite Chemical Based on GO@Fe2O3/Y2O3 Nanocomposite by Electrochemical Approach for Environmental Assessment. J. Ind. Eng. Chem. 2025, 144, 552–564. [Google Scholar] [CrossRef]
- Saravanan, T.; Anandan, P.; Azhagurajan, M.; Arivanandhan, M.; Pazhanivel, K.; Hayakawa, Y.; Jayavel, R. Synthesis and Characterization of Y2O3 -Reduced Graphene Oxide Nanocomposites for Photocatalytic Applications. Mater. Res. Express 2016, 3, 075502. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Kukharenko, A.I.; Maksimov, R.N.; Finkelstein, L.D.; Cholakh, S.O.; Osipov, V.V.; Kurmaev, E.Z. Optical Transparency and Local Electronic Structure of Yb-Doped Y2O3 Ceramics with Tetravalent Additives. Symmetry 2019, 11, 243. [Google Scholar] [CrossRef]
- Kandasamy, K.; Kumpati, P. In Vitro Antioxidant and Antibacterial Potential of Biosynthesized Yttrium Oxide Nanoparticles Using Floral Extract of Illicium Verum. J. Appl. Biol. Biotechnol. 2023, 11, 112–118. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.-G. Hydrothermally Reduced Graphene Oxide as a Supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Alanazi, A.A.; Aldawsari, A.M.; Alotaibi, M.A.; Aladeemy, S.A.; Abdulaziz, F.; Aljohani, Y.S. Multifunctional Nitrogen Doped Carbon Nanotube-Encapsulated Defect-Rich Cobalt-Iron Oxide Catalyst for Efficient Water Electrolysis and and Dye Removal from Water. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mishra, K.; Thombal, R.S.; Kaliraj, K.; Lee, Y.R. Sonochemical Green Synthesis of Yttrium Oxide (Y2O3) Nanoparticles as a Novel Heterogeneous Catalyst for the Construction of Biologically Interesting 1,3-Thiazolidin-4-Ones. Catal. Lett. 2017, 147, 2630–2639. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Zhen, F.; Wang, L.; Zhang, Q.; Sun, R.; Selim, F.A.; Wong, C.; Chen, H. High Sinterability Nano-Y2O3 Powders Prepared via Decomposition of Hydroxyl-Carbonate Precursors for Transparent Ceramics. J. Mater. Sci. 2017, 52, 8556–8567. [Google Scholar] [CrossRef]
- Shashikumara, J.K.; Kalaburgi, B.; Swamy, B.E.K.; Nagabhushana, H.; Sharma, S.C.; Lalitha, P. Effect of RGO-Y2O3 and RGO-Y2O3:Cr3+ Nanocomposite Sensor for Dopamine. Sci. Rep. 2021, 11, 9372. [Google Scholar] [CrossRef]
- Iqbal, T.; Khan, M.A.R.; Tahir, H.M. Green Synthesis of Yttrium Oxide (Y2O3) Nanoparticles by Agathosma Betulina Leaf Extract: Structural, Optical and Antimicrobial Properties. Int. J. Mod. Phys. B 2024, 38, 2450278. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Fayad, A.M. Optical Band Gap and Structural Study on GeO2- and Y2O3-Doped Barium Aluminoborate Glasses. Appl. Phys. A Mater. Sci. Process 2016, 122, 931. [Google Scholar] [CrossRef]
- Johnson, A.; Welsher, K.D. Single-Nanoparticle Electrophoretic Mobility Determination and Trapping Using Active-Feedback 3D Tracking. bioRxiv 2024. [Google Scholar] [CrossRef]
- Narayan, H.; Alemu, H.; Macheli, L.; Thakurdesai, M.; Rao, T.K.G. Synthesis and Characterization of Y3+-Doped TiO2 Nanocomposites for Photocatalytic Applications. Nanotechnology 2009, 20, 255601. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, S.; Thangamani, C.; Thiyagarajulu, N.; Thangaraj, K.; Paramasivam, D.; Thangavel, S.; Kannan, K.; Parvathiraja, C.; Vibala, B.V.; Velmurugan, P.; et al. Facile Synthesis of ZnO-Y2O3 Nanocomposite for Photocatalytic and Biological Applications. Catal. Commun. 2023, 184, 106786. [Google Scholar] [CrossRef]
- Ali Baig, A.B.; Rathinam, V.; Palaninathan, J. Photodegradation Activity of Yttrium-Doped SnO2 Nanoparticles against Methylene Blue Dye and Antibacterial Effects. Appl. Water Sci. 2020, 10, 76. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W.; Fujishima, A.; Terashima, C. Enhanced Photocatalytic Degradation Activity Using the V2O5/RGO Composite. Nanomaterials 2023, 13, 338. [Google Scholar] [CrossRef]
- Aragaw, B.A.; Dagnaw, A. Copper/Reduced Graphene Oxide Nanocomposite for High Performance Photocatalytic Methylene Blue Dye Degradation. Ethiop. J. Sci. Technol. 2020, 12, 125–137. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Ahmed, E.; Akhtar, M.S.; Khalid, N.R. Facile and Inexpensive Synthesis of Ag Doped ZnO/CNTs Composite: Study on the Efficient Photocatalytic Activity and Photocatalytic Mechanism. J. Mol. Liq. 2020, 311, 113326. [Google Scholar] [CrossRef]
- Alomayrah, N.; Ikram, M.; Zulfiqar, S.; Alomairy, S.; Al-Buriahi, M.S.; Shakir, I.; Warsi, M.F.; Cochran, E.W. Fabrication of a Highly Efficient CuO/ZnCo2O4/CNTs Ternary Composite for Photocatalytic Degradation of Hazardous Pollutants. RSC Adv. 2024, 14, 24874–24897. [Google Scholar] [CrossRef]
- Govindasamy, R.; Govindarasu, M.; Alharthi, S.S.; Mani, P.; Bernaurdshaw, N.; Gomathi, T.; Ansari, M.A.; Alomary, M.N.; Atwah, B.; Malik, M.S.; et al. Sustainable Green Synthesis of Yttrium Oxide (Y2O3) Nanoparticles Using Lantana Camara Leaf Extracts: Physicochemical Characterization, Photocatalytic Degradation, Antibacterial, and Anticancer Potency. Nanomaterials 2022, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alrokayan, S.A.; Ramamoorthy, M.M.; Alaizeri, Z.A.M. High Surface Reactivity and Biocompatibility of Y2O3 NPs in Human MCF-7 Epithelial and HT-1080 Fibro Blast Cells. Molecules 2020, 25, 1137. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jin, Y.; Jia, G.; Suo, X.; Liu, H.; Liu, D.; Yang, X.; Ge, K.; Liang, X.; Wang, S.; et al. Y2O3 Nanoparticles Caused Bone Tissue Damage by Breaking the Intracellular Phosphate Balance in Bone Marrow Stromal Cells. ACS Nano 2018, 13, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Mao, L.; Bao, T.; Wen, W.; Wang, S.; Gomathi, T.; Gnanasundaram, N.; Rebezov, M.; Shariati, M.A.; Chung, I.-M.; et al. Yttrium Oxide Nanoparticle Synthesis: An Overview of Methods of Preparation and Biomedical Applications. Appl. Sci. 2021, 11, 2172. [Google Scholar] [CrossRef]






| Samples | D (nm) | δ (Lines/m2) × 10−3 | ε × 10−3 | (J/m2) |
|---|---|---|---|---|
| Pure Y2O3 NPs | 10.12 ± 1.8 | 3.21 ± 0.6 | 9.74 ± 0.2 | 4.52 ± 1.6 |
| Y2O3/RGO NCs | 9.10 ± 1.3 | 4.12 ± 0.3 | 12.10 ± 0.7 | 7.01 ± 1.1 |
| Y2O3/CNTs NCs | 11.11 ± 2.3 | 1.27 ± 0.1 | 8.30 ± 0.5 | 2.16 ± 0.8 |
| Samples | Zeta Potential (mV ± SD) | Electrophonic Mobility (µmcm/Vs ± SD) |
|---|---|---|
| Pure Y2O3 NPs | −19.6 ± 3.79 | −1.54 ± 0.68 |
| Y2O3/RGO NCs | −24.5 ± 5.16 | −1.92 ± 1.02 |
| Y2O3/CNTs NCs | −22.1 ± 4.53 | −1.75 ± 0.97 |
| Sample Type | Average Size (nm) | Time (min) | Light Source | D (%) | Ref. |
|---|---|---|---|---|---|
| Y2O3 RGO NCs | 9.10 | 120 | UV light | 87.83 | This work |
| Y2O3/TiO2 NCs | 73.0 | 180 | UV light | 86.00 | [53] |
| ZnO/Y2O3 NCs | 15.3 | 120 | UV light | 96.00 | [51] |
| Y/SnO2 NPs | 27.1 | 180 | Visible light | 92.34 | [46] |
| V2O5/RGO NCs | .---- | 100 | Visible light | 63.00 | [54] |
| Cu/GO NCs | 12.2 | 50 | Visible light | 94.00 | [55] |
| Ag/Zn/CNTs NCs | 37.0 | 120 | Visible light | 100.00 | [56] |
| CuO/ZnCo2O4/CNTs NCs | 9.8 | 90 | Sunlight | 87.00 | [57] |
| Sample | In Vitro Cytotoxicity (IC50) Values for 24 h | ||
|---|---|---|---|
| IC50 | Log IC50 | R2 | |
| Y2O3 NPs | 158.12 | 2.19 | 0.9671 |
| Y2O3/RGO NCs | 45.70 | 1.66 | 0.9975 |
| Y2O3/CNTs NCs | 73.45 | 1.86 | 0.9764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaizeri, Z.M.; Ali, S.M.; Alhadlaq, H.A. Enhancement of Photocatalytic and Anticancer Properties in Y2O3 Nanocomposites Embedded in Reduced Graphene Oxide and Carbon Nanotubes. Catalysts 2025, 15, 960. https://doi.org/10.3390/catal15100960
Alaizeri ZM, Ali SM, Alhadlaq HA. Enhancement of Photocatalytic and Anticancer Properties in Y2O3 Nanocomposites Embedded in Reduced Graphene Oxide and Carbon Nanotubes. Catalysts. 2025; 15(10):960. https://doi.org/10.3390/catal15100960
Chicago/Turabian StyleAlaizeri, ZabnAllah M., Syed Mansoor Ali, and Hisham A. Alhadlaq. 2025. "Enhancement of Photocatalytic and Anticancer Properties in Y2O3 Nanocomposites Embedded in Reduced Graphene Oxide and Carbon Nanotubes" Catalysts 15, no. 10: 960. https://doi.org/10.3390/catal15100960
APA StyleAlaizeri, Z. M., Ali, S. M., & Alhadlaq, H. A. (2025). Enhancement of Photocatalytic and Anticancer Properties in Y2O3 Nanocomposites Embedded in Reduced Graphene Oxide and Carbon Nanotubes. Catalysts, 15(10), 960. https://doi.org/10.3390/catal15100960







