Highly Efficient 1-Iodination of Terminal Alkynes Catalyzed by Inorganic or Organic Bases
Abstract
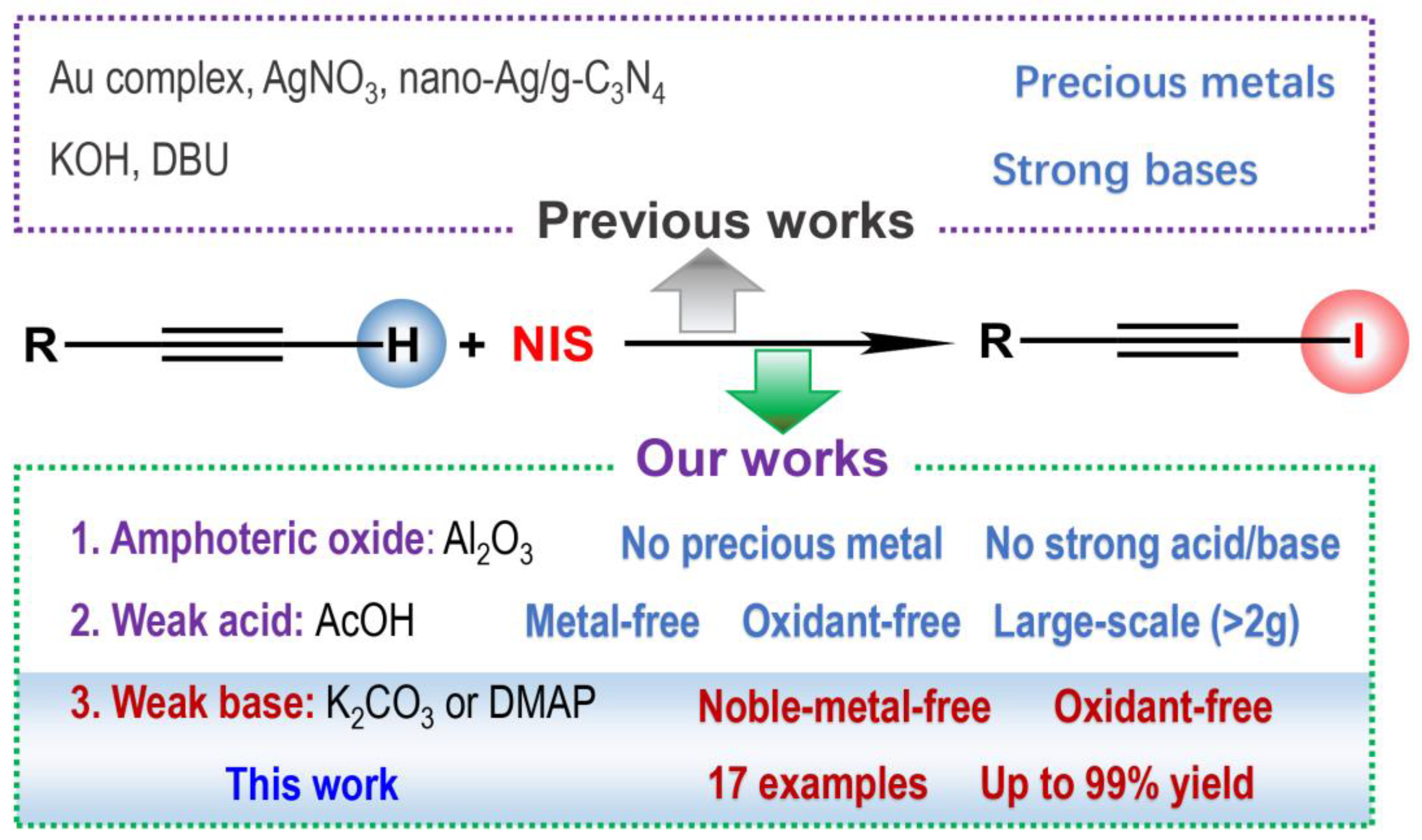
1. Introduction
2. Results and Discussion
2.1. Screening of Reaction Conditions
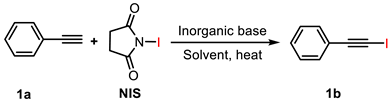
2.1.1. Inorganic Alkali Activators
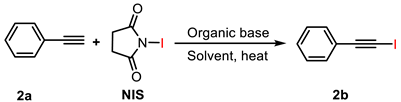
2.1.2. Organic Alkali Activators
2.2. Substrate Scope Analyses
2.3. Characterization of Products
- 1-Iodo-2-phenylacetylene (3b-1): Yellow oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.41–7.39 (m, 2H), 7.27–7.24 (m, 3H). 13C NMR(CDCl3, 100 MHz): δ (ppm) 132.42, 128.91, 128.35, 123.44, 94.25, 6.61.
- 1-(Iodoethynyl)-4-methylbenzene (3b-2): Colorless oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.33 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 2.36 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) 139.12, 132.31, 129.10, 120.46, 94.36, 21.66, 5.14.
- 1-(Iodoethynyl)-4-(trifluoromethyl)benzene (3b-3): White solid. m.p. 118–120 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.59–7.52 (m, 4H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 132.75, 130.62 (q, J = 32.8 Hz), 127.18, 125.34 (q, J = 3.8 Hz), 123.94 (q, J = 273.3 Hz.), 92.96, 10.31.
- Methyl 4-(iodoethynyl)benzoate (3b-4): White solid. m.p. 133–135 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.98 (d, J = 8.8 Hz, 2H), 7.49 (d, J = 8.8 Hz, 2H), 3.91 (s, 3H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 166.52, 132.41, 130.15, 129.54, 128.02, 93.58, 52.43, 10.60.
- 4-(Iodoethynyl)benzonitrile (3b-5): White solid. m.p. 171.0–171.6 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.60 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 8.8 Hz, 2H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 132.99, 132.07, 128.18, 118.38, 112.24, 92.67, 13.20.
- 1-(Iodoethynyl)-4-nitrobenzene (3b-6): Light yellow powdery solid. m.p. 183.5–187.2 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 8.18 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.8 Hz, 2H).
- 1-(Iodoethynyl)-2-methoxybenzene (3b-7): Light yellow oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.40 (dd, J = 7.6, 1.6 Hz, 1H), 7.31–7.27 (m, 1H), 6.92–6.86 (m, 2H), 3.88 (s, 3H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 160.97, 134.40, 130.29, 120.35, 112.50, 110.61, 90.41, 55.84, 9.60.
- 1-(Iodoethynyl)-3-methoxybenzene (3b-8): White solid. m.p. 50.3–51.5 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.24–7.20 (m, 1H), 7.05–7.02 (m, 1H), 6.97–6.96 (m, 1H), 6.90–6.87 (m, 1H), 3.79 (s, 3H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 159.26, 129.41, 124.96, 124.40, 117.15, 115.68, 94.16, 55.39, 6.39.
- 1-Fluoro-2-(iodoethynyl)benzene (3b-9): Light yellow oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.45–7.41 (m, 1H), 7.33–7.28 (m, 1H), 7.11–7.04 (m, 2H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 163.83 (d, J = 253 Hz), 134.31 (d, J = 1.3 Hz), 130.62 (d, J = 8.0 Hz), 123.99 (d, J = 3.7 Hz), 115.61 (d, J = 20.8 Hz), 112.01 (d, J = 15.8 Hz), 87.48, 12.01 (d, J = 3.2 Hz).
- 1-Fluoro-3-(iodoethynyl)benzene (3b-10): Light yellow oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.30–7.20 (m, 2H), 7.14–7.11 (m, 1H), 7.04–7.01 (m, 1H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 162.30 (d, J = 248 Hz), 129.94 (d, J = 8.7 Hz), 128.35 (d, J = 3.1 Hz), 125.19 (d, J = 9.5 Hz), 119.26 (d, J = 23 Hz), 116.40 (d, J = 21.3 Hz), 92.99 (d, J = 3.4 Hz), 8.40.
- 1-Fluoro-4-(iodoethynyl)benzene (3b-11): Light brown oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.44–7.40 (m, 2H), 7.03–6.98 (m, 2H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 162.80 (d, J = 251.4 Hz), 134.35 (d, J = 8.6 Hz), 119.52 (d, J = 3.5 Hz), 115.65 (d, J = 22.3 Hz), 93.14, 6.44.
- 1-Chloro-2-(iodoethynyl)benzene (3b-12): Yellow oil. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.47 (dd, J = 7.6, 2.0 Hz, 1H), 7.39 (dd, J = 8.0, 1.2 Hz, 1H), 7.28–7.19 (m, 2H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 136.77, 134.26, 129.87, 129.31, 126.47, 123.29, 90.99, 12.50.
- 1-Chloro-3-(iodoethynyl)benzene (3b-13): Light brown oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.42–7.41 (m, 1H), 7.33–7.29 (m, 2H), 7.26–7.22 (m, 1H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 134.12, 132.28, 130.52, 129.54, 129.21, 125.02, 92.81, 8.88.
- 1-Chloro-4-(iodoethynyl)benzene (3b-14): Pale yellow solid. m.p. 83.5–85.8 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.36 (d, J = 8.8 Hz, 2H), 7.29 (d, J = 8.8 Hz, 2H). 13C NMR (CDCl3, 100 MHz): δ (ppm) 135.04, 133.66, 128.73, 121.96, 93.10, 7.86.
- 1-Iodo-1-dodecyne (3b-15): Pale yellow oily liquid. 1H NMR (CDCl3, 400 MHz): δ (ppm) 2.35 (t, J = 7.0 Hz, 2H), 1.51–1.49 (m, 2H), 1.29–1.26 (m, 14H), 0.88–0.86 (m, 3H).
- 3-Iodoithynylthiophene (3b-16): Pale yellow solid. m.p. 44.2–45.8 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 8.53 (dd, J = 2.8, 1.2 Hz, 1H), 7.71 (dd, J = 5.0, 2.8 Hz, 1H), 7.68 (dd, J = 5.0, 1.2 Hz, 1H).
- 3-Iodoithynylpyridine (3b-17): Yellowish brown powdery solid. m.p. 132.8–136.6 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 8.67 (dd, J = 2.0, 1.2 Hz, 1H), 8.53 (dd, J = 4.8, 1.6 Hz, 1H), 7.71 (dt, J = 8.0, 2.0 Hz, 1H), 7.27–7.23 (m, 1H).
3. Material and Method
3.1. Instruments and Reagents
3.2. Synthesis of Iodide Alkynes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasson, Y.; Webster, O.W. Quaternary ammonium fluoride catalysed halogenation of carbon acids by polyhaloalkanes. J. Chem. Soc. Chem. Commun. 1992, 1200–1201. [Google Scholar] [CrossRef]
- Hofmeister, H.; Annen, K.; Laurent, H.; Wiechert, R. A Novel Entry to 17α-Bromo- and 17α-Iodoethynyl Steroids. Angew. Chem. Int. Ed. Engl. 1984, 23, 727–729. [Google Scholar] [CrossRef]
- Jarszak-Tyl, A.; Pigulski, B.; Szafert, S. Solvent-free C–H alkynylation of azulenes. Org. Chem. Front. 2021, 8, 5674–5680. [Google Scholar] [CrossRef]
- Gulia, N.; Pigulski, B.; Szafert, S. Base-Promoted Double Amination of 1-Haloalkynes: Direct Synthesis of Ene-1,1-diamines. Eur. J. Org. Chem. 2020, 2020, 5610–5615. [Google Scholar] [CrossRef]
- Vaughn, T.H.; Nieuwland, J.A. The Direct Iodination of Monosubstituted Acetylenes. J. Am. Chem. Soc. 1933, 55, 2150–2153. [Google Scholar] [CrossRef]
- Jeffery, T. Copper(I)- and phase-transfer-catalysed iodination of terminal alkynes. J. Chem. Soc. Chem. Commun. 1988, 909–910. [Google Scholar] [CrossRef]
- Narayana Rao, M.L.; Periasamy, M. A simple convenient method for the synthesis of 1-iodoalkynes. Synth. Commun. 1995, 25, 2295–2299. [Google Scholar] [CrossRef]
- He, G.-W.; Liu, F.-W.; Xu, X.-H. Cesium Carbonate Catalyzed Iodination of Terminal Alkynes to Synthesize 1-Iodoalkynes. Chin. J. Org. Chem. 2007, 27, 663–665. [Google Scholar]
- Meng, L.G.; Cai, P.J.; Guo, Q.X.; Xue, S. Direct Iodination of Monosubstituted Aryl Acetylenes and Acetylenic Ketones. Synth. Commun. 2008, 38, 225–231. [Google Scholar] [CrossRef]
- Chen, S.-N.; Hung, T.-T.; Lin, T.-C.; Tsai, F.-Y. Reusable and Efficient Cul/TBAB-Catalyzed Iodination of Terminal Alkynes in Water under Air. J. Chin. Chem. Soc. 2009, 56, 1078–1081. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Cheng, D. Novel and Efficient Synthesis of 1-Iodoalkynes. Synlett 2007, 2007, 2442–2444. [Google Scholar] [CrossRef]
- Rajender Reddy, K.; Venkateshwar, M.; Uma Maheswari, C.; Santhosh Kumar, P. Mild and efficient oxy-iodination of alkynes and phenols with potassium iodide and tert-butyl hydroperoxide. Tetrahedron Lett. 2010, 51, 2170–2173. [Google Scholar] [CrossRef]
- Ferris, T.; Carroll, L.; Mease, R.C.; Spivey, A.C.; Aboagye, E.O. Iodination of terminal alkynes using KI/CuSO4—A facile method with potential for radio-iodination. Tetrahedron Lett. 2019, 60, 936–939. [Google Scholar] [CrossRef]
- Nishiguchi, I.; Kanbe, O.; Itoh, K.; Maekawa, H. Facile Iodination of Terminal Acetylenes by Anodic Oxidation in the Presence of NaI. Synlett 2000, 2000, 89–91. [Google Scholar]
- Casarini, A.; Dembech, P.; Reginato, G.; Ricci, A.; Seconi, G. Terminal 1-halo- 1 and 1-pseudohalo-1-alkynes via bis(trimethylsilyl)peroxide (BTMSPO) promoted Umpolung transfer of Halides and pseudohalides. Tetrahedron Lett. 1991, 32, 2169–2170. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Zhao, H.; Guo, X.; Hu, X. Switchable Synthesis of Iodoalkynes and Diiodoalkenes from Terminal Alkynes. Chin. J. Org. Chem. 2018, 38, 1172–1176. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.; Huang, J.; Maruoka, K. Hypervalent Iodine Mediated Chemoselective Iodination of Alkynes. J. Org. Chem. 2017, 82, 11865–11871. [Google Scholar] [CrossRef]
- Srujana, K.; Swamy, P.; Naresh, M.; Durgaiah, C.; Rammurthy, B.; Sai, G.K.; Sony, T.; Narender, N. A Quaternary Ammonium Salt Promoted Regioselective Iodination of Terminal Alkynes: A Convenient Access to 1-Iodoalkynes in Aqueous Media. ChemistrySelect 2017, 2, 748–752. [Google Scholar] [CrossRef]
- Brunel, Y.; Rousseau, G. An easy preparation of iodoacetylenes. Tetrahedron Lett. 1995, 36, 2619–2622. [Google Scholar] [CrossRef]
- Abele, E.; Fleisher, M.; Rubina, K.; Abele, R.; Lukevics, E. Halogenation of terminal acetylenes by perhalogenoalkanes under phase transfer catalysis conditions. J. Mol. Catal. A Chem. 2001, 165, 121–126. [Google Scholar] [CrossRef]
- Nouzarian, M.; Hosseinzadeh, R.; Golchoubian, H. Ionic Liquid Iodinating Reagent for Mild and Efficient Iodination of Aromatic and Heteroaromatic Amines and Terminal Alkynes. Synth. Commun. 2013, 43, 2913–2925. [Google Scholar] [CrossRef]
- Hein, J.E.; Tripp, J.C.; Krasnova, L.B.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Cycloaddition of Organic Azides and 1-Iodoalkynes. Angew. Chem. Int. Ed. 2009, 48, 8018–8021. [Google Scholar] [CrossRef] [PubMed]
- Starkov, P.; Rota, F.; D’Oyley, J.M.; Sheppard, T.D. Catalytic Electrophilic Halogenation of Silyl-Protected and Terminal Alkynes: Trapping Gold(I) Acetylides vs. a Brønsted Acid-Promoted Reaction. Adv. Synth. Catal. 2012, 354, 3217–3224. [Google Scholar] [CrossRef]
- Nösel, P.; Lauterbach, T.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Synthesis of Iodofulvenes. Chem. Eur. J. 2013, 19, 8634–8641. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Zhao, B.; Liang, F.; Jin, L.-Y. Facile and efficient synthesis of 1-haloalkynes via DBU-mediated reaction of terminal alkynes and N-haloimides under mild conditions. RSC Adv. 2014, 4, 30046–30049. [Google Scholar] [CrossRef]
- Gómez-Herrera, A.; Nahra, F.; Brill, M.; Nolan, S.P.; Cazin, C.S.J. Sequential Functionalization of Alkynes and Alkenes Catalyzed by Gold(I) and Palladium(II) N-Heterocyclic Carbene Complexes. ChemCatChem 2016, 8, 3381–3388. [Google Scholar] [CrossRef]
- Shi, W.; Guan, Z.; Cai, P.; Chen, H. Highly efficient and recyclable catalyst for the direct chlorination, bromination and iodination of terminal alkynes. J. Catal. 2017, 353, 199–204. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, J.; Yang, S.; Xiong, H.; Li, L.; Liu, E.; Shi, H. Efficient synthesis of 1-iodoalkynes via Al₂O₃ mediated reaction of terminal alkynes and N-iodosuccinimide. RSC Adv. 2020, 10, 3946–3950. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, J.; Yang, S.; Liu, E.; Xiong, H. Acetic Acid Promoted Direct Iodination of Terminal Alkynes with N-Iodosuccinimide: Efficient Preparation of 1-Iodoalkynes. Synlett 2020, 31, 1102–1106. [Google Scholar] [CrossRef]
| Year | Iodine Source | Catalyst | Solvent/Condition | Ref. |
|---|---|---|---|---|
| 1933 | I2 | — | Liquid NH3 | Vaughn [5] |
| 1988 | I2 | CuI, K2CO3 or Na2CO3, TBAC | DMF | Jeffery [6] |
| 1995 | I2 | EtMgBr | THF | Rao [7] |
| 2007 | I2 | Cs2CO3, KOH | THF-HMPA | He [8] |
| 2008 | I2 | DMAP | CH2Cl2 | Meng [9] |
| 2009 | I2 | CuI, Et3N, TBAB | H2O | Chen [10] |
| 2007 | KI | CuI, Et3N, PhI(OAc)2 | MeCN | Yan [11] |
| 2010 | KI | tBHP | MeOH | Reddy [12] |
| 2019 | KI | CuSO4ˑ5H2O, BPDS | NaOAc buffer | Ferris [13] |
| 2000 | NaI | — | MeOH divided cell | Nishiguchi [14] |
| 1991 | CuI or ZnI2 | Me3SiOOSiMe3 | THF | Casarini [15] |
| 2018 | ZnI2 | TBN, Et3N | CHCl3 | Chen [16] |
| 2017 | TBAI | PhI(OAc)2 | MeCN | Liu [17] |
| 2017 | TBAI | KHSO5 | Water | Srujana [18] |
| 1995 | (collidine)2I+PF6− | — | CH2Cl2 | Brunel [19] |
| 2001 | CI4 | KOH, 18-crown-6 | Benzene | Abele [20] |
| 2013 | HMBMIBDCI IL | DBU | THF | Nouzarian [21] |
| 2009 | N-iodomorpholine | CuI | THF | Hein [22] |
| 2012 | NIS | Ph3PAuNTf2 | CH2Cl2 | Starkov [23] |
| 2013 | NIS | AgNO3 or KOH | Acetone/H2O or MeOH | Nösel [24] |
| 2014 | NIS | DBU | MeCN | Li [25] |
| 2016 | NIS | [Au(SIPr)(NEt3)][HF2] | Toluene | Gómez-Herrera [26] |
| 2017 | NIS | Ag/g-C3N4 | Acetone | Shi [27] |
| 2020 | NIS | γ-Al2O3, 4 Å MS | MeCN | Our group [28] |
| 2020 | NIS | AcOH | MeCN | Our group [29] |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Inorganic Base | Base (Equiv.) | Solvent | T (°C) | T (Min) | Yield b (%) |
| 1 | — | 0 | MeCN | 40 | 40 | 21.5 c |
| 2 | K2CO3 | 0.25 | MeCN | 40 | 40 | 30.6 |
| 3 | Na2CO3 | 0.25 | MeCN | 40 | 40 | 28.4 |
| 4 | NaHCO3 | 0.25 | MeCN | 40 | 40 | — d |
| 5 | KHCO3 | 0.25 | MeCN | 40 | 40 | — d |
| 6 | K2CO3 | 0.25 | THF | 40 | 40 | — d |
| 7 | K2CO3 | 0.25 | DMSO | 40 | 40 | 80.3 |
| 8 | K2CO3 | 0.25 | DMF | 40 | 40 | 84.0 |
| 9 | K2CO3 | 0.25 | MeOH | 40 | 40 | 90.6 |
| 10 | K2CO3 | 0.03 | MeOH | 40 | 40 | 92.5 |
| 11 | K2CO3 | 0.06 | MeOH | 40 | 40 | 91.1 |
| 12 | K2CO3 | 0.12 | MeOH | 40 | 40 | 89.3 |
| 13 | K2CO3 | 0.5 | MeOH | 40 | 40 | 86.2 |
| 14 | K2CO3 | 0.03 | MeOH | 20 | 40 | 87.5 |
| 15 | K2CO3 | 0.03 | MeOH | 30 | 40 | 88.4 |
| 16 | K2CO3 | 0.03 | MeOH | 50 | 40 | 94.7 |
| 17 | K2CO3 | 0.03 | MeOH | 40 | 5 | 93.8 |
| 18 | K2CO3 | 0.03 | MeOH | 40 | 10 | 97.6 |
| 19 | K2CO3 | 0.03 | MeOH | 40 | 20 | 95.2 |
| 20 | K2CO3 | 0.03 | MeOH | 40 | 30 | 94.0 |
 | ||||
|---|---|---|---|---|
| Entry | Base (Equiv.) | Solvent | T (°C) | Yield b (%) |
| 1 | Et3N (0.05) | CH3CN | 15 | 28 |
| 2 | Et3N (0.1) | CH3CN | 15 | 30 |
| 3 | Et3N (0.25) | CH3CN | 15 | 43 |
| 4 | Et3N (0.5) | CH3CN | 15 | 32 |
| 5 | Et3N (0.25) | CH3OH | 15 | 49 |
| 6 | Et3N (0.25) | CH3OH | 30 | 79 |
| 7 | Et3N (0.25) | CH3OH | 45 | 87 |
| 8 | Et3N (0.25) | CH3OH | 60 | 75 |
| 9 | DBU (0.5) | CH3CN | 15 | 46 |
| 10 | DBU (0.25) | CH3CN | 15 | 39 |
| 11 | DBU (0.6) | CH3CN | 15 | 42 |
| 12 | DBU (0.5) | CH3CN | 30 | 59 |
| 13 | DBU (0.5) | CH3CN | 45 | 65 |
| 14 | DBU (0.5) | CH3OH | 15 | 75 |
| 15 | DBU (0.5) | CH3OH | 30 | 81 |
| 16 | DBU (0.5) | CH3OH | 45 | 83 |
| 17 | DMAP (0.5) | CH3CN | 15 | 71 |
| 18 | DMAP (0.25) | CH3CN | 15 | 76 |
| 19 | DMAP (0.1) | CH3CN | 15 | 68 |
| 20 | DMAP (0.25) | CH3CN | 30 | 95 |
| 21 | DMAP (0.25) | CH3CN | 45 | 97 |
| 22 | DMAP (0.25) | CH3CN | 60 | 92 |
| 23 | DMAP (0.25) | CH3OH | 45 | 85 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Yield Under K2CO3 a/% | Yield Under DMAP b/% |
| 1 | 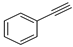 |  | 99 | 97 |
| 2 | 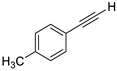 | 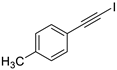 | 87 | 92 |
| 3 |  | 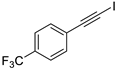 | 87 | 82 |
| 4 | 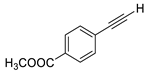 | 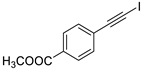 | 89 | 80 |
| 5 | 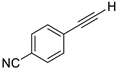 | 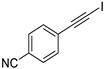 | 89 | 94 |
| 6 | 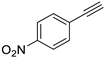 | 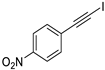 | 99 | 91 |
| 7 |  | 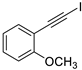 | 90 | 74 |
| 8 |  |  | 85 | 91 |
| 9 | 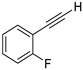 | 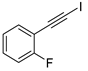 | 87 | 76 |
| 10 |  |  | 84 | 72 |
| 11 | 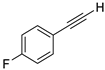 |  | 90 | 77 |
| 12 | 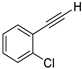 |  | 85 | 81 |
| 13 | 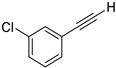 |  | 88 | 72 |
| 14 | 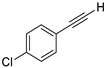 |  | 92 | 73 |
| 15 | 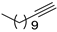 | 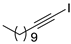 | 92 | 86 |
| 16 |  |  | 87 | 80 |
| 17 | 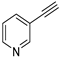 |  | 98 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, J.; Wan, F.; Liu, E.; Zhang, H. Highly Efficient 1-Iodination of Terminal Alkynes Catalyzed by Inorganic or Organic Bases. Catalysts 2024, 14, 610. https://doi.org/10.3390/catal14090610
Yang Y, Zhang J, Wan F, Liu E, Zhang H. Highly Efficient 1-Iodination of Terminal Alkynes Catalyzed by Inorganic or Organic Bases. Catalysts. 2024; 14(9):610. https://doi.org/10.3390/catal14090610
Chicago/Turabian StyleYang, Yang, Jian Zhang, Fang Wan, E Liu, and Huaxin Zhang. 2024. "Highly Efficient 1-Iodination of Terminal Alkynes Catalyzed by Inorganic or Organic Bases" Catalysts 14, no. 9: 610. https://doi.org/10.3390/catal14090610
APA StyleYang, Y., Zhang, J., Wan, F., Liu, E., & Zhang, H. (2024). Highly Efficient 1-Iodination of Terminal Alkynes Catalyzed by Inorganic or Organic Bases. Catalysts, 14(9), 610. https://doi.org/10.3390/catal14090610






