Metal-Organic Frameworks and Covalent Organic Frameworks for CO2 Electrocatalytic Reduction: Research Progress and Challenges
Abstract
1. Introduction
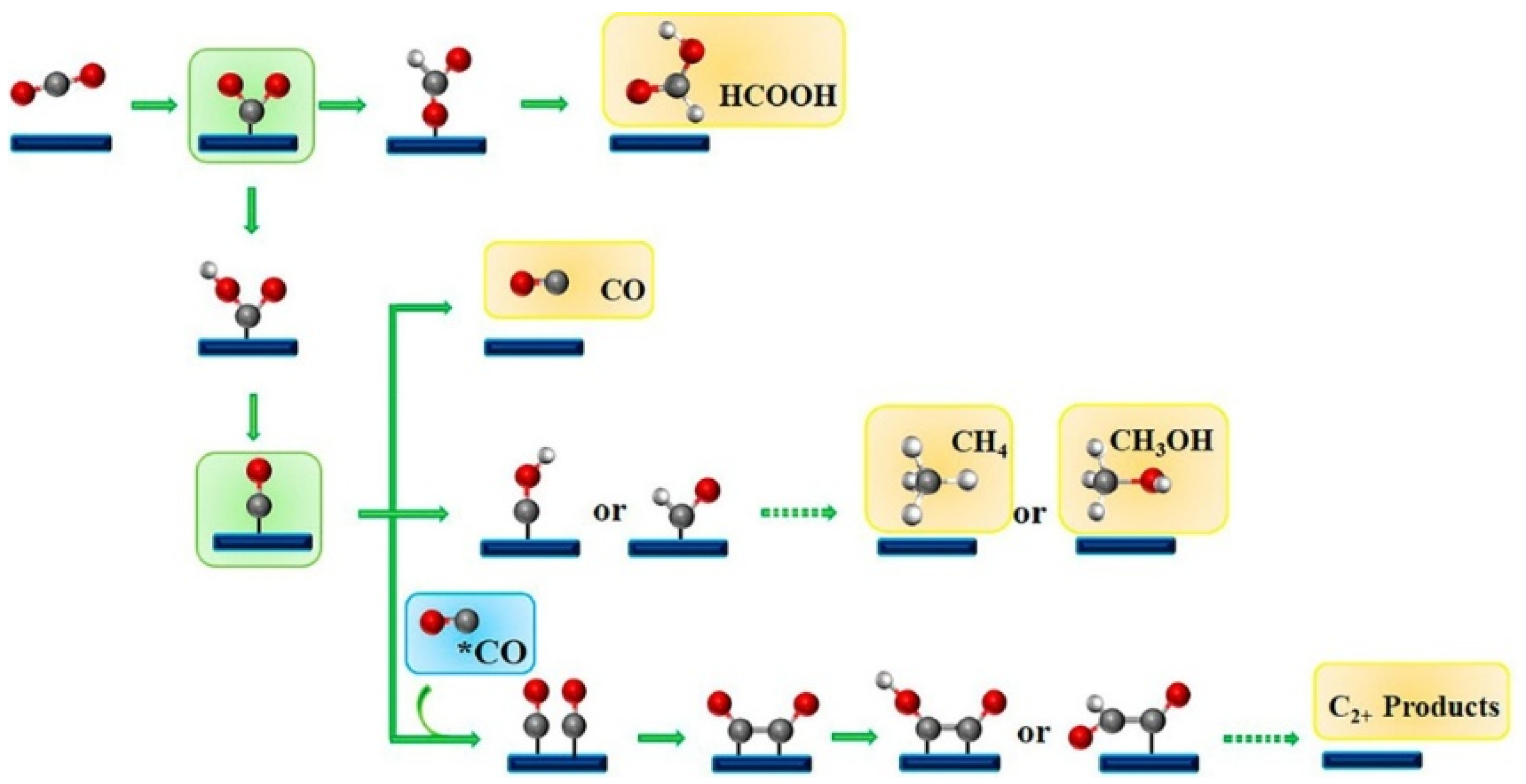
2. The Mechanism of Electrocatalytic Reduction of CO2
- (1).
- Initial carbon coordination: Adsorption and activation of CO2
- (2).
- Key Transformation: Protonation drives oxygen-terminal binding (formation of oxygen coordination intermediates)
- (3).
- Subsequent evolution: Oxygen coordination intermediates determine product branching
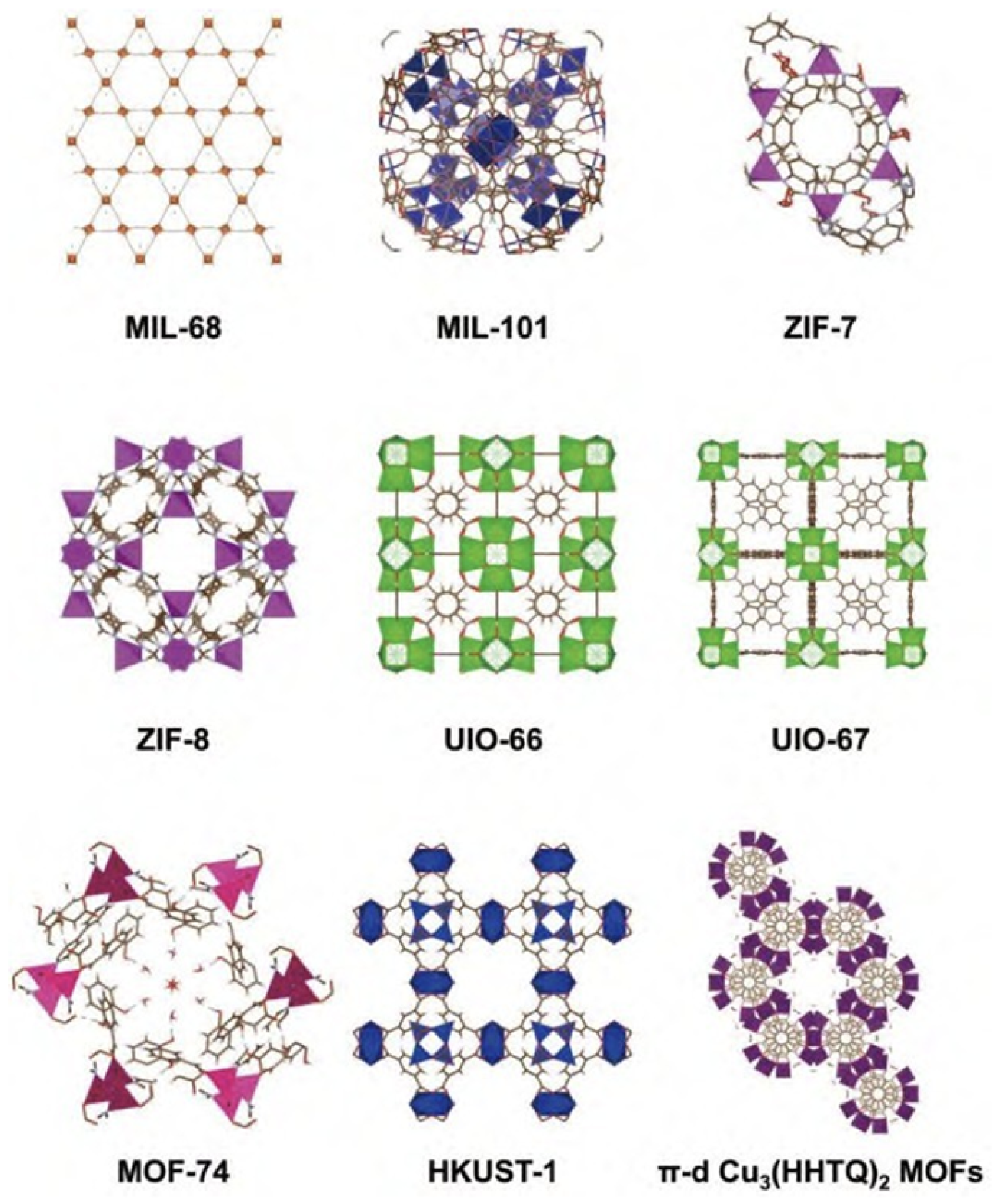
3. The Application of MOFs in CO2 Electrocatalysis
3.1. Improve Electrical Conductivity
3.2. Improve Stability
- (1)
- Selection of Stable Metal Nodes and Intrinsically Robust MOF Platforms
- (2)
- Ligand Design for Mechanical and Chemical Stability
- (3)
- Compositional Modification and Structural Engineering
- a.
- Doping and Ion Exchange
- b.
- Surface Modification
- c.
- Core-Shell Architectures
3.3. Difficulties in Selectively Regulating Products
3.3.1. Pulse Potential Strategy
3.3.2. Bimetallic Catalyst
3.3.3. Series Catalyst
3.3.4. Structural Innovation
- Water as solvent yields Cu-MMT nanoribbons that primarily expose (100) facets, achieving 55.22% FE for CH4.
- Isopropanol as solvent produces Cu-MMT cross-linked nanosheets with exposed (001) faces, exhibiting 73.75% FE for C2 products (especially ethylene).
- Ce doping: Chen et al. [50] doped Cu lattices with Ce (larger ionic radius) to form atomically doped Ce-CuOx catalysts, which boost eCO2RR performance and CH4 selectivity. Within −1.4 to −1.75 V vs. RHE, FE_CH4 remains above 62% (peaking at 67.4%), with a partial current density of 293 mA/cm2 at −1.6 V vs. RHE.
- Co doping: Sun et al. [51] prepared atomically dispersed Co-Cu alloys via electrochemical reconstruction of Co-doped Cu-MOFs. This catalyst exhibits outstanding eCO2RR activity and CH4 selectivity: during CH4 conversion, it achieves 60 ± 1% FE and 303 ± 5 mA/cm2 partial current density, outperforming most reported Cu-based catalysts.
4. Application of COFs in CO2 Electrocatalysis
4.1. Metal Center Design
4.1.1. Single-Atom Active Sites
4.1.2. Dual-/Multi-Metal Synergy Sites
4.1.3. Coordinate Environment and Metal Valence Control
4.2. Framework Optimization and CO2 Adsorption Activation
4.2.1. π-Conjugated Frameworks for Enhanced Electron Transport
4.2.2. Pore Size Control and Molecular Sieving Effect
4.2.3. Functional Group Incorporation to Enhance Adsorption and Activation
4.3. Construction of Efficient Charge Transport Networks
4.3.1. Compositing with Conductive Substrates to Build 3D Electron Pathways
4.3.2. In Situ Carbonization to Form Conductive Frameworks
4.3.3. Heterogeneous Atom Doping Regulates Electronic Structure
4.4. Multifunctional Synergy and Heterostructure Design
4.4.1. Heterojunction Catalyst Design
4.4.2. Photoelectrochemical Synergistic CO2 Reduction Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Laurenceson, J.; Liu, Y.; Zhou, D.Y. The potential impact of EU’s carbon border adjustment mechanism (CBAM): An Australia-China relationship perspective. J. Chin. Econ. Foreign Trade Stud. 2024, 17, 75–91. [Google Scholar]
- Zhang, Z.; Hu, G.; Mu, X.; Kong, L. From low carbon to carbon neutrality: A bibliometric analysis of the status, evolution and development trend. J. Environ. Manag. 2022, 322, 116087. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Xu, P.; Chen, L. CO2 electrolysis: Advances and challenges in electrocatalyst engineering and reactor design. Mater. Rep. Energy 2023, 3, 82–102. [Google Scholar] [CrossRef]
- Li, C.; Ji, Y.; Wang, Y.; Liu, C.; Chen, Z.; Tang, J.; Hong, Y.; Li, X.; Zheng, T.; Jiang, Q.; et al. Applications of Metal–Organic Frameworks and Their Derivatives in Electrochemical CO2 Reduction. Nano-Micro Lett. 2023, 15, 113. [Google Scholar] [CrossRef]
- Kuai, Y.; Wang, Y. Innovative COF@MXene composites for high-performance energy applications. Carbon Neutrality 2024, 3, 10. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X. Carbon-Based Materials for Electrochemical Reduction of CO2 to C2+ Oxygenates: Recent Progress and Remaining Challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Chang, B.; Pang, H.; Raziq, F.; Wang, S.; Huang, K.-W.; Ye, J.; Zhang, H. Electrochemical reduction of carbon dioxide to multicarbon (C2+) products: Challenges and perspectives. Energy Environ. Sci. 2023, 16, 46. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Sarkar, P.; Chowdhury, I.H.; Das, S.; Islam, S.M. Recent trends in covalent organic frameworks (COFs) for carbon dioxide reduction. Mater. Adv. 2022, 3, 8063–8080. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent advances on electrocatalytic CO2 reduction to resources: Target products, reaction pathways and typical catalysts. Chem. Eng. J. 2023, 453, 139663. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Zakharov, V. Triazine derivatives as metal-free electrocatalysts:do three nitrogen atoms mimic a metal? Sustain. Energy Fuels 2025, 9, 1464–1479. [Google Scholar] [CrossRef]
- Li, C.; Yan, H.; Yang, H. Recent advances and future perspectives of metal-organic frameworks as efficient electrocatalysts for CO2 reduction. Sci. China Mater. 2025, 68, 21–38. [Google Scholar] [CrossRef]
- Gao, H.; Yang, T.; Nie, W.; Gao, Y.; Wang, Z.; Dong, A. Recent Advances in Cu-Based Metal–Organic Framework Electrocatalysts for CO2 Reduction Reactions. Catalysts 2025, 15, 328. [Google Scholar] [CrossRef]
- Xin, Z.; Yuan, Z.; Liu, J.; Wang, X.; Shen, K.; Chen, Y.; Lan, Y.-Q. Cu cluster embedded porous nanofibers for high-performance CO2 electroreduction. Chin. Chem. Lett. 2023, 34, 107456. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Wang, K.; Yu, F.; Xie, J.; Zhang, Q. Multi-thiol-supported dicarboxylate based metal–organic framework with excellent performance for lithium-ion battery. Chem. Eng. J. 2022, 431, 133234. [Google Scholar] [CrossRef]
- Wang, M.; Dong, R.; Feng, X. Two-dimensional conjugated metal–organic frameworks (2D c-MOFs): Chemistry and function for MOFtronics. Chem. Soc. Rev. 2021, 50, 2764–2793. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, C.; Gao, G. Recent progress of advanced conductive metal–organic frameworks: Precise synthesis, electrochemical energy storage applications, and future challenges. Small 2022, 18, 2203140. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, S.-L.; Zhong, Y.-H.; Hu, J.; Chung, L.-H.; He, J. Conductive MOFs based on thiol-functionalized linkers: Challenges, opportunities, and recent advances. Coord. Chem. Rev. 2022, 450, 214235. [Google Scholar] [CrossRef]
- Meng, H.; Han, Y.; Zhou, C.; Jiang, Q.; Shi, X.; Zhan, C.; Zhang, R. Conductive Metal–Organic Frameworks: Design, Synthesis, and Applications. Small Methods 2020, 4, 2000396. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically conductive metal–organic frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef]
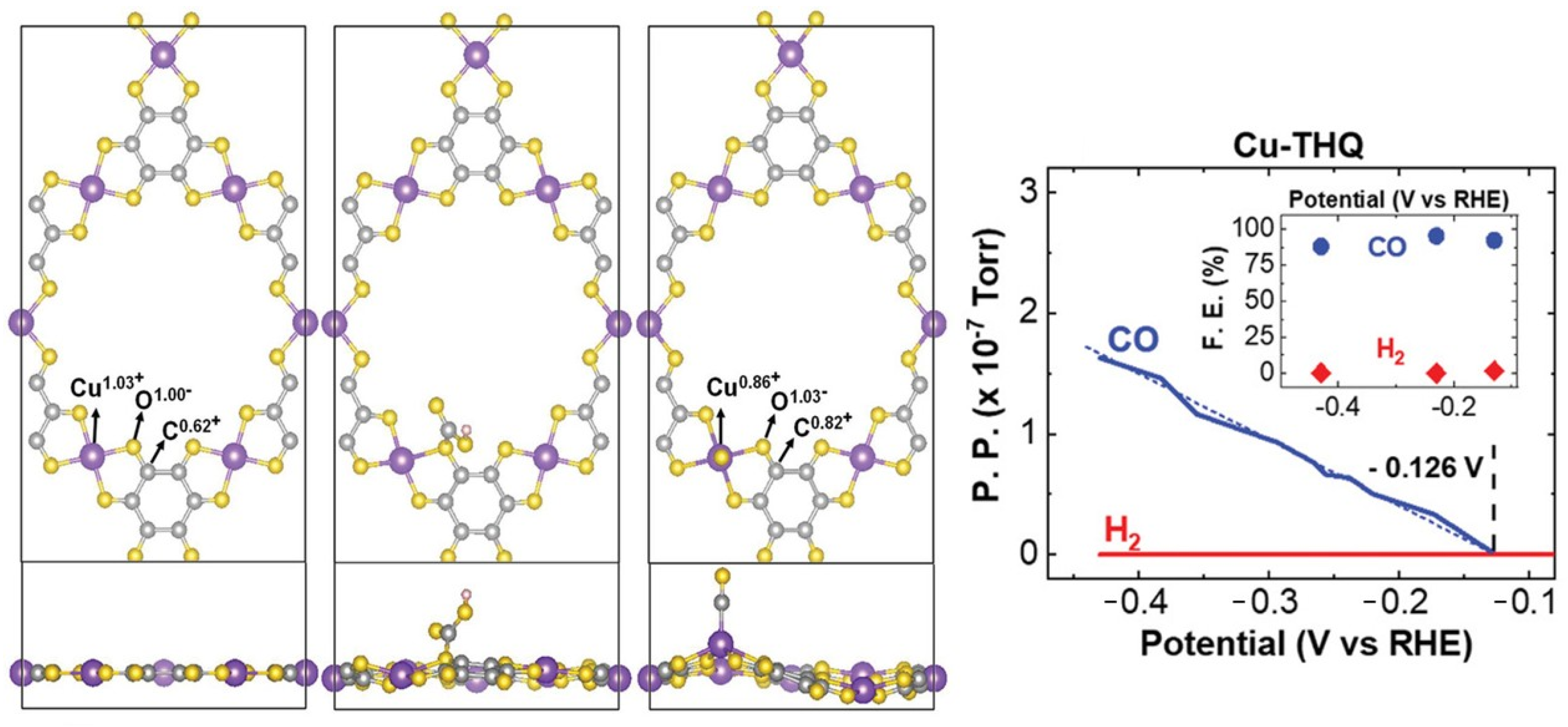
- Majidi, L.; Ahmadiparidari, A.; Shan, N.; Misal, S.N.; Kumar, K.; Huang, Z.; Rastegar, S.; Hemmat, Z.; Zou, X.; Zapol, P.; et al. 2D copper tetrahydroxyquinone conductive metal–organic framework for selective CO2 electrocatalysis at low overpotentials. Adv. Mater. 2021, 33, 2004393. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gong, Y.; Zhu, Y.; Li, J.; Li, L.; Shi, Y.; Hou, M.; Gao, X.J.; Zhang, Z.; Hu, W. Large π-conjugated indium-based metal–organic frameworks for high-performance electrochemical conversion of CO2. Nano Res. 2023, 16, 8743–8750. [Google Scholar] [CrossRef]
- Yang, D.; Zuo, S.; Yang, H.; Zhou, Y.; Wang, X. Freestanding millimeter-scale porphyrin-based monoatomic layers with 0.28 nm thickness for CO2 electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 18954–18959. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-X.; Hou, S.-Z.; Zhang, X.-D.; Xu, M.; Yang, H.-F.; Cao, P.-S.; Gu, Z.-Y. Cathodized copper porphyrin metal–organic framework nanosheets for selective formate and acetate production from CO2 electroreduction. Chem. Sci. 2019, 10, 2199–2205. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X. 2D π-conjugated metal–organic frameworks for CO2 electroreduction. SmartMat 2022, 3, 54–67. [Google Scholar] [CrossRef]
- Kadhom, M.; Al-Furaiji, M.; Salih, S.; Al-Obaidi, M.A.; Abdullah, G.H.; Albayati, N. A review on UiO-66 applications in membrane-based water treatment processes. J. Water Process Eng. 2023, 51, 103402. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, J.; Guo, L.; Xie, Z.; Wang, K.; Wang, Y.; Hao, F.; Ma, Y.; Zhou, J.; Lu, P.; et al. In situ phase transformation-enabled metal–organic frameworks for efficient CO2 electroreduction to multicarbon products in strong acidic media. ACS Nano 2024, 18, 33602–33613. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Ding, P.; Huang, J.; Dierolf, M.; Kelly, S.D.; Qiu, X.; Chen, Y.; Hussain, M.Z.; Li, W.; et al. Engineering a Cu-Pd paddle-wheel metal–organic framework for selective CO2 electroreduction. Angew. Chem. Int. Ed. 2024, 63, e202414600. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, W.; Wang, Y.; Shen, F.; Lan, Y. Tailoring the hydrophobic interface of core–shell HKUST-1@Cu2O nanocomposites for efficiently selective CO2 electroreduction. Small 2024, 20, e2307467. [Google Scholar] [CrossRef]
- Ma, M.; Xiong, L.; Dong, Y.; Bai, Q.; Hua, W.; Zheng, Z.; Lyu, F.; Lian, Y.; Wei, Z.; Yuan, H.; et al. Metalloporphyrin frameworks to encapsulate copper oxides for boosting ethylene production in neutral electrolyte. Adv. Funct. Mater. 2024, 34, 2315667. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Liu, J.; Bai, J.; Li, Y.; Chen, H.; Zhou, L.; Gao, J.; Jiang, Y. Hierarchically porous metal–organic framework immobilized formate dehydrogenase for enzyme electrocatalytic CO2 reduction. Chem. Eng. J. 2022, 450, 138164. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Naeem, M.S.; Shanker, G.S.; Ghatak, A.; Kottaichamy, A.R.; Shimoni, R.; Avram, L.; Liberman, I.; Balilty, R.; Ifraemov, R.; et al. Local CO2 reservoir layer promotes rapid and selective electrochemical CO2 reduction. Nat. Commun. 2024, 15, 3397. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, W.; Jiang, W.; Yang, J.; Zhu, J.; Wang, L.; Ou, H.; Zhuang, Z.; Chen, M.; Sun, X.; et al. MOF encapsulating N-heterocyclic carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis. Angew. Chem. 2022, 134, e202114450. [Google Scholar] [CrossRef]
- Huang, J.-Y. Pulsed strategy steers the structural evolution of Cu metal–organic framework for CO2 reduction to methane. Chem. Eur. J. 2025, 31, e202500744. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, C.; Hong, S.H.; Jang, H.Y.; Back, S.; Seo, M.; Lee, M.; Min, H.; Choi, Y.; Jang, Y.J.; et al. Transition metal ion doping on ZIF-8 enhances the electrochemical CO2 reduction reaction. Adv. Mater. 2023, 35, e2208224. [Google Scholar] [CrossRef]
- Zou, Y.-H.; Wang, X.; Ning, F.; Yi, J.; Liu, Y. Implanting MWCNTs in BiCu-MOFs to enhance electrocatalytic CO2 reduction to formate. Sep. Purif. Technol. 2023, 317, 123456. [Google Scholar] [CrossRef]
- Huang, H.; Yue, K.; Liu, C.; Zhan, K.; Dong, H.; Yan, Y. CuO (111) microcrystalline evoked indium–organic framework for efficient electroreduction of CO2 to formate. Small 2024, 20, 2400441. [Google Scholar] [CrossRef]
- Xue, H.; Zhu, H.; Huang, J.; Liao, P.; Chen, X. Ultrathin two-dimensional triptycene-based metal–organic framework for highly selective CO2 electroreduction to CO. Chin. Chem. Lett. 2023, 34, 107134. [Google Scholar] [CrossRef]
- Su, W.; Guo, W.; Fan, Y. CuAg bimetallic catalysts derived from an Ag-anchored Cu-based metal–organic framework for CO2 electroreduction to ethanol. Chem. Eng. J. 2023, 477, 147204. [Google Scholar] [CrossRef]
- He, Q.; Li, H.; Hu, Z.; Lei, L.; Wang, D.; Li, T. Highly selective CO2 electroreduction to C2H4 using a dual-sites Cu(II) porphyrin framework coupled with Cu2O nanoparticles via a synergetic-tandem strategy. Angew. Chem. Int. Ed. 2024, 63, e202407090. [Google Scholar] [CrossRef]
- Shao, P.; Wan, Y.; Yi, L.; Chen, S.; Zhang, H.; Zhang, J. Enhancing electroreduction CO2 to hydrocarbons via tandem electrocatalysis by incorporation Cu NPs in boron imidazolate frameworks. Small 2024, 20, 2305199. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Yu, C.; Tan, X.; Li, W.; Zhang, Y.; Qiu, J. A tandem catalyst with high CO2 capture capability to achieve a promoted CO2-to-CH4 electrochemical conversion. Chem. Eng. J. 2023, 470, 144083. [Google Scholar] [CrossRef]
- Sun, B.; Hu, H.; Liu, H.; Guan, J.; Song, K.; Shi, C.; Cheng, H. Highly-exposed copper and ZIF-8 interface enables synthesis of hydrocarbons by electrocatalytic reduction of CO2. J. Colloid Interface Sci. 2024, 661, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, P.; Sun, W.-Y. Single-Site Metal-Organic Framework and Copper Foil Tandem Catalyst for Highly Selective CO2 Electroreduction to C2H4. Small 2023, 19, e2206070. [Google Scholar] [CrossRef]
- Lv, J.; Li, W.; Li, J.; Zhu, Z.; Dong, A.; Lv, H.; Li, P.; Wang, B. A Triptycene-Based 2D MOF with Vertically Extended Structure for Improving the Electrocatalytic Performance of CO2 to Methane. Angew. Chem. Int. Ed. 2023, 62, e202217958. [Google Scholar] [CrossRef]
- Zheng, K.; Hu, D.-Y.; Zhang, X.-W.; Xiao, X.-X.; Liang, Z.-J.; Wu, J.-X.; Lin, D.-Y.; Zhuo, L.-L.; Yi, H.; Gong, L.; et al. Bending two-dimensional Cu(I)-based coordination networks to inverse electrocatalytic HER/CO2RR selectivity. J. Mater. Chem. A 2024, 12, 16396–16402. [Google Scholar] [CrossRef]
- Lu, P.; Lv, J.; Chen, Y.; Ma, Y.; Wang, Y.; Lyu, W.; Yu, J.; Zhou, J.; Yin, J.; Xiong, Y.; et al. Steering the Selectivity of Carbon Dioxide Electroreduction from Single-Carbon to Multicarbon Products on Metal-Organic Frameworks via Facet Engineering. Nano Lett. 2024, 24, 1553–1562. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Song, Y.; Geng, S.; Peng, Z.; Yu, J.; Liu, F.; Wang, Y.; Xi, S.; Zhang, Z.; et al. Simultaneous Defect and Size Control of Metal-Organic Framework Nanostructures for Highly Efficient Carbon Dioxide Electroreduction to Multicarbon Products. ACS Mater. Lett. 2023, 5, 2121–2130. [Google Scholar] [CrossRef]
- Chen, X.; Xu, A.; Wei, D.; Huang, F.; Ma, J.; He, H.; Xu, J. Atomic cerium-doped CuOx catalysts for efficient electrocatalytic CO2 reduction to CH4. Chin. Chem. Lett. 2025, 36, 110175. [Google Scholar] [CrossRef]
- Sun, H.; Lin, L.; Hua, W.; Xie, X.; Mu, Q.; Feng, K.; Zhong, J.; Lyu, F.; Deng, Z.; Peng, Y. Atomically dispersed Co-Cu alloy reconstructed from metal-organic framework to promote electrochemical CO2 methanation. Nano Res. 2023, 16, 3680–3686. [Google Scholar] [CrossRef]
- Jing, Z.; Su, W.; Fan, Y. Increasing electrochemical carbon dioxide reduction to methane via a novel copper-based conductive metal organic framework. J. Colloid Interface Sci. 2025, 678, 251–260. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Z.; Liu, Y.; Dai, L.; Liu, R.; Sun, C.; Liu, W.; Feng, X.; Yang, W.; Wang, B. Asymmetric Cu-N1O3 Sites Coupling Atop-type and Bridge-type Adsorbed *C1 for Electrocatalytic CO2-to-C2 Conversion. Angew. Chem. Int. Ed. 2024, 63, e202411216. [Google Scholar] [CrossRef]
- Liu, W.; Tang, B.; Huang, K.; Zhang, Z.; Wang, Z.; An, G.; Zhang, M.; Wang, K.; Fu, S.; Guo, H.; et al. Radiation-Synthesized Metal–Organic Frameworks with Ligand-Induced Lewis Pairs for Selective CO2 Electroreduction. Small 2024, 20, e2408688. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yuan, B.; Liu, D. Editorial: Materials for electroanalysis and electrocatalysis based on advanced frameworks. Front. Chem. 2022, 10, 987654. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lv, J.; Xu, S.; Zong, J.; Liang, L.; Wang, B.; Li, P. Construction of Interlayered Single-Atom Active Sites on Bipyridine-Based 2D Conjugated Covalent-Organic Frameworks for Boosting the C2 Products of Electrochemical CO2 Reduction. ACS Appl. Mater. Interfaces 2024, 16, 67813–67820. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Das, R.; Banerjee, R. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki–Miyaura Coupling Reaction. ACS Appl. Mater. Interfaces 2014, 6, 3381–3388. [Google Scholar] [CrossRef]
- Vardhan, H.; Verma, G.; Ramani, S.; Nafady, A.; Al-Enizi, A.M.; Pan, Y.; Yang, Z.; Yang, H.; Ma, S. Covalent Organic Framework Decorated with Vanadium as a New Platform for Prins Reaction and Sulfide Oxidation. ACS Appl. Mater. Interfaces 2019, 11, 3070–3079. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Ren, W.; Xu, Y.; Liu, Q. Schottky-mediated porphyrin-metal-organic framework/Ti3C2-MXene heterojunction for water decontamination via photonic-thermal-enzyme synergistic catalysis. J. Colloid Interface Sci. 2025, 691, 137462. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, X.; Yang, S.; Yang, X.; Li, X.; He, J.; Chen, G.Z.; Xu, Q.; Zeng, G. Modulating the Density of Catalytic Sites in Multiple-Component Covalent Organic Frameworks for Electrocatalytic Carbon Dioxide Reduction. ACS Appl. Mater. Interfaces 2023, 15, 44384–44393. [Google Scholar] [CrossRef]
- Dong, X.; Chen, H.; Wang, S.; Zou, R.; Zang, S.; Cai, J. Introducing La into a Customized Dual Cu Covalent Organic Framework to Steer CO2 Electroreduction Selectivity from C2H4 to CH4. Adv. Mater. 2025, 37, 2413710. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Tao, S.; Chong, M.; Shi, Z.; Liu, X.; Cheng, D.; Chen, F. Ag Nanoparticles-Confined Doped within Triazine-Based Covalent Organic Frameworks for Syngas Production from Electrocatalytic Reduction of CO2. ACS Appl. Mater. Interfaces 2024, 16, 55267–55275. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wei, W.; Ma, D.-D.; Wu, X.-T.; Zhu, Q.-L. Efficient Carbon Dioxide Electroreduction over Ultrathin Covalent Organic Framework Nanolayers with Isolated Cobalt Porphyrin Units. ACS Appl. Mater. Interfaces 2020, 12, 37986–37992. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Ahmad, A.; Wang, X.; Khan, U.; Gao, J. MOFs/COFs Hybrids as Next-Generation Materials for Electrocatalytic CO2 Reduction Reaction. Chem. Eng. J. 2024, 486, 150098. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Si, D.-H.; Ye, S.; Dong, Y.-L.; Cao, R.; Huang, Y.-B. Photocoupled Electroreduction of CO2 over Photosensitizer-Decorated Covalent Organic Frameworks. J. Am. Chem. Soc. 2023, 145, 19856–19865. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, S.; Zhang, Y.; Li, R.; Zhang, J.; Peng, T. Structural Regulation of Coupled Phthalocyanine-Porphyrin Covalent Organic Frameworks to Highly Active and Selective Electrocatalytic CO2 Reduction. Adv. Mater. 2022, 34, 2203139. [Google Scholar] [CrossRef]
- Li, X.; Fang, Z.; Feng, X.; Wang, Z.; Xu, Y.; He, Y.; Li, H. Enhanced Electrocatalytic Carbon Dioxide Reduction Activity via Local Charge Environment Regulation of Active Sites with Rational Functionalization. Inorg. Chem. 2025, 64, 9852–9862. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Li, M.; Zhu, S.; Li, T.; Yang, J.; Lin, T.; Delmo, E.P.; Wang, Y.; Jang, J.; et al. Organic Frameworks Confined Cu Single Atoms and Nanoclusters for Tandem Electrocatalytic CO2 Reduction to Methane. SmartMat 2022, 3, 183–193. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Liu, P.; Xu, Q.; Zeng, Q.; Chen, Y.; Yang, Y.; Yang, H.; Yu, F.; Wang, Y.; et al. Subtle Tuning of Catalytic Well Effect in Phthalocyanine Covalent Organic Frameworks for Selective CO2 Electroreduction into C2H4. Adv. Mater. 2025, 37, 2415799. [Google Scholar] [CrossRef]
- Liu, R.; Tan, K.T.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent organic frameworks: An ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 2021, 50, 120–242. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 2013, 4, 2736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guan, J. Single-Atom Catalysts for Electrocatalytic Applications. Adv. Funct. Mater. 2020, 30, 2001234. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Liang, J.; Huang, Y.-B.; Cao, R. Thermo-, Electro-, and Photocatalytic CO2 Conversion to Value-Added Products over Porous Metal/Covalent Organic Frameworks. Acc. Chem. Res. 2022, 55, 2978–2997. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Bondue, C.J.; Graf, M.; Koper, M.T.M. Effect of Pore Diameter and Length on Electrochemical CO2 Reduction Reaction at Nanoporous Gold Catalysts. Chem. Sci. 2022, 13, 3288–3298. [Google Scholar] [CrossRef]
- Liu, J.; Hao, C.; Zhang, H.; Chen, H.; Zhang, L.; Chen, X.; Wang, J.; Wang, M.; Wei, S.; Lu, X.; et al. Alkali Metal-Functionalized Covalent Organic Frameworks for CO2 Adsorption and CO2/N2 Separation. ACS Appl. Polym. Mater. 2025, 7, 1729–1740. [Google Scholar] [CrossRef]
- Xie, T.; Chen, S.; Yue, Y.; Sheng, T.; Huang, N.; Xiong, Y. Biomimetic Phthalocyanine-Based Covalent Organic Frameworks with Tunable Pendant Groups for Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2024, 63, e202411188. [Google Scholar] [CrossRef]
- Han, B.; Ding, X.; Yu, B.; Wu, H.; Zhou, W.; Liu, W.; Wei, C.; Chen, B.; Qi, D.; Wang, H.; et al. Two-Dimensional Covalent Organic Frameworks with Cobalt(II)-Phthalocyanine Sites for Efficient Electrocatalytic Carbon Dioxide Reduction. J. Am. Chem. Soc. 2021, 143, 7104–7113. [Google Scholar] [CrossRef]
- Hussain, I.; Kathiresan, M.; Singh, K.; Kalidasan, B.; Mendhe, A.C.; Islam, M.N.; Meng, K.; Aslam, M.K.; Hanif, M.B.; Al Zoubi, W.; et al. Interface and Surface Engineering of MXenes and COFs for Energy Storage and Conversion. InfoMat 2025, 7, e70011. [Google Scholar] [CrossRef]
- Xin, Z.; Liu, J.; Wang, X.; Shen, K.; Yuan, Z.; Chen, Y.; Lan, Y.-Q. Implanting Polypyrrole in Metal-Porphyrin MOFs: Enhanced Electrocatalytic Performance for CO2RR. ACS Appl. Mater. Interfaces 2021, 13, 54959–54966. [Google Scholar] [CrossRef]
- Seo, J.-M.; Noh, H.-J.; Jeon, J.-P.; Kim, H.; Han, G.-F.; Kwak, S.K.; Jeong, H.Y.; Wang, L.; Li, F.; Baek, J.-B. Conductive and Ultrastable Covalent Organic Framework/Carbon Hybrid as an Ideal Electrocatalytic Platform. J. Am. Chem. Soc. 2022, 144, 19973–19980. [Google Scholar] [CrossRef]
- Yuan, W.; Weng, J.; Ding, M.; Jiang, H.-M.; Fan, Z.; Zhao, Z.; Zhang, P.; Xu, L.-P.; Zhou, P. Novel covalent organic framework/carbon nanotube composites with multiple redox-active sites for high-performance Na storage. Energy Storage Mater. 2024, 65, 103142. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, D.; Xia, C.; Shahid, Z.; Chen, S.; Xia, B.Y. Copper-Organic Frameworks for Electrocatalytic Carbon Dioxide Reduction. Coord. Chem. Rev. 2024, 517, 216021. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, W.; Yang, Q.; Kaneti, Y.V.; Xu, X.; Alshehri, S.M.; Ahamad, T.; Hossain, S.A.; Na, J.; Tang, J.; et al. Core-shell motif construction: Highly graphitic nitrogen-doped porous carbon electrocatalysts using MOF-derived carbon@COF heterostructures as sacrificial templates. Chem. Eng. J. 2020, 396, 125154. [Google Scholar] [CrossRef]
- Di Girolamo, A.; Monti, F.; Mazzanti, A.; Matteucci, E.; Armaroli, N.; Sambri, L.; Baschieri, A. 4-Phenyl-1,2,3-triazoles as Versatile Ligands for Cationic Cyclometalated Iridium(III) Complexes. Inorg. Chem. 2022, 61, 8509–8520. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, L.; Honsho, Y.; Feng, X.; Saengsawang, O.; Guo, J.; Saeki, A.; Seki, S.; Irle, S.; Nagase, S.; et al. An n-Channel Two-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2011, 133, 14510–14513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wun, C.K.T.; Lo, T.W.B. Harnessing Synergistic Cooperation of Neighboring Active Motifs in Heterogeneous Catalysts for Enhanced Catalytic Performance. Adv. Mater. 2024, 36, 2501960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, M.; Yuan, C.-Y.; Sun, Q.-W.; Huang, B.; Dong, H.; Zhang, Y.-W. Strong Electronic Coupling Effects at the Heterojunction Interface of SnO2 Nanodots and g-C3N4 for Enhanced CO2 Electroreduction. ACS Catal. 2023, 13, 7055–7066. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Wang, J.; Shang, X.; Zhang, C.; Wang, Q. Advances of functionalized bipyridine-based covalent-organic frameworks for boosting photocatalysis. Coord. Chem. Rev. 2024, 516, 215997. [Google Scholar] [CrossRef]
- Zhong, W.; Sa, R.; Li, L.; He, Y.; Li, L.; Bi, J.; Zhuang, Z.; Yu, Y.; Zou, Z. A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO. J. Am. Chem. Soc. 2020, 142, 21899–21907. [Google Scholar] [CrossRef]


| Catalyst Type | Specific Catalyst | Catalytic Performance | References |
|---|---|---|---|
| MOF | MOF-545-Cu/PAN (MCP)-500 | CO2RR for CO production; FE for CO (FE_CO) reaches 98% (−0.8 V vs. RHE), maintains FE_CO > 95% after 10 h of continuous catalysis; FE_CO remains 80–98% at voltages ranging from −0.6 V to −1.0 V vs. RHE | [15] |
| MOF | Cu-THQ 2D c-MOF | CO2RR for CO production; average FE_CO of 91% with a conversion rate of 20.82 s−1; in a flow battery, the current density reaches 173 mA/cm2 at 0.45 V vs. RHE | [22] |
| MOF | In-TCPP (In-based porphyrin π-d MOF) | CO2RR for HCOO− production; FE_HCOO− reaches 90% with a cathodic efficiency of 63.8% | [23] |
| MOF | 2D PML-Cu | CO2RR for HCOO− production; FE_HCOO− reaches 80.86% at −0.7 V vs. RHE | [24] |
| MOF | Cu2(CuTCPP) nanosheets | CO2RR for HCOO− production; FE_HCOO− reaches 68.4% at −0.94 V vs. RHE | [25] |
| MOF | HP-UiO-66-NH2 (hierarchical porous, with polar amino groups) | CO2RR for HCOO− production; HCOO− yield exceeds 6000 μmol gcat−1h−1 within 3 h (5.6 times that of the free enzyme system), and HCOO− concentration reaches 1.83 mM; retains over 90% CO2 adsorption capacity after pore structure modification | [32] |
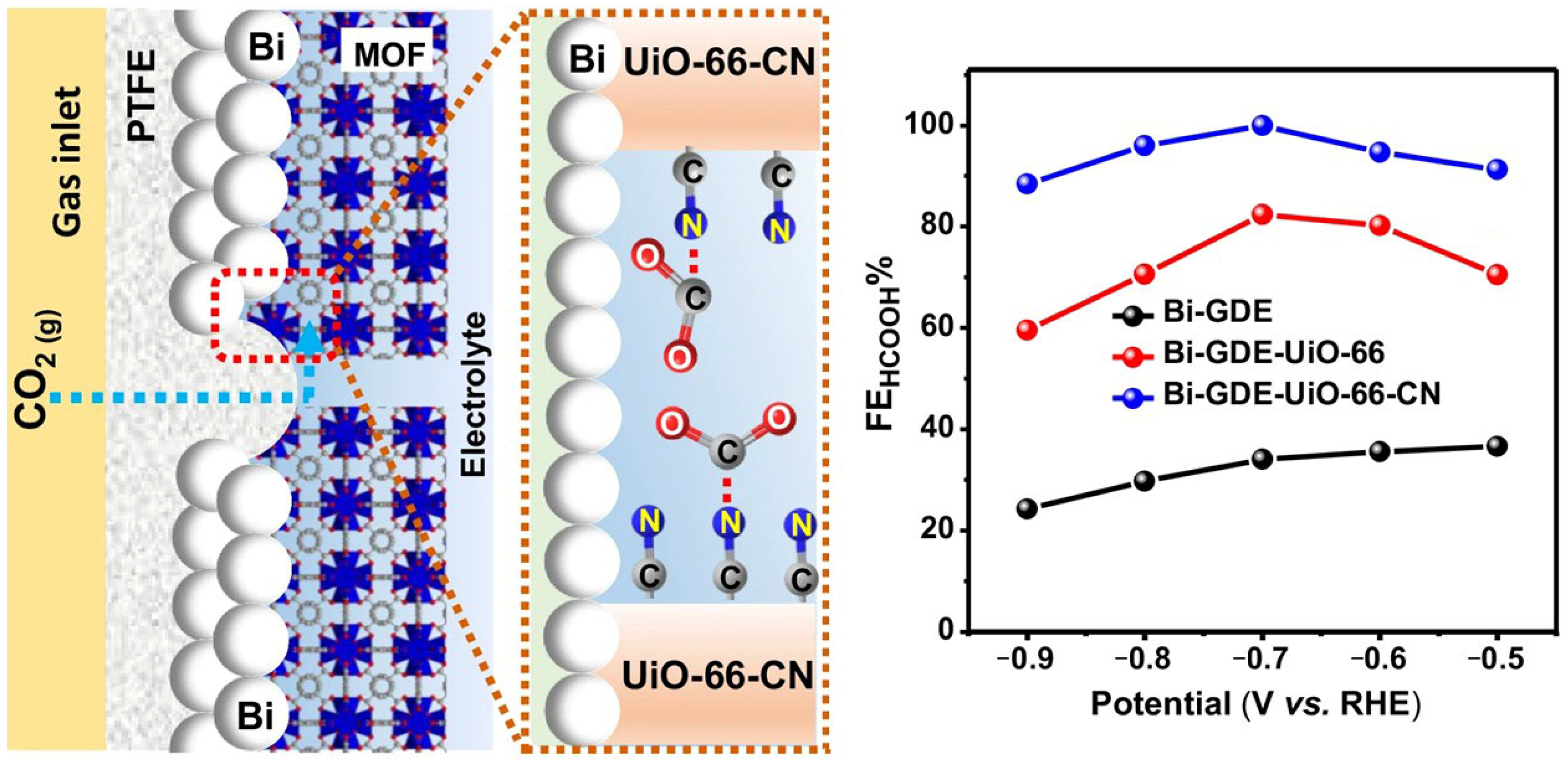
| MOF | UiO-66-CN (CN-functionalized UiO-66) | CO2RR for HCOOH production; FE_HCOOH reaches 93% at −0.75 V vs. RHE | [33] |
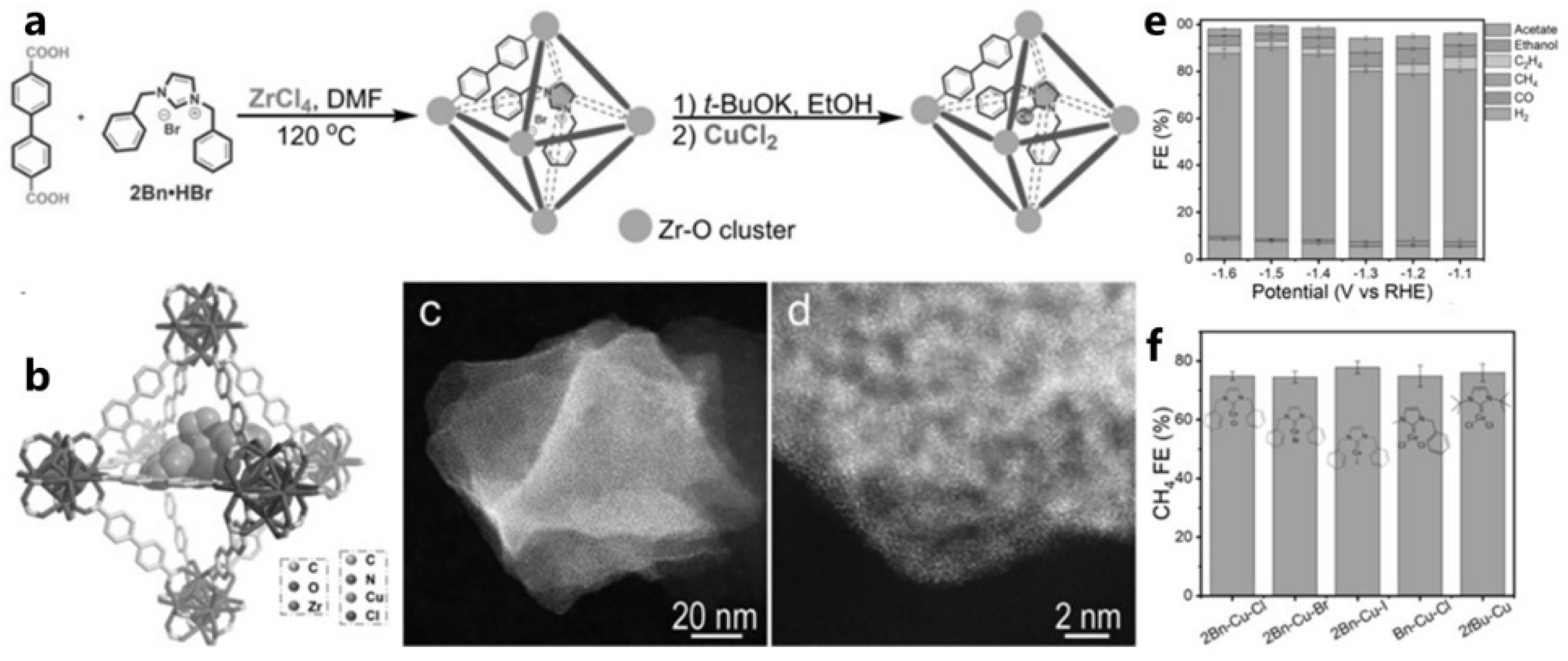
| MOF | 2Bn-Cu@UiO-67 (N-heterocyclic carbene-linked Cu single-atom catalyst) | CO2RR for CH4 production; FE_CH4 reaches 81% at −1.5 V vs. RHE, corresponding to a current density of 420 mA/cm2; TOF reaches 16.3 s−1, and FE_CH4 > 70% over the entire potential range | [34] |
| MOF | Pd-doped HKUST-1 (forms Cu-Pd paddlewheel dimers) | CO2RR; in 0.5 M CO2-saturated KHCO3, FE increases from 28.7% to 84.8% at −0.77 V vs. RHE | [29] |
| MOF | Cu-btca | CO2RR under acidic conditions; at 300 mA/cm2, FE_C2H4 reaches 51.2%, and FE of multi-carbon products reaches 81.9%; porous structure promotes mass transfer and inhibits HER | [28] |
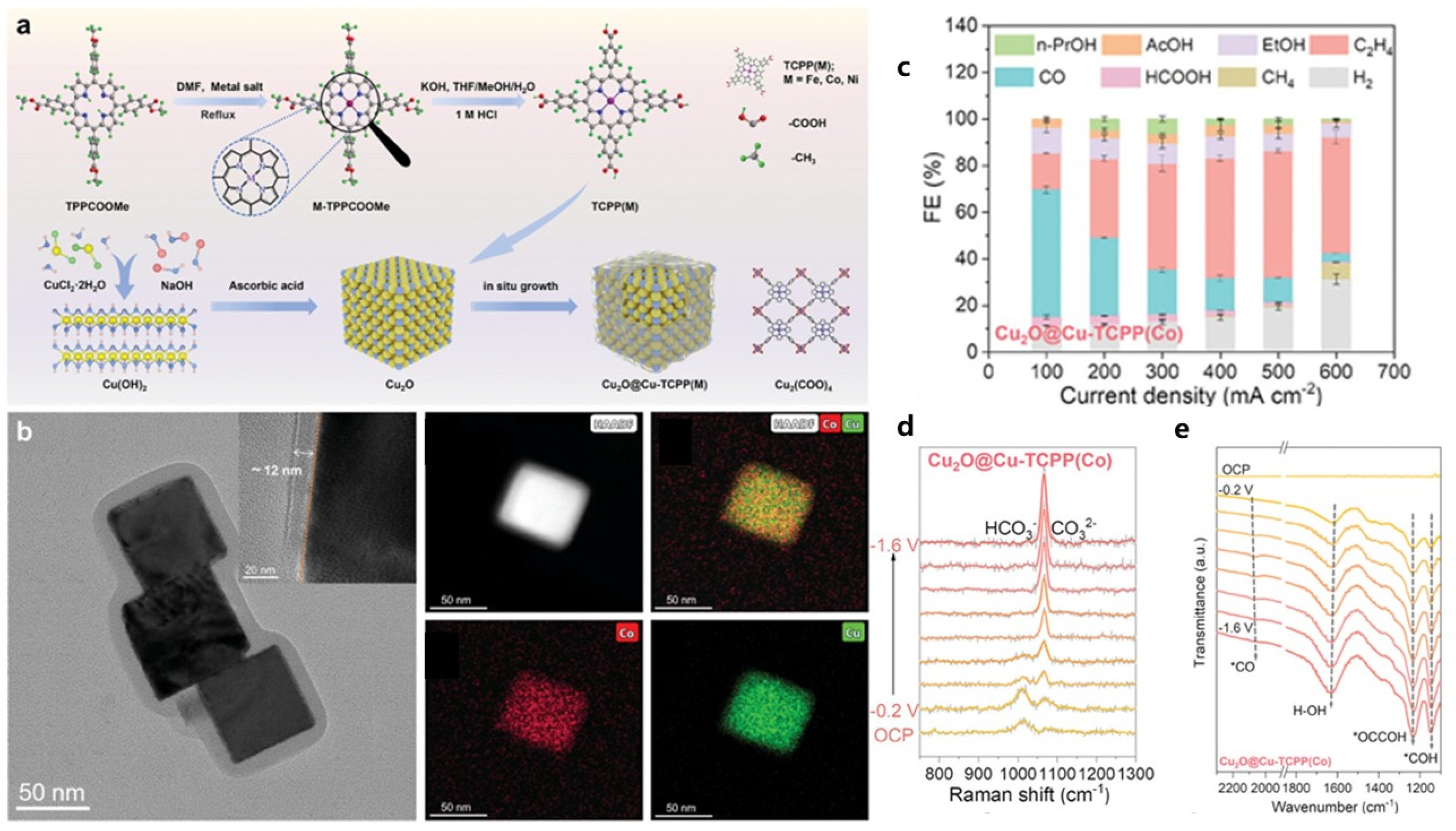
| MOF | Cu2O@Cu-TCPP(M) (M = Co, Fe, Ni; core-shell structure) | CO2RR for C2H4 production; in 1 M KCl, at 500 mA/cm2, FE_C2H4 reaches 54 ± 2%, and FE of C2 products reaches 69 ± 4%; retains Cu2O crystallinity and valence state, improving stability | [31] |
| MOF | HKUST-1@Cu2O/PTFE-1 (PTFE-modified core-shell structure) | CO2RR for C2H4 production; FE_C2H4 reaches 46.08% at −1.0 V vs. RHE, with a hydrocarbon fuel efficiency of 67.41%; no significant current decay and stable ethylene FE during 12-h continuous testing | [30] |
| MOF | Cu-DMTZ MOF (pulsed potential regulation) | CO2RR for CH4 production; FE_CH4 reaches 82.9% under pulsed electrolysis (much higher than 47.4% under constant potential); FE_CH4 remains > 60% after 12-h continuous operation | [35] |
| MOF | Cu0.5Zn0.5/ZIF-8 (Cu-doped ZIF-8) | CO2RR for CO production; FE reaches 88.5% at −1.0 V vs. RHE, with a partial current density of 11.57 mA/cm2; Cu doping modifies the electronic structure of organic ligands and promotes *COOH formation | [36] |
| MOF | Cu-HHTT (ultrathin 2D triphenyl MOF) | CO2RR for CO production; FE_CO reaches 96.6% at −0.6 V vs. RHE, with a current density of 18 mA/cm2; DFT calculations show low CO desorption energy barrier | [39] |
| MOF | SU-101-Cu@2.5 C (BiCu bimetallic organic framework, MWCNTs composite) | CO2RR for formic acid production; initial potential of −0.46 V vs. RHE, partial current density > 100 mA/cm2; FE of formic acid is nearly 100% at −0.96 V vs. RHE, and FE > 80% in the potential range of −0.86 V to −1.06 V vs. RHE; good stability during 30-h continuous catalysis | [37] |
| MOF | MIL-68(In)/CuO (In-Cu dual active sites) | CO2RR for formic acid production; FE of formic acid reaches 89.7% at −0.7 V vs. RHE in a flow battery; maintains activity within 180 h in an MEA battery and inhibits HER | [38] |
| MOF | CuAg5@NC (Ag-anchored Cu-based MOF-derived CuAg bimetal) | CO2RR for ethanol production; FE_ethanol reaches 51.8% at −1.0 V vs. RHE, and FE of C2 products reaches 82.6% at −1.2 V vs. RHE; Cu-Ag biphasic interface promotes CO migration and C–C coupling | [40] |
| MOF | CuPOF-Bpy/Cu2O@CNT (dual-site Cu(II) porphyrin framework composite with Cu2O) | CO2RR for C2H4 production; FE_C2H4 reaches 71.0% at −1.1 V vs. RHE, and FE_C2H4 > 40% over the entire potential range; CuPor/CuBpy sites produce *CO, and Cu2O sites promote C–C coupling | [41] |
| MOF | Cu@BIF-144(Zn) (tandem catalyst) | CO2RR for CH4 and C2H4 production; FE_CH4 reaches 41.8% at −1.6 V vs. RHE, and FE_C2H4 reaches 12.9% at −1.5 V vs. RHE; Zn sites create a CO-enriched environment to promote deep CO2 reduction on Cu NPs | [42] |
| MOF | R-Cu-TAl-CNTs1 (tandem catalyst) | CO2RR for CH4 production; FE_CH4 reaches 54% at −1.56 V vs. RHE (30% without CNTs); FE ratio of CH4/C2H4 reaches 9.5 in the potential range of −0.96 V to −1.76 V vs. RHE (12 times that without CNTs) | [43] |
| MOF | Cu@ZIF-8 NW (Cu-ZIF-8 interface-exposed nanowires) | CO2RR for hydrocarbons (CH4, C2H4); FE of hydrocarbons reaches 57.5% at −0.7 V vs. RHE; Cu-ZIF-8 interface optimizes intermediate adsorption and stabilizes *CHO | [44] |
| MOF | Cu-MOF-CF (Cu-MOF anchored on copper foil) | CO2RR for C2H4 production; FE_C2H4 reaches 48.6% at −1.11 V vs. RHE (22.4% for pure copper foil); partial current density of C2H4 is more than 4 times that of pure copper foil | [45] |
| MOF | 2D-vc-MOF(Cu) (2D vertically conductive MOF) | CO2RR for CH4 production; FE_CH4 reaches 65% at −1.4 V vs. RHE (20% at −1.2 V, 33% at −1.6 V); vertical structure reduces energy barrier and improves reaction kinetics | [46] |
| MOF | Curved 2D Cu(I)-based coordination polymer | CO2RR for C2H4 production; electrocatalytic selectivity shifts from HER (80%) to CO2RR (76%), with FE_C2H4 reaching 52%; wave-like structure promotes hydrogen bonding between amino groups and *CO2RR intermediates, inhibiting HER | [47] |
| MOF | Cu-MMT nanoribbons (water as solvent, exposing (100) planes) | CO2RR for CH4 production; FE_CH4 reaches 55.22% | [48] |
| MOF | Cu-MMT cross-linked nanosheets (isopropanol as solvent, exposing (001) planes) | CO2RR for C2 products (mainly C2H4); FE_C2 reaches 73.75% | [48] |
| MOF | Ce-CuOx (atomic-level Ce doping) | CO2RR for CH4 production; FE_CH4 > 62% in the potential range of −1.4 V to −1.75 V vs. RHE, FE_CH4 reaches 67.4% at −1.6 V vs. RHE, with a partial current density of 293 mA/cm2 | [50] |
| MOF | Co-Cu alloy (Co-doped Cu-MOF via electrochemical reconstruction) | CO2RR for CH4 production; FE_CH4 reaches 60 ± 1%, corresponding to a partial current density of 303 ± 5 mA/cm2 | [51] |
| MOF | Cu-PD-2-MBI (Cu-based c-MOF containing 2-methylbenzimidazole) | CO2RR for CH4 production; FE_CH4 reaches 73.7% at −1.3 V vs. RHE, with a partial current density of −428.3 mA/cm2; FE_CH4 increases in the potential range of −1.1 V to −1.3 V vs. RHE, inhibiting HER | [52] |
| MOF | CuTrz-109 nm (small-sized CuTrz nanostructure) | CO2RR for C2 products; FE_C2H4 reaches 55.4% at −1.15 V vs. RHE, and FE of C2 products reaches 81.8%; rich in grain boundaries and defects, improving catalytic performance | [49] |
| COF | Cu@BTT-BPy-COF (bipyridine-based 2D conjugated COF with interlayer single-atom Cu sites) | CO2RR for C2 products; FE_C2 reaches 46.7% at −0.8 V vs. RHE, outperforming most framework-based electrocatalysts | [56] |
| COF | Pd/COF-LZU1 (Pd-supported COF-LZU1) | Suzuki-Miura coupling reaction; reaction yield of 96–98%, wide substrate applicability, good stability, and easy recovery | [57] |
| COF | Vanadium-modified COF (2D COF modified with vanadyl acetoacetonate) | Prins condensation reaction, sulfide oxidation reaction; high catalytic activity, retains framework crystallinity, reusable; possesses eclipsed stacking structure, macroporosity, hydroxyl functionality, high thermal and chemical stability | [58] |
| COF | 0.75NiPc-COF (multi-component synthesized NiPc-COF) | CO2RR for CO production; TOF reaches 4909.87 h−1, FE_CO reaches 95.37%, exhibiting optimal CO2RR activity and selectivity | [60] |
| COF | La-doped dual-Cu COF | CO2RR; La doping modifies the coordination environment of Cu centers with N/O, adjusts Cu oxidation state and electronic properties, shifting product selectivity from C2H4 to CH4 | [61,62] |
| COF | COF containing triazine/carboxyl/fluoro-substituted groups | CO2 adsorption and activation; CO2 adsorption capacity of 174 mg/g, adsorption heat Qst = 43.5 kJ/mol; reduces *COOH formation energy barrier by 0.54 eV; dual-pore topology (1.27/1.55 nm) generates a sub-nanometer molecular sieve effect, FE > 95% under 10% CO2 gas flow | [70] |
| COF | CoPc-2H2Por (1:2 phthalocyanine-porphyrin COF) | CO2RR for CO production; FE_CO > 90%, outperforming 1:1 COF and monomeric CoPc; larger pore size and optimized conjugated structure promote CO2RR kinetics and reduce charge transfer resistance | [66] |
| COF | HFPTP-BPDA-COF (sub-nanometer dual-pore structure) | Molecular sieving; 1.27/1.55 nm dual pores, with “zigzag” C-H sequences on the inner wall of triangular pores forming molecular “checkpoints”; completely adsorbs Nile Red (1.50 nm) within 2 min, while adsorption of DAPC (1.55 nm) is negligible, achieving single-atom level selective sieving | [74] |
| COF | Piperazine-linked conductive COF containing -CH2NH2 | CO2RR; -CH2NH2 (electron-donating group) reduces COF surface work function and enhances *COOH adsorption energy (superior to electron-withdrawing groups such as -CN and -COOH); high chemical stability and conductivity, improving CO2RR activity and selectivity | [77] |
| COF | [HO2C]X%-H2P-COFs (carboxyl-functionalized COF) | CO2 adsorption; CO2 adsorption capacity is positively correlated with carboxyl content; carboxyl density is regulated via ring-opening reaction between phenolic hydroxyl groups and succinic anhydride (X = 25, 50, 75, 100) | [78] |
| COF | Pt1@BCOF-600C (BCOF thermally annealed carbon hybrid) | HER; in 0.5 M H2SO4, mass activity reaches 8.56 A mg_Pt−1 (30 times that of commercial Pt/C); retains N-anchoring sites and 2.1 nm mesoporous channels; partial carbonization at grain boundaries forms a continuous conductive network | [82] |
| COF | PPy/MOF-545-Co (PPy embedded in MOF-545-Co pores) | CO2RR for CO production; FE_CO doubles compared to pure MOF, reaching 98%; PPy acts as an electron transport “cable” to promote electron transfer | [82] |
| COF | TP-OH-COF@CNT50 (redox-active group COF/CNT composite) | Sodium battery cathode; specific capacity of 256.4 mAh/g at 0.1 A/g, 100% capacity retention after 3000 cycles at 2 A/g, and 103 mAh/g at 10 A/g; surface-dominated sodium storage mechanism with low sodium diffusion energy barrier | [83] |
| COF | CNP-900 (N,P co-doped carbon material) | CO2RR for CO production; FE_CO reaches 80.8% at an overpotential of 0.44 V; suitable pyrrolic N/graphitic N ratio and low P-N content are more favorable for CO2RR | [86] |
| COF | 2D-NiPc-BTDA COF (phthalocyanine and benzothiadiazole copolymerized COF) | Photoelectric performance; wide band gap, absorption extended to 1000 nm, full-color photoelectric effect, high near-infrared light sensitivity; electron mobility reaches 0.6 cm2/(V·s), charge transport mode shifts from hole transport to electron transport | [64] |
| COF | SnO2/g-C3N4 (0D/2D heterojunction) | CO2RR for formic acid production; FE of formic acid reaches 91.7% at −0.88 V vs. RHE; strong metal oxide-support interaction between SnO2 nanodots and g-C3N4, p-p orbital coupling promotes electron transfer | [89] |
| COF | Ni-TpBpy (COF with single Ni sites) | Photocatalytic CO2RR for CO production; CO yield of 4057 μmol/g within 5 h under visible light, CO selectivity of 96%; maintains 76% CO selectivity even at 0.1 atm CO2 partial pressure; TpBpy promotes CO2 activation and inhibits HER | [89] |
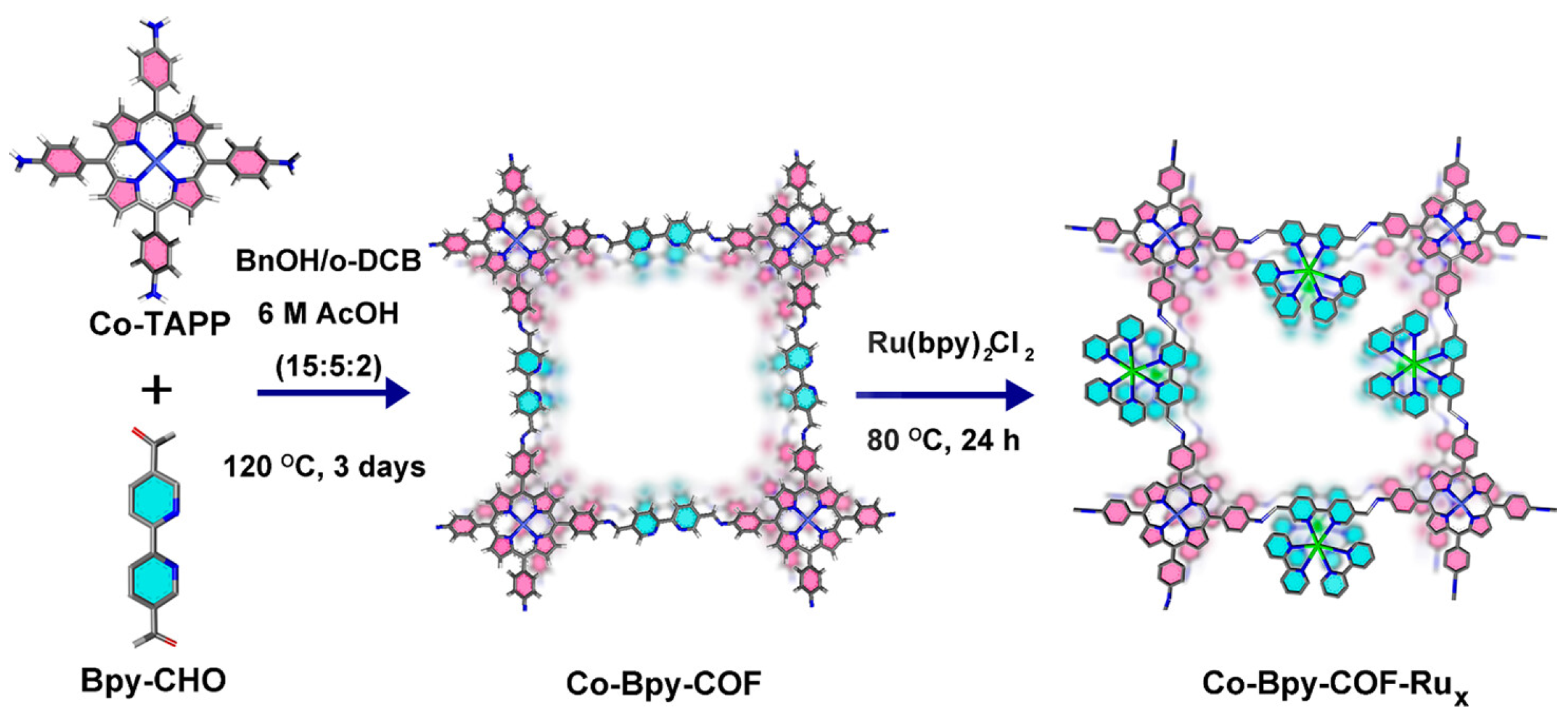
| COF | Co-Bpy-COF-Rux (Co-porphyrin COF modified with Ru(bpy)3Cl2) | Photoelectrochemical CO2RR for CO production; FE_CO reaches 96.7% under light irradiation, with significantly improved current density; Ru photosensitizer promotes electron transfer to Co-porphyrin, extends the excited state lifetime of active sites, and reduces reaction energy barrier | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhu, H.; Wang, Y.; Yin, G.; Chen, S.; Li, T.; Wu, C.; Jia, S.; Shang, J.; Ren, Z.; et al. Metal-Organic Frameworks and Covalent Organic Frameworks for CO2 Electrocatalytic Reduction: Research Progress and Challenges. Catalysts 2025, 15, 936. https://doi.org/10.3390/catal15100936
Huang Y, Zhu H, Wang Y, Yin G, Chen S, Li T, Wu C, Jia S, Shang J, Ren Z, et al. Metal-Organic Frameworks and Covalent Organic Frameworks for CO2 Electrocatalytic Reduction: Research Progress and Challenges. Catalysts. 2025; 15(10):936. https://doi.org/10.3390/catal15100936
Chicago/Turabian StyleHuang, Yuyuan, Haiyan Zhu, Yongle Wang, Guohao Yin, Shanlin Chen, Tingting Li, Chou Wu, Shaobo Jia, Jianxiao Shang, Zhequn Ren, and et al. 2025. "Metal-Organic Frameworks and Covalent Organic Frameworks for CO2 Electrocatalytic Reduction: Research Progress and Challenges" Catalysts 15, no. 10: 936. https://doi.org/10.3390/catal15100936
APA StyleHuang, Y., Zhu, H., Wang, Y., Yin, G., Chen, S., Li, T., Wu, C., Jia, S., Shang, J., Ren, Z., Ding, T., & Li, Y. (2025). Metal-Organic Frameworks and Covalent Organic Frameworks for CO2 Electrocatalytic Reduction: Research Progress and Challenges. Catalysts, 15(10), 936. https://doi.org/10.3390/catal15100936






