Removal of Cu(II) from Aqueous Medium with LDH-Mg/Fe and Its Subsequent Application as a Sustainable Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of LDH-Mg/Fe and LDH-Cu-Mg/Fe
2.1.1. X-Ray Diffraction
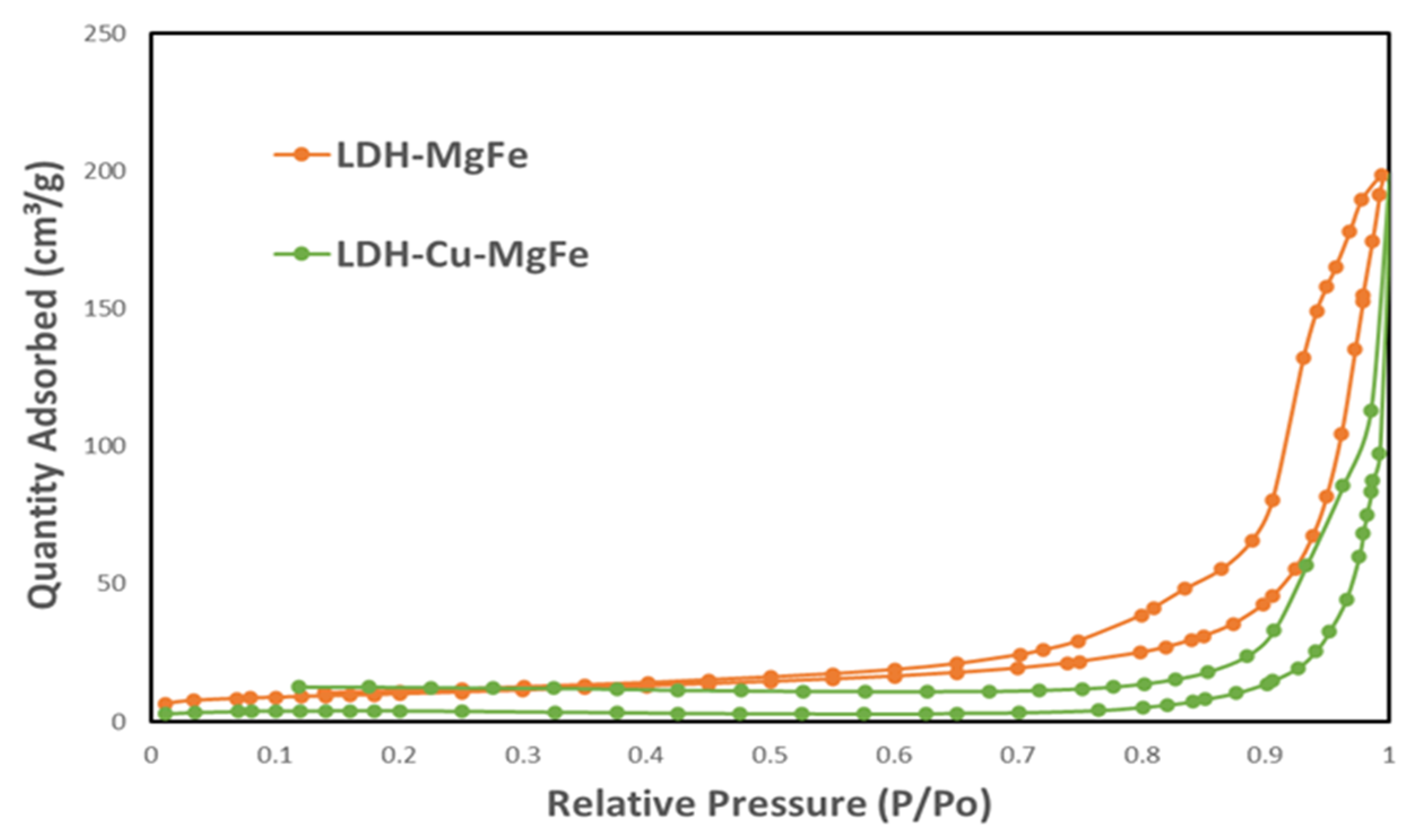
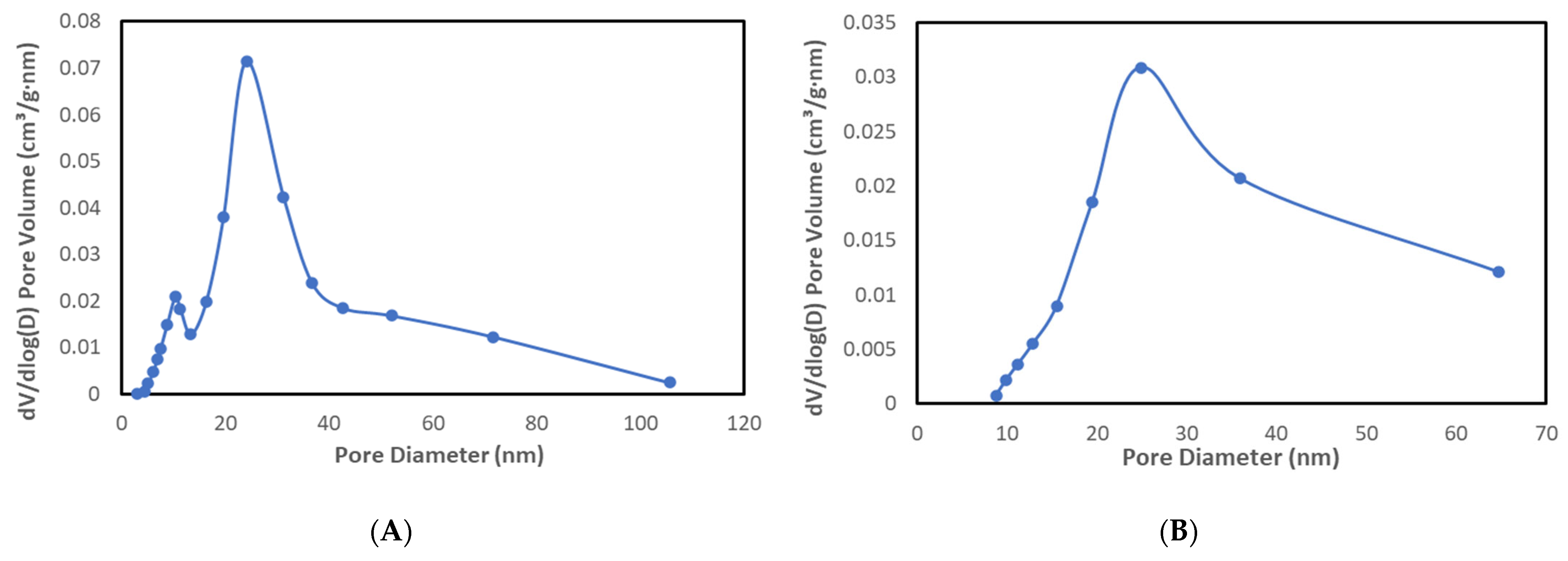
2.1.2. Textural Characterization from N2 Adsorption–Desorption Isotherms
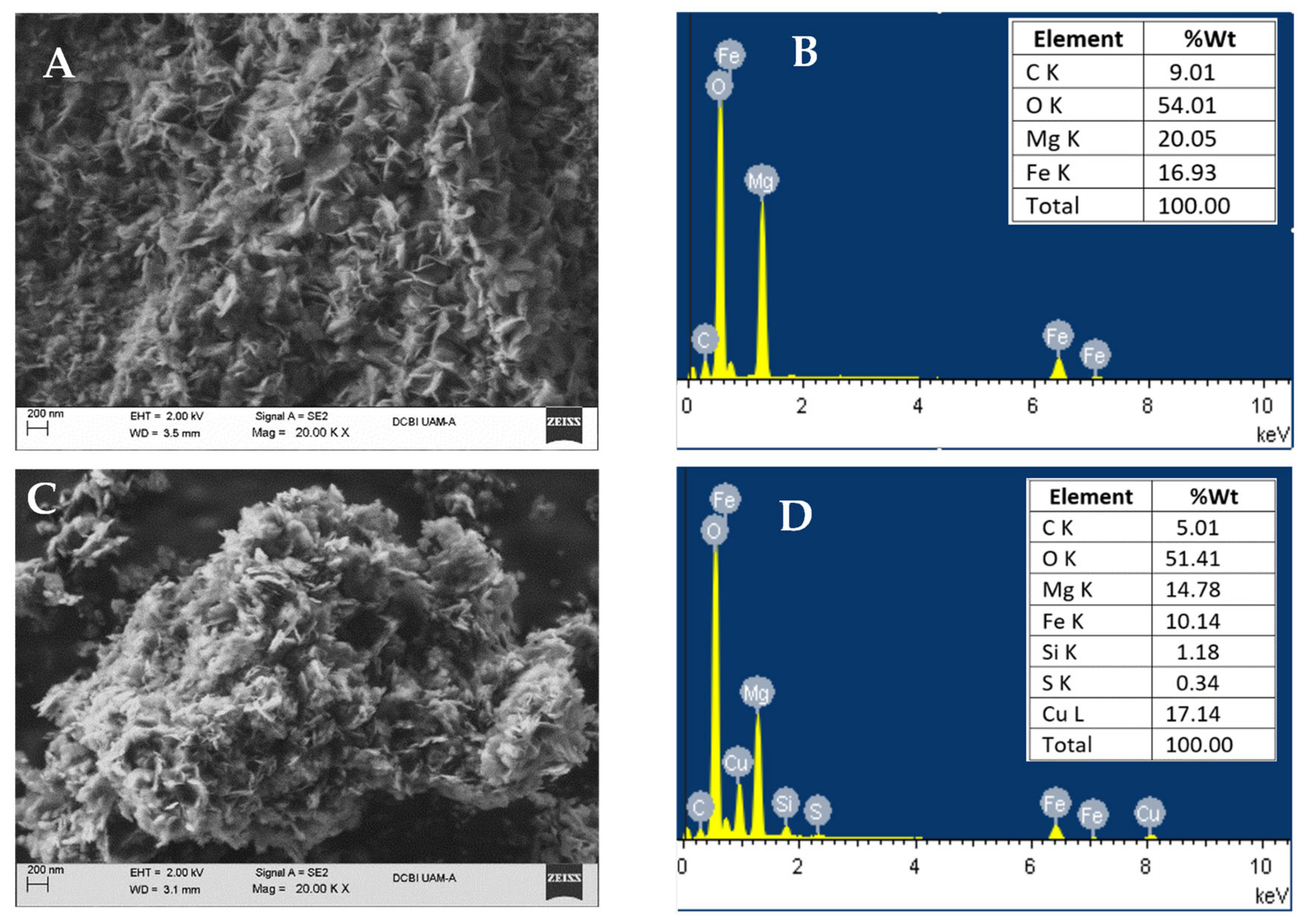
2.1.3. Scanning Electron Microscopy and Chemical Analysis (SEM-EDS)
2.1.4. UV-Vis Spectrophotometry
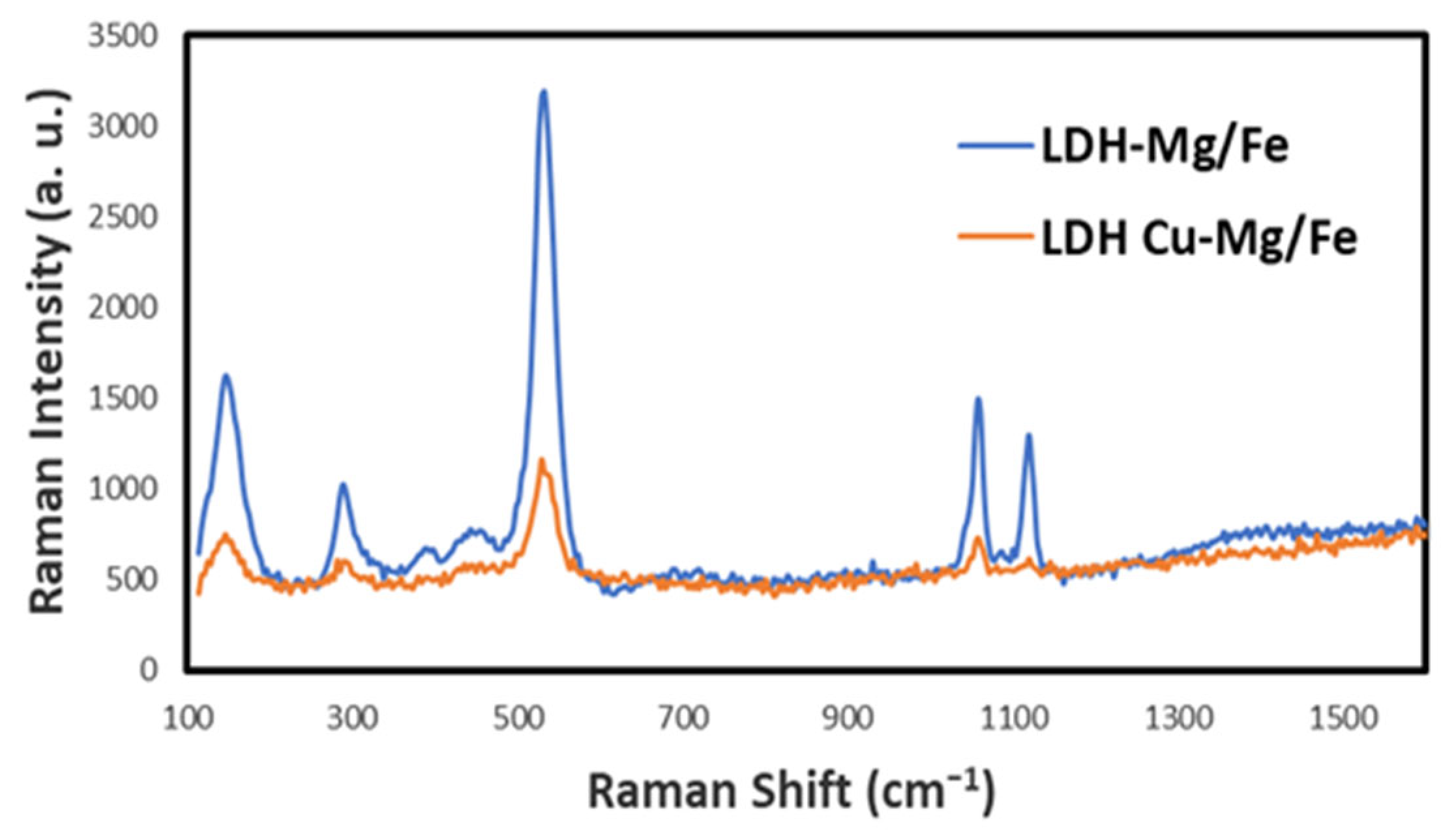
2.1.5. Raman Spectroscopy
2.1.6. Infrared Spectroscopy
2.2. Adsorption Experiments of Cu(II) on LDH-Mg/Fe
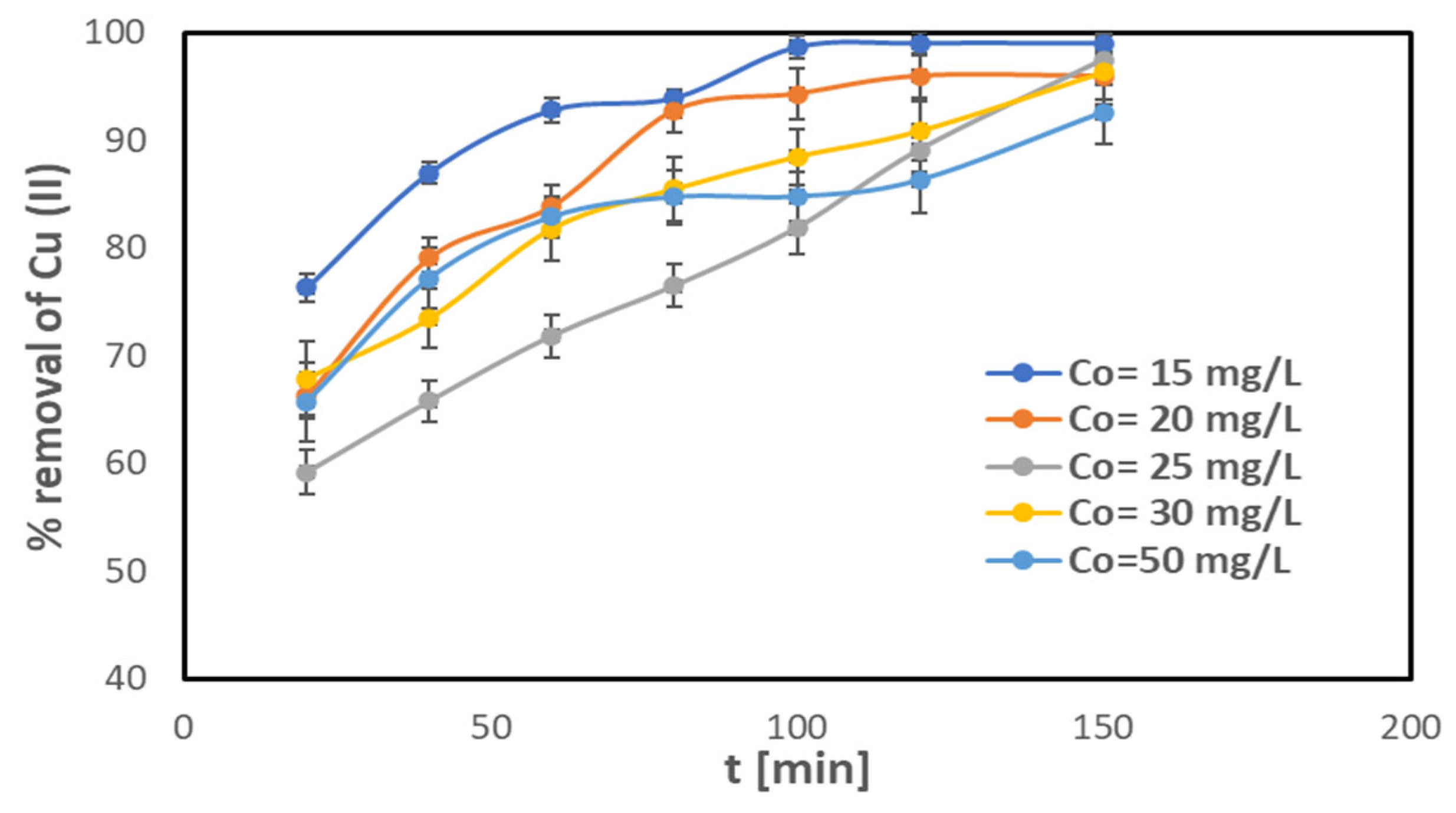
2.2.1. Removal of Cu(II) from Aqueous Solution
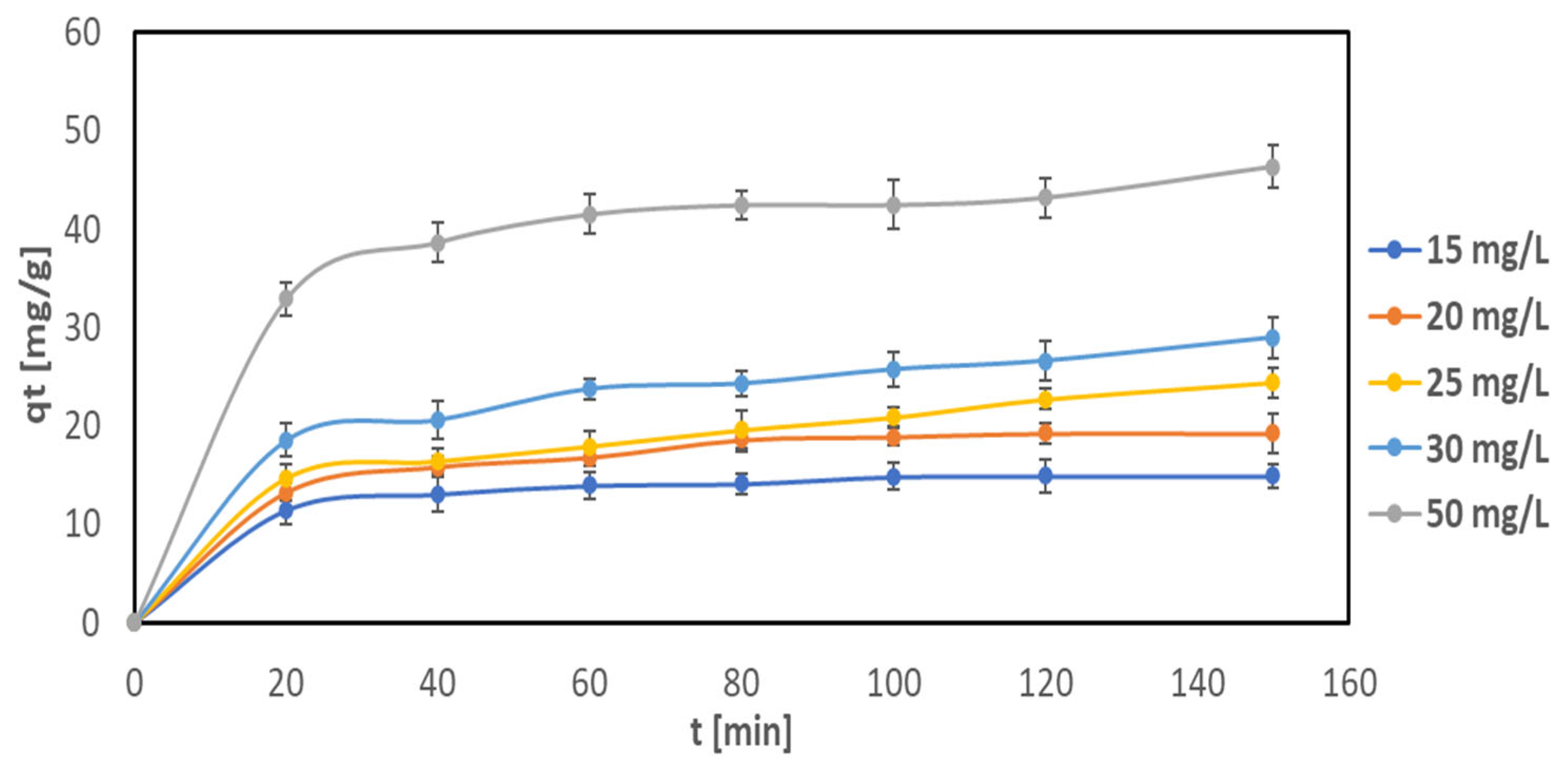
2.2.2. Influence of Contact Time on Removal
2.2.3. Kinetics of Cu(II) Adsorption
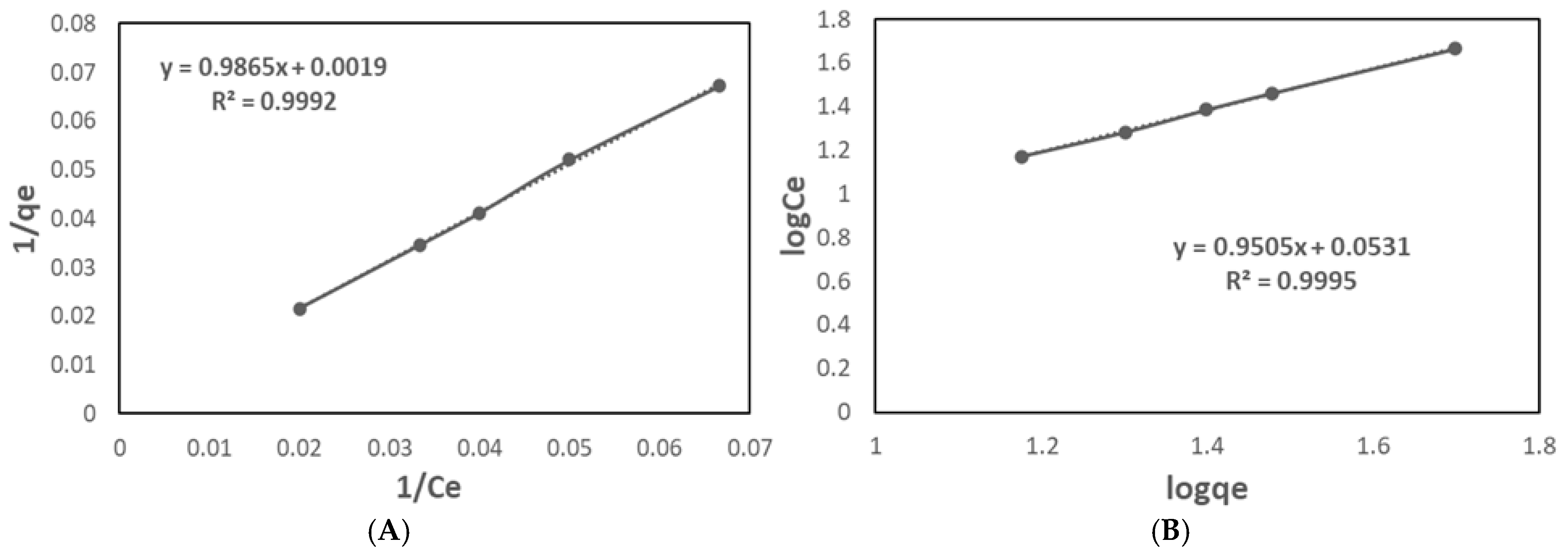
2.2.4. Adsorption Equilibrium
2.2.5. Elovich Model
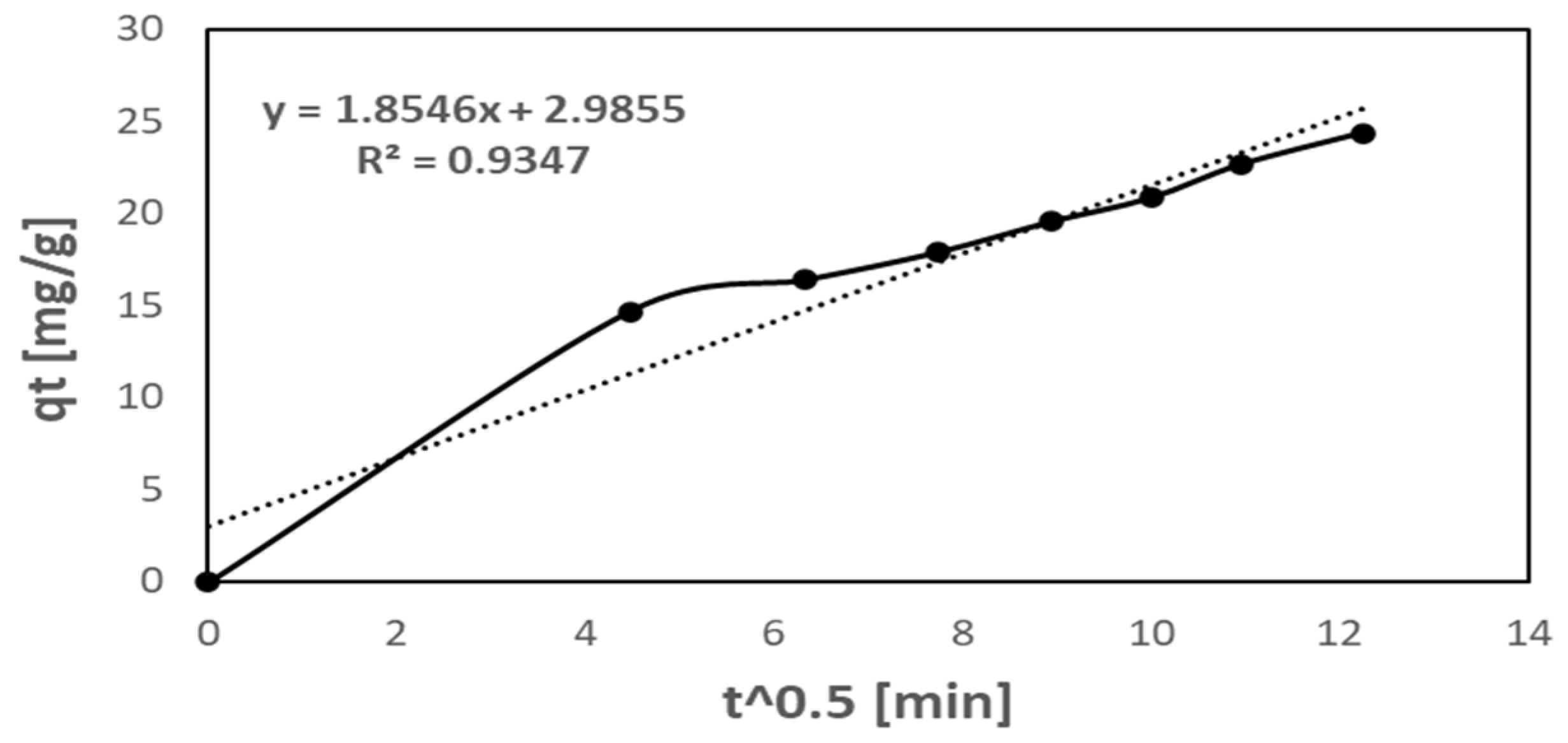
2.2.6. Intra-Particle Diffusion (IP) Model
2.3. Catalytic Activity of Sustainable Catalyst LDH-Cu-Mg/Fe
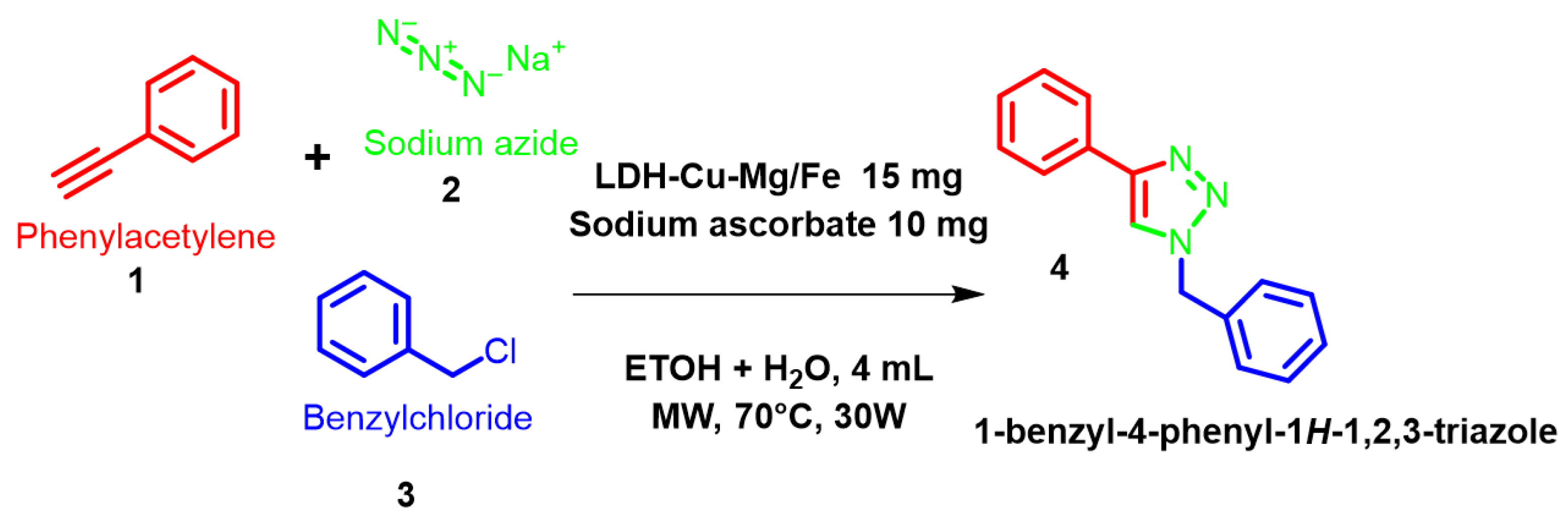
2.3.1. Catalytic Evaluation in Organic Synthesis Reaction
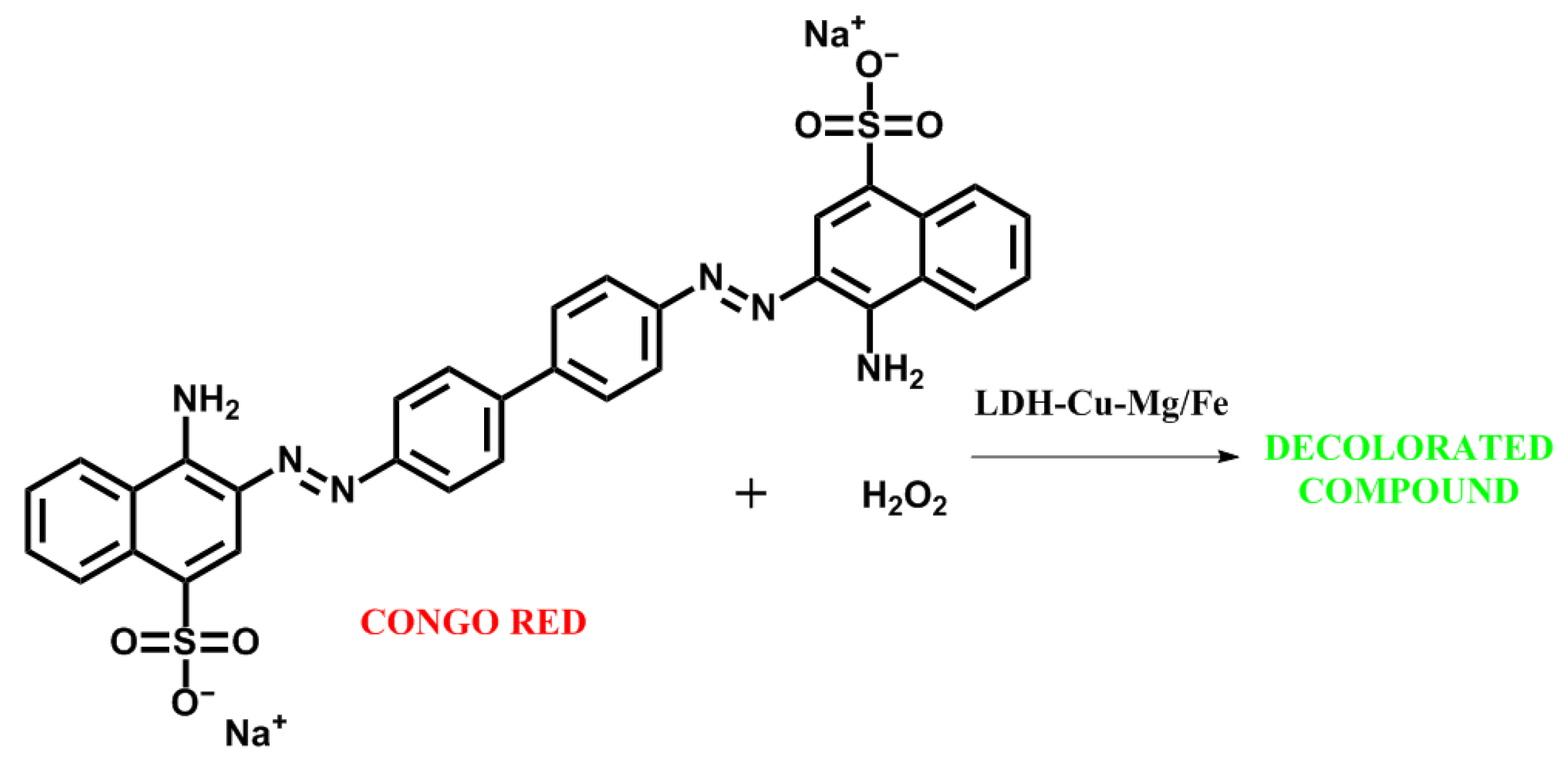
2.3.2. Catalytic Evaluation for Fenton-like Reaction
3. Materials and Methods
3.1. Synthesis of Mg/Fe-LDH
3.2. Cu(II) Adsorption Experiments
3.3. Organic Synthesis—Catalytic Evaluation of LDH-Cu-Mg/Fe
3.4. Catalytic Evaluation of LDH-Cu-Mg/Fe in a Fenton-like Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis Of Variance |
| ATR | Attenuated total reflectance |
| BET | Brunauer–Emmett–Teller |
| EPA | Environmental Protection Agency |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| FID | Flame Ionization Detection |
| GC | Gas Chromatography |
| ICDD | International Centre for Diffraction Data |
| LDH | Layered Double Hydroxide |
| MCM | Mobil Composition of Matter |
| SEM | Scanning Electron Microscopy |
| UV-Vis | Ultraviolet-Visible |
| WHO | World Health Organization |
References
- Karpińska, J.; Kotowska, U. Removal of Organic Pollution in the Water Environment. Water 2019, 11, 2017. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud. Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Moore, J.M.; Fout, A.R. Synthetic Strategies for Oxyanion Reduction: Metal-Based Insights and Innovations. Coord. Chem. Rev. 2025, 541, 216692. [Google Scholar]
- Chandra, K.; Proshad, R.; Dey, H.C.; Idris, A.M. A Review on Radionuclide Pollution in Global Soils with Environmental and Health Hazards Evaluation. Environ. Geochem. Health 2023, 45, 9245–9266. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar]
- Awual, M.R. New Type Mesoporous Conjugate Material for Selective Optical Copper(II) Ions Monitoring & Removal from Polluted Waters. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar] [CrossRef]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the Impact of Heavy Metals from Industrial Wastewater Effluent and Removal Technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Méndez-Ramírez, M.; Armienta-Hernández, M.A. Distribución de Fe, Zn, Pb, Cu, Cd y As originada por residuos mineros y aguas residuales en un transecto del Río Taxco en Guerrero, México. Rev. Mex. Cien. Geol 2012, 29, 450–462. [Google Scholar]
- Corral-Bermúdez, M.L.; Rivera-Quintero, N.; Sánchez-Ortiz, E. Perceptions and Realities about Pollution in the Mining Community of San José de Avino, Durango. Tecnol. Cienc. Agua 2014, 5, 119–134. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. npj Clean Water 2021, 4, 36. [Google Scholar]
- Hussain, F.; Kim, L.H.; Oh, S.E.; Kim, S. Neutralization of PH and Removal of Heavy Metals from Acid Mine Water by Using Low-Cost Biosorbents in Batch and Column Studies. Groundw. Sustain. Dev. 2025, 31, 101506. [Google Scholar] [CrossRef]
- Jawad, N.H.; Darwesh, T.M.; Abbas, A.F.; Yahya, A.A.; Kamel, A.H.; Rashid, K.T.; Abood, T.W.; Al-Juboori, R.A.; Meskher, H.; Al-Saadi, S.; et al. MXene Synthesis, Surface Functionalization, and Membrane Integration for Photocatalytic Removal of Heavy Metals from Wastewater: A Comprehensive Review. Mater. Today Sustain. 2025, 32, 101208. [Google Scholar] [CrossRef]
- Xing, W.; Chen, X.; Liu, J.; Meng, X.; Zhang, J. Removal of Heavy Metals from Sewage Sludge by Sequential Acidification with HCl–H3PO4 and Precipitation with Na2S–Ca(OH)2. Waste Manag. 2025, 203, 114897. [Google Scholar] [CrossRef] [PubMed]
- Taharia, M.; Das, K.; Sukul, U.; Chao, H.C.; Banerjee, P.; Dey, G.; Sharma, R.K.; Lin, P.Y.; Hung, T.C.; Chen, C.Y. Impact of Bacterial Cell Concentration on Microbial-Mediated Cerium Carbonate Precipitation for Efficient Heavy Metal Removal: Insights from Adsorption Isotherm, Kinetics, and Thermodynamics. Bioresour. Technol. 2025, 421, 132151. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xie, X.; Li, C.; Xu, Y.; Zhu, W.; Wang, L. Regulation of Structure and Anion-Exchange Performance of Layered Double Hydroxide: Function of the Metal Cation Composition of a Brucite-like Layer. Materials 2022, 15, 7983. [Google Scholar] [CrossRef]
- Xiong, W.; Yang, M.; Wang, J.; Wang, H.; Zhao, P.; Li, Z.; Liu, B.; Kong, X.; Duan, H.; Zhao, Y. Removal, Recycle and Reutilization of Multiple Heavy Metal Ions from Electroplating Wastewater Using Super-Stable Mineralizer Ca-Based Layered Double Hydroxides. Chem. Eng. Sci. 2023, 279, 118928. [Google Scholar] [CrossRef]
- Nemati, S.S.; Dehghan, G.; Khataee, A.; Alidokht, L.; Kudaibergenov, N. Layered Double Hydroxides as Versatile Materials for Detoxification of Hexavalent Chromium: Mechanism, Kinetics, and Environmental Factors. J. Environ. Chem. Eng. 2024, 12, 114742. [Google Scholar] [CrossRef]
- Shi, D.; Ren, Y.; Wang, X.; Han, X.; Zhu, X.; Xu, N. Efficient Removal of Aqueous Heavy Metal Cd2+ by Alanine Intercalation of MgAl–LDH: Adsorbent Preparation, Characterization, Adsorption Performance, and Mechanisms. ChemistrySelect 2025, 10, e202405400. [Google Scholar] [CrossRef]
- Awang, N.A.; Wan Salleh, W.N.; Ahmad, S.Z.N.; Mohd Razali, N.A.; Sazali, N.; Ismail, A.F. Synergistic Effect of Molar Ratio on Lead Removal by Zn-Al Layered Double Hydroxide. Inorg. Chem. Commun. 2025, 179, 114660. [Google Scholar] [CrossRef]
- Wu, K.; Xing, L.; Ji, Y. Synthesis and Applications of Copper-Based Catalysts. Catalysts 2023, 13, 973. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Yang, J.; Yu, J.; Chen, Q.; Peng, L. Recent Progress on Copper-Based Bimetallic Heterojunction Catalysts for CO2 Electrocatalysis: Unlocking the Mystery of Product Selectivity. Adv. Sci. 2024, 11, e2309865. [Google Scholar] [CrossRef] [PubMed]
- Drelinkiewicz, D.; Whitby, R.J. A Practical Flow Synthesis of 1,2,3-Triazoles. RSC Adv. 2022, 12, 28910–28915. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Feng, Y.; Li, F.; Su, W.; Fang, Y.; Zhao, B. Cu/CeO2 Catalysts for Reverse Water Gas Shift Reactions: The Effect of the Preparation Method. RSC Adv. 2024, 14, 16736–16746. [Google Scholar] [CrossRef]
- Zubair, Y.O.; Fuchida, S.; Oyama, K.; Tokoro, C. Morphologically Controlled Synthesis of MgFe-LDH Using MgO and Succinic Acid for Enhanced Arsenic Adsorption: Kinetics, Equilibrium, and Mechanism Studies. J. Envion. Sci. 2025, 148, 637–649. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Petranovskii, V.P.; Kryazhov, A.; Ozhereliev, O.; Pfänder, N.; Knop-Gericke, A. Study of Copper Nanoparticles Formation on Supports of Different Nature by UV-Vis Diffuse Reflectance Spectroscopy. Chem. Phys. Lett. 2004, 385, 173–176. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Raeisi, A. FTIR Characterization of Layered Double Hydroxides and Modified Layered Double Hydroxides. In Woodhead Publishing Series in Composites Science and Engineering, Layered Double Hydroxide Polymer Nanocomposites; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar] [CrossRef]
- Puzyrnaya, L.N.; Pshinko, G.N.; Zub, V.Y.; Zuy, O.V. Removal of Cu(II), Co(II) and Cd(II) from Water Solutions by Layered-Double Hydroxides with Different [Mg(II)]/[Fe(III)] Molar Ratios. Bull. Mater. Sci. 2020, 43, 3. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of Apparent Pseudo-Second-Order Adsorption Kinetics onto Cellulosic Materials. Bioresources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Lv, D.; Liu, Y.; Zhou, J.; Yang, K.; Lou, Z.; Baig, S.A.; Xu, X. Application of EDTA-Functionalized Bamboo Activated Carbon (BAC) for Pb(II) and Cu(II) Removal from Aqueous Solutions. Appl. Surf. Sci. 2018, 428, 648–658. [Google Scholar] [CrossRef]
- Benazouz, K.; Bouchelkia, N.; Moussa, H.; Boutheldja, R.; Zamouche, M.; Amrane, A.; Parvathiraja, C.; Al-Lohedan, H.A.; Bollinger, J.C.; Mouni, L. Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study. Water 2025, 17, 851. [Google Scholar] [CrossRef]
- Mohammadnezhad, G.; Soltani, R.; Abad, S.; Dinari, M. A Novel Porous Nanocomposite of Aminated Silica MCM-41 and Nylon-6: Isotherm, Kinetic, and Thermodynamic Studies on Adsorption of Cu(II) and Cd(II). J. Appl. Polym. Sci. 2017, 134, 45383. [Google Scholar] [CrossRef]
- Huang, G.; Wang, D.; Ma, S.; Chen, J.; Jiang, L.; Wang, P. A New, Low-Cost Adsorbent: Preparation, Characterization, and Adsorption Behavior of Pb(II) and Cu(II). J. Colloid Interface Sci. 2015, 445, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Lin, C.; Chao, H. Amino Acids-Intercalated Mg/Al Layered Double Hydroxides as Dual-Electronic Adsorbent for Effective Removal of Cationic and Oxyanionic Metal Ions. Sep. Purif. Technol. 2018, 192, 36–45. [Google Scholar] [CrossRef]
- Ghanavati Nasab, S.; Semnani, A.; Karimi, M.; Javaheran Yazd, M.; Cheshmekhezr, S. Synthesis of Ion-Imprinted Polymer-Decorated SBA-15 as a Selective and Efficient System for the Removal and Extraction of Cu(II) with Focus on Optimization by Response Surface Methodology. Analyst 2019, 144, 4596–4612. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M.; Tahmasebi, E. Highly Selective and Efficient Removal and Extraction of Heavy Metals by Layered Double Hydroxides Intercalated with the Diphenylamine-4- Sulfonate: A Comparative Study. Chem. Eng. J. 2017, 323, 212–223. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy Metal Ion Removal of Wastewater by Zeolite-Imidazolate Frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2018; Volume I, p. 13. [Google Scholar]
- Hernández, M.; Edgar, J.; Beltrán, D.Á.; Negrón, S.; Guillermo, E.; Olvera, R.G.; Castro, U.; Ivette, C.; Romero, L.L. Hidrotalcitas Cu/Al como catalizadores en la reacción de Huisgen. Rev. Tend. Docencia Investig. Química 2015, 659–667. [Google Scholar]
- González-Olvera, R.; Urquiza-Castro, C.I.; Negrón-Silva, G.E.; Ángeles-Beltrán, D.; Lomas-Romero, L.; Gutiérrez-Carrillo, A.; Lara, V.H.; Santillan, R.; Morales-Serna, J.A. Cu-Al Mixed Oxide Catalysts for Azide-Alkyne 1,3-Cycloaddition in Ethanol-Water. RSC Adv. 2016, 6, 63660–63666. [Google Scholar] [CrossRef]




| No. Peak | Pos. [°2Th.] | FWHM [°2Th.] | Crystallite Size [nm] | d-Spacing [nm] |
|---|---|---|---|---|
| 1 | 11.7262 | 0.3936 | 20.6139 | 0.7547 |
| 2 | 23.2812 | 0.3936 | 21.9728 | 0.3820 |
| 3 | 34.2403 | 0.2755 | 34.8814 | 0.2619 |
| 4 | 59.7959 | 0.3149 | 50.1469 | 0.1547 |
| 5 | 61.0826 | 0.3149 | 52.1726 | 0.1517 |
| 6 | 64.9338 | 0.6298 | 29.7735 | 0.1436 |
| 7 | 70.8608 | 1.152 | 21.0333 | 0.1329 |
| Average size | 32.9420 |
| No. Peak | Pos. [°2Th.] | FWHM [°2Th.] | Crystallite Size [nm] | d-Spacing [nm] |
|---|---|---|---|---|
| 1 | 11.5445 | 0.4723 | 17.1677 | 0.7665 |
| 2 | 23.1127 | 0.4723 | 18.2884 | 0.3848 |
| 3 | 34.2041 | 0.4723 | 20.3381 | 0.2622 |
| 4 | 59.5789 | 0.3542 | 44.2950 | 0.1552 |
| 5 | 60.8854 | 0.432 | 37.7952 | 0.1520 |
| Average size | 27.5769 |
| Material | BET Surface [m2/g] | Pore Volume [cm3/g] | Pore Size [nm] |
|---|---|---|---|
| LDH-Mg/Fe | 35.1 | 0.27 | 30.833 |
| LDH-Cu-Mg/Fe | 13.3 | 0.13 | 38.793 |
| Initial Concentration of Cu(II) [mg/L] | R2 Pseudo-First-Order | R2 Pseudo-Second-Order | Kinetic Constant “k2” [g mg−1 min−1] | Amount of Cu(II) Adsorbed “qe,exp” [mg g−1] | Amount of Cu(II) Adsorbed “qe,theo” [mg g−1] | % Maximum Removal |
|---|---|---|---|---|---|---|
| 15 | 0.5336 | 0.9978 | 0.01499 | 14.87 | 15.04 | 99.10 |
| 20 | 0.6189 | 0.9948 | 0.00707 | 19.20 | 20.04 | 96.01 |
| 25 | 0.7676 | 0.9756 | 0.00277 | 24.38 | 24.94 | 95.26 |
| 30 | 0.6969 | 0.9849 | 0.00314 | 28.91 | 29.24 | 96.05 |
| 50 | 0.5696 | 0.9945 | 0.00358 | 46.32 | 46.30 | 92.63 |
| Isotherm | Parameter | Cu(II) |
|---|---|---|
| Langmuir | qmax [mg/g] | 526.32 |
| KL [L/mg] | 0.0019 | |
| RL | 0.9122 | |
| R2 | 0.9992 | |
| Freundlich | KF [(mg/g)/(mg/L)1/n] | 1.1301 |
| n | 1.0520 | |
| 1/n | 0.9505 | |
| R2 | 0.9995 |
| Material | qmax [mg/g] | T [°C] | Relation Adsorbent Dose/Volume Solution W[mg]:V[ml] | Reference |
|---|---|---|---|---|
| EDTA-functionalized bamboo activated carbon (BAC) | 42 | 30 | 20:25 | [33] |
| Chitosan | 468 | 25 | 100:100 | [34] |
| Nanocomposite of aminated MCM-41/nylon-6 | 36 | 25 | 25:30 | [35] |
| Sulfonated Lignin- Mg/Al-LDH 2:1 | 64 | RT | 25:50 | [36] |
| LDH-Mg/Fe 3:1 | 500 | 20 | 50:50 | [28] |
| Phenylalanine-Mg/Al-LDH | 459 | 30 | 25:25 | [37] |
| SBA-15 supported 1-(3(trimethoxysilyl) propyl)-1H-imidazole-copper complex | 323 | 25 | 18:100 | [38] |
| Diphenylamine-4-sulfonate-Ni/Cr-LDH 3:1 | 282 | 25 | 10:20 | [39] |
| 2-methylimidazole-ZIF | 617 | 25 | 50:50 | [40] |
| LDH-Mg/Fe 3:1 | 526 | 30 | 10:50 | This work |
| Catalyst | Performance (%) 4 § |
|---|---|
| ----------- | 1 |
| Mg/Fe-LDH | 1 |
| LDH-Cu-Mg/Fe | 85 |
| Cu/Al-LDH [42] | 44 |
| Calcinated Cu/Al-LDH [42] | 86 |
| Time (min) | % Decoloration LDH-Mg/Fe | % Decoloration LDH-Cu-Mg/Fe |
|---|---|---|
| 0 | 0 | 0 |
| 30 | 23.66 | 80.06 |
| 60 | 30.38 | 83.92 |
| 90 | 34.41 | 84.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva Cruz, E.O.; Lopez-Medina, R.; Angeles-Beltrán, D.; Rodríguez-Vázquez, R. Removal of Cu(II) from Aqueous Medium with LDH-Mg/Fe and Its Subsequent Application as a Sustainable Catalyst. Catalysts 2025, 15, 930. https://doi.org/10.3390/catal15100930
Leyva Cruz EO, Lopez-Medina R, Angeles-Beltrán D, Rodríguez-Vázquez R. Removal of Cu(II) from Aqueous Medium with LDH-Mg/Fe and Its Subsequent Application as a Sustainable Catalyst. Catalysts. 2025; 15(10):930. https://doi.org/10.3390/catal15100930
Chicago/Turabian StyleLeyva Cruz, Edgar Oswaldo, Ricardo Lopez-Medina, Deyanira Angeles-Beltrán, and Refugio Rodríguez-Vázquez. 2025. "Removal of Cu(II) from Aqueous Medium with LDH-Mg/Fe and Its Subsequent Application as a Sustainable Catalyst" Catalysts 15, no. 10: 930. https://doi.org/10.3390/catal15100930
APA StyleLeyva Cruz, E. O., Lopez-Medina, R., Angeles-Beltrán, D., & Rodríguez-Vázquez, R. (2025). Removal of Cu(II) from Aqueous Medium with LDH-Mg/Fe and Its Subsequent Application as a Sustainable Catalyst. Catalysts, 15(10), 930. https://doi.org/10.3390/catal15100930







