Catalytic Application of Ionic Liquids for the Green Synthesis of Aromatic Five-Membered Nitrogen Heterocycles
Abstract
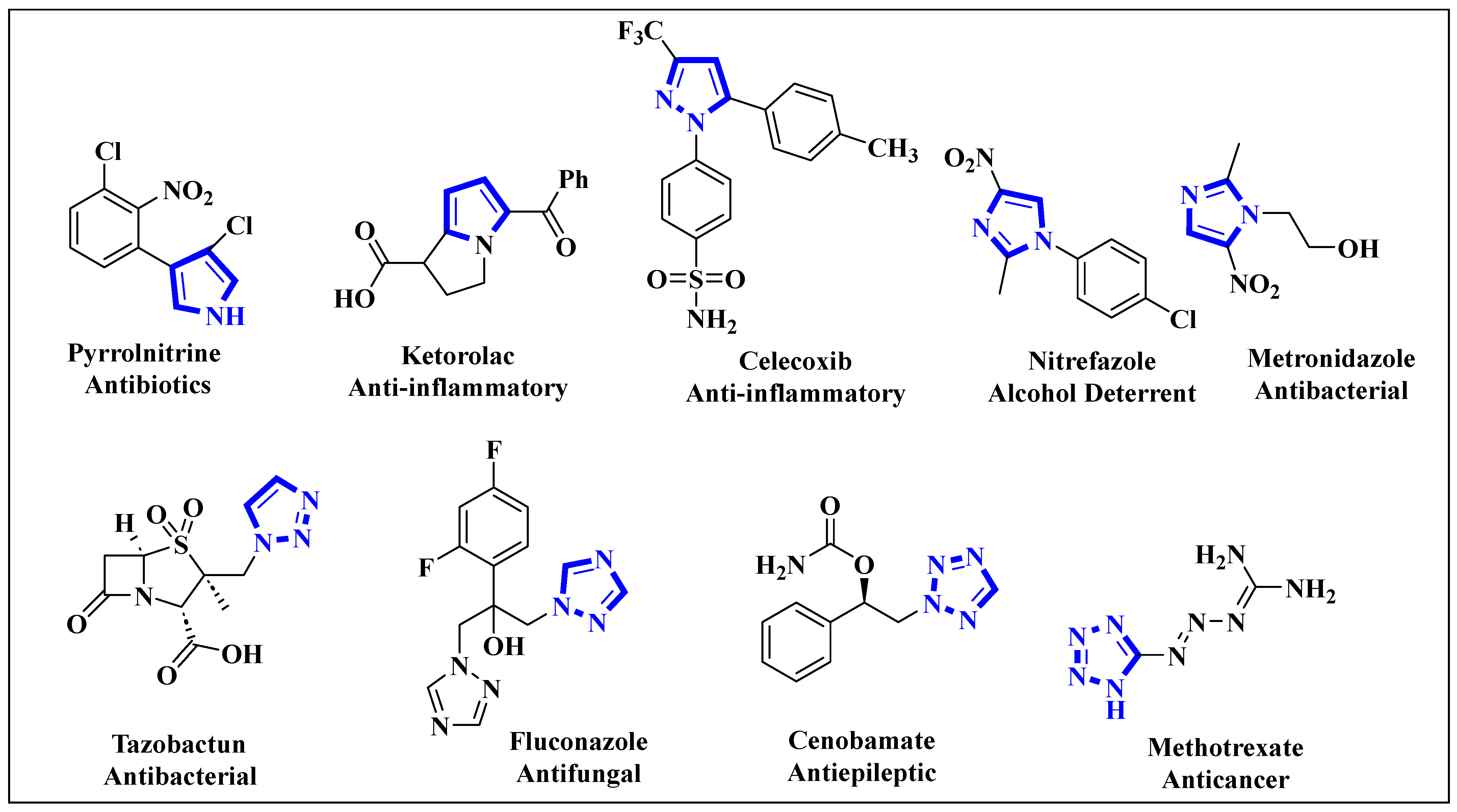
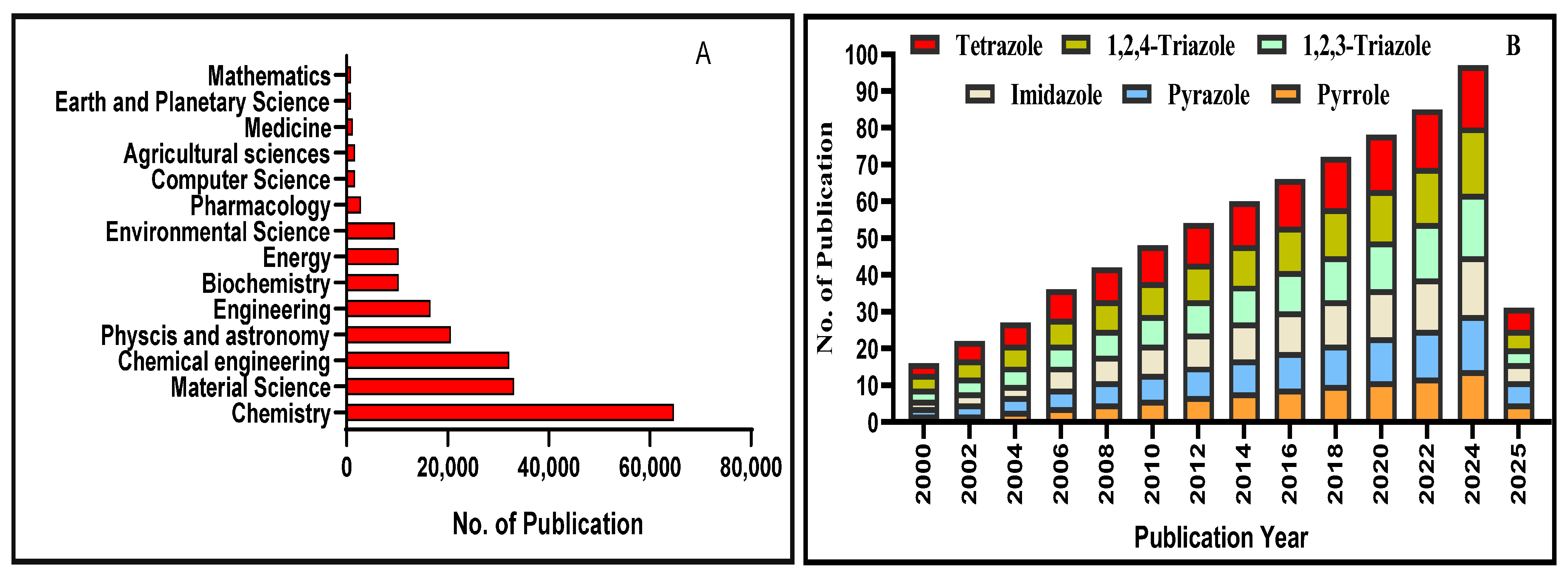
1. Introduction
2. Structure and Properties of Ionic Liquids
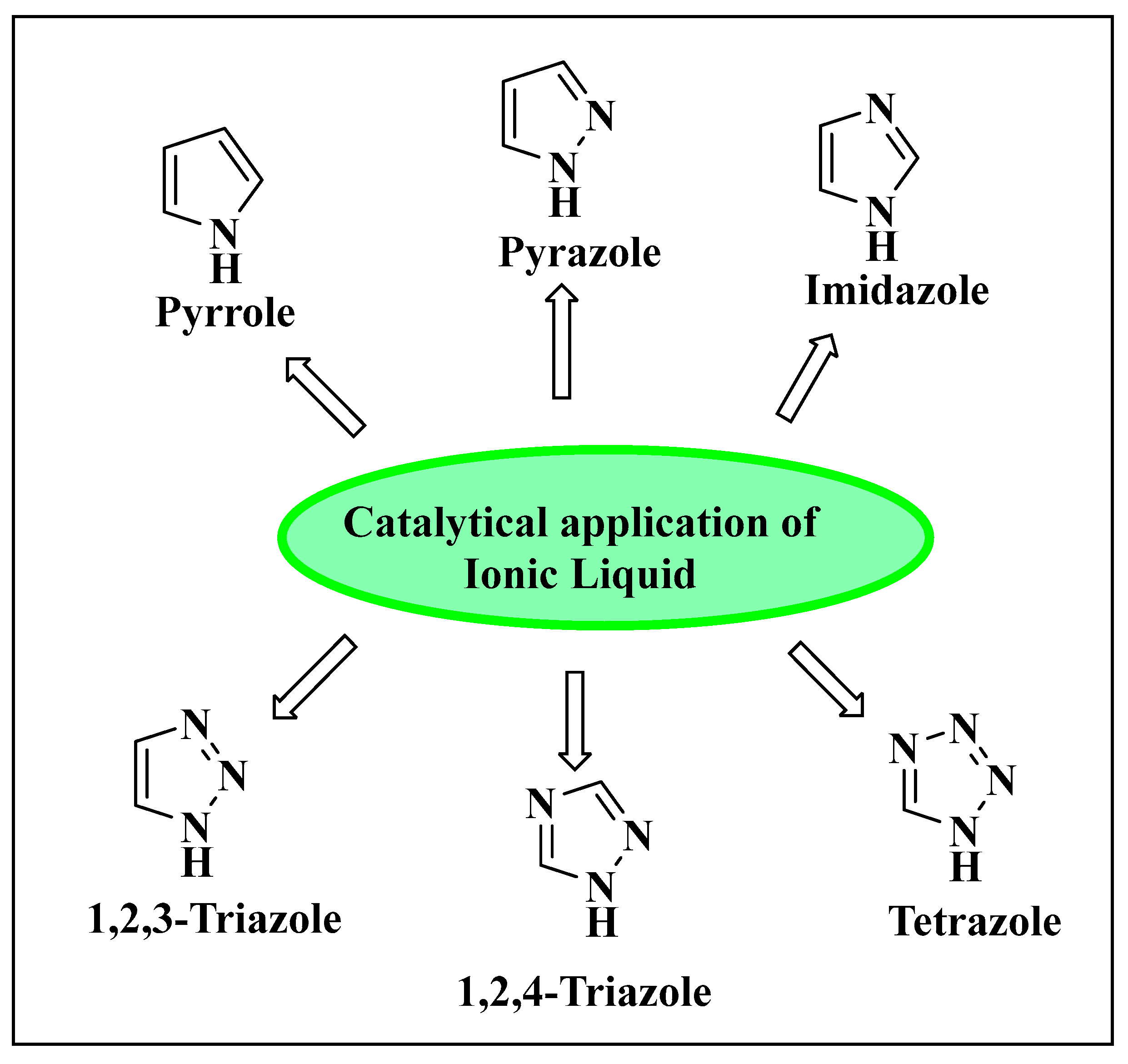
3. Catalytic Application of Ionic Liquids in Organic Reactions
3.1. Synthesis of Pyrrole Derivatives Using Ionic Liquids
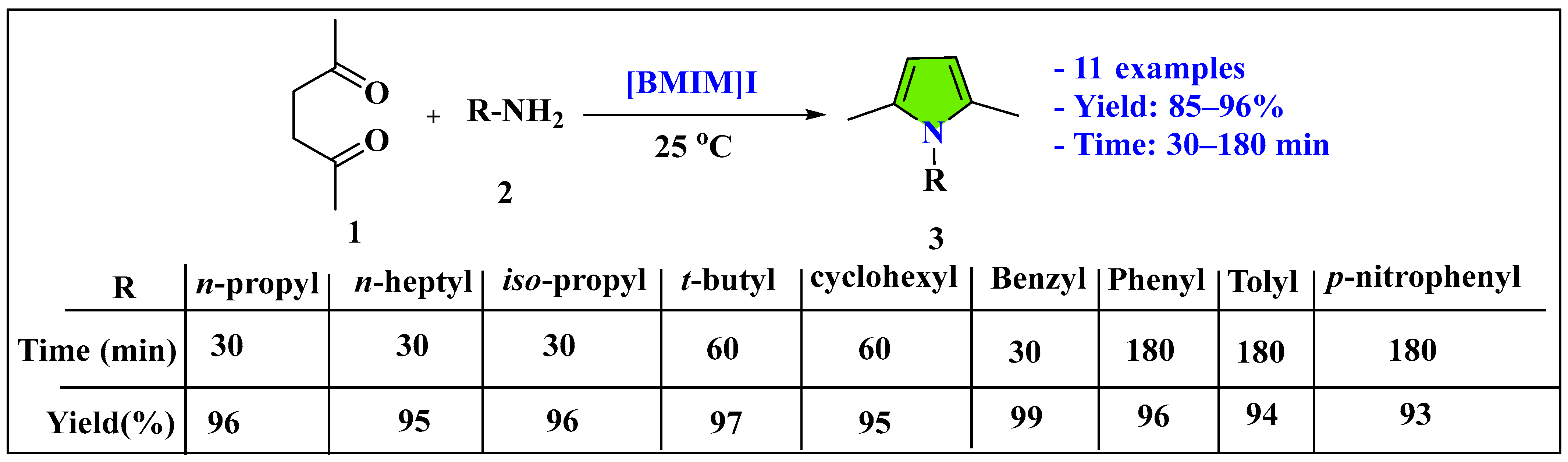
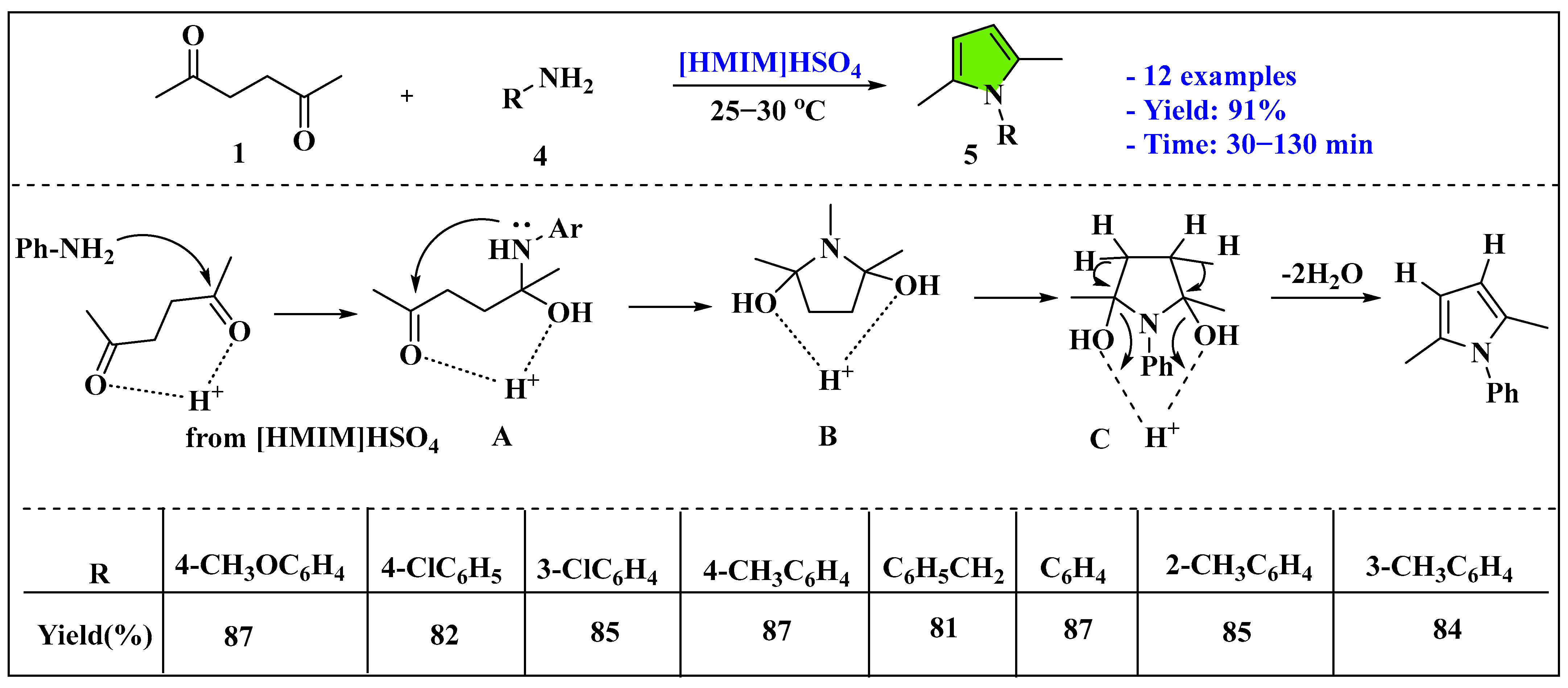
3.1.1. Ionic Liquids Mediated Paal–Knorr Reaction in the Homogenous Phase
3.1.2. Ionic Liquids Mediated Paal–Knorr Reaction in Heterogeneous Phase
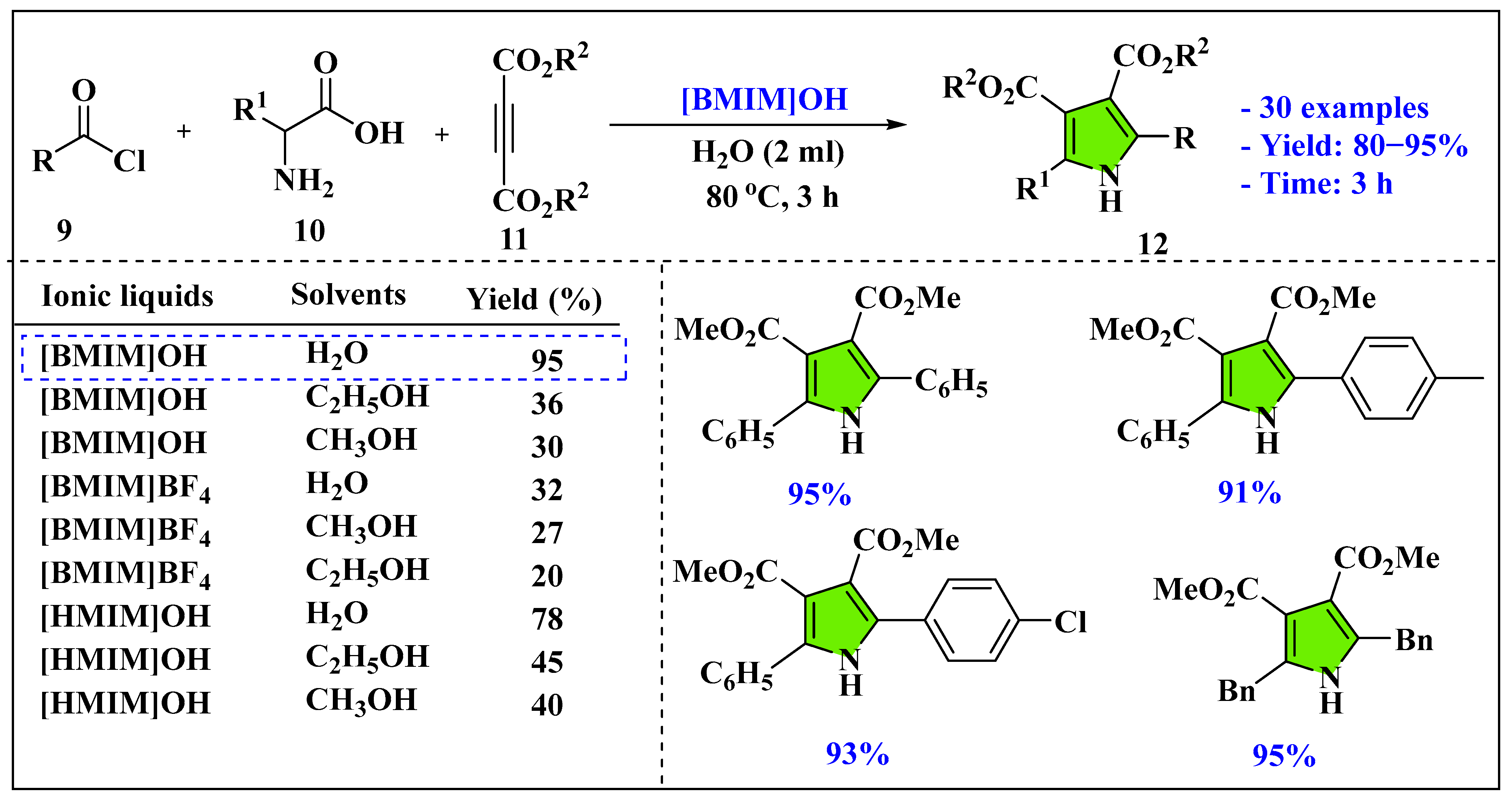
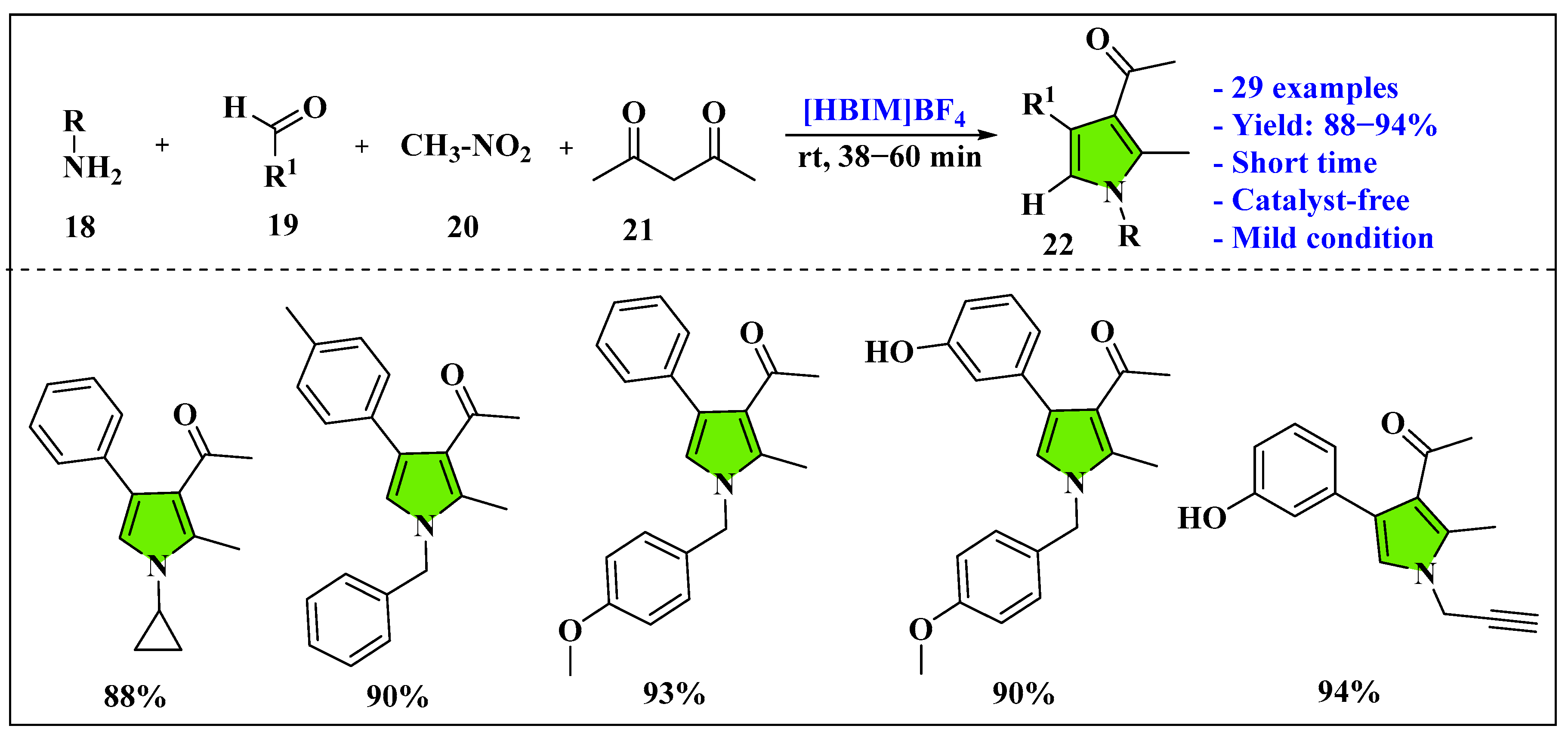
3.1.3. Ionic Liquids Mediated Multicomponent Reaction for Pyrrole Synthesis in Homogenous Phase
3.1.4. Ionic Liquids Mediated Clauson-Kass Reaction in Homogenous Phase
3.1.5. Ionic Liquids Mediated Functionalization of Pyrrole in Homogeneous Phase
3.2. Synthesis of Pyrazole Derivatives Using Ionic Liquids
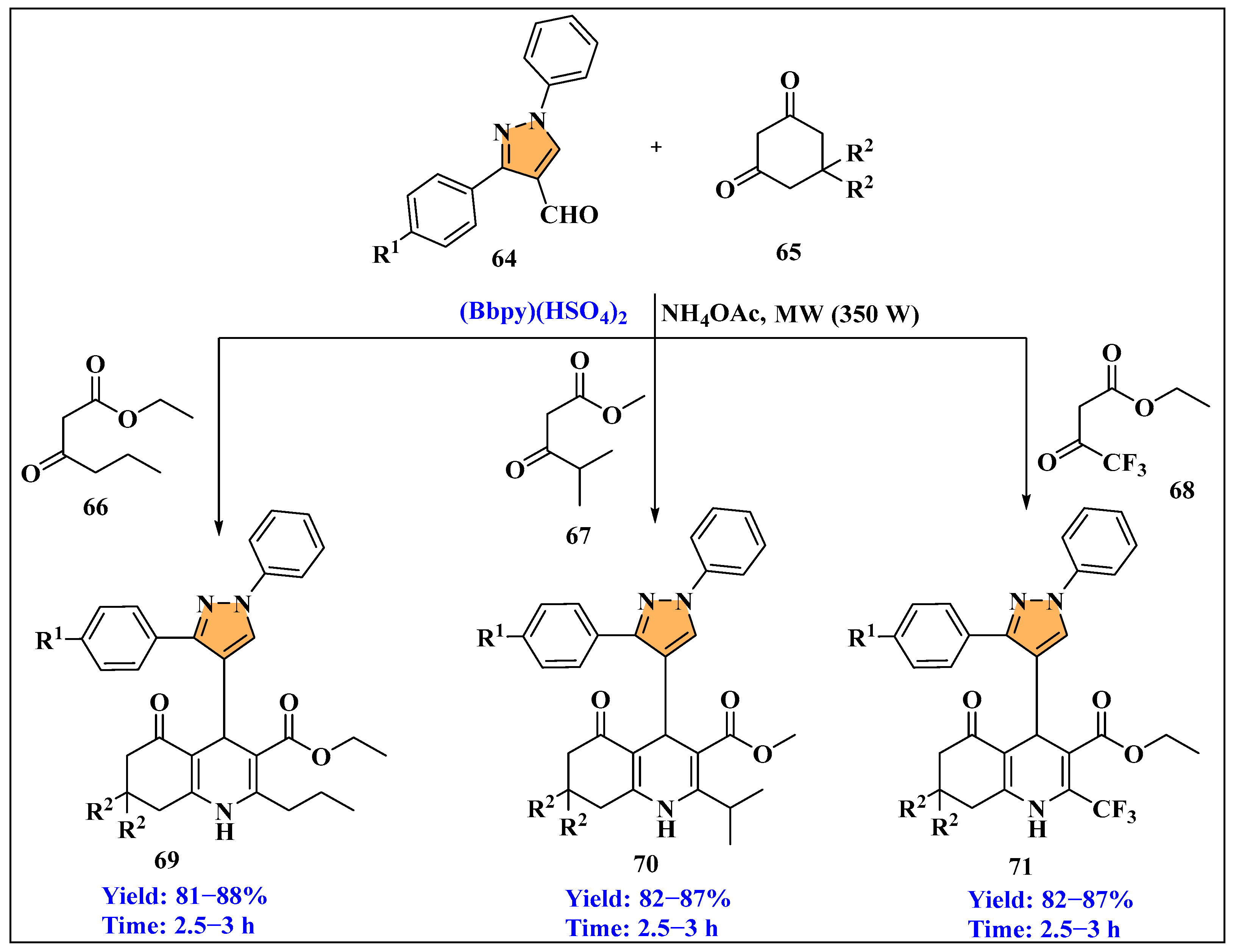
3.2.1. Ionic Liquids Mediated Paal–Knorr-Reaction in Homogenous Phase


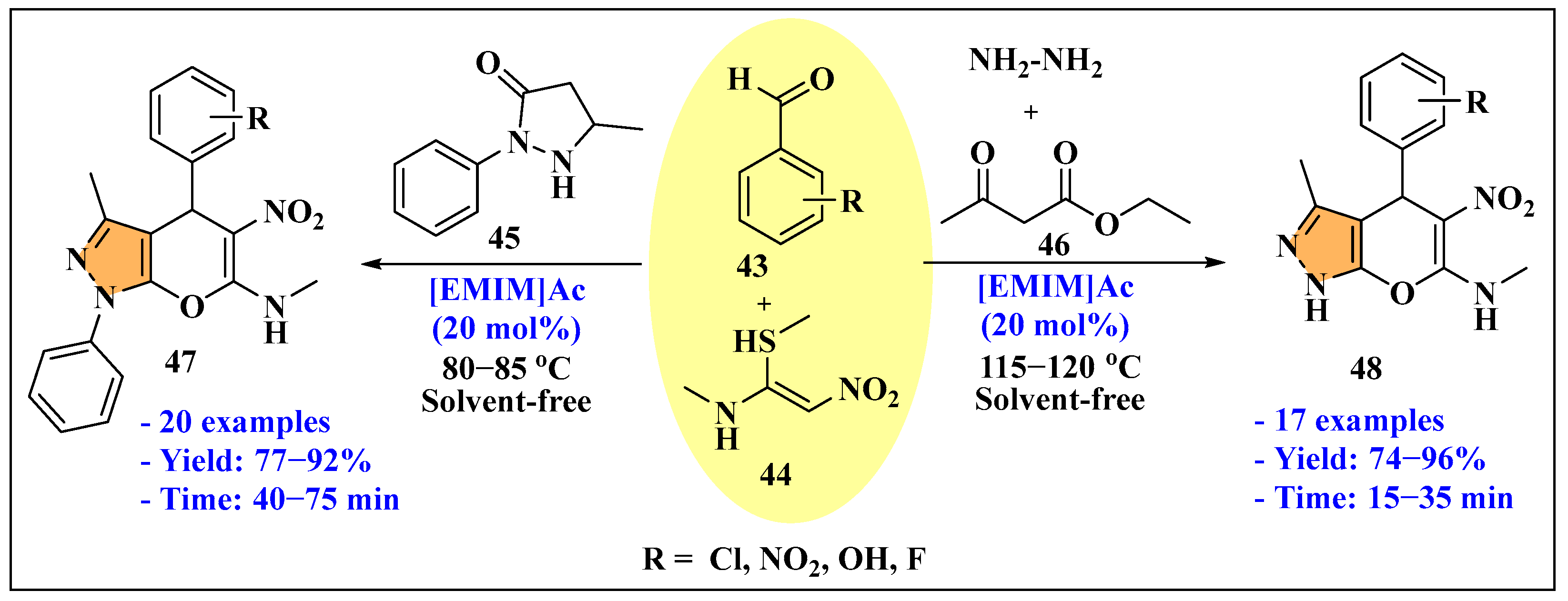
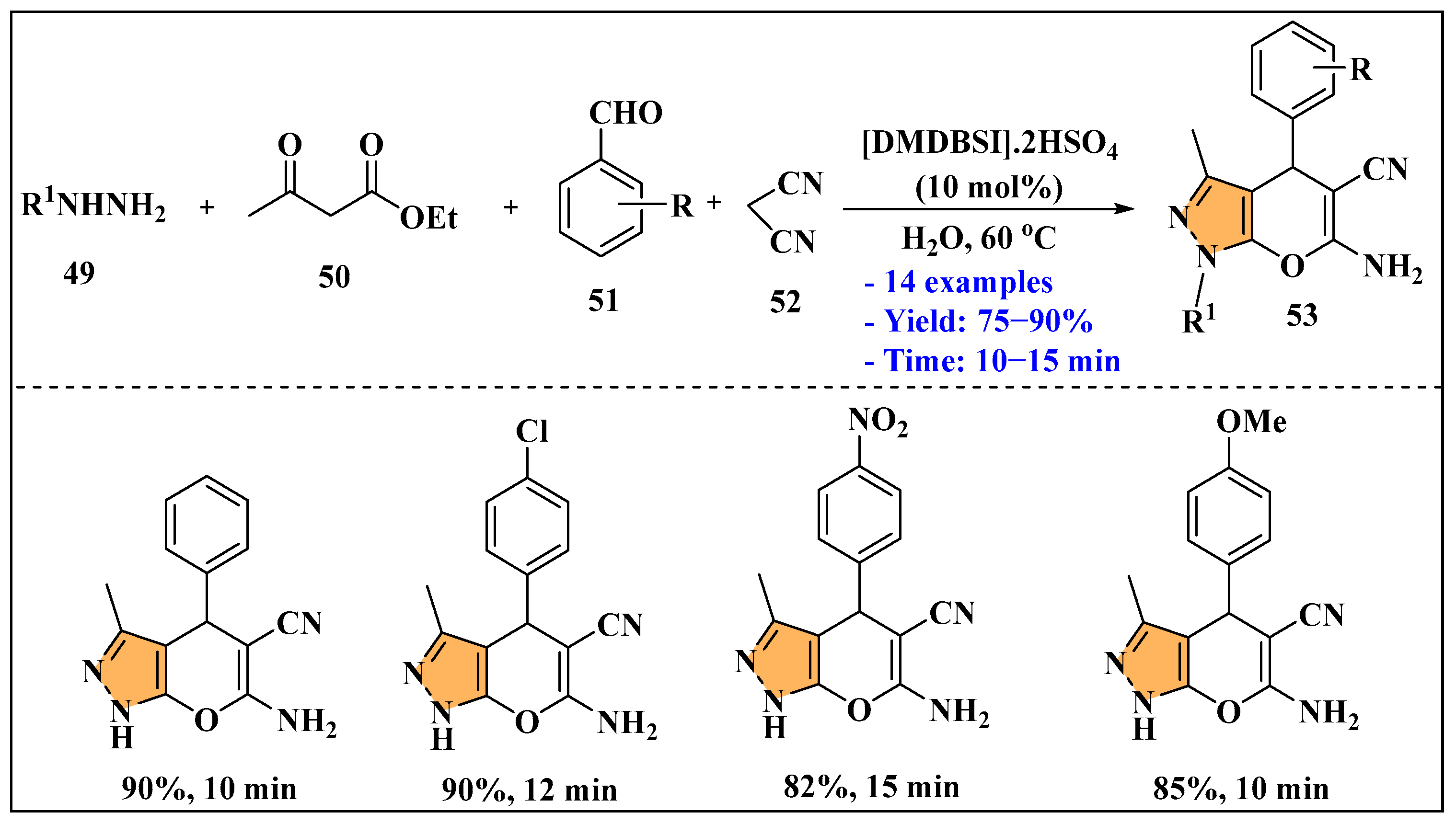
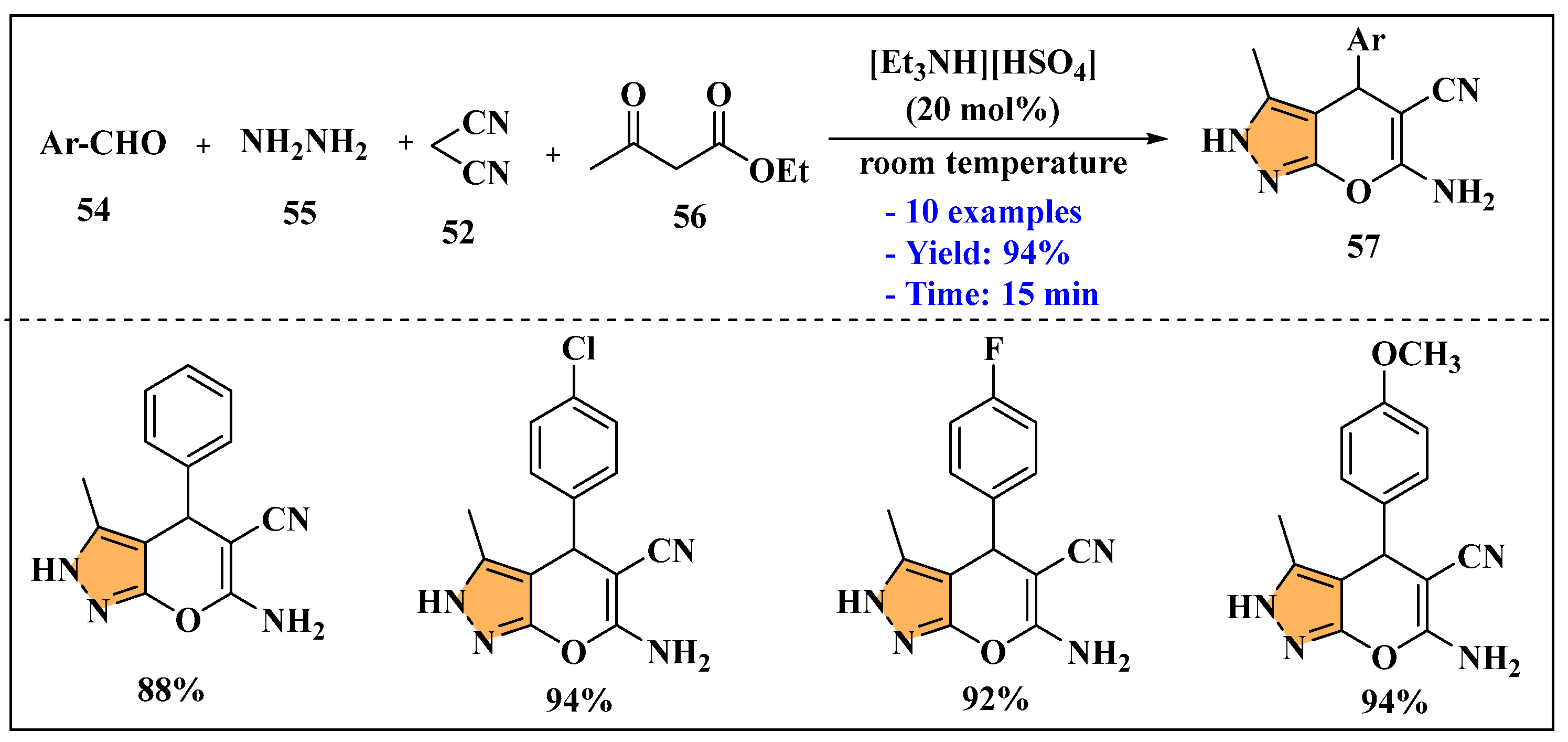
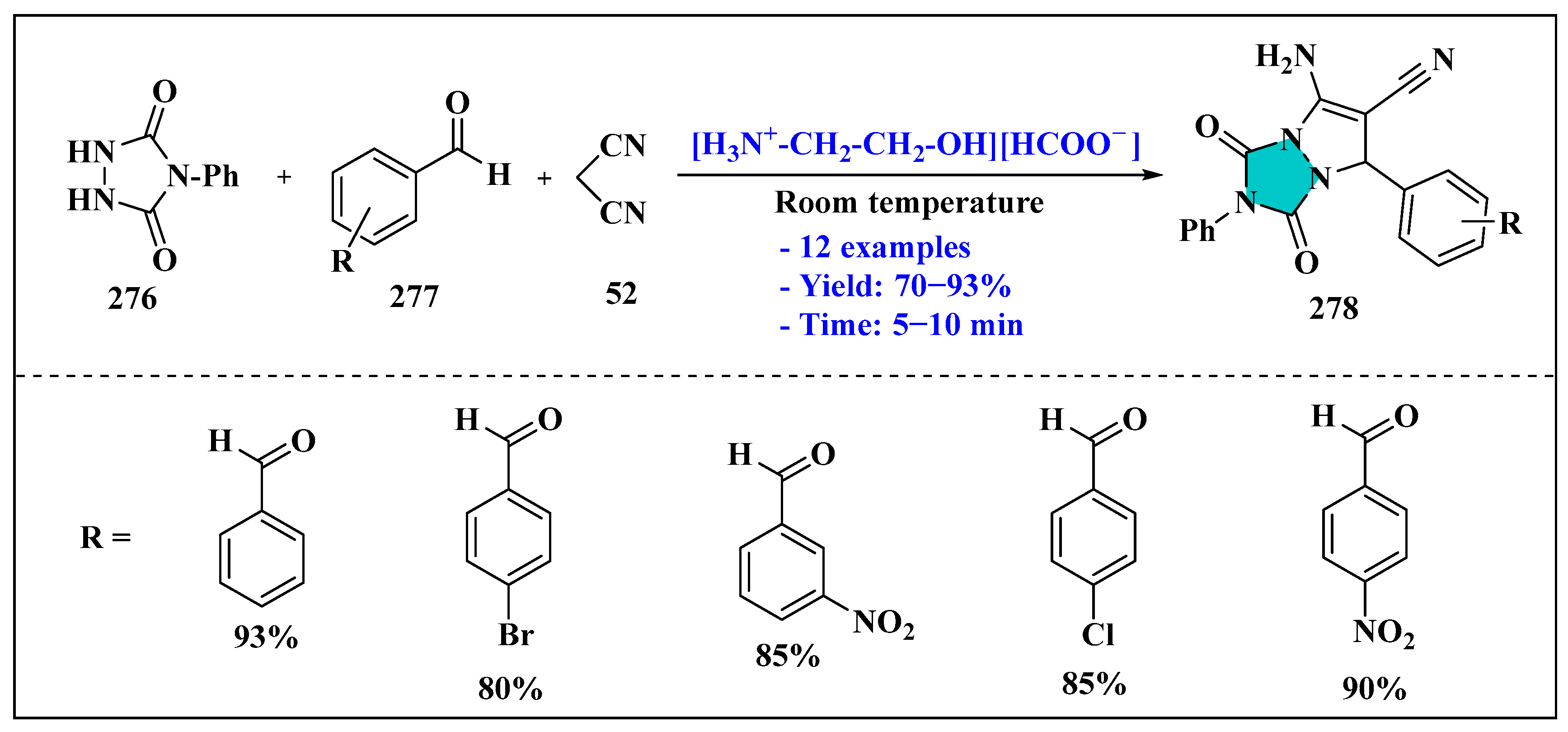
3.2.2. Ionic Liquids Mediated Multicomponent Reaction for Pyrazole Synthesis in Homogenous Phase


3.2.3. Ionic Liquids Mediated Functionalization of Pyrazole in Homogeneous Phase


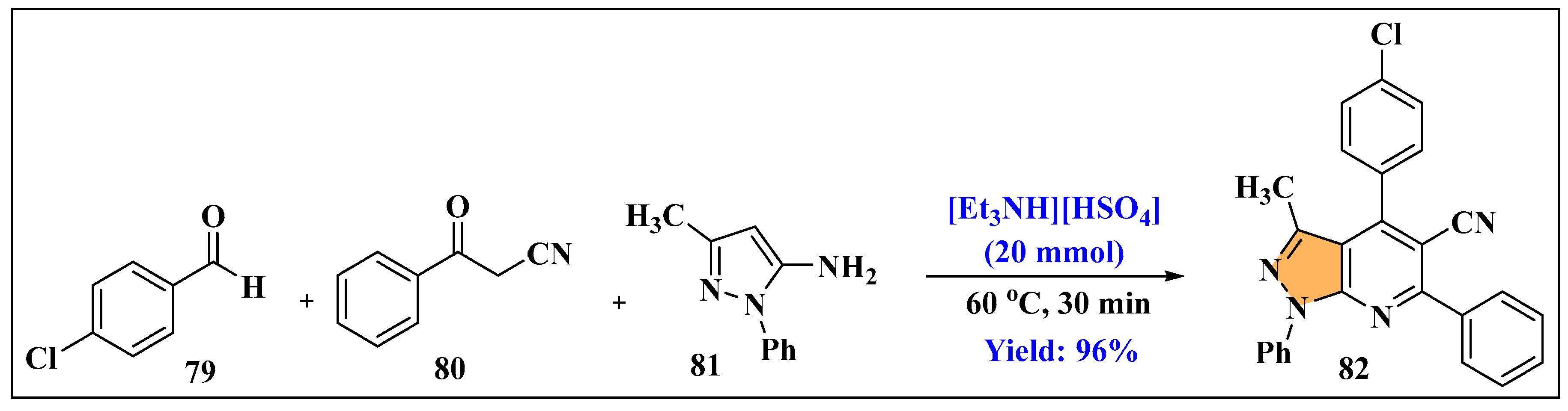
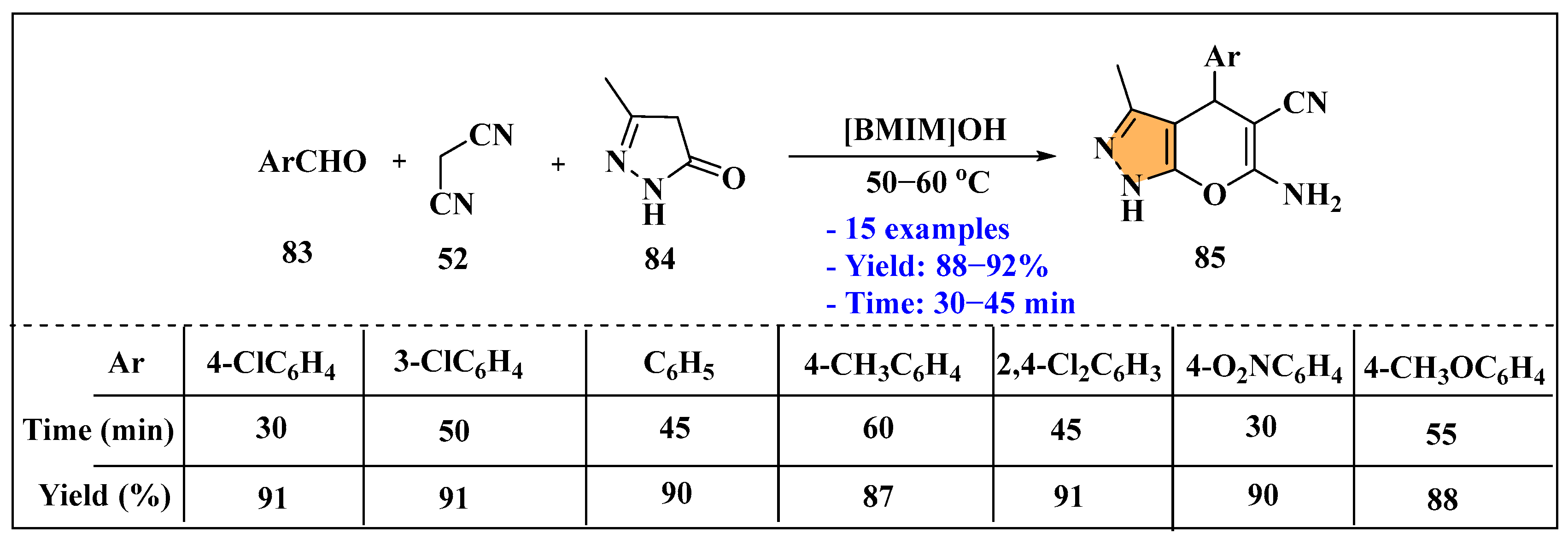

3.2.4. Ionic Liquids Mediated Paal–Knorr Reaction in Heterogeneous Phase
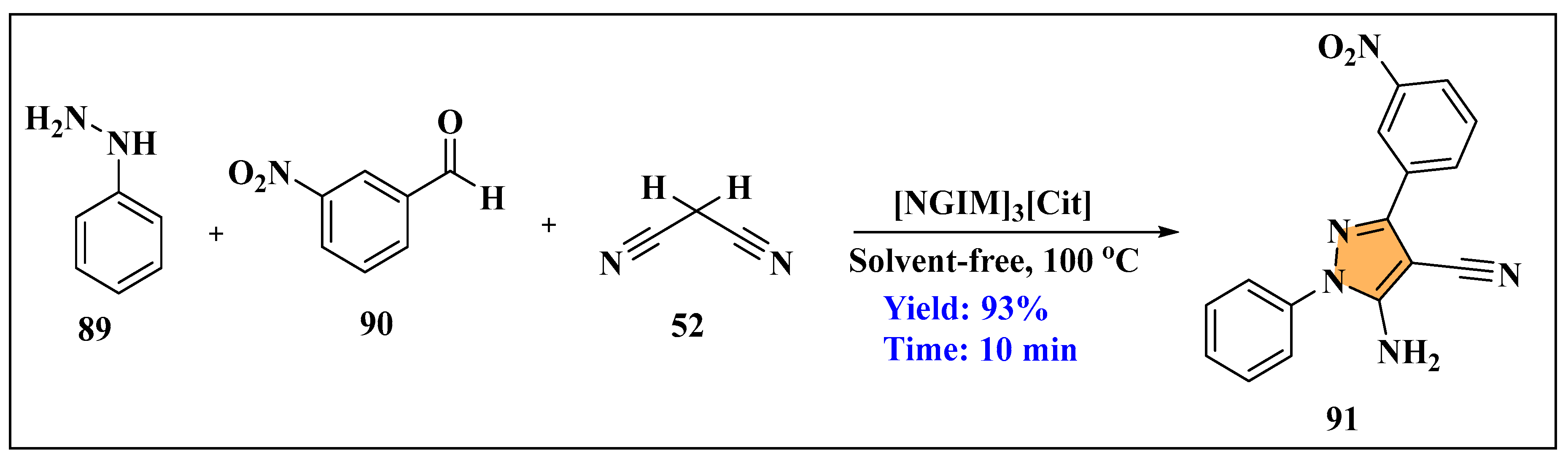


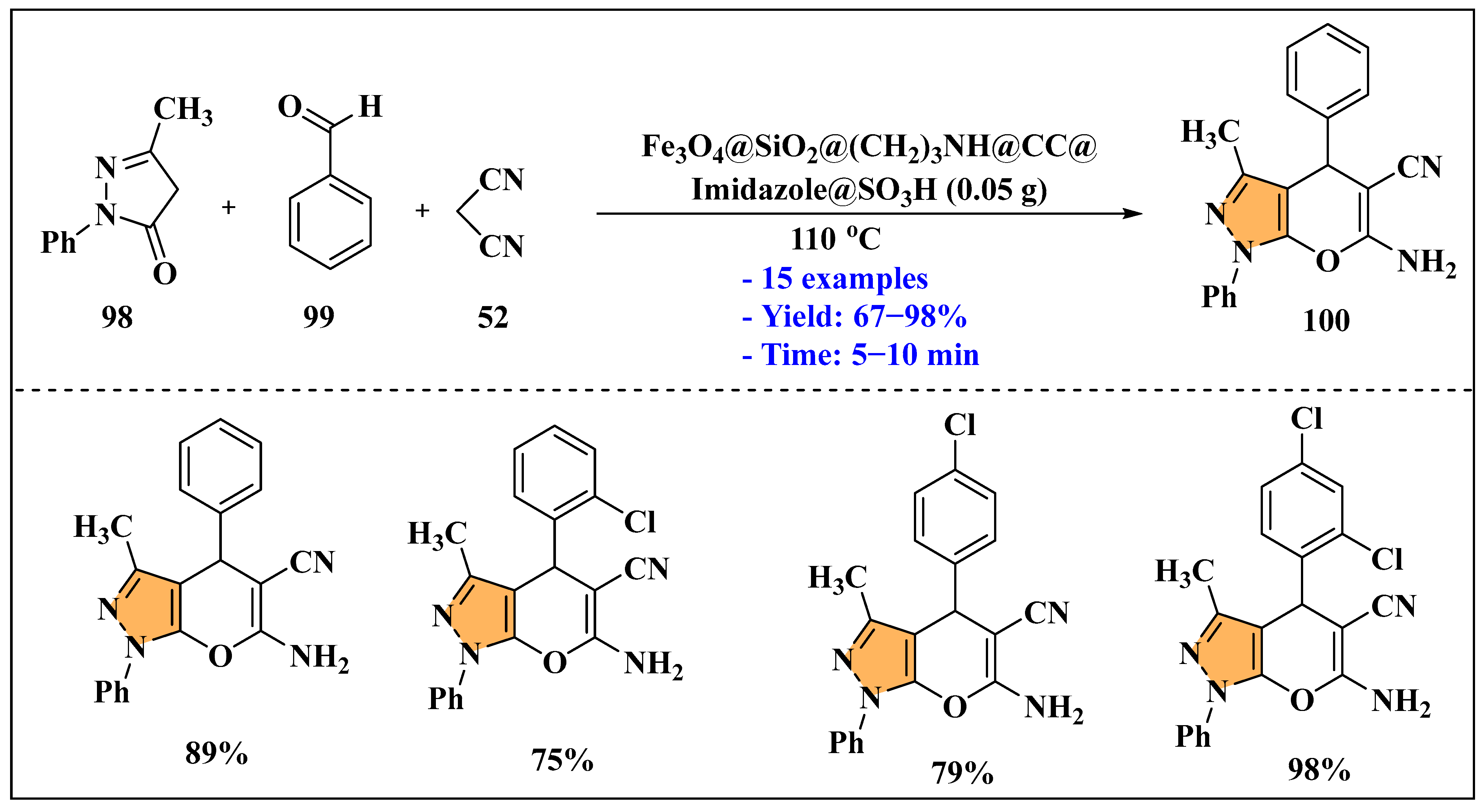
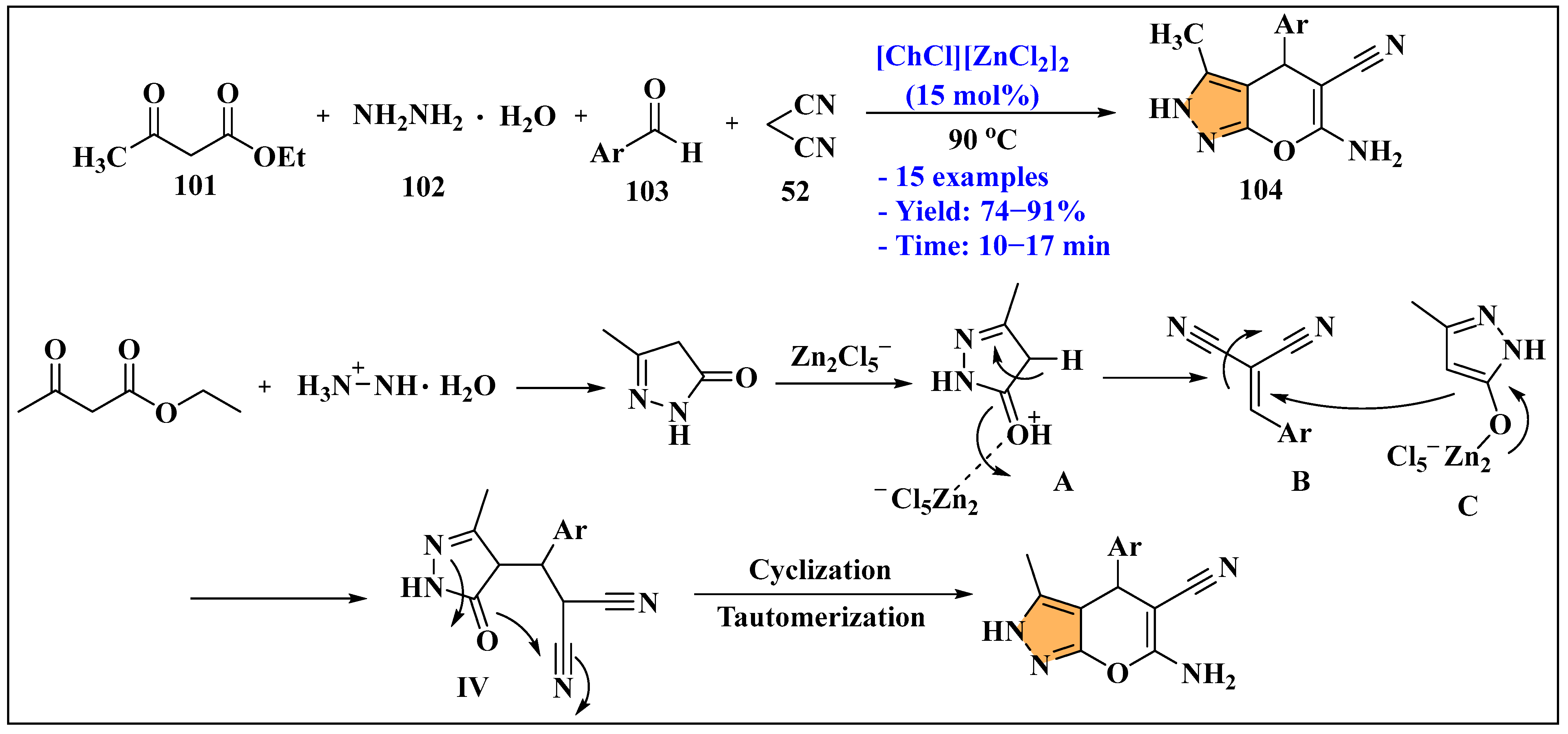

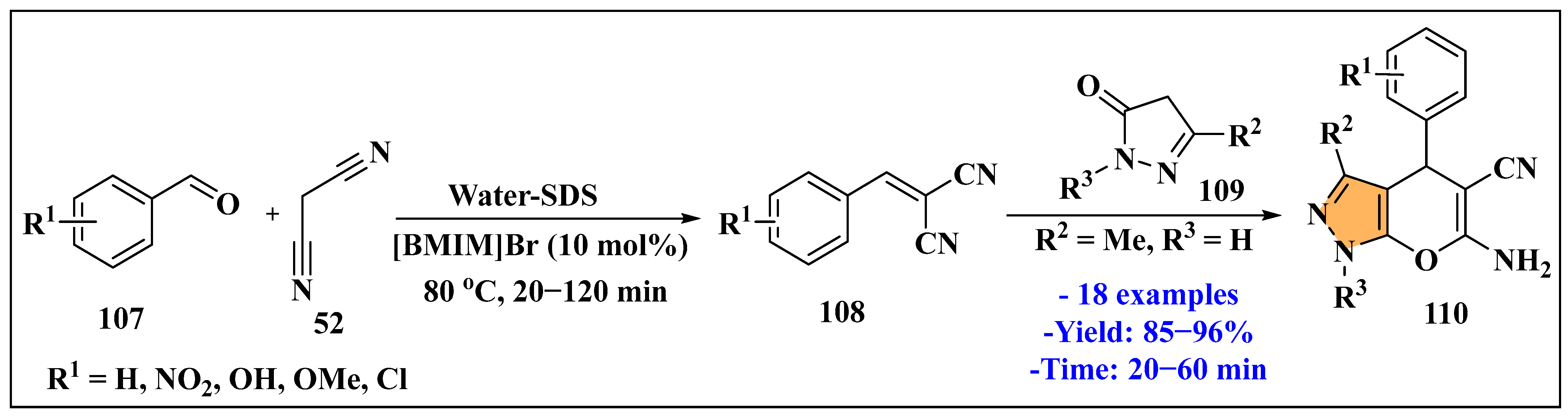
3.3. Synthesis of Imidazole Derivatives Using Ionic Liquids
3.3.1. Synthesis of Imidazole Derivatives in Homogeneous Phase
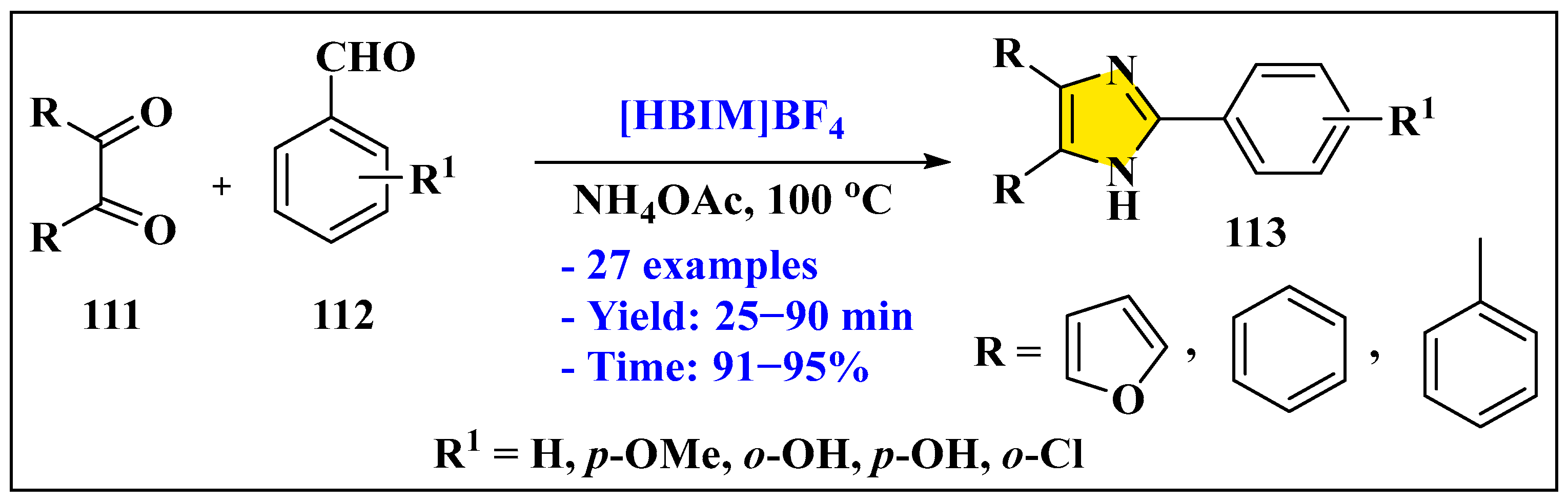
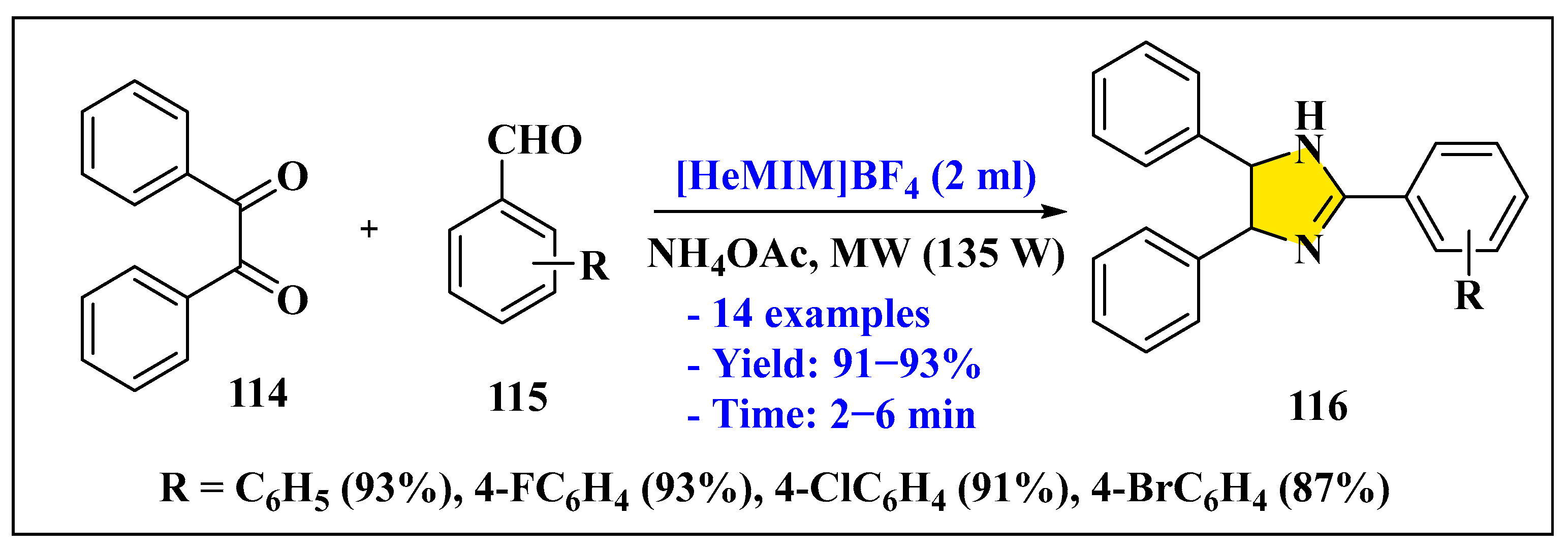
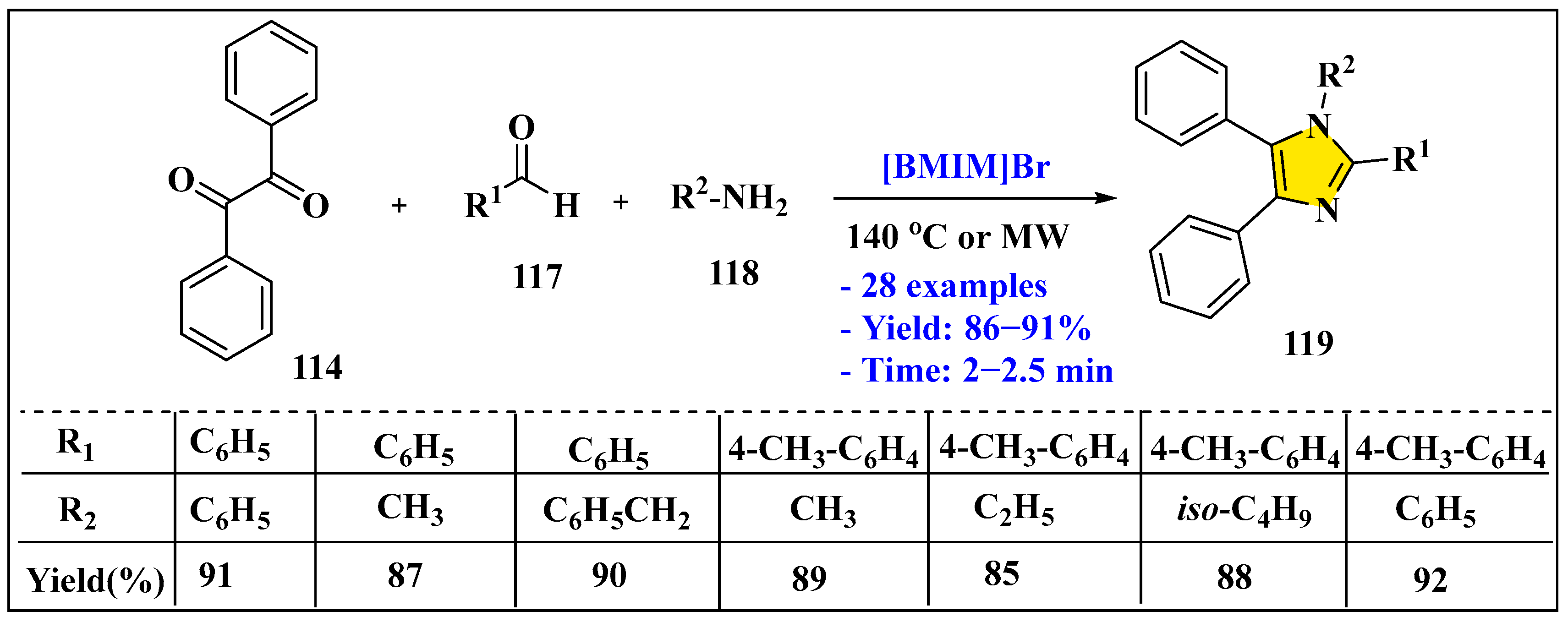


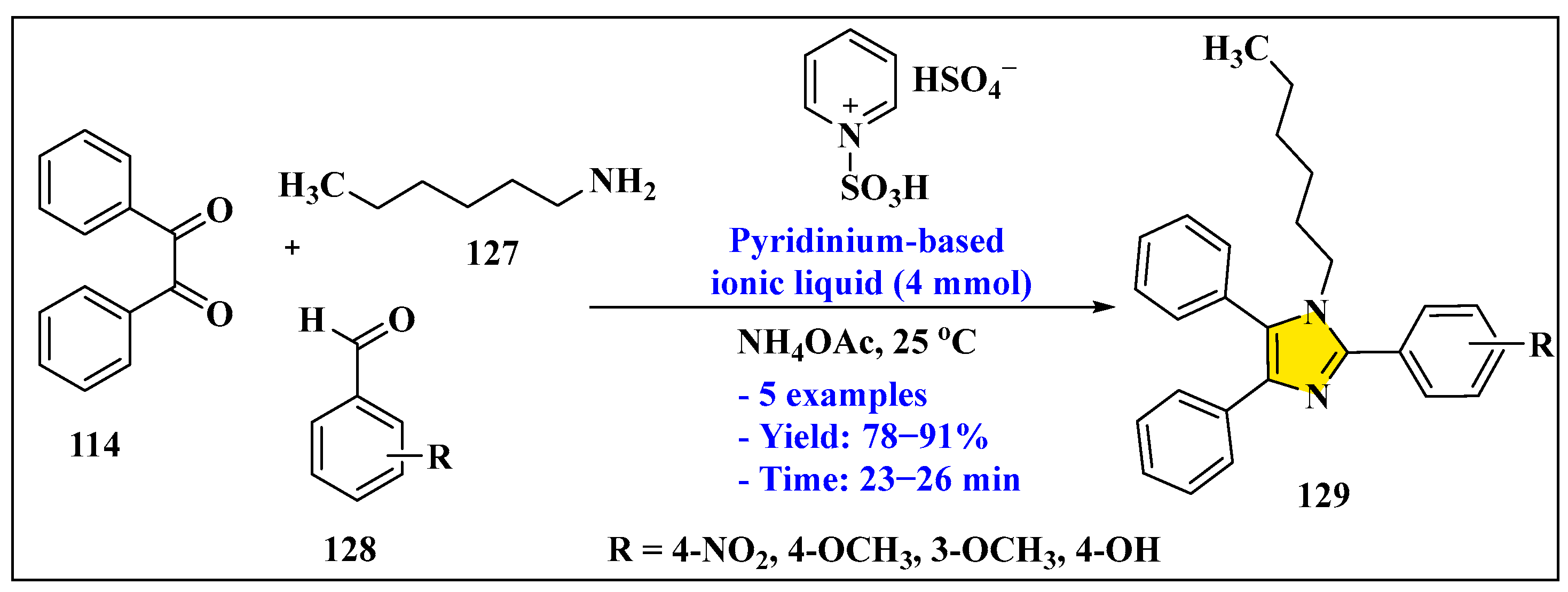
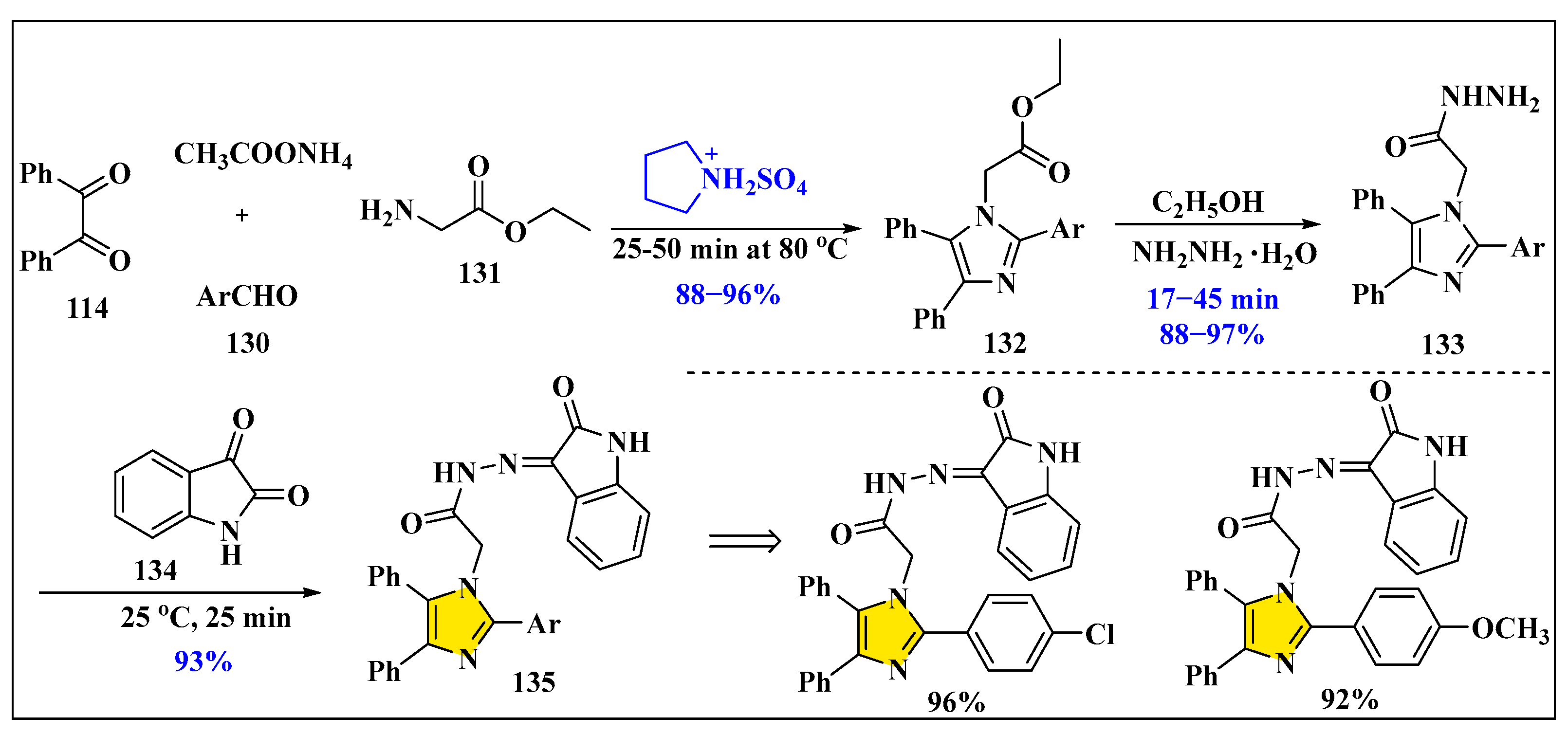
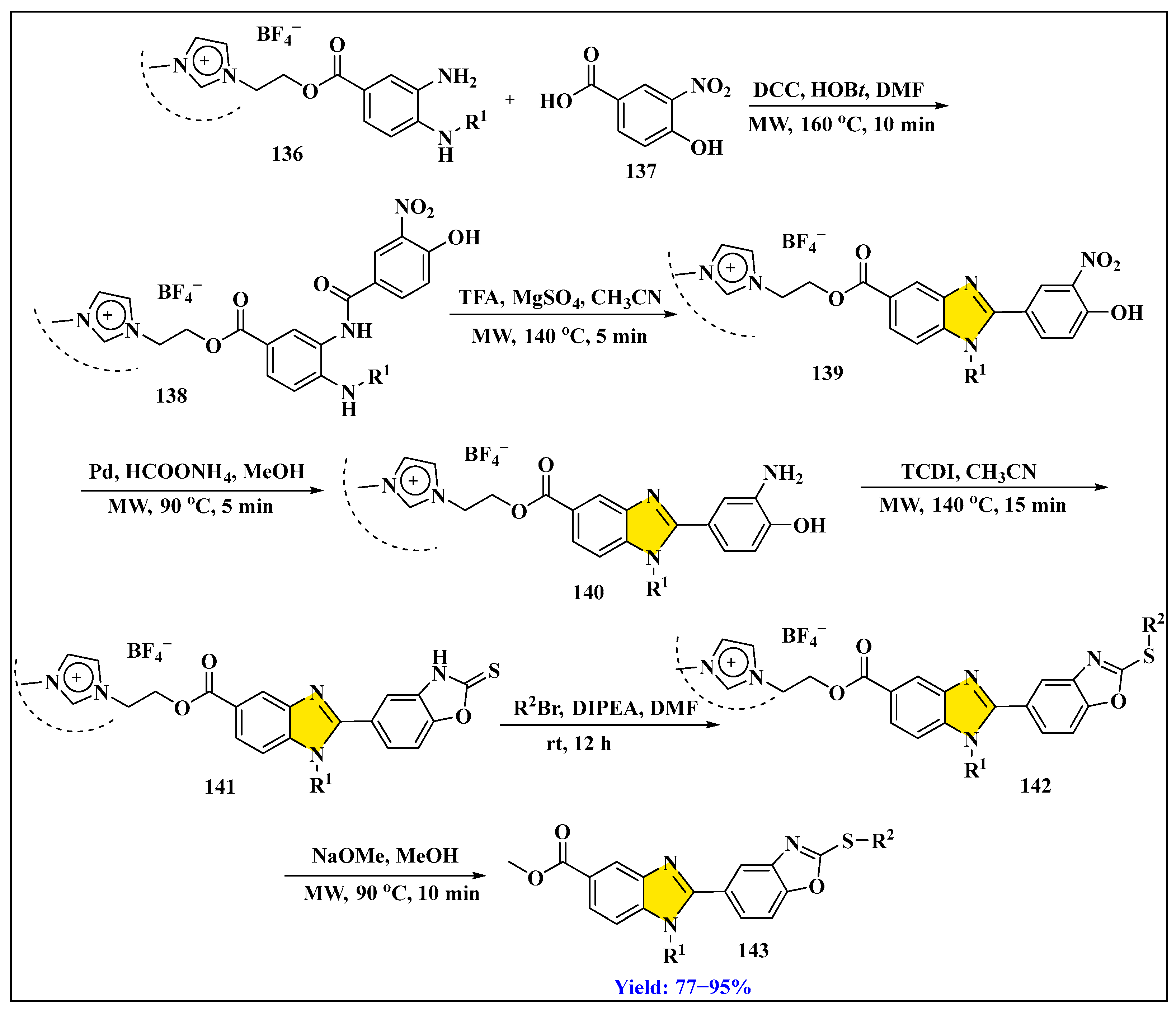
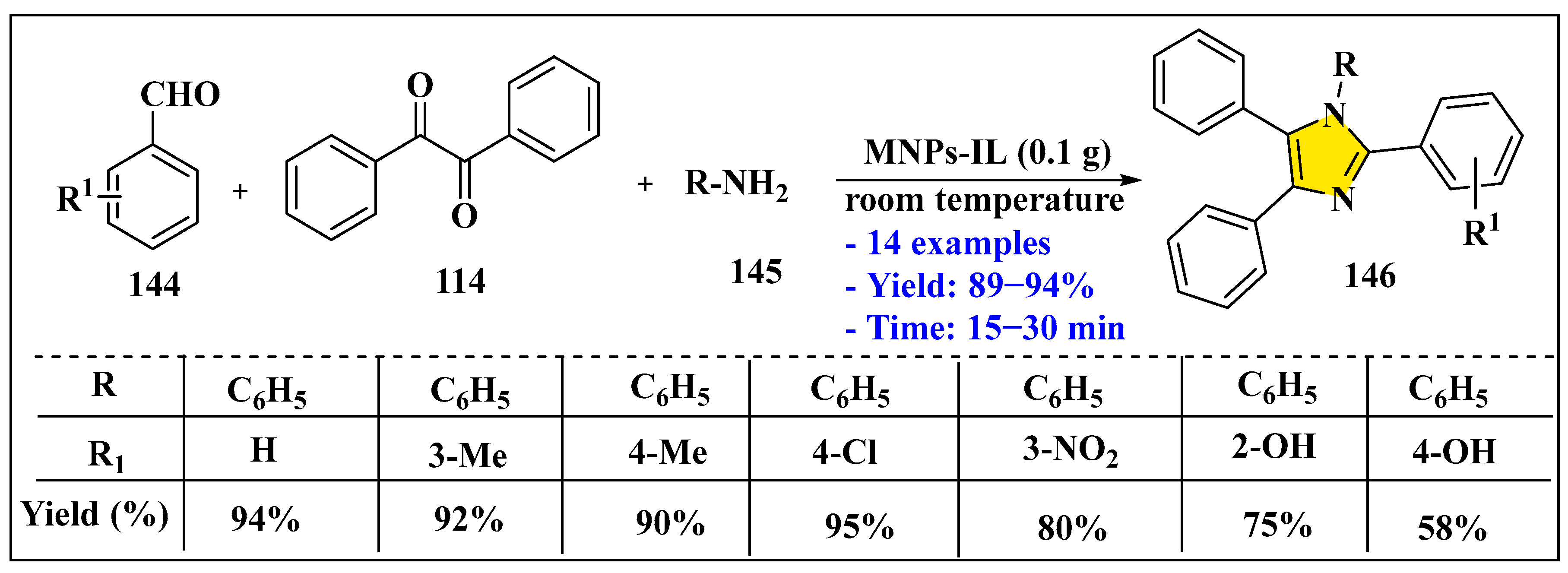
3.3.2. Synthesis of Imidazole Derivatives in Heterogeneous Phase


3.4. Synthesis of 1,2,3-Triazole Derivatives Using Ionic Liquids
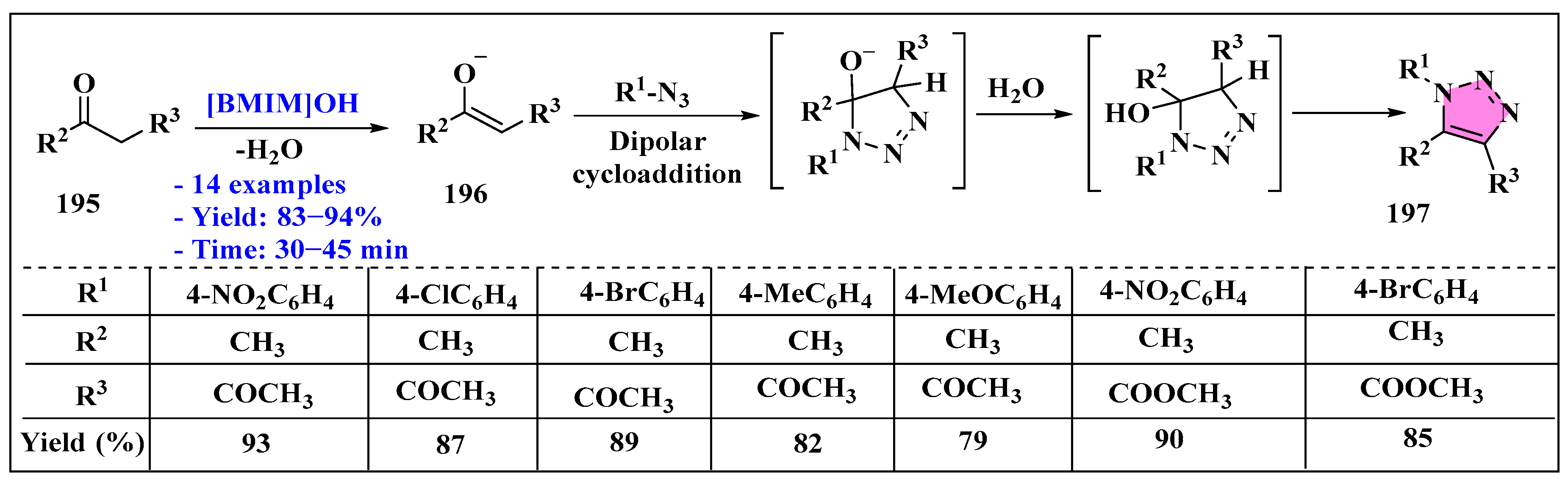
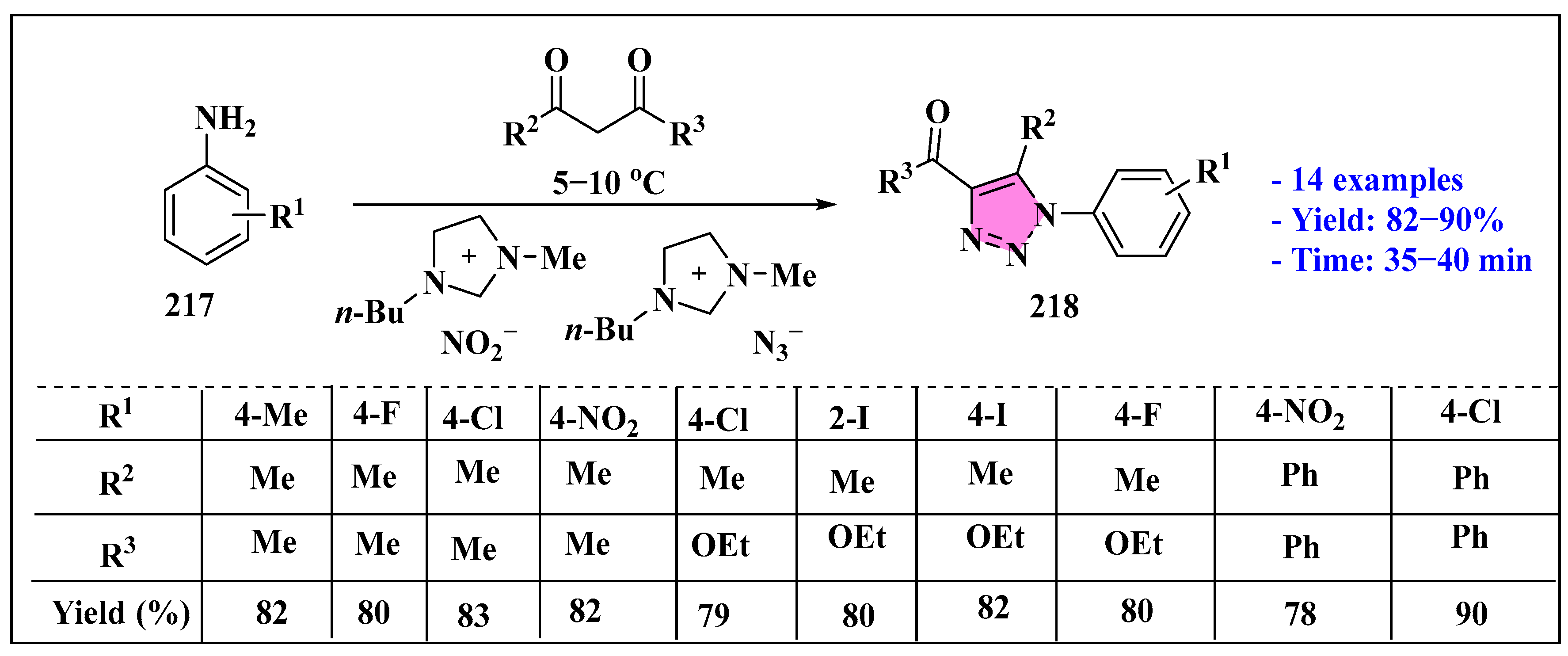
3.4.1. Ionic Liquids Mediated Cycloaddition Reaction in Homogeneous Phase
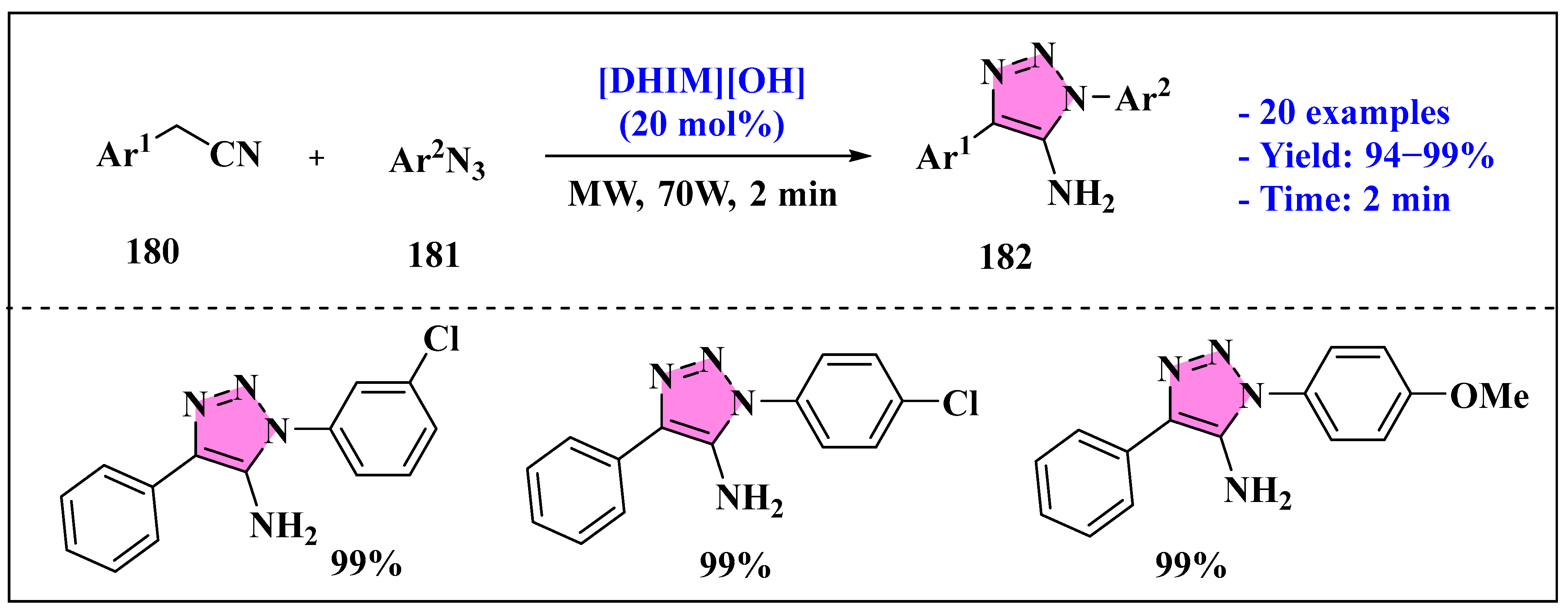
3.4.2. Ionic Liquids Mediated Miscellaneous Reaction for Triazole Synthesis in Homogeneous Phase
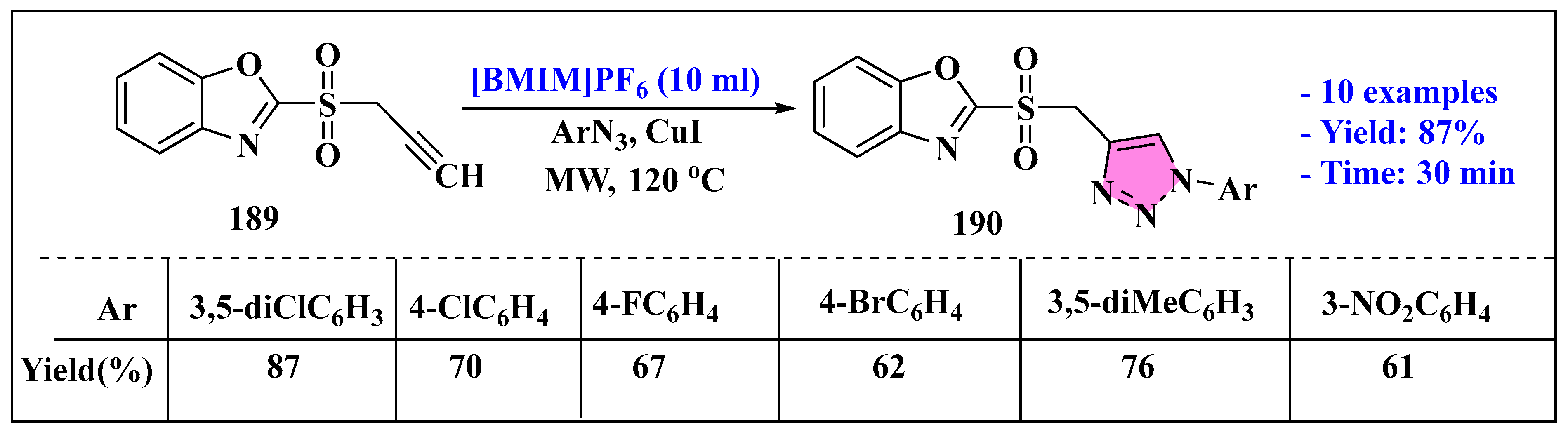
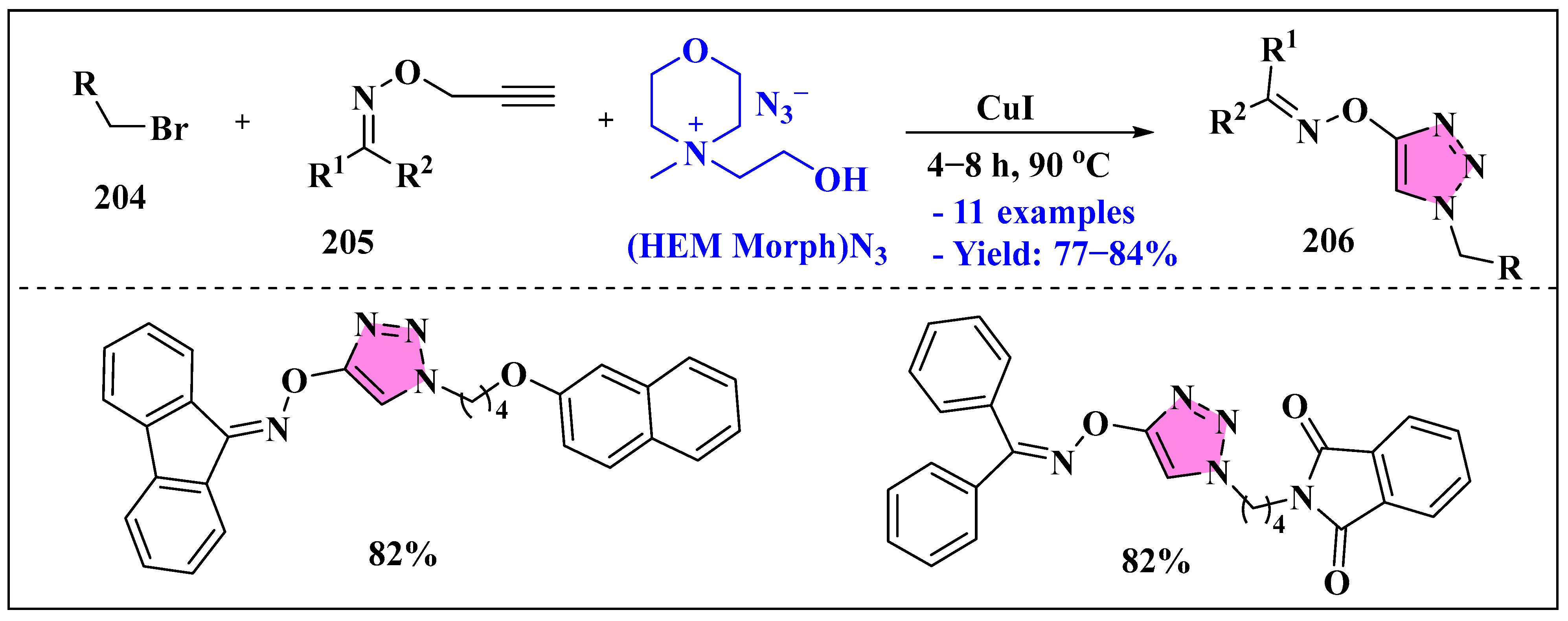
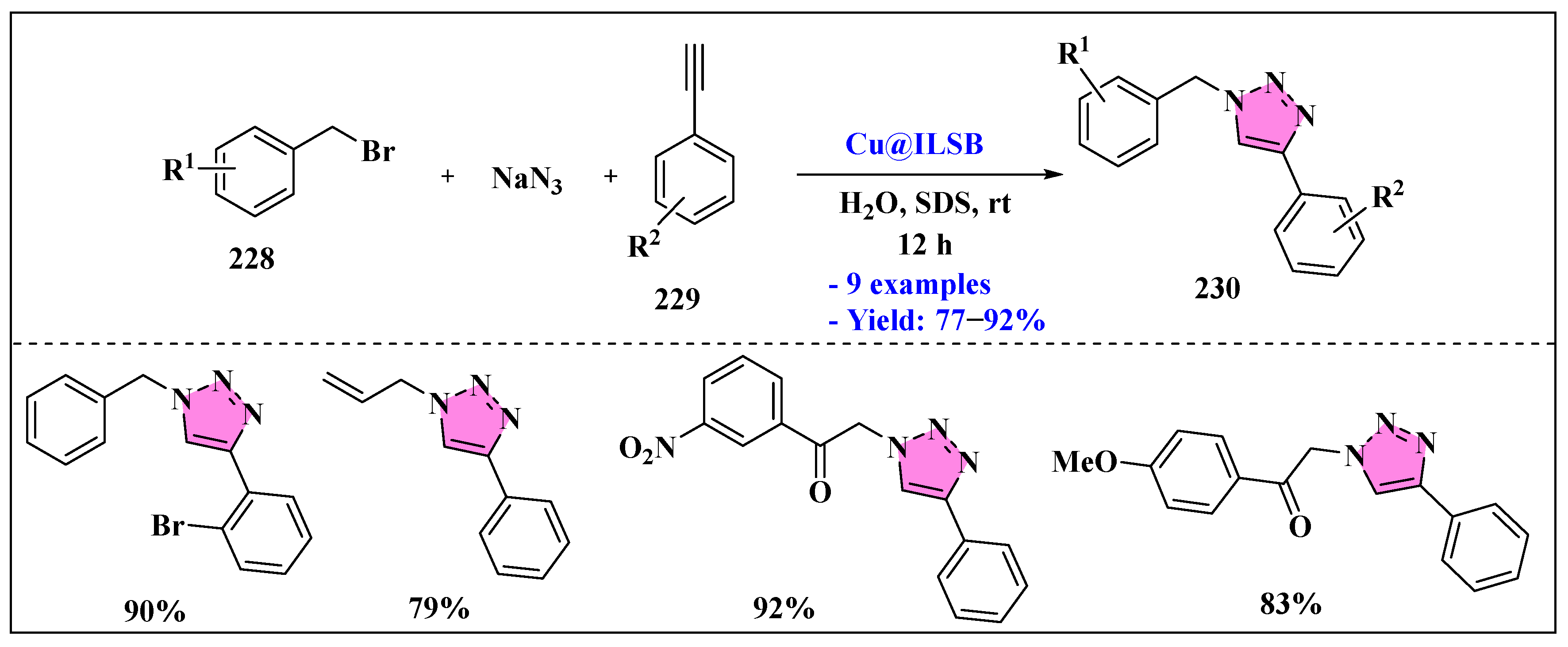
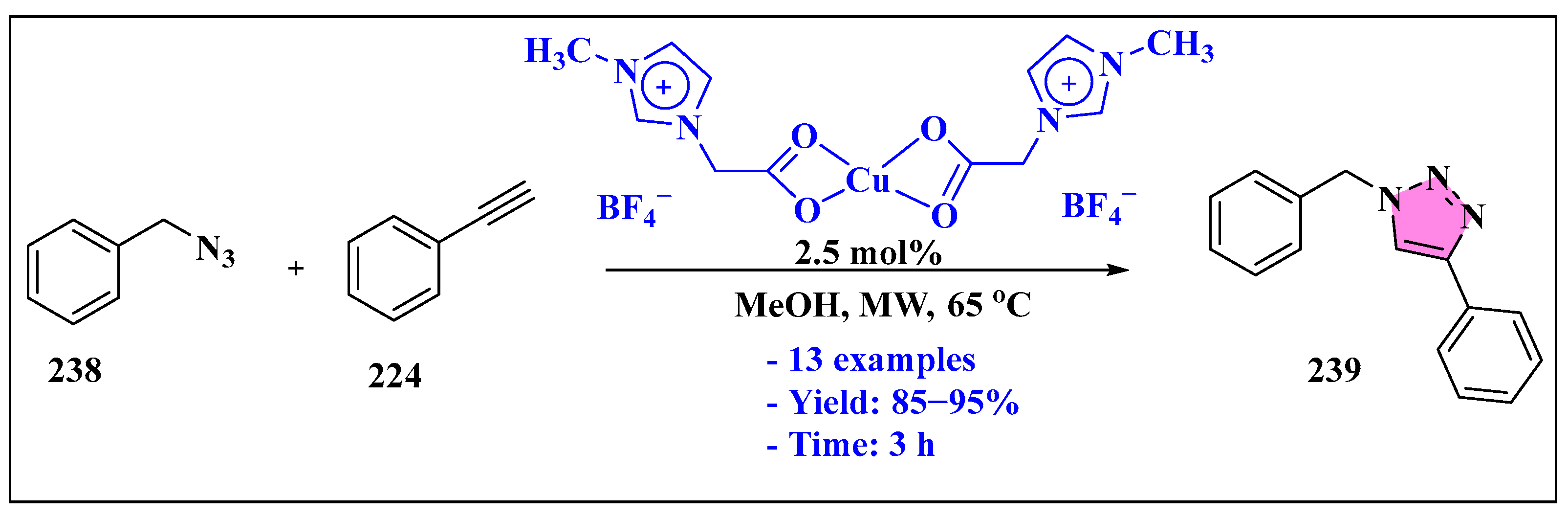
3.4.3. Ionic Liquids Mediated Functionalization of Triazole in Homogenous Phase
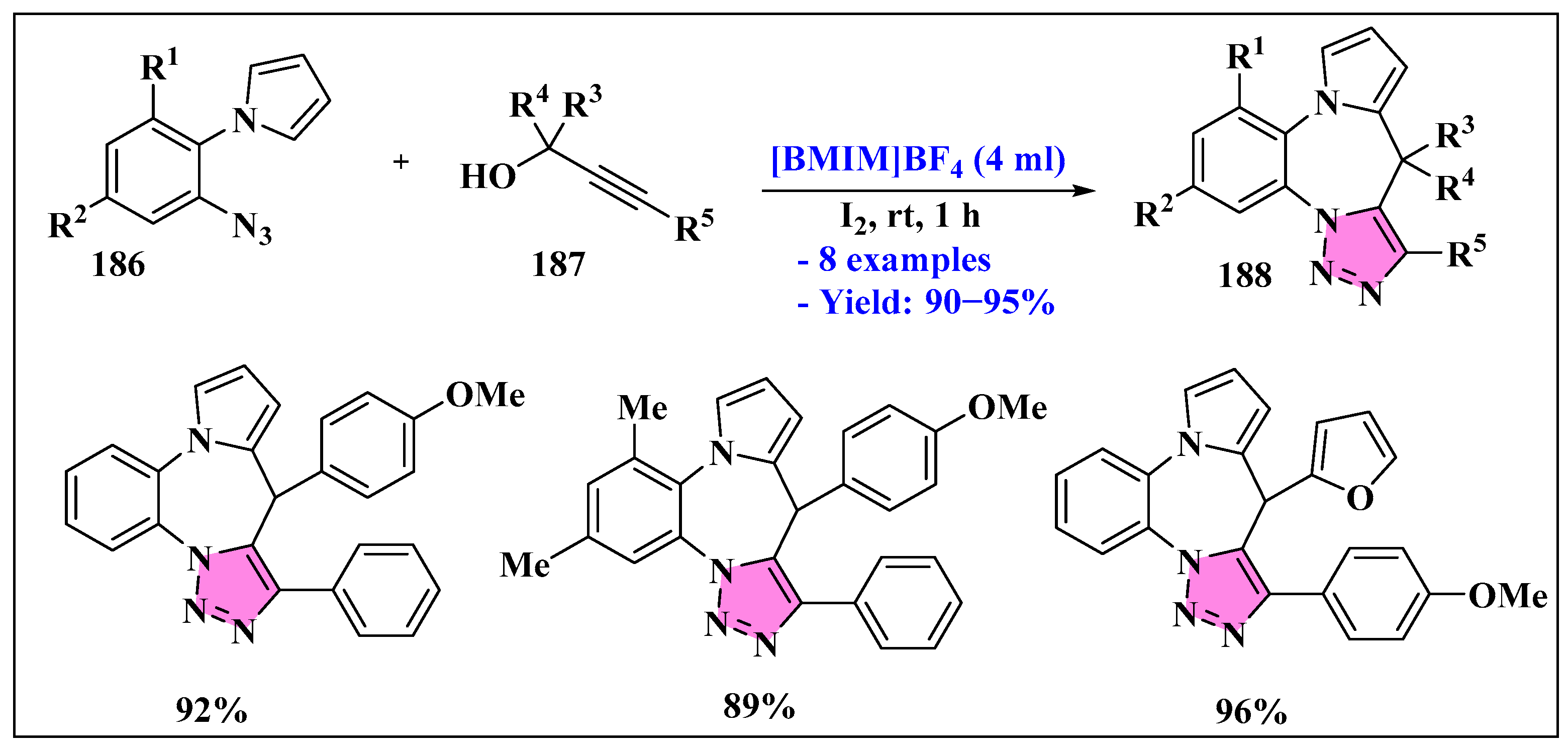
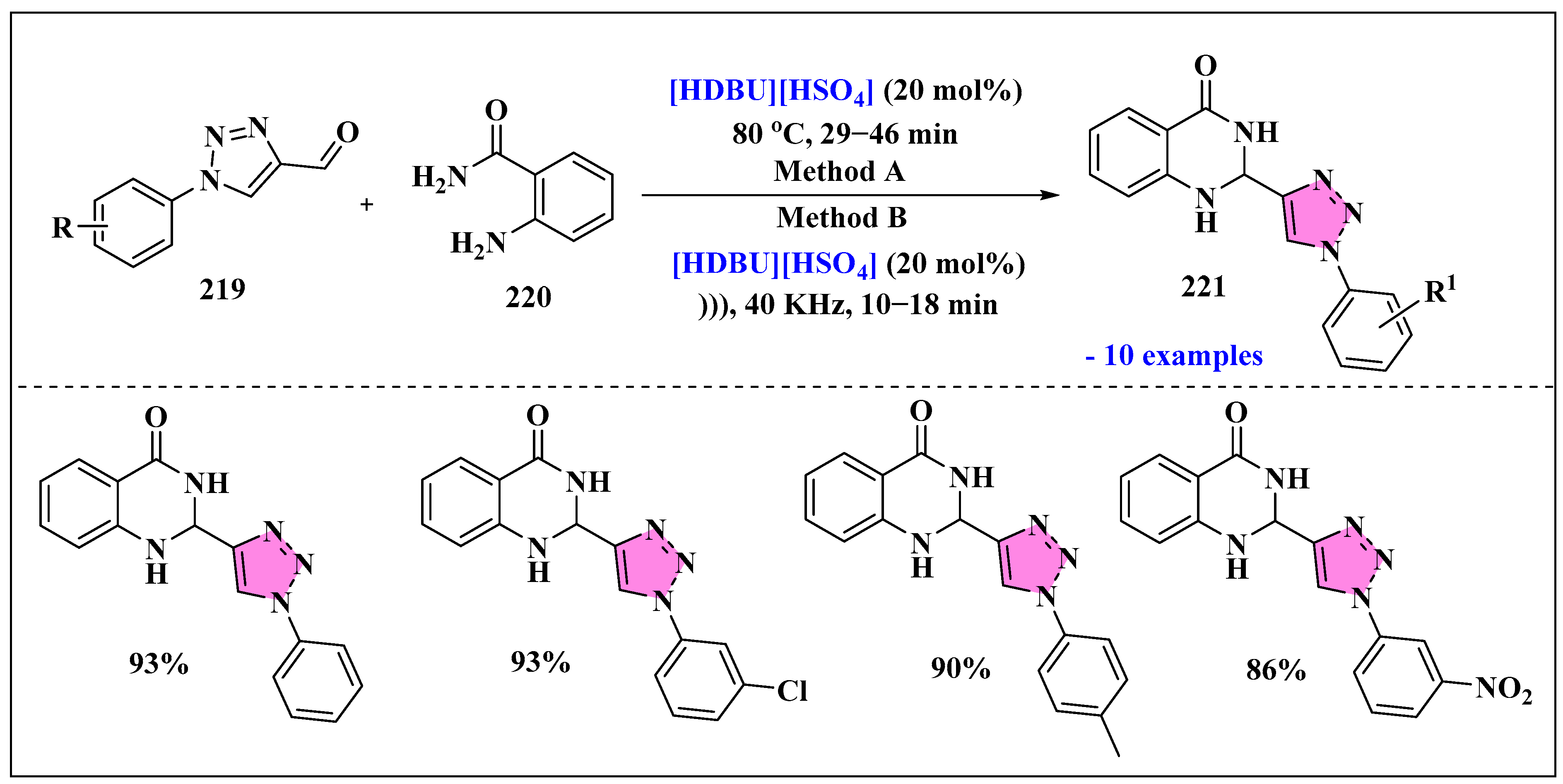
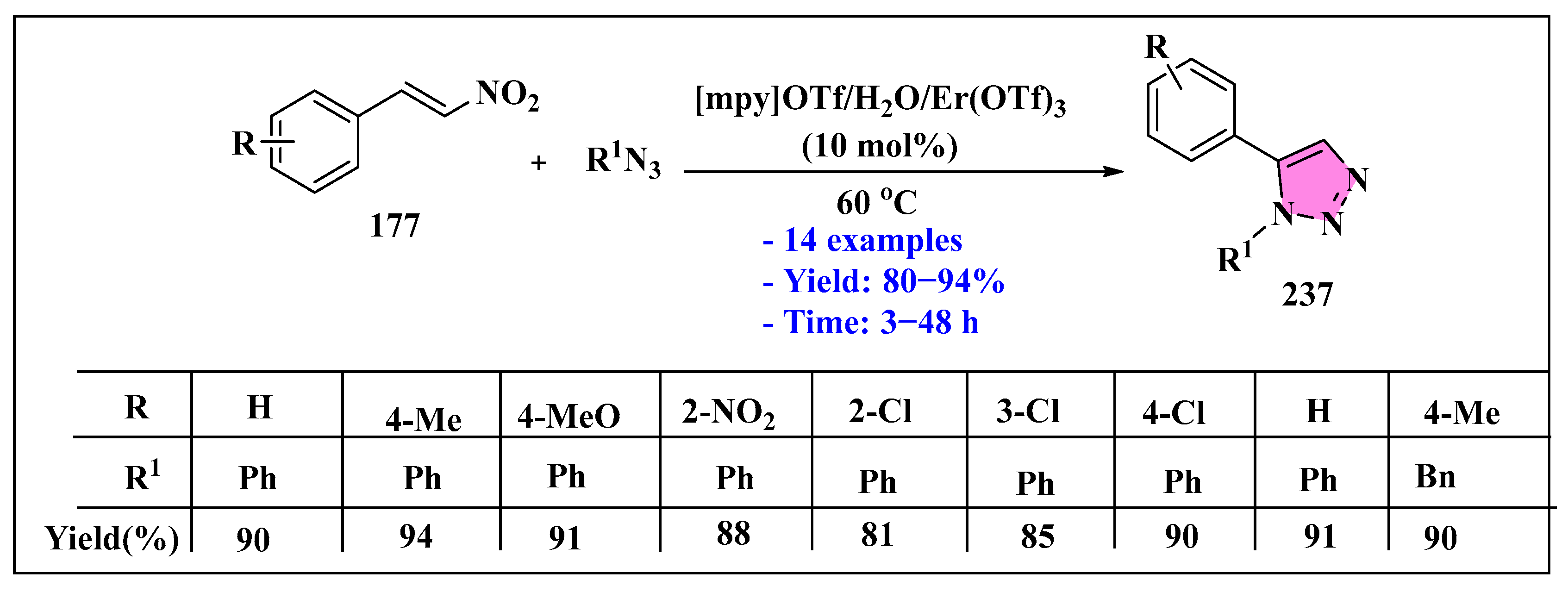
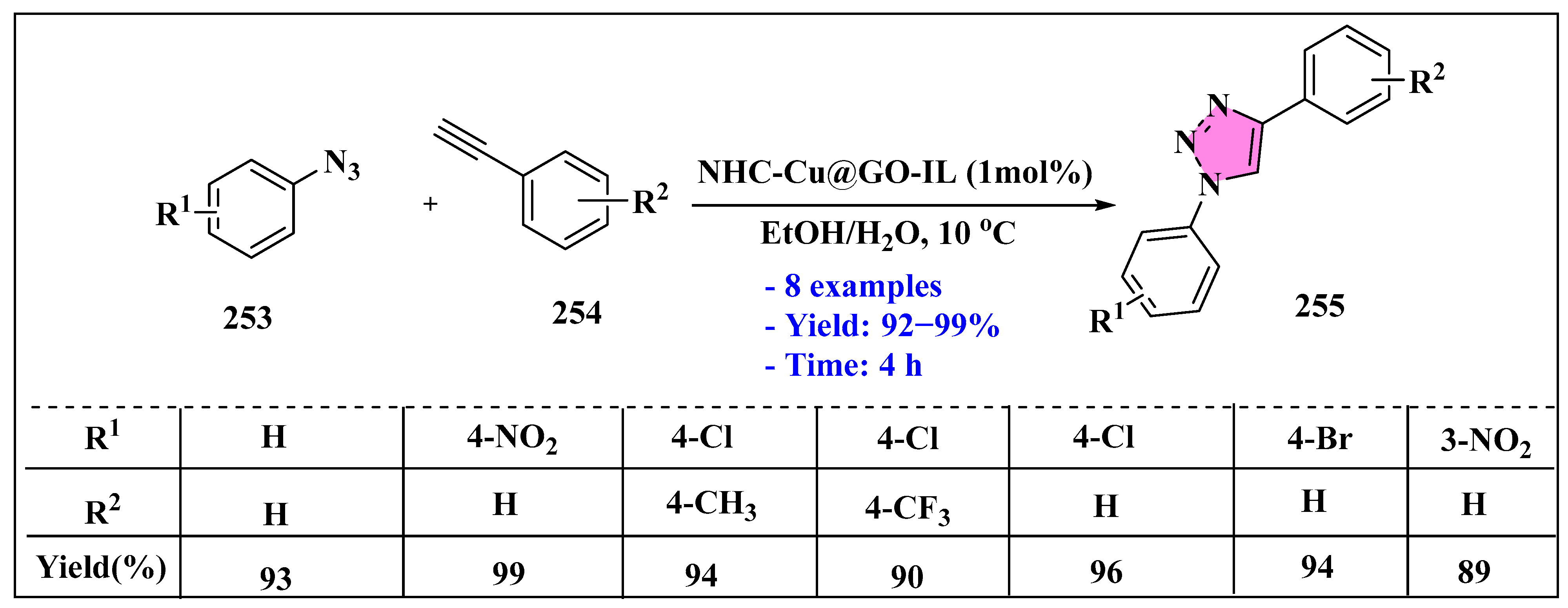
3.4.4. Ionic Liquids Mediated Cycloaddition Reaction in Heterogeneous Phase
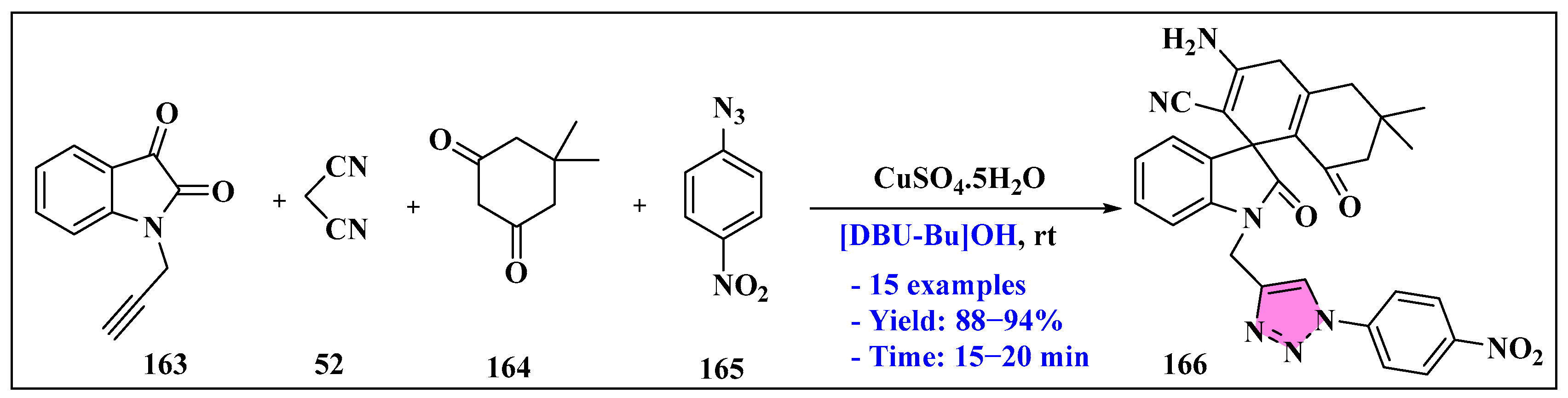
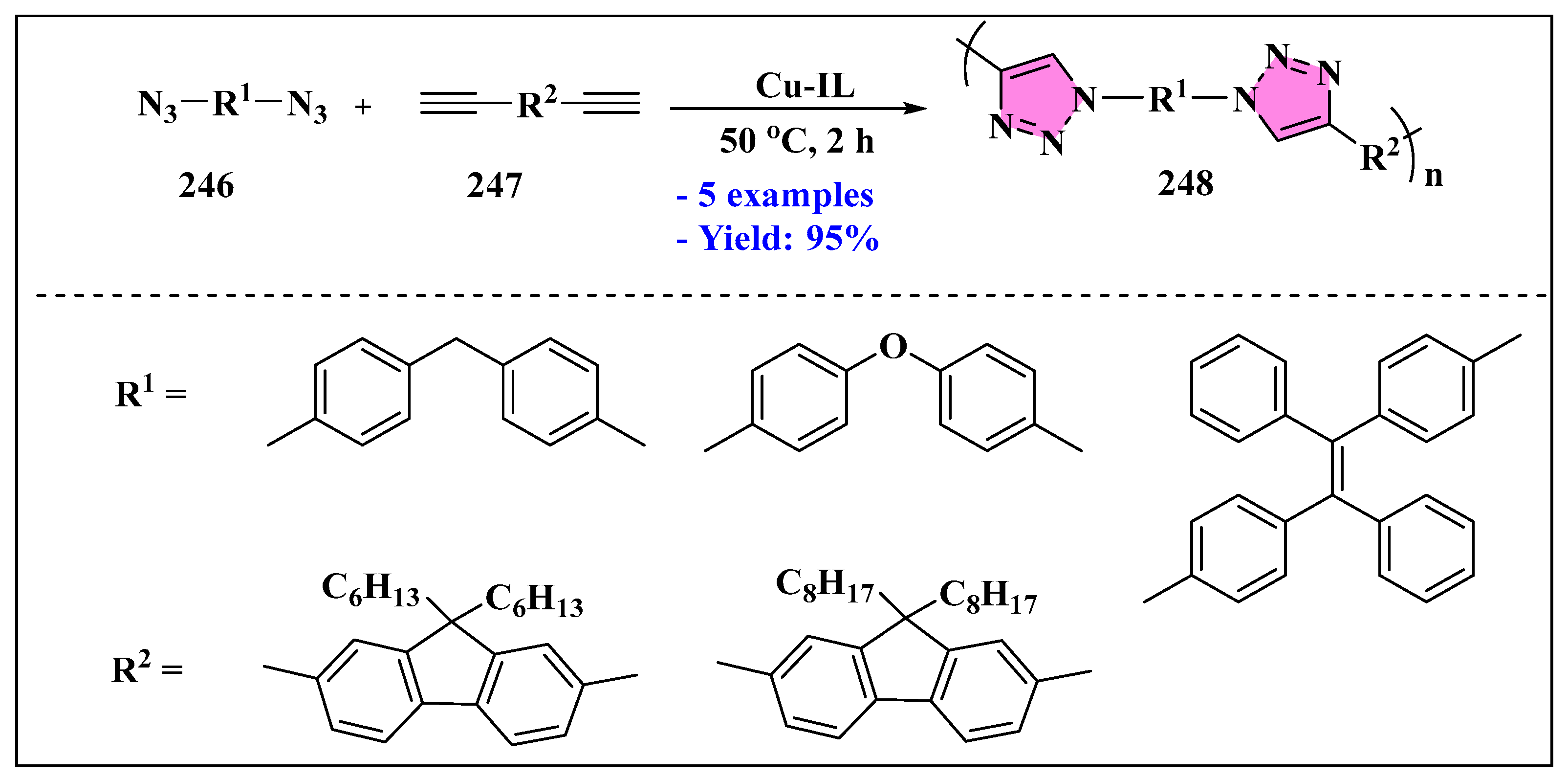
3.4.5. Ionic Liquids Mediated Miscellaneous Reaction in Heterogeneous Phase
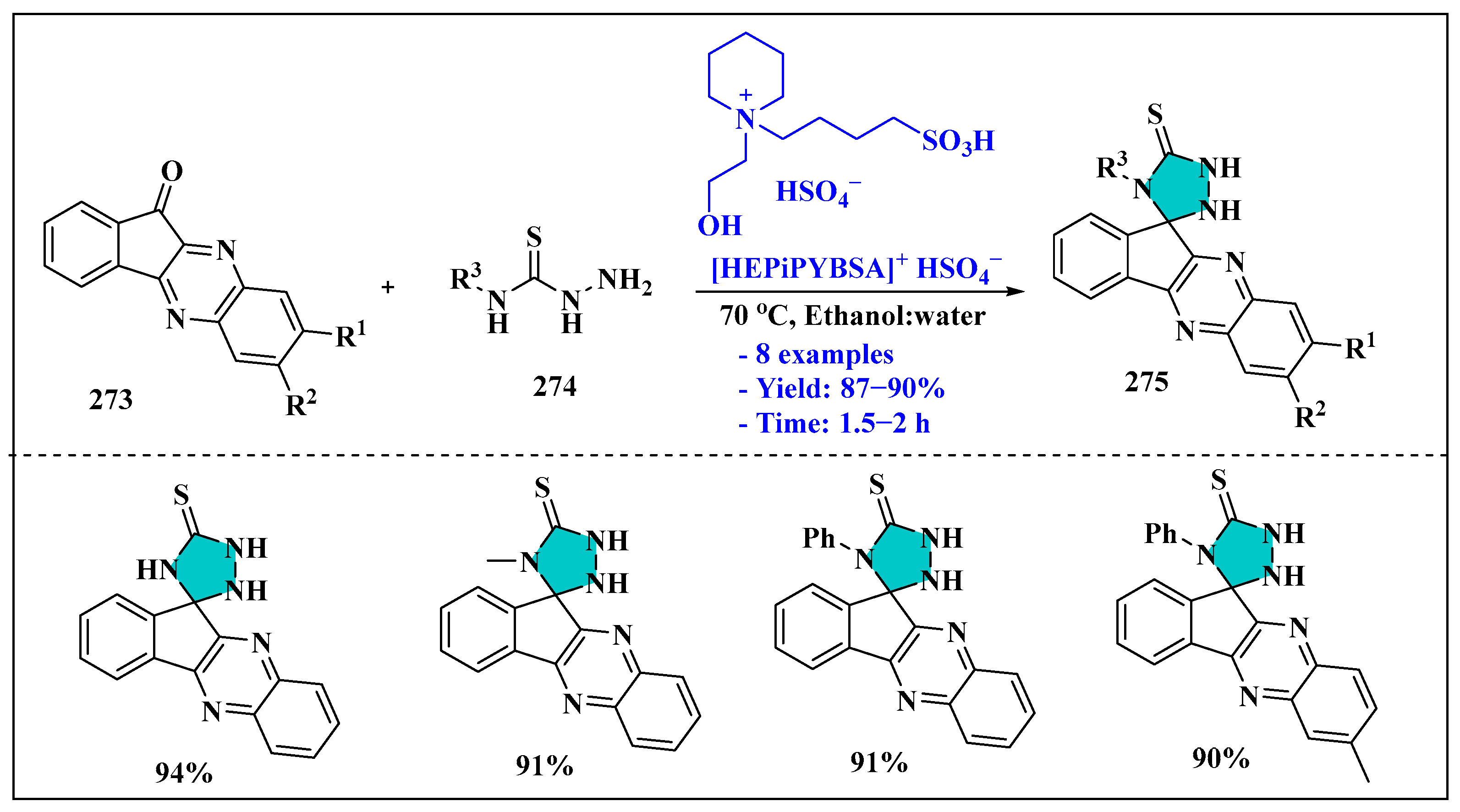
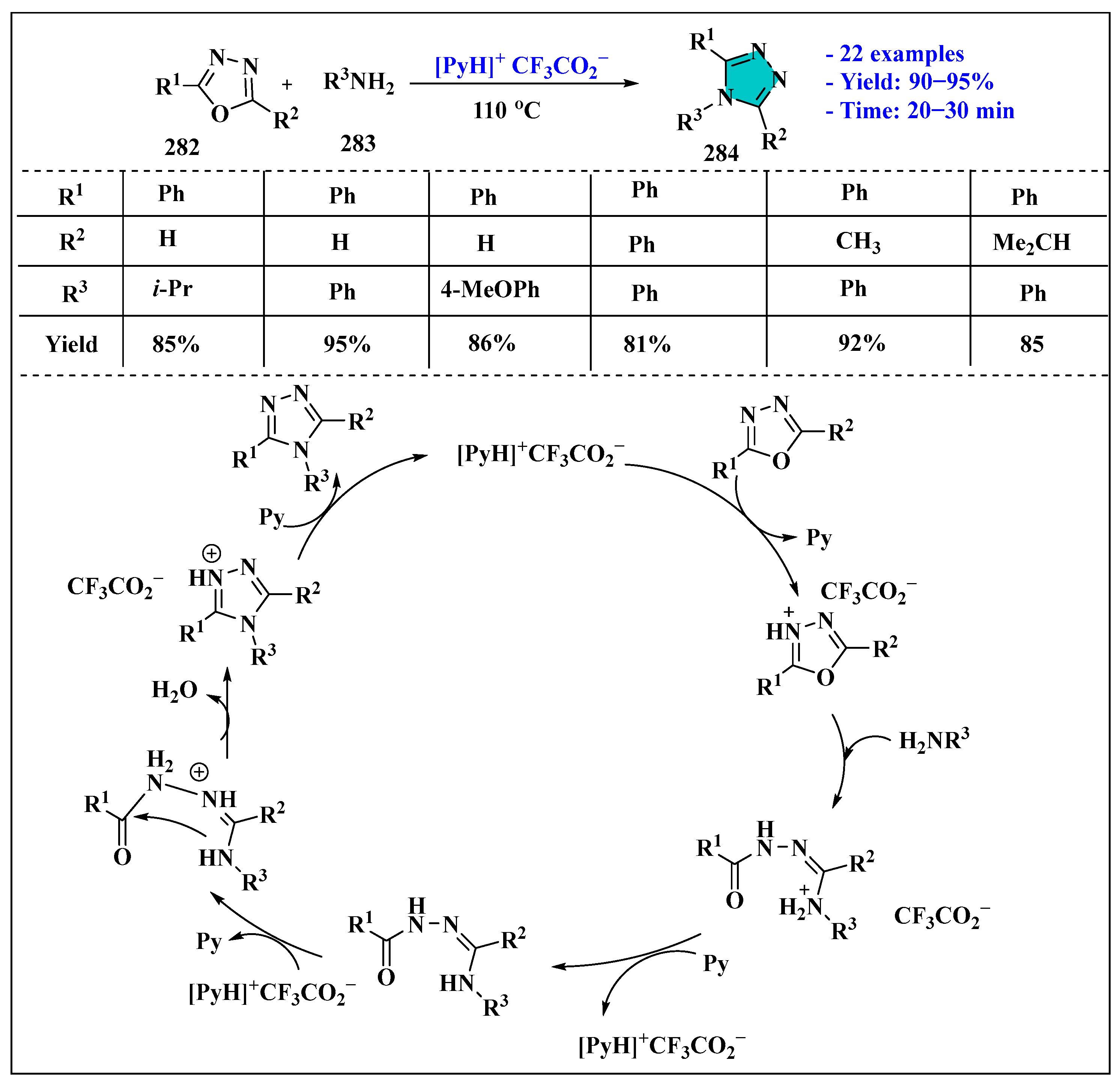
3.5. Synthesis of 1,2,4-Triazole Using Ionic Liquids
Ionic Liquids Mediated Synthesis of 1,2,4-Triazole Derivatives in Homogenous Phase
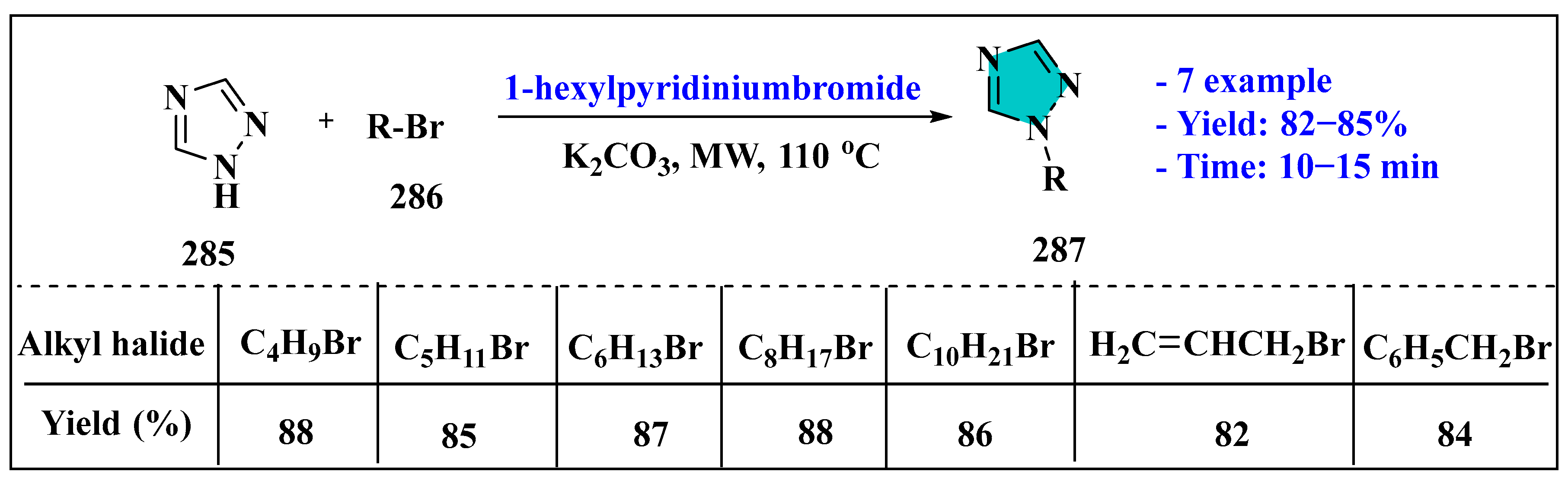
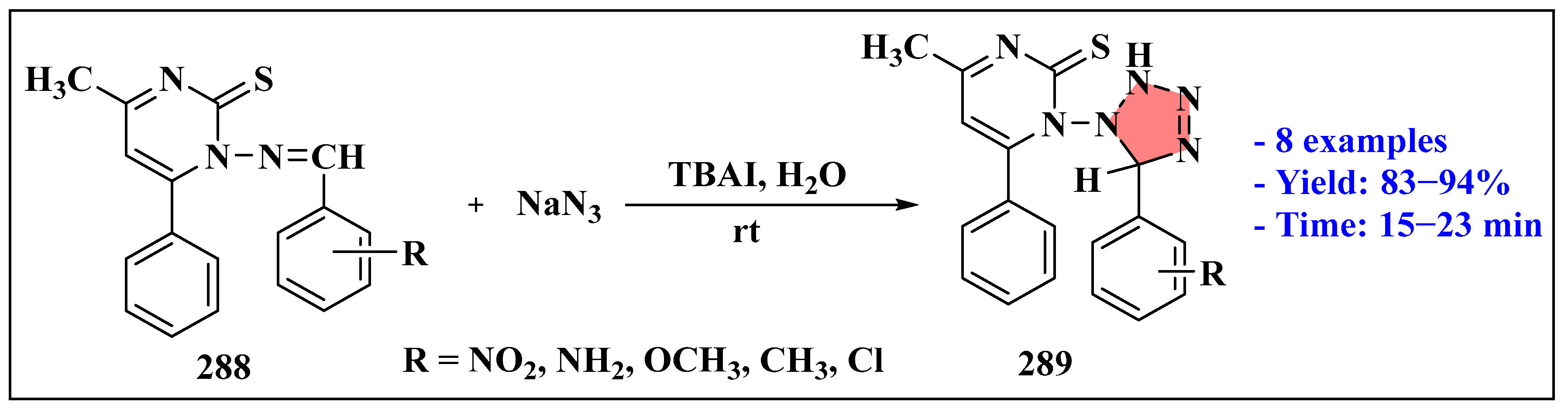
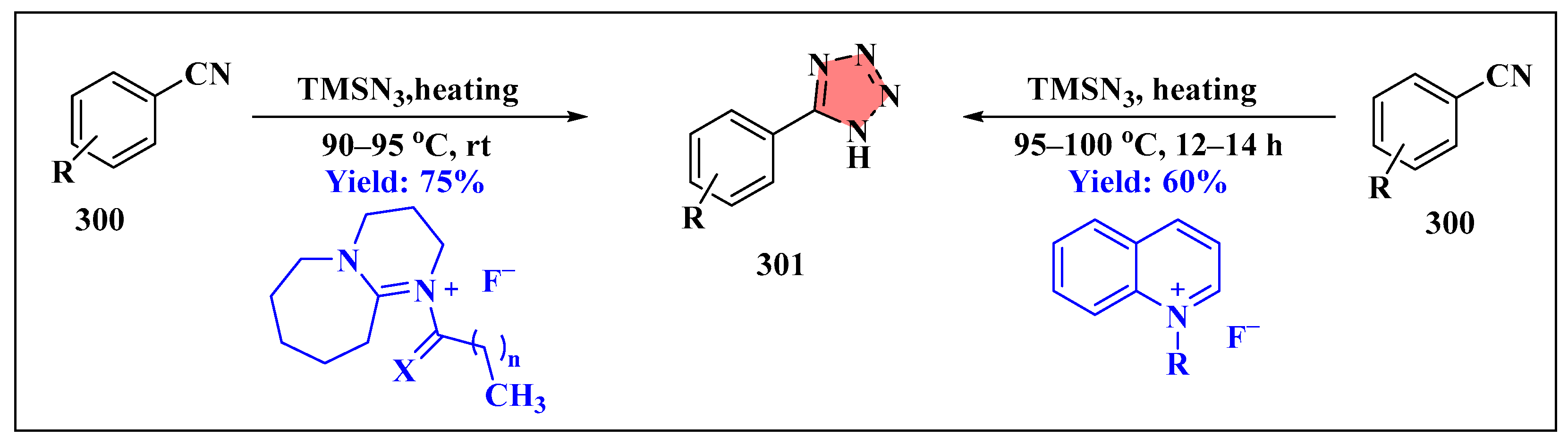
3.6. Synthesis of Tetrazole Derivatives Using Ionic Liquids
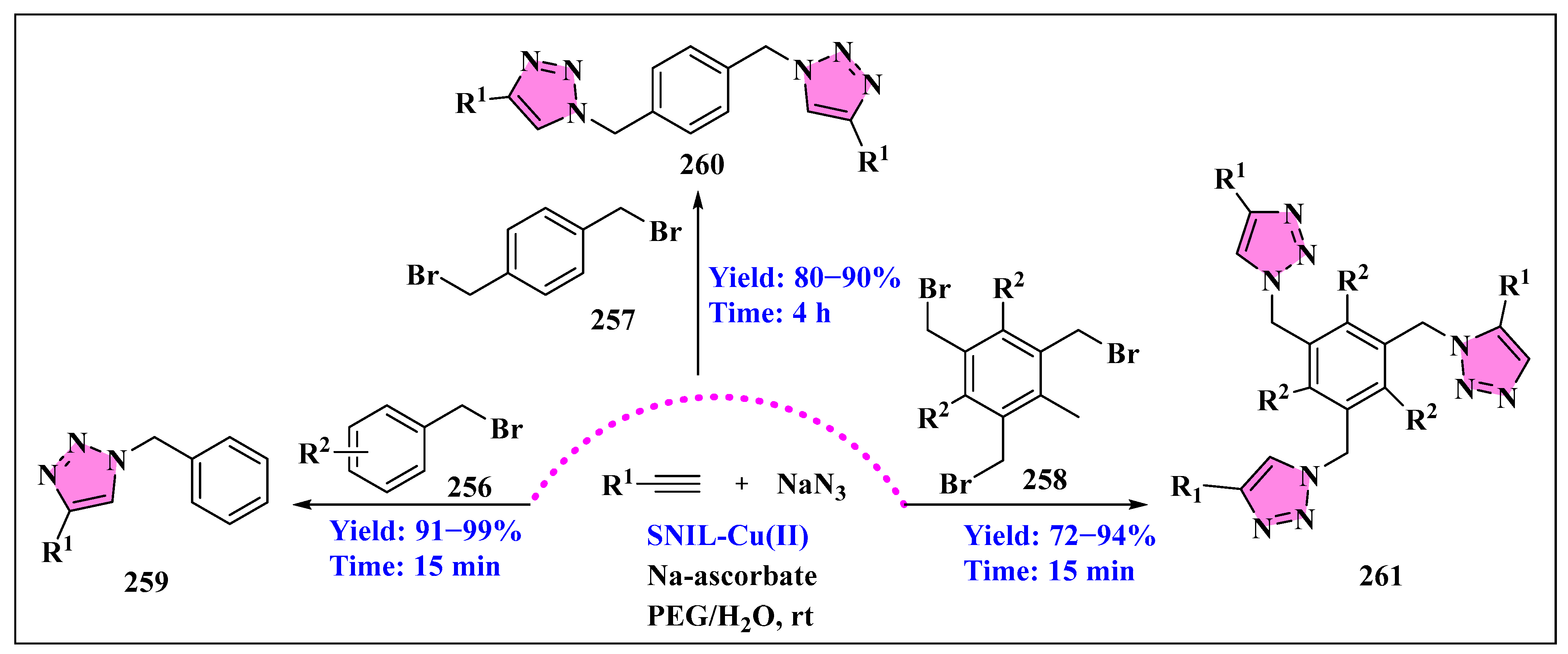
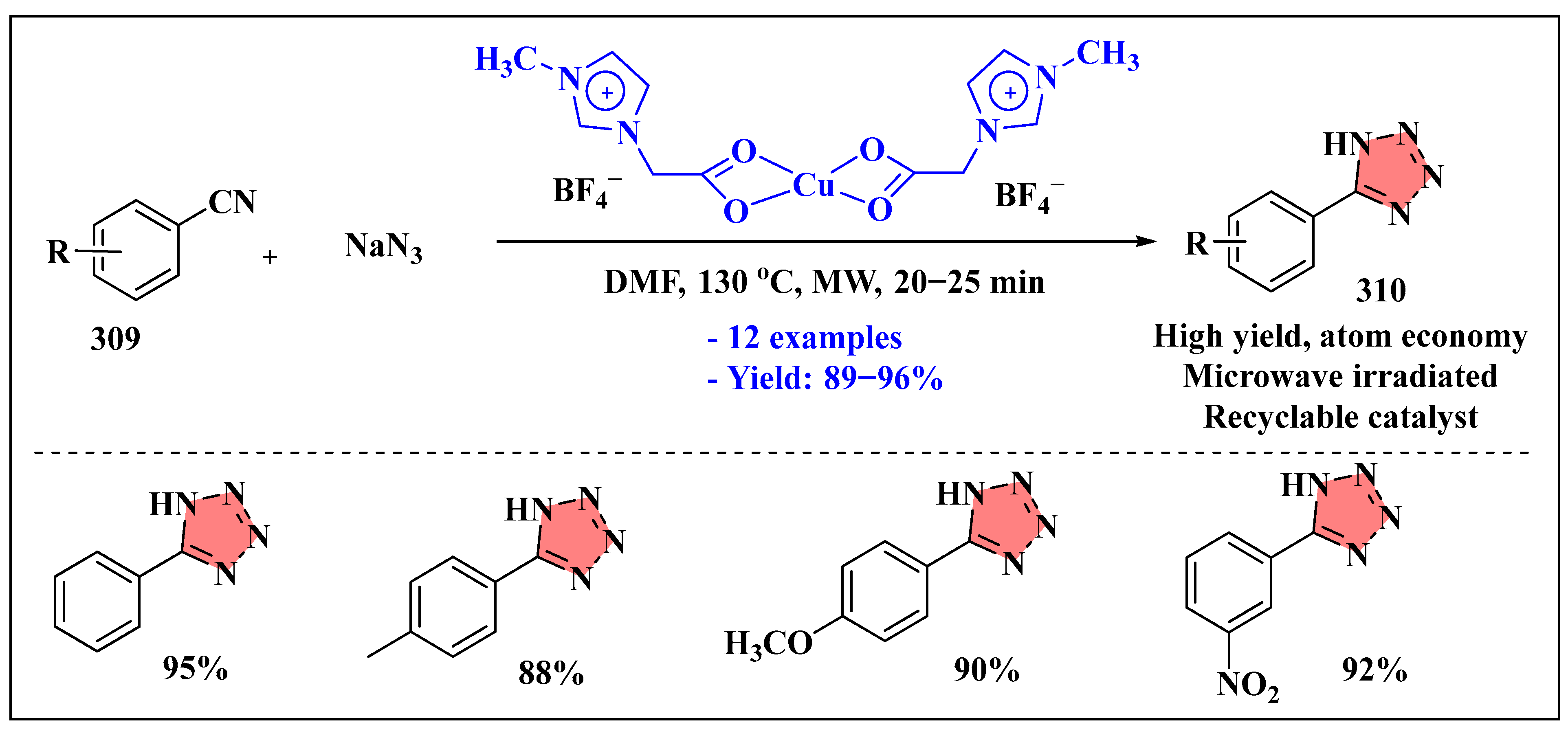
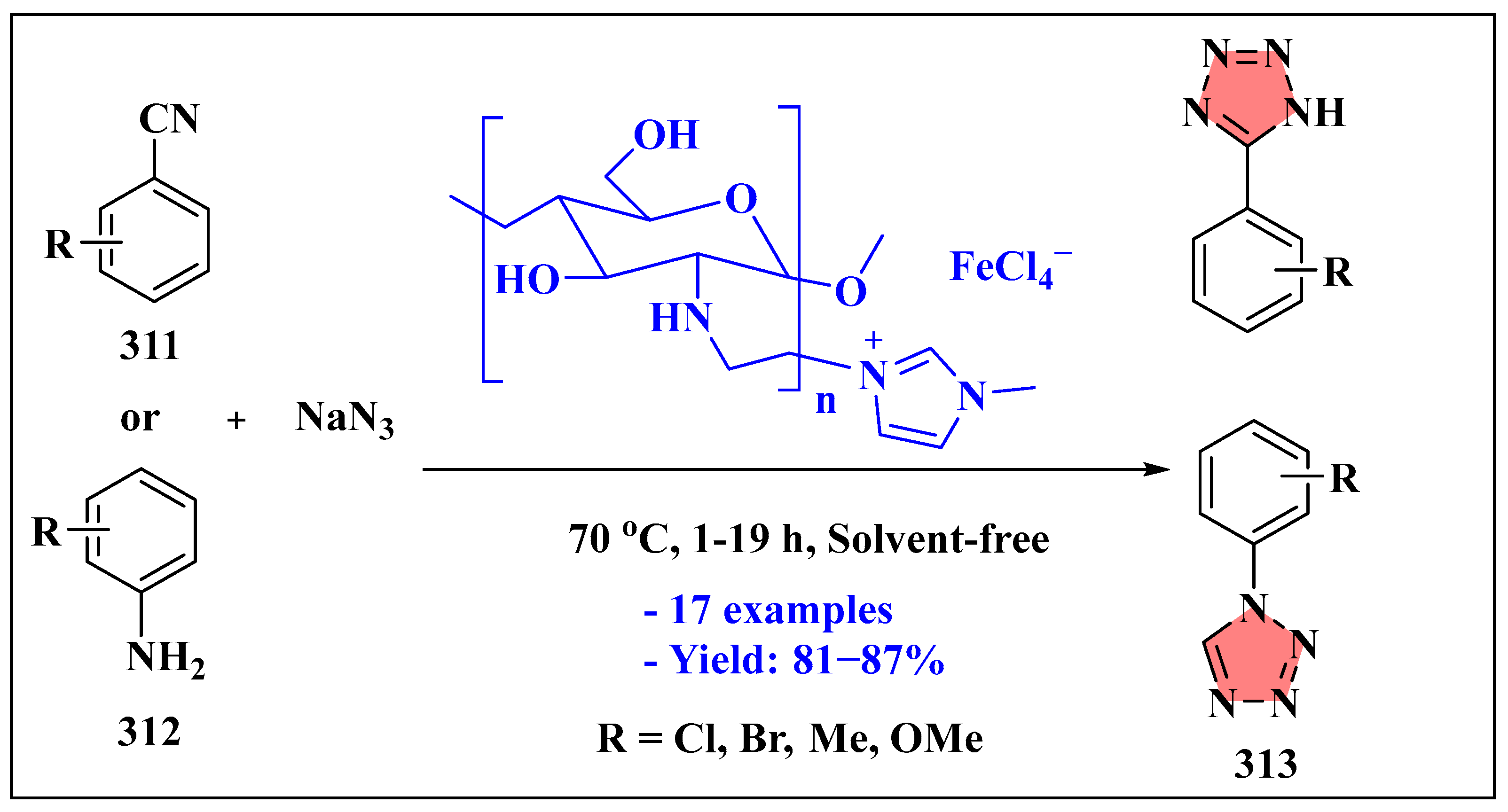
3.6.1. Ionic Liquids Mediated Click Reaction in Homogenous Phase
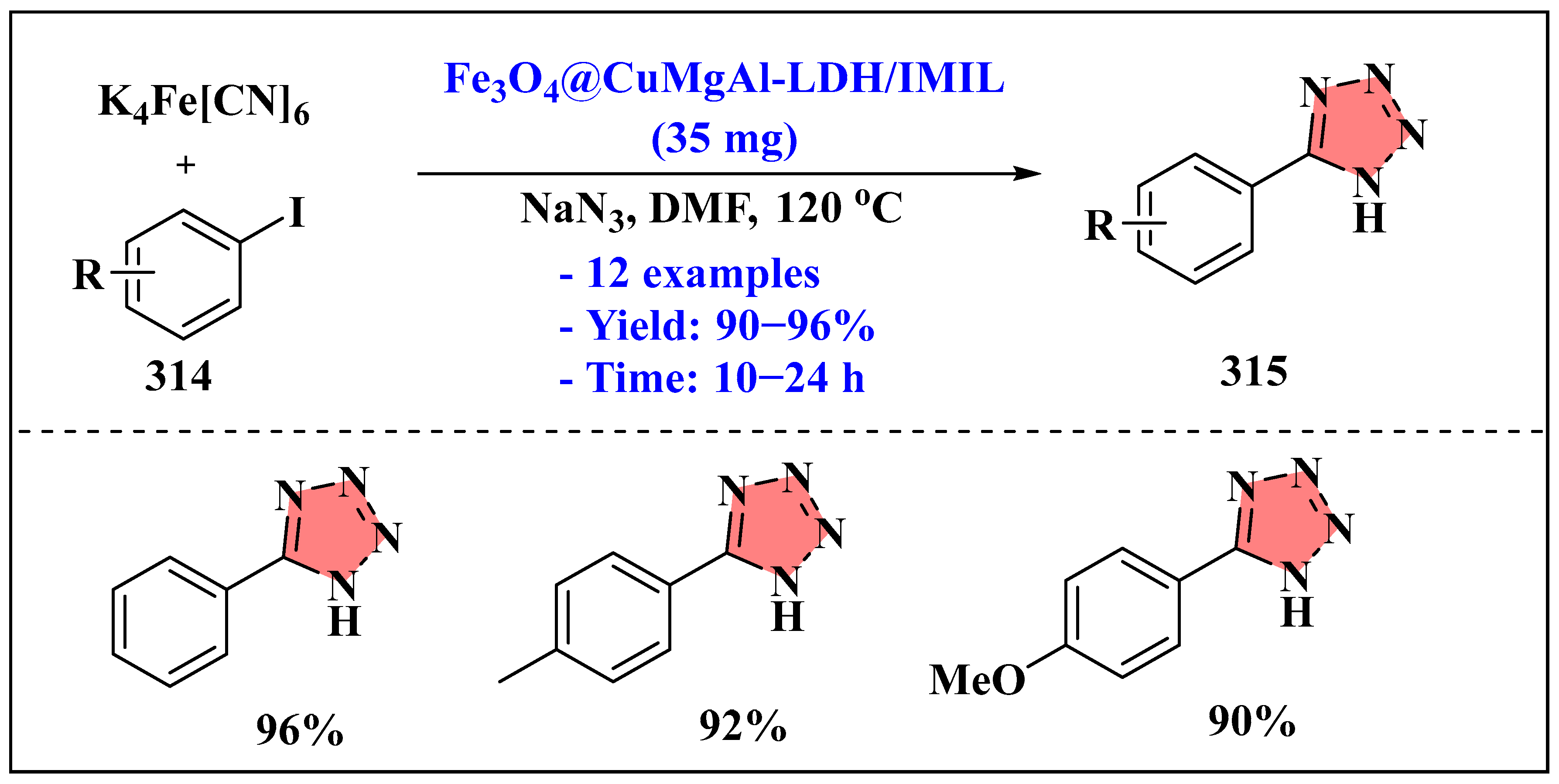
3.6.2. Ionic Liquids Mediated Click Reaction in Heterogenous Phase
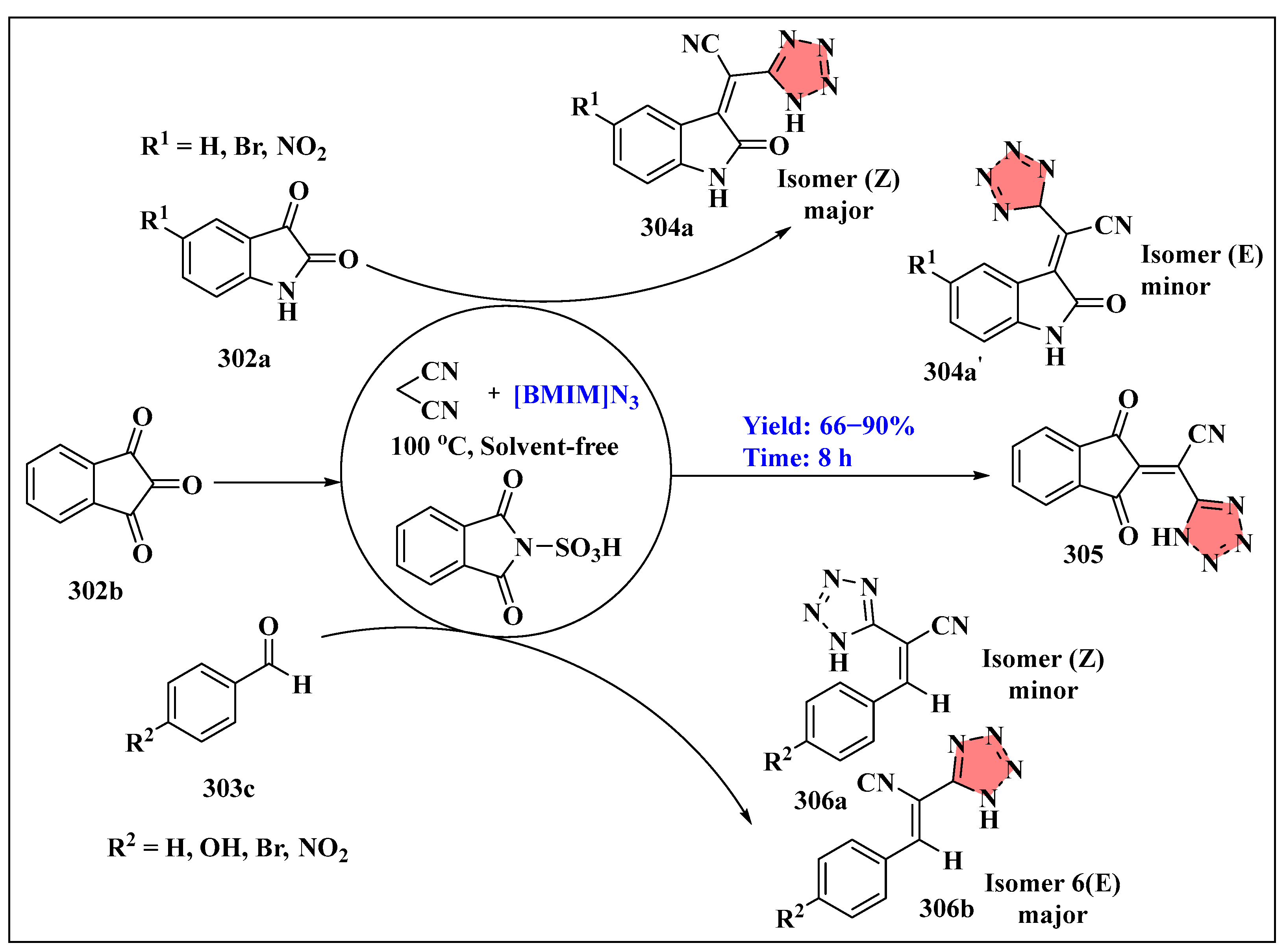
3.6.3. Miscellaneous Reactions
4. Research Gap and Novelty
5. Conclusions
6. Challenges and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| DBU | 1,8-Diazabicyclo [5.4.0]undec-7-ene |
| DABCO | 1,4-diazabicyclo [2.2.2]octane |
| DSC | Differential Scanning Calorimetry |
| NMR | Nuclear Magnetic Resonance |
| CAN | Ceric Ammonium Nitrate |
| FDA | Food and Drug Administration |
| PEG:400 | Polyethylene Glycol 400; |
| DMAD | dialkyacetylenedicarboxylates |
| HOMO | Highest Occupied Molecular Orbital; |
| LUMO | Lowest Unoccupied Molecular Orbital; |
| NMSM | N-methyl-1-(methylthio)-2-nitroethenamine); |
| AAIL | Amino acid ionic liquid |
| DMF | Dimethylformamide |
| TEA | Triethanolamine |
| TGA | Thermogravimetric analysis |
| MsO | Methanesulfonate |
| DTG | Differential Thermogravimetry |
References
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An update on the nitrogen heterocycle compositions and properties of US FDA-approved pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Verma, K.; Dwivedi, J.; Sharma, S. Tetrazole derivatives in the management of neurological disorders: Recent advances on synthesis and pharmacological aspects. Eur. J. Med. Chem. 2024, 271, 116388. [Google Scholar] [CrossRef]
- Behera, S.; Kanti Bera, S.; Basoccu, F.; Cuccu, F.; Caboni, P.; De Luca, L.; Porcheddu, A. Application of Bertagnini’s Salts in a Mechanochemical Approach Toward Aza-Heterocycles and Reductive Aminations via Imine Formation. Adv. Synth. Catal. 2024, 366, 2035–2043. [Google Scholar] [CrossRef]
- Behera, B.K.; Ghosh, P.; Saikia, A.K. Recent Advances in the Synthesis of N-Heterocycles via Lewis acid-Catalyzed/Mediated Cyclization of Propargyl-and Homopropargyl-amines and their Derivatives. Tetrahedron 2024, 162, 134123. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Devi, M.; Jaiswal, S.; Yaduvanshi, N.; Kaur, N.; Kishore, D.; Dwivedi, J.; Sharma, S. Design, synthesis, antibacterial evaluation and docking studies of triazole and tetrazole linked 1,4-benzodiazepine nucleus via click approach. ChemistrySelect 2023, 8, 202204710. [Google Scholar] [CrossRef]
- Jaiswal, S.; Arya, N.; Kishore, D.; Dwivedi, J.; Sharma, S. Exploring azetidine containing heterocycles: From green synthesis to applications. Tetrahedron 2025, 174, 134491. [Google Scholar] [CrossRef]
- Łowicki, D.; Przybylski, P. Tandem construction of biological relevant aliphatic 5-membered N-heterocycles. Eur. J. Med. Chem. 2022, 235, 114303. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Jordaan, M.A.; Carena, G.; Bono, T.; Shapi, M.; Tuszynski, J.A. An overview of the biological evaluation of selected nitrogen-containing heterocycle medicinal chemistry compounds. Int. J. Mol. Sci. 2022, 23, 8117. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Jaiswal, S.; Bhardwaj, A.; Arora, D.; Jain, S.; Kumar, G.; Dwivedi, J.; Sharma, S. Unveiling novel thiadiazolo-annulated azepine hybrids as peptide deformylase inhibitor: DFT, molecular docking and ADMET studies. J. Mol. Struct. 2025, 1336, 142105. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef]
- Balakrishna, A.; Aguiar, A.; Sobral, P.J.; Wani, M.Y.; Almeida e Silva, J.; Sobral, A.J. Paal–Knorr synthesis of pyrroles: From conventional to green synthesis. Catal. Rev. 2019, 61, 84–110. [Google Scholar] [CrossRef]
- Shiri, P. An overview on the copper-promoted synthesis of five-membered heterocyclic systems. Appl. Organomet. Chem. 2020, 34, 5600. [Google Scholar] [CrossRef]
- Jaiswal, S.; Devi, M.; Yaduvanshi, N.; Jain, S.; Dwivedi, J.; Kishore, D.; Kuznetsov, A.E.; Sharma, S. Identification of New Triazolo Annulated Dipyridodiazepine Derivatives as HIV-1 Reverse Transcriptase Inhibitors: Design, Synthesis, DFT, Molecular Modelling and In silico Studies. J. Mol. Struct. 2024, 1314, 138734. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Vlasenko, Y.A.; Postnikov, P.S.; Trusova, M.E.; Shafir, A.; Zhdankin, V.V.; Yoshimura, A.; Yusubov, M.S. Synthesis of five-membered iodine–nitrogen heterocycles from benzimidazole-based iodonium salts. J. Org. Chem. 2018, 83, 12056–12070. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.H.; Liu, Z.F.; Wu, M.F.; Xiao, Y.; Zheng, F.K.; Guo, G.C.; Huang, J.S. Anion-directed self-assembly of Cu(II) coordination compounds with tetrazole-1-acetic acid: Syntheses in ionic liquids and crystal structures. New J. Chem. 2014, 38, 269–276. [Google Scholar] [CrossRef]
- Kedimar, N.; Rao, P.; Rao, S.A. Imidazole-based ionic liquid as sustainable green inhibitor for corrosion control of steel alloys: A review. J. Mol. Liq. 2024, 411, 125789. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Budhalakoti, B.; Kumar, A.; Singh, K.; Kumar Kamboj, R.; Bhatrola, K. Exploring the Impact of Ionic Liquids on Pyrazole Derivatives Synthesis: A Critical Review. ChemistrySelect 2024, 9, 202401925. [Google Scholar] [CrossRef]
- Jaiswal, S.; Bhardwaj, K.; Dwivedi, J.; Sharma, S. New Trends in Click Reactions for Synthesis of 1,2,3-Triazole Derivatives. Chem. Biodivers. 2025, e00387. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial applications of ionic liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Singh, K.S.; Majik, M.S. Pyrrole-derived alkaloids of marine sponges and their biological properties. Stud. Nat. Prod. Chem. 2019, 62, 377–409. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Luo, C.; Yang, T.; Yang, L.; Suo, J. Pyrrole synthesis in ionic liquids by Paal–Knorr condensation under mild conditions. Tetrahedron Lett. 2004, 45, 3417–3419. [Google Scholar] [CrossRef]
- Li, D.; Zang, H.; Wu, C.; Yu, N. 1-Methylimidazolium hydrogen sulfate catalyzed convenient synthesis of 2,5-dimethyl-N-substituted pyrroles under ultrasonic irradiation. Ultrason. Sonochem. 2013, 20, 1144–1148. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Eeshwaraiah, B.; Gupta, M.K. Bi (OTf)3/[bmim]BF4 as novel and reusable catalytic system for the synthesis of furan, pyrrole and thiophene derivatives. Tetrahedron Lett. 2004, 45, 5873–5876. [Google Scholar] [CrossRef]
- Yavari, I.; Kowsari, E. Efficient and green synthesis of tetrasubstituted pyrroles promoted by task-specific basic ionic liquids as catalyst in aqueous media. Mol. Divers. 2009, 13, 519–528. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, K.N.; Singh, J. Ionic liquid catalyzed one pot four-component coupling reaction for the synthesis of functionalized pyrroles. J. Mol. Liq. 2014, 199, 470–473. [Google Scholar] [CrossRef]
- Meshram, H.M.; Babu, B.M.; Kumar, G.S.; Thakur, P.B.; Bangade, V.M. Catalyst-free four-component protocol for the synthesis of substituted pyrroles under reusable reaction media. Tetrahedron Lett. 2013, 54, 2296–2302. [Google Scholar] [CrossRef]
- Zand, H.R.E.; Ghafuri, H. Basic GO-Imidazolium ionic liquid as a recoverable nanocatalyst for the synthesis of pyrrole derivatives. Synthesis 2017, 13, 14. [Google Scholar]
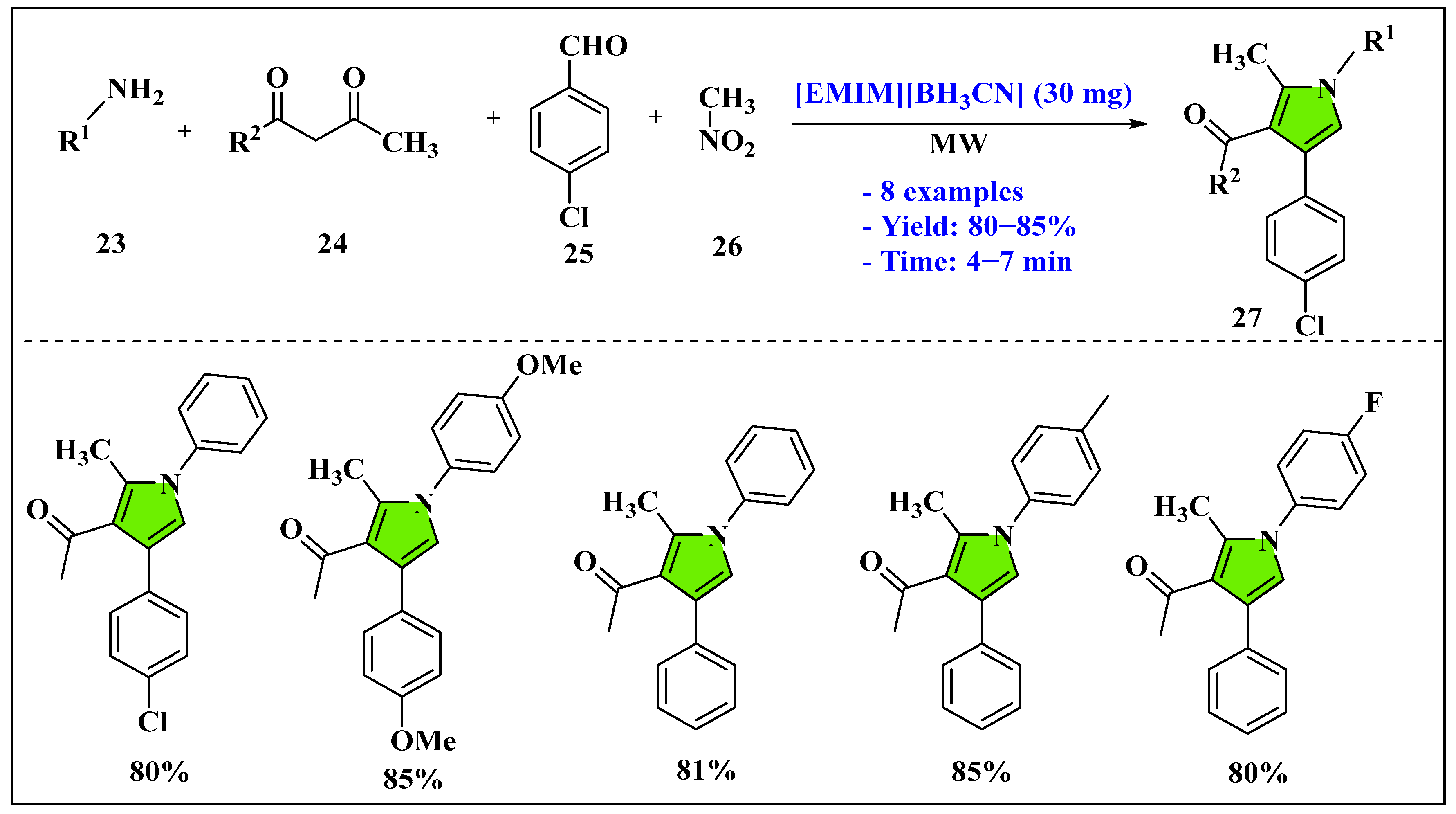
- Manjul, R.K.; Gaikwad, S.T.; Gade, V.B.; Rajbhoj, A.S.; Jopale, M.K.; Patil, S.M.; Gaikwad, D.N.; Suryavanshi, D.M.; Goskulwad, S.P.; Shinde, S.D. [EMIm][BH3CN] Ionic Liquid as an Efficient Catalyst for the Microwave-Assisted One-Pot Synthesis of Triaryl Imidazole Derivatives. Lett. Org. Chem. 2023, 20, 967–975. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.Q.; He, Y.H.; Guan, Z. The acidic ionic liquid [BSO3HMIm] HSO4: A novel and efficient catalyst for one-pot, three-component syntheses of substituted pyrroles. Z. Naturforschung B 2015, 70, 29–38. [Google Scholar] [CrossRef]
- Aydogan, F.; Yolacan, C. Clauson Kaas pyrrole synthesis catalyzed by acidic ionic liquid under microwave irradiation. J. Chem. 2013, 2013, 976724. [Google Scholar] [CrossRef]
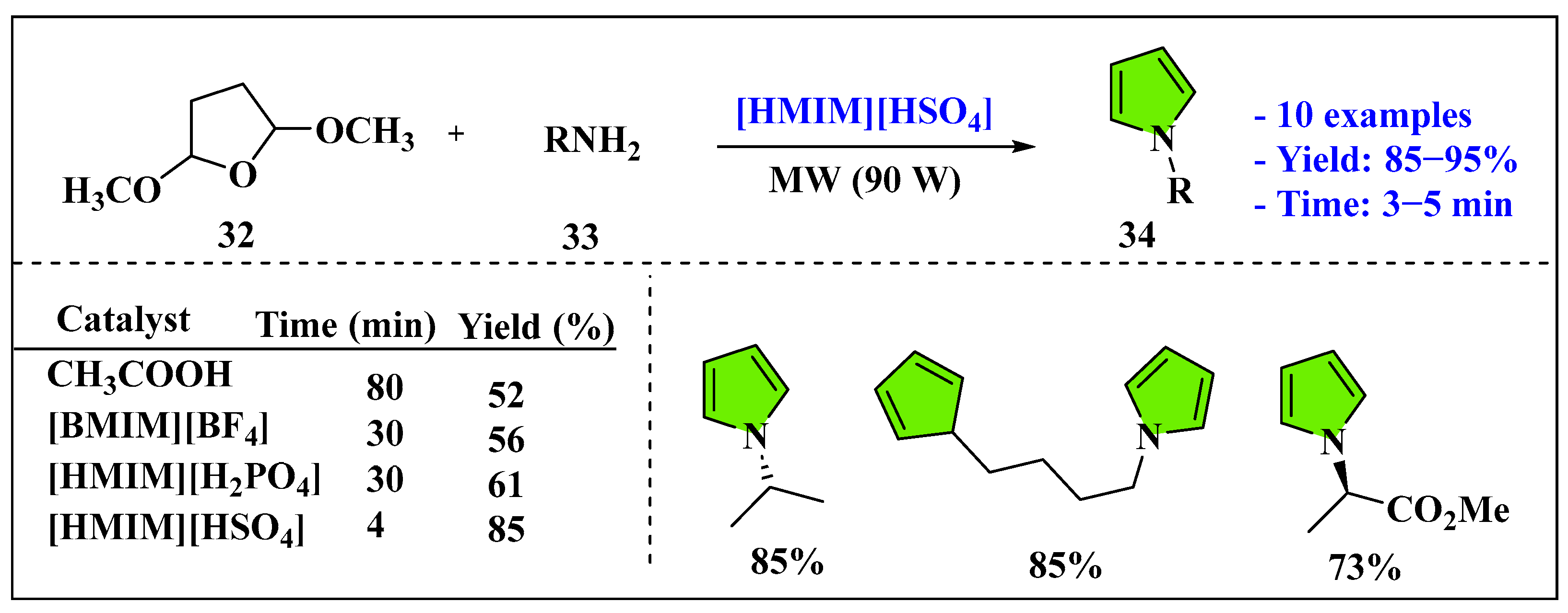
- Le, Z.G.; Chen, Z.C.; Hu, Y.; Zheng, Q.G. Organic reactions in ionic liquids: A simple and highly regioselective N-substitution of pyrrole. Synthesis 2004, 2004, 1951–1954. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M.H. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, C.P.; Marzari, M.R.; Buriol, L.; Moreira, D.N.; Rosa, F.A.; Vargas, P.S.; Zanatta, N.; Bonacorso, H.G.; Martins, M.A. Ionic liquid effects on the reaction of β-enaminones and tert-butylhydrazine and applications for the synthesis of pyrazoles. Catal. Commun. 2009, 10, 1967–1970. [Google Scholar] [CrossRef]
- Buriol, L.; Frizzo, C.P.; Prola, L.D.; Moreira, D.N.; Marzari, M.R.; Scapin, E.; Zanatta, N.; Bonacorso, H.G.; Martins, M.A. Synergic effects of ionic liquid and microwave irradiation in promoting trifluoromethylpyrazole synthesis. Catal. Lett. 2011, 141, 1130–1135. [Google Scholar] [CrossRef]
- Katariya, A.P.; Shirsath, P.D.; Narode, H.; Gaikwad, P.B.; Kadam, G.G.; Katariya, M.V.; Deshmukh, S.U. Unraveling the access to the regioselective synthesis of highly functionalized pyranopyrazoles using an ionic liquid catalyst. Mol. Divers. 2023, 27, 2633–2649. [Google Scholar] [CrossRef]
- Zakeri, M.; Nasef, M.M.; Kargaran, T.; Ahmad, A.; Abouzari-Lotf, E.; Asadi, J. Synthesis of pyrano[2,3-c]pyrazoles by ionic liquids under green and eco-safe conditions. Res. Chem. Intermed. 2017, 43, 717–728. [Google Scholar] [CrossRef]
- Nimbalkar, U.D.; Seijas, J.A.; Vazquez-Tato, M.P.; Damale, M.G.; Sangshetti, J.N.; Nikalje, A.P.G. Ionic liquid-catalyzed green protocol for multi-component synthesis of dihydropyrano[2,3-c]pyrazoles as potential anticancer scaffolds. Molecules 2017, 22, 1628. [Google Scholar] [CrossRef]
- Pogaku, V.; Krishna, V.S.; Sriram, D.; Rangan, K.; Basavoju, S. Ultrasonication-ionic liquid synergy for the synthesis of new potent anti-tuberculosis 1,2,4-triazol-1-yl-pyrazole based spirooxindolopyrrolizidines. Bioorg. Med. Chem. Lett. 2019, 29, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Baddepuri, S.; Gamidi, R.K.; Kumari, J.; Sriram, D.; Basavoju, S. Ultrasound-assisted ionic liquid-mediated green method for synthesis of 1,3-diphenylpyrazole-based spirooxindolopyrrolizidines, their anti-tubercular activity, molecular docking study and ADME predictions. New J. Chem. 2024, 48, 9970–9980. [Google Scholar] [CrossRef]
- Bhatt, J.D.; Patel, T.S.; Chudasama, C.J.; Patel, K.D. Microwave-Assisted Synthesis of Novel Pyrazole Clubbed Polyhydroquinolines in an Ionic-Liquid and their Biological Perspective. ChemistrySelect 2018, 3, 3632–3640. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Moreira, D.N.; Guarda, E.A.; Fiss, G.F.; Marzari, M.R.; Zanatta, N.; Bonacorso, H.G.; Martins, M.A. Ionic liquid as catalyst in the synthesis of N-alkyl trifluoromethyl pyrazoles. Catal. Commun. 2009, 10, 1153–1156. [Google Scholar] [CrossRef]
- Sridhar, R.; Perumal, P.T. A new protocol to synthesize 1, 4-dihydropyridines by using 3,4,5-trifluorobenzeneboronic acid as a catalyst in ionic liquid: Synthesis of novel 4-(3-carboxyl-1H-pyrazol-4-yl)-1,4-dihydropyridines. Tetrahedron 2005, 61, 2465–2470. [Google Scholar] [CrossRef]
- Jadhav, C.; Nipate, A.; Chate, A.; Gill, C. Triethylammonium Hydrogen Sulfate [Et3NH][HSO4]-Catalyzed Rapid and Efficient Multicomponent Synthesis of Pyrido [2,3-d] pyrimidine and Pyrazolo[3,4-b]pyridine Hybrids. ACS Omega 2021, 6, 18215–18225. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chaudhary, A. Efficient and Green Synthesis of 4H-pyrans and 4H-pyrano[2,3-c] Pyrazoles Catalyzed by Task-specific Ionic Liquid [bmim] OH under Solvent-free Conditions. Green Chem. Lett. Rev. 2012, 5, 633–638. [Google Scholar] [CrossRef]
- Aliabadi, R.S.; Mahmoodi, N.O. Green and efficient synthesis of pyranopyrazoles using [bmim][OH−] as an ionic liquid catalyst in water under microwave irradiation and investigation of their antioxidant activity. RSC Adv. 2016, 6, 85877–85884. [Google Scholar] [CrossRef]
- Noura, S.; Ghorbani, M.; Zolfigol, M.A.; Narimani, M.; Yarie, M.; Oftadeh, M. Biological based (nano) gelatoric ionic liquids (NGILs): Application as catalysts in the synthesis of a substituted pyrazole via vinylogous anomeric based oxidation. J. Mol. Liq. 2019, 271, 778–785. [Google Scholar] [CrossRef]
- Bakhtiarian, M.; Khodaei, M.M. Pyridinium-based dual acidic ionic liquid supported on the pectin for efficient synthesis of pyrazoles. J. Mol. Liq. 2022, 363, 119883. [Google Scholar] [CrossRef]
- Konwar, M.; Elnagdy, H.M.; Gehlot, P.S.; Khupse, N.D.; Kumar, A.; Sarma, D. Transition metal containing ionic liquid-assisted one-pot synthesis of pyrazoles at room temperature. J. Chem. Sci. 2019, 131, 80. [Google Scholar] [CrossRef]
- Akbarpour, T.; Yousefi Seyf, J.; Khazaei, A.; Sarmasti, N. Synthesis of pyrano [2,3-c] pyrazole derivatives using a novel ionic-liquid based nano-magnetic catalyst (Fe3O4@SiO2@(CH2)3NH@CC@Imidazole@ SO3H+Cl−). Polycycl. Aromat. Compd. 2022, 42, 3844–3864. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimzadeh, M.; Tavallaei, H. Fast synthesis of pyrano[2,3-c]pyrazoles: Strong effect of Brönsted and Lewis acidic ionic liquids. J. Iran. Chem. Soc. 2015, 12, 987–991. [Google Scholar] [CrossRef]
- Niknam, K.; Piran, A. Silica-Grafted Ionic Liquids as Recyclable Catalysts for the Synthesis of 3,4-Dihydropyrano[c]chromenes and Pyra-no[2,3-c]pyrazoles. Green Sustain. Chem. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Chakraborty, S.; Paul, B.; De, U.C.; Natarajan, R.; Majumdar, S. Water–SDS–[BMIm] Br composite system for one-pot multicomponent synthesis of pyrano[2,3-c]pyrazole derivatives and their structural assessment by NMR, X-ray, and DFT studies. RSC Adv. 2023, 13, 6747–6759. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having versatile biological activities. J. Chem. 2013, 2013, 329412. [Google Scholar] [CrossRef]
- Soni, J.; Sethiya, A.; Sahiba, N.; Agarwal, D.K.; Agarwal, S. Contemporary progress in the synthetic strategies of imidazole and its biological activities. Curr. Org. Synth. 2019, 16, 1078–1104. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Narkhede, U.C.; Palimkar, S.S.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or α-hydroxyketone. Tetrahedron 2005, 61, 3539–3546. [Google Scholar] [CrossRef]
- Xia, M.; Lu, Y.D. A novel neutral ionic liquid-catalyzed solvent-free synthesis of 2,4,5-trisubstituted imidazoles under microwave irradiation. J. Mol. Catal. A Chem. 2007, 265, 205–208. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Ameri Rad, J. Catalyst-free one-pot four component synthesis of polysubstituted imidazoles in neutral ionic liquid 1-butyl-3-methylimidazolium bromide. J. Comb. Chem. 2010, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Shirole, G.D.; Kadnor, V.A.; Tambe, A.S.; Shelke, S.N. Brønsted-acidic ionic liquid: Green protocol for synthesis of novel tetrasubstituted imidazole derivatives under microwave irradiation via multicomponent strategy. Res. Chem. Intermed. 2017, 43, 1089–1098. [Google Scholar] [CrossRef]
- Abdullayev, Y.; Abbasov, V.; Ducati, L.C.; Talybov, A.; Autschbach, J. Ionic liquid solvation versus catalysis: Computational insight from a multisubstituted imidazole synthesis in [Et2NH2][HSO4]. ChemistryOpen 2016, 5, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Lateef, H.M.; Shalabi, K.; Abdelhamid, A.A. One-pot synthesis of novel triphenyl hexyl imidazole derivatives catalyzed by ionic liquid for acid corrosion inhibition of C1018 steel: Experimental and computational perspectives. J. Mol. Liq. 2021, 334, 116081. [Google Scholar] [CrossRef]
- Abdelhamid, A.A.; Salah, H.A.; Marzouk, A.A. Synthesis of imidazole derivatives: Ester and hydrazide compounds with antioxidant activity using ionic liquid as an efficient catalyst. J. Heterocycl. Chem. 2020, 57, 676–685. [Google Scholar] [CrossRef]
- Chanda, K.; Maiti, B.; Tseng, C.C.; Sun, C.M. Microwave-assisted linear approach toward highly substituted benzo[d]oxazol-5-yl-1H-benz [d]imidazole on ionic liquid support. ACS Comb. Sci. 2012, 14, 115–123. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Magnetic nanoparticle supported ionic liquid as novel and effective heterogeneous catalyst for synthesis of substituted imidazoles under ultrasonic irradiation. Monatsh. Chem. 2013, 144, 1389–1396. [Google Scholar] [CrossRef]
- Khalifeh, R.; Naseri, V.; Rajabzadeh, M. Synthesis of Imidazolium-Based Ionic Liquid on Modified Magnetic Nanoparticles for Application in One-Pot Synthesis of Trisubstituted Imidazoles. ChemistrySelect 2020, 5, 11453–11462. [Google Scholar] [CrossRef]
- Jourshari, M.S.; Mamaghani, M.; Shirini, F.; Tabatabaeian, K.; Rassa, M.; Langari, H. An expedient one-pot synthesis of highly substituted imidazoles using supported ionic liquid-like phase (SILLP) as a green and efficient catalyst and evaluation of their anti-microbial activity. Chin. Chem. Lett. 2013, 24, 993–996. [Google Scholar] [CrossRef]
- Dwivedi, J.; Sharma, S.; Jaiswal, S. Structural Insights in Development of Antiviral Agents Derived from the Condensed Nitrogen Heterocycles: Synthesis and Biological Properties. J. Mol. Struct. 2025, 1350, 143948. [Google Scholar] [CrossRef]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzym. Inhib. 2011, 26, 1–21. [Google Scholar] [CrossRef]
- Ali, A.A.; Konwar, M.; Chetia, M.; Sarma, D. [Bmim] OH mediated Cu-catalyzed azide–alkyne cycloaddition reaction: A potential green route to 1,4-disubstituted 1,2,3-triazoles. Tetrahedron Lett. 2016, 57, 5661–5665. [Google Scholar] [CrossRef]
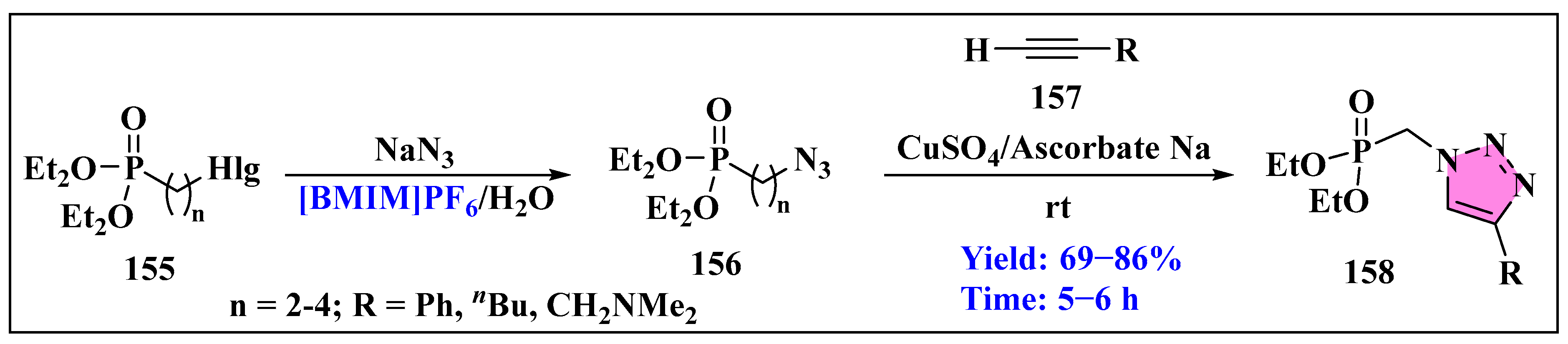
- Artyushin, O.I.; Vorob’eva, D.V.; Vasil’eva, T.P.; Osipov, S.N.; Röschenthaler, G.V.; Odinets, I.L. Facile synthesis of phosphorylated azides in ionic liquids and their use in the preparation of 1,2,3-triazoles. Heteroat. Chem. Int. J. Main Group Elem. 2008, 19, 293–300. [Google Scholar] [CrossRef]
- Sucharitha, E.R.; Krishna, T.M.; Manchal, R.; Ramesh, G.; Narsimha, S. Fused benzo [1, 3] thiazine-1,2,3-triazole hybrids: Microwave-assisted one-pot synthesis, in vitro antibacterial, antibiofilm, and in silico ADME studies. Bioorg. Med. Chem. Lett. 2021, 47, 128201. [Google Scholar] [CrossRef]
- Singh, H.; Sindhu, J.; Khurana, J.M.; Sharma, C.; Aneja, K.R. Ultrasound promoted one pot synthesis of novel fluorescent triazolyl spirocyclic oxindoles using DBU based task specific ionic liquids and their antimicrobial activity. Eur. J. Med. Chem. 2014, 77, 145–154. [Google Scholar] [CrossRef]
- Ketsomboon, N.; Saeeng, R.; Srisook, K.; Sirion, U. Convenient synthesis of long alkyl-chain triazolylglycosides using ionic liquid as dual promoter-solvent: Readily access to non-ionic triazolylglycoside surfactants for evaluation of cytotoxic activity. Tetrahedron Lett. 2021, 80, 153325. [Google Scholar] [CrossRef]
- Marra, A.; Vecchi, A.; Chiappe, C.; Melai, B.; Dondoni, A. Validation of the copper (I)-catalyzed azide–alkyne coupling in ionic liquids. Synthesis of a triazole-linked C-disaccharide as a case study. J. Org. Chem. 2008, 73, 2458–2461. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L. Synthesis of 1, 4-disubstituted 1,2,3-triazoles by use of copper (I) and amino acids ionic liquid catalytic system. Synthesis 2010, 2010, 447–452. [Google Scholar] [CrossRef]
- De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-functionalized 1,2,3-triazoles using ionic liquid/iron (III) chloride as an efficient and reusable homogeneous catalyst. Catalysts 2018, 8, 364. [Google Scholar] [CrossRef]
- Dutta, B.; Garg, A.; Phukan, P.; Kulshrestha, A.; Kumar, A.; Sarma, D. Designing a new basic ionic liquid [DHIM][OH] as a task specific bifunctional catalyst for facile microwave assisted metal free synthesis of 5-amino-1,2,3-triazoles. New J. Chem. 2021, 45, 12792–12797. [Google Scholar] [CrossRef]
- Dutta, B.; Hazarika, P.K.; Saikia, P.; Konwer, S.; Borthakur, L.; Sarma, D. [DDQM][TFSI]: A room temperature ionic liquid as an active electrode material for supercapacitor devices and a catalyst for rapid synthesis of 4-aryl-NH-1,2,3-triazoles under microwave irradiation. New J. Chem. 2023, 47, 11389–11393. [Google Scholar] [CrossRef]
- Saquib, M.; Ahamad, S.; Khan, M.F.; Khan, M.I.; Hussain, M.K. An ultrasound assisted, ionic liquid-molecular iodine synergy driven efficient green synthesis of pyrrolobenzodiazepine-triazole hybrids as potential anticancer agents. Front. Pharmacol. 2023, 14, 1168566. [Google Scholar] [CrossRef]
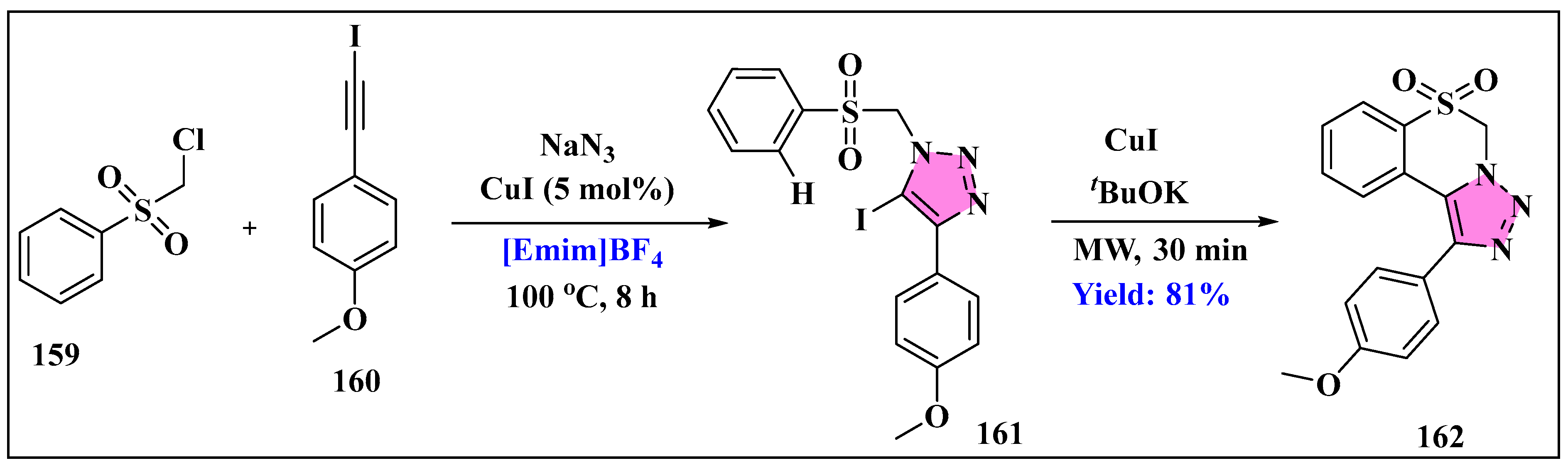
- Babu, H.R.; Ravinder, M.; Narsimha, S. Synthesis and Biological Evaluation of New 1,2,3-Triazole Based 2-Sulfonylbenzoxazoles as Potent Anti-inflammatory and Antibacterial Agents. Indian J. Heterocycl. Chem 2019, 29, 389–395. [Google Scholar]
- Narsimha, S.; Battula, K.S.; Reddy, Y.N.; Nagavelli, V.R. Microwave-assisted Cu-catalyzed C–C bond formation: One-pot synthesis of fully substituted 1,2,3-triazoles using nonsymmetrical iodoalkynes and their biological evaluation. Chem. Heterocycl. Compd. 2018, 54, 1161–1167. [Google Scholar] [CrossRef]
- Dabiri, M.; Kazemi Movahed, S.; MaGee, D.I. One-pot synthesis of 2,4,5-triaryl-1H-imidazoles linked 1,4-disubstituted 1,2,3-triazoles based on a merging multicomponent condensation with Huisgen 1,3-dipolar cycloaddition in ionic liquid. Res. Chem. Intermed. 2015, 41, 3335–3347. [Google Scholar] [CrossRef]
- Singh, H.; Sindhu, J.; Khurana, J.M. Efficient, green and regioselective synthesis of 1,4,5-trisubstituted-1,2,3-triazoles in ionic liquid [bmim] BF4 and in task-specific basic ionic liquid [bmim] OH. J. Iran. Chem. Soc. 2013, 10, 883–888. [Google Scholar] [CrossRef]
- Seregin, I.V.; Batog, L.V.; Makhova, N.N. Synthesis of 1-aryl(hetaryl)-1,2,3-triazoles with the use of ionic liquids. Mendeleev Commun. 2002, 12, 83–84. [Google Scholar] [CrossRef]
- De Nino, A.; Algieri, V.; Tallarida, M.A.; Costanzo, P.; Pedrón, M.; Tejero, T.; Merino, P.; Maiuolo, L. Regioselective synthesis of 1,4,5-trisubstituted-1,2,3-triazoles from aryl azides and enaminones. Eur. J. Org. Chem. 2019, 2019, 5725–5731. [Google Scholar] [CrossRef]
- Rad, M.N.S.; Behrouz, S.; Saremi, H.; Mohammadtaghi-Nezhad, J. Synthesis of hydroxyethyl methyl morpholinium azide (HEM Morph) N3: A highly efficient new task specific azide-based ionic liquid and its dual application as an azide source and media for synthesis of some novel aromatic O-oxime ethers-1,2,3-triazole conjugates as a potential antihistaminic agents. J. Mol. Liq. 2020, 299, 112245. [Google Scholar] [CrossRef]
- Koguchi, S.; Izawa, K. Ionic liquid-phase synthesis of 1,5-disubstituted 1,2,3-triazoles. ACS Comb. Sci. 2014, 16, 381–385. [Google Scholar] [CrossRef]
- Zare, P.; Mahrova, M.; Tojo, E.; Stojanovic, A.; Binder, W.H. Ethylene glycol-based ionic liquids via azide/alkyne click chemistry. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 190–202. [Google Scholar] [CrossRef]
- Singh, H.; Khanna, G.; Nand, B.; Khurana, J.M. Metal-free synthesis of 1,2,3-triazoles by azide–aldehyde cycloaddition under ultrasonic irradiation in TSIL [DBU-Bu]OH and in hydrated IL Bu4NOH under heating. Monatsh. Chem. 2016, 147, 1215–1219. [Google Scholar] [CrossRef]
- Valizadeh, H.; Amiri, M.; Khalili, E. Task-specific nitrite and azide ionic liquids for the efficient one-pot synthesis of 1,2,3-triazoles from the aniline derivatives. Mol. Divers. 2012, 16, 319–323. [Google Scholar] [CrossRef]
- Siddiqui, M.M.; Nagargoje, A.A.; Akolkar, S.V.; Sangshetti, J.N.; Khedkar, V.M.; Pisal, P.M.; Shingate, B.B. [HDBU][HSO4]-catalyzed facile synthesis of new 1,2,3-triazole-tethered 2,3-dihydroquinazolin-4[1H]-one derivatives and their DPPH radical scavenging activity. Res. Chem. Intermed. 2022, 48, 1199–1225. [Google Scholar] [CrossRef]
- Hosseini, H.G.; Doustkhah, E.; Kirillova, M.V.; Rostamnia, S.; Mahmoudi, G.; Kirillov, A.M. Combining ethylenediamine and ionic liquid functionalities within SBA-15: A promising catalytic pair for tandem Cu–AAC reaction. Appl. Catal. A Gen. 2017, 548, 96–102. [Google Scholar] [CrossRef]
- Tavassoli, M.; Landarani-Isfahani, A.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Polystyrene-supported ionic liquid copper complex: A reusable catalyst for one-pot three-component click reaction. Appl. Catal. A Gen. 2015, 503, 186–195. [Google Scholar] [CrossRef]
- Thakur, M.; Choudhury, P.; Kejriwal, A.; Mukherjee, A.; Biswas, K. A novel benzimidazole based ionic liquid-tagged Schiff base copper catalyst: Synthesis, characterization and application toward the synthesis of 1,4-disubstituted 1,2,3-triazoles. Inorg. Chim. Acta. 2023, 549, 121405. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, M.; Chowhan, B.; Frontera, A. Magnetically separable nanocatalyst (IL@ CuFe2O4-L-Tyr-TiO2/TiTCIL): Preparation, characterization and its applications in 1,2,3-triazole synthesis and in photodegradation of MB. J. Mol. Struct. 2021, 1224, 129029. [Google Scholar] [CrossRef]
- Daraie, M.; Heravi, M.M. A biocompatible chitosan-ionic liquid hybrid catalyst for regioselective synthesis of 1,2,3-triazols. Int. J. Biol. Macromol. 2019, 140, 939–948. [Google Scholar] [CrossRef]
- Maiuolo, L.; Russo, B.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Tallarida, M.A.; De Nino, A. Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles by 1,3-dipolar cycloaddition: Role of Er(OTf)3, ionic liquid and water. Tetrahedron Lett. 2019, 60, 672–674. [Google Scholar] [CrossRef]
- Saikia, A.A.; Rao, R.N.; Das, S.; Jena, S.; Rej, S.; Maiti, B.; Chanda, K. Sequencing [3+2]-cycloaddition and multicomponent reactions: A regioselective microwave-assisted synthesis of 1,4-disubstituted 1,2,3-triazoles using ionic liquid supported Cu(II) precatalysts in methanol. Tetrahedron Lett. 2020, 61, 152273. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tajbakhsh, M.; Farhang, M.; Hosseini, S.H. Copper-loaded polymeric magnetic nanocatalysts as retrievable and robust heterogeneous catalysts for click reactions. New J. Chem. 2015, 39, 4591–4600. [Google Scholar] [CrossRef]
- Patil, J.D.; Patil, S.A.; Pore, D.M. A polymer supported ascorbate functionalized task specific ionic liquid: An efficient reusable catalyst for 1, 3-dipolar cycloaddition. RSC Adv. 2015, 5, 21396–21404. [Google Scholar] [CrossRef]
- Li, B.; Hu, R.; Qin, A.; Tang, B.Z. Copper-based ionic liquid-catalyzed click polymerization of diazides and diynes toward functional polytriazoles for sensing applications. Polym. Chem. 2020, 11, 2006–2014. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sasaki, H.; Hoshi, T.; Suzuki, T. Sustainable click reaction catalyzed by supported ionic liquid catalyst (Cu-SILC). Synlett 2009, 2009, 643–647. [Google Scholar] [CrossRef]
- Phukan, P.; Kulshrestha, A.; Kumar, A.; Chakraborti, S.; Chattopadhyay, P.; Sarma, D. Cu(II) ionic liquid promoted Simple and Economical Synthesis of 1,4-disubstituted-1,2,3-triazoles with Low Catalyst Loading. J. Chem. Sci. 2021, 133, 131. [Google Scholar] [CrossRef]
- Dabiri, M.; Alavioon, S.I.; Movahed, S.K. N-Heterocyclic carbene–copper complex supported on ionic liquid-modified graphene oxide: Versatile catalyst for synthesis of (i) 1,2,3-triazole and (ii) propargylamine derivatives. J. Iran. Chem. Soc. 2018, 15, 2463–2474. [Google Scholar] [CrossRef]
- Tavassoli, M.; Landarani-Isfahani, A.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Copper dithiol complex supported on silica nanoparticles: A sustainable, efficient, and eco-friendly catalyst for multicomponent click reaction. ACS Sustain. Chem. Eng. 2016, 4, 1454–1462. [Google Scholar] [CrossRef]
- Singh, N. A concise and simple click reaction catalyzed by immobilized Cu (I) in an ionic liquid leading to the synthesis of β-hydroxy triazoles. Comptes Rendus Chim. 2015, 18, 1257–1263. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent advances bioactive 1,2,4-triazole-3-thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Devi, M.; Sharma, N.; Rathi, K.; Dwivedi, J.; Sharma, S. Emerging approaches for synthesis of 1,2,3-triazole derivatives. A review. Org. Prep. Proced. Int. 2022, 54, 387–422. [Google Scholar] [CrossRef]
- Patil, P.B.; Patil, J.D.; Korade, S.N.; Kshirsagar, S.D.; Govindwar, S.P.; Pore, D.M. An efficient synthesis of anti-microbial 1,2,4-triazole-3-thiones promoted by acidic ionic liquid. Res. Chem. Intermed. 2016, 42, 4171–4180. [Google Scholar] [CrossRef]
- Dige, N.C.; Randive, C.S.; Tamhane, O.S.; Ghorpade, R.S.; Kale, S.R.; Mahajan, P.G. Morpholine-Based Novel Ionic Liquid for Synthesis and Characterization of Triazolidinethiones and Their Biological Properties. Catal. Res. 2023, 3, 1–15. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Alsayari, A.; Asiri, Y.I.; Bin Muhsinah, A. 1,2,4-Triazole-3-Thiones: Greener, One-Pot, Ionic Liquid Mediated Synthesis and Antifungal Activity. Polycycl. Aromat. Compd. 2023, 43, 167–175. [Google Scholar] [CrossRef]
- Shinde, V.B.; Mhaldar, P.M.; Chhowala, T.N.; Mirzaei, M.; Ghotekar, S.K.; Rashinkar, G.S.; Pore, D.M. An efficient Brønsted acid ionic liquid catalyzed synthesis of novel spiro1,2,4-triazolidine-5-thiones and their photoluminescence study. J. Mol. Struct. 2022, 1249, 131528. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Kangani, M. Mild Brønsted basic ionic liquids catalyzed three component synthesis of pyrazolo [1,2-a][1,2,4] triazole-1,3-dione and 2-amino-3-cyano-5,10-dioxo-4-phenyl-5,10-dihydro-4H-benzo [g] chromene derivatives. Sci. Iran. 2013, 20, 571–579. [Google Scholar]
- Sarhandi, S.; Zare Fekri, L.; Vessally, E. Ultrasound Assisted Chromatography-Free Synthesis of Triazolo [1,2-a] Indazole-Triones in the Presence of 1,4-Diazabicyclo [2.2.2] Octanium Diacetate as an Environmentally Friendly Green Media. Polycycl. Aromat. Compd. 2021, 41, 963–973. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Xu, Y.; Zou, G. Tunable protic ionic liquids as solvent-catalysts for improved synthesis of multiply substituted 1,2,4-triazoles from oxadiazoles and organoamines. Tetrahedron 2012, 68, 4813–4819. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, B.; Dwivedi, A.R.; Kumar, V. Regioselective alkylation of 1, 2, 4-triazole using ionic liquids under microwave conditions. Green Process. Synth. 2016, 5, 233–237. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dwivedi, J.; Kishore, D.; Sharma, S. Green Methodologies for Tetrazole Synthesis from Different Starting Materials: A Recent Update. Curr. Org. Chem. 2024, 28, 134–160. [Google Scholar] [CrossRef]
- Popova, E.A.; Protas, A.V.; Trifonov, R.E. Tetrazole derivatives as promising anticancer agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 1856–1868. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Gadipelly, C.; Mannepalli, L.K. Advances in tetrazole synthesis–An overview. ChemistrySelect 2022, 7, 202200706. [Google Scholar] [CrossRef]
- Singh, L.P.; Gugulothu, S.; Perusomula, R.; Mishra, A.; Bhavani, P.D.; Singh, S.; Sharma, H.; Dwivedi, M. Synthesis of Some Tetrazole and Thiazolidine-4-One Derivatives of Schiff base by using Ionic Liquids as Catalyst and Evaluation of their Antifungal and Antibacterial Activity. Eur. Chem. Bull. 2023, 12, 281–297. [Google Scholar]
- Aali, E.; Gholizadeh, M.; Noroozi-Shad, N. 1-Disulfo-[2,2-bipyridine]-1,1-diium chloride ionic liquid as an efficient catalyst for the green synthesis of 5-substituted 1H-tetrazoles. J. Mol. Struct. 2022, 1247, 131289. [Google Scholar] [CrossRef]
- Schmidt, B.; Meid, D.; Kieser, D. Safe and fast tetrazole formation in ionic liquids. Tetrahedron 2007, 63, 492–496. [Google Scholar] [CrossRef]
- Jasim, S.A.; Tanjung, F.A.; Sharma, S.; Mahmoud, M.Z.; Kadhim, S.B.; Kazemnejadi, M. Ultrasound and microwave irradiated sustainable synthesis of 5-and 1-substituted tetrazoles in TAIm[I] ionic liquid. Res. Chem. Intermed. 2022, 48, 3547–3566. [Google Scholar] [CrossRef]
- Hameed, A.; Ali, S.A.; Khan, A.A.; Moin, S.T.; Khan, K.M.; Hashim, J.; Basha, F.Z.; Malik, M.I. Solvent-free click chemistry for tetrazole synthesis from 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU)-Based fluorinated ionic liquids, their micellization, and density functional theory studies. RSC Adv. 2014, 4, 64128–64137. [Google Scholar] [CrossRef]
- Iqbal, N.; Hashim, J.; Ali, S.A.; Al-Rashida, M.; Alharthy, R.D.; Ahmad, S.; Khan, K.M.; Basha, F.Z.; Moin, S.T.; Hameed, A. Solvent-free 1H-tetrazole,1,2,5,6-tetrahydronicotinonitrile and pyrazole synthesis using quinoline based ionic fluoride salts (QuFs): Thermal and theoretical studies. RSC Adv. 2015, 5, 95061–95072. [Google Scholar] [CrossRef]
- Khaghaninejad, S.; Heravi, M.M.; Hosseinnejad, T.; Oskooie, H.A.; Bakavoli, M. Regio-selective synthesis of 5-substituted 1H-tetrazoles using ionic liquid [BMIM]N3 in solvent-free conditions: A click reaction. Res. Chem. Intermed. 2016, 42, 1593–1610. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Motahharifar, N.; Nezafat, Z.; Shokouhimehr, M. Chitosan supported 1-phenyl-1H-tetrazole-5-thiol ionic liquid copper(II) complex as an efficient catalyst for the synthesis of arylaminotetrazoles. J. Mol. Liq. 2021, 341, 117398. [Google Scholar] [CrossRef]
- Padmaja, R.D.; Chanda, K. A robust and recyclable ionic liquid-supported copper(II) catalyst for the synthesis of 5-substituted-1H-tetrazoles using microwave irradiation. Res. Chem. Intermed. 2020, 46, 1307–1317. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Mohammadi, S. Highly efficient synthesis of 1-and 5-substituted 1H-tetrazoles using chitosan derived magnetic ionic liquid as a recyclable biopolymer-supported catalyst. RSC Adv. 2013, 3, 4362–4371. [Google Scholar] [CrossRef]
- Leilan, A.H.; Babazadeh, M.; Hekmati, M.; Ghasemi, E. Application of modified copper-doped Fe3O4@ CuMgAl-LDH nanoreactor with imidazolium ionic liquid as a reusable catalyst for synthesis of tetrazoles from aryl halides. Inorg. Chem. Commun. 2023, 155, 111044. [Google Scholar] [CrossRef]
- Norouzi, M.; Moradi, P.; Khanmoradi, M. Aluminium-based ionic liquid grafted on biochar as a heterogeneous catalyst for the selective synthesis of tetrazole and 2,3-dihydroquinazolin 4(1H)-one derivatives. RSC Adv. 2023, 13, 35569–35582. [Google Scholar] [CrossRef]
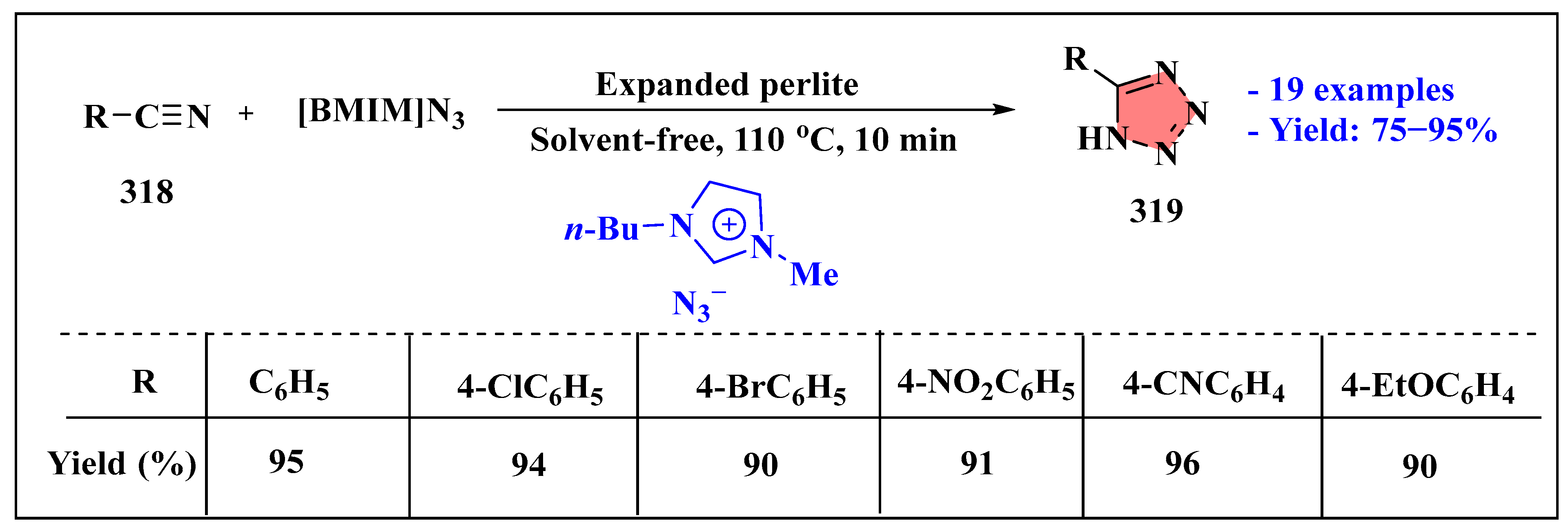
- Jahanshahi, R.; Akhlaghinia, B. Expanded perlite: An inexpensive natural efficient heterogeneous catalyst for the green and highly accelerated solvent-free synthesis of 5-substituted-1H-tetrazoles using [bmim]N3 and nitriles. RSC Adv. 2025, 5, 104087–104094. [Google Scholar] [CrossRef]
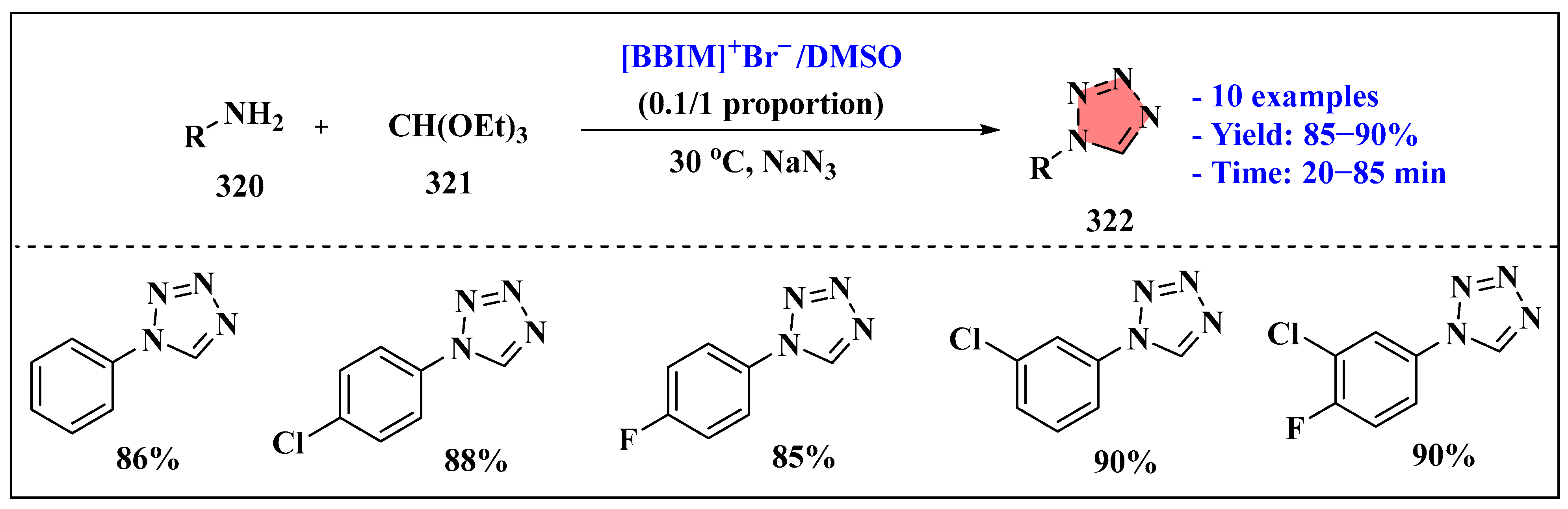
- Dighe, S.N.; Jain, K.S.; Srinivasan, K.V. A novel synthesis of 1-aryl tetrazoles promoted by employing the synergy of the combined use of DMSO and an ionic liquid as the solvent system at ambient temperature. Tetrahedron Lett. 2009, 50, 6139–6142. [Google Scholar] [CrossRef]
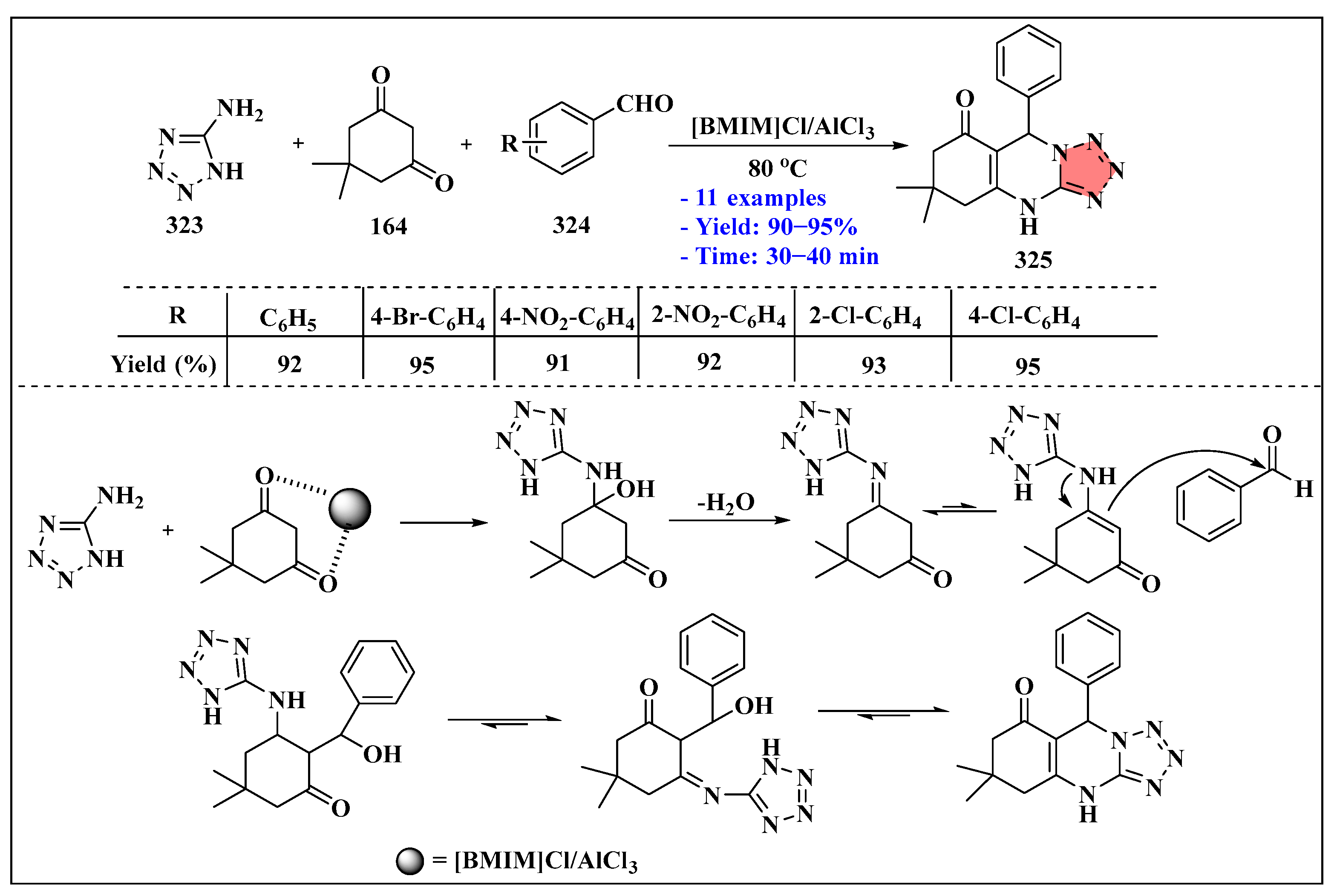
- Hassankhani, A.; Mosaddegh, E. One-pot, Environmentally Benign Procedure for the Synthesis of tetrahydrotetrazolo [1,5-a] quinazolines Using [Bmim]Cl/AlCl3 as a Task-specific Ionic Liquid. J. Appl. Chem. Res. 2019, 13, 89–96. [Google Scholar]
- Mishra, B.B.; Kumar, D.; Singh, A.S.; Tripathi, R.P.; Tiwari, V.K. Ionic liquids-prompted synthesis of biologically relevant five-and six-membered heterocyclic skeletons: An update. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Elsevier: Amsterdam, The Netherlands, 2025; pp. 437–493. [Google Scholar] [CrossRef]
- Chudasama, S.J.; Shah, B.J.; Patel, K.M.; Dhameliya, T.M. The spotlight review on ionic liquids catalyzed synthesis of aza-and oxa-heterocycles reported in 2021. J. Mol. Liq. 2022, 361, 119664. [Google Scholar] [CrossRef]
- Kaur, N.; Bhardwaj, P.; Devi, M.; Verma, Y.; Ahlawat, N.; Grewal, P. Ionic liquids for the synthesis of five-membered N,N-, N,N,N- and N,N,N,N-heterocycles. Curr. Org. Chem. 2019, 23, 1214–1238. [Google Scholar] [CrossRef]




































| Abbreviated Name | Structure | Ref. |
|---|---|---|
| [BMIM]I |  1-butyl-3-methylimidazolium iodide | [25] |
| [HMIM]HSO4 | 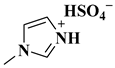 1-Methylimidazolium hydrogen sulphate | [26] |
| Bi(OTf)3/[BMIM]BF4 | 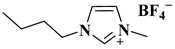 1-butyl-3-methylimidazolium tetrafluoroborate | [27] |
| [BMIM]OH | 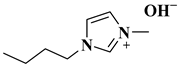 1-butyl-3-methylimidazolium hydroxide | [28] |
| [BMIM]HSO4 | 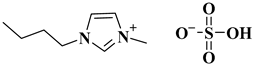 1-butyl-3-methylimidazolium hydrogen sulphate | [29] |
| [HBIM]BF4 | 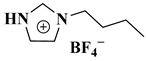 1-n-butylimidazolium tetrafluoroborate | [30] |
| [EMIM][BH3CN] | 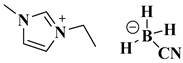 1-ethyl-3 methylimidazolium cyanoborohydride | [31] |
| [BSO3HMIM]HSO4 | 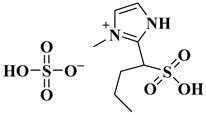 3-methyl-2-(1-sulfobutyl) 1H-imidazolium hydrogensulphate | [33] |
| [BMIM]PF6 | 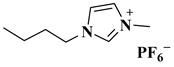 1-butyl-3-methylimidazolium hexafluorophosphate | [35] |
| [BMIM][BF4] | 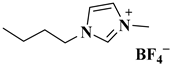 1-butyl-3-methyl imidazolium tetrafluoroborate | [38] |
| [EMIM]Ac | 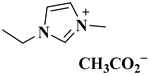 1-Ethyl-3-methylimidazolium acetate | [40] |
| [DMDBSI].2HSO4 | 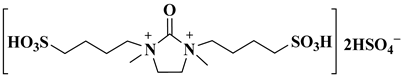 1,3-dimethyl-2-oxo-1,3-bis(4-sulfobutyl) imidazolidine-1,3-diium hydrogen sulphate | [41] |
| [Et3NH][HSO4] | 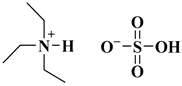 Triethylammonium hydrogen sulphate | [42] |
| (Bbpy)(HSO4)2 | 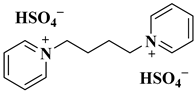 1,1′-butylenebispyridiniumhydrogen sulphate | [45] |
| [BMIM]Cl | 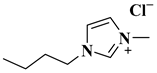 1-Butyl-3-methylimidazolium chloride | [47] |
| [NGIM]3[Cit] | 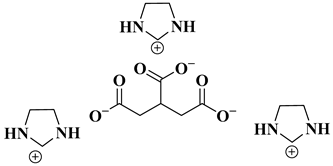 Biological-based(nano) gelatoric ionic liquids | [51] |
| Pec-DAP-BS | 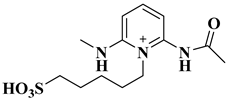 Pectin-supported Pyridinium-based ionic liquids | [52] |
| [C4mim][FeCl4] |  Transition metal-based ionic liquids | [53] |
| Ionic-liquid-based nano-magnetic solid acid heterogeneous catalyst | 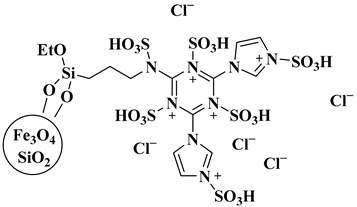 Fe3O4@SiO2@(CH2)3NH@CC@Imidazole@SO3H | [54] |
| Choline chloride salt/N-methyl-2-pyrrolidonum hydrogen sulphate | 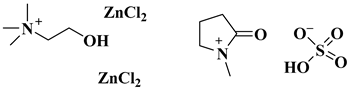 [ChCl][ZnCl2]2 and H-NMP | [55] |
| water-SDS [BMIM]Br |  Sodium dodecyl sulphate/1-butyl-3-methylimidazolium chloride | [57] |
| [HeMIM]BF4 | 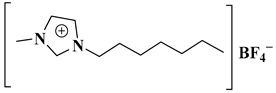 1-methyl-3-heptyl-imidazolium tetrafluoroborate | [62] |
| [BMIM]Br | 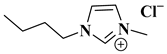 1-butyl-3-methylimidazolium bromide | [63] |
| [Et2NH2][HSO4] | 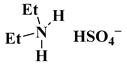 2-ethyl imidazolium hydrogen sulphate | [65] |
| MNPs-IL | 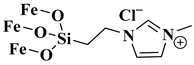 1-methyl-3-(3-trimethoxysi lylpropyl)imidazolium chloride was immobilized on Fe3O4 nanoparticles | [69] |
| Fe3O4@SiO2EPIM | 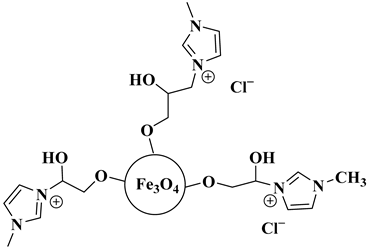 Fe3O4@SiO2 modified by epichlorohydrin and 1-methyl-imidazole | [70] |
| SILLP was prepared by using Merrifield resin |  Supported ionic liquids-like phase | [71] |
| DBU-based ionic liquids | 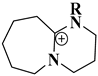 1,8-Diazabicyclo [5.4.0]undec-7-ene ionic liquids | [77] |
| [bpy][Br] | 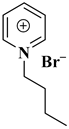 Pyridinium bromide | [78] |
| [C8dabco][N(CN)2] | 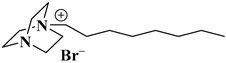 N-octyl dabco-cation-based dicyanamide | [79] |
| [DHIM][OH] |  1,3-dihexadecyl-1H-imidazol-3-ium bromide | [82] |
| [DDQM][TFSI] | 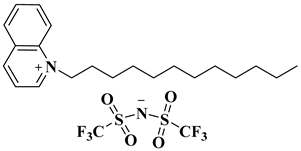 1-dodecylquinolin-1-ium bis(trifluoro methane)sulfonimide | [83] |
| [HMIM]TFA | 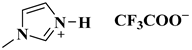 1-methylimidazolium trifluoroacetate | [87] |
| [DBU-Bu]OH | 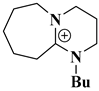 Butyl-substituted 1,8-diazabicyclo [5.4.0]undec-7-ene cation combined with a hydroxide anion | [94] |
| [HDBU][HSO4] | 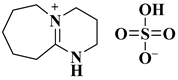 1,8-diazabicyclo [5.4.0]undec-7-ene cation combined with a hydrogen sulphate anions | [96] |
| CuI@SBA-15/PrEn/ImPF6 | 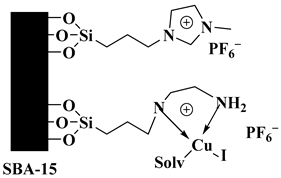 Mesoporous silica SBA-15 skeleton which bears a supported ethylenediamine/CuI complex and covalently anchored imidazolium/PF6 ionic liquid | [97] |
| MR-IMZ-As | 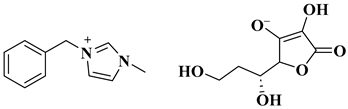 Merrifield resin (polymer)-supported ascorbate functionalized task specific ionic liquid | [105] |
| SNIL-Cu(II) | 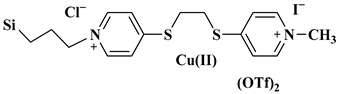 Silica nanoparticles-supported copper containing ionic liquid | [110] |
| [(Py)2SO][HSO4]2 | 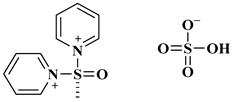 1,10 sulfinyldipyridinium bis (hydrogen sulphate) | [115] |
| [NBMMorph]+Br− | 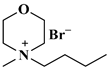 N-methyl morpholine | [116] |
| DABCO-diacetate | 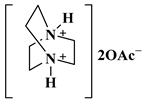 1,4-Diazabicyclo [2.2.2] Octanium Diacetate | [120] |
| OMIM-Cl | 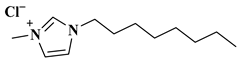 1-octyl-3-methylimidazolium | [128] |
| [CS@Tel-IL-Cu(II)] | 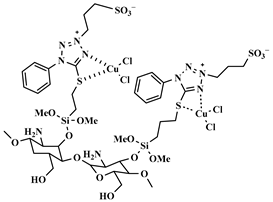 Chitosan-supported 1-phenyl-1H tetrazole-5-thiol ionic liquid copper(II) complex | [133] |
| BC/[TESPMI]AlCl4 | 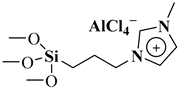 Aluminium-based ionic liquid grafted onto a biochar surface | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwivedi, J.; Jaiswal, S.; Kapoor, D.U.; Sharma, S. Catalytic Application of Ionic Liquids for the Green Synthesis of Aromatic Five-Membered Nitrogen Heterocycles. Catalysts 2025, 15, 931. https://doi.org/10.3390/catal15100931
Dwivedi J, Jaiswal S, Kapoor DU, Sharma S. Catalytic Application of Ionic Liquids for the Green Synthesis of Aromatic Five-Membered Nitrogen Heterocycles. Catalysts. 2025; 15(10):931. https://doi.org/10.3390/catal15100931
Chicago/Turabian StyleDwivedi, Jaya, Shivangi Jaiswal, Devesh U. Kapoor, and Swapnil Sharma. 2025. "Catalytic Application of Ionic Liquids for the Green Synthesis of Aromatic Five-Membered Nitrogen Heterocycles" Catalysts 15, no. 10: 931. https://doi.org/10.3390/catal15100931
APA StyleDwivedi, J., Jaiswal, S., Kapoor, D. U., & Sharma, S. (2025). Catalytic Application of Ionic Liquids for the Green Synthesis of Aromatic Five-Membered Nitrogen Heterocycles. Catalysts, 15(10), 931. https://doi.org/10.3390/catal15100931







