Abstract
The formation of cross-linked enzyme aggregates (CLEAs) using macromolecular cross-linkers improves substrate accessibility and enhances enzyme retention. However, there have been few studies exploring the use of macromolecular cross-linkers due to the challenges related to cross-linker screening. In compliance with our previous computational and experimental screening, dextran is the optimal macromolecular cross-linker to develop CLEAs of endolevanase from Bacillus lehensis G1 (rlevblg1-dex-CLEA) for levan-type-fructooligosaccharides (L-FOS) production. In this study, rlevblg1-dex-CLEAs was optimized, and the activity recovery continued to increase and reached 90.5%. Subsequently, the rlevblg1-dex-CLEAs were characterized and they displayed higher thermal stability after 1 h of incubation in comparison to the free enzyme. Moreover, the rlevblg1-dex-CLEAs were reusable for five cycles and exhibited greater storage stability over 180 days at 4 °C (60.9%) than that of free rlevblg1. In addition, the rlevblg1-dex-CLEAs demonstrated similar catalytic efficiency as the free enzyme and generated a substantial amount of L-FOS with a longer degree of polymerization, which is more beneficial for industrial use.
1. Introduction
Fructooligosaccharides (FOSs) have been widely recognized as functional oligosaccharides due to their prebiotic role [1]. FOSs provide moderate sweetness, reduce constipation, are of low carcinogenicity and promote control of body weight due to their low level of calories [2,3]. In addition, they improve the absorption of minerals and prohibit the growth of harmful bacteria in the host intestines, thus positively influencing human health [4].
FOSs are fructose oligomers that are composed of one glucose unit followed by up to 60 fructose monomers, which are linked together by glycosidic bonds [5]. Upon the nature of the glycosyl bonds (β (2–1) or β (2–6)) among the monomers, FOSs are categorized to inulin-type FOSs or levan-type FOSs (L-FOSs), respectively [5,6]. Compared to the inulin-type FOSs, L-FOSs enhance greater growth and activity in the beneficial microbes of the host intestines [7,8]. Therefore, L-FOSs have attracted a high interest as food ingredients that can improve the quality of food and human health.
Endolevanase from Bacillus lehensis G1 (rlevblg1) is a biocatalyst that exhibits high specific activity towards levan for the synthesis of L-FOS with various degrees of polymerization (from 2 to 10) [9]. However, free enzymes demonstrate poor stability and a lack of reusability under the harsh industrial conditions [10]. Fortunately, these two shortcomings could be overcome via enzyme immobilization, which generates continuous economic activities while providing straightforward isolation, stability, purity, high product recovery, and excellent reusability [11].
Enzyme immobilization via carrier-free, cross-linked enzyme aggregates (CLEAs) has become a prevalent strategy for enzyme stabilization. CLEAs technology develops biocatalysts that exhibit high activity and are robust, reusable as well as operationally stable [12,13]. In CLEA formation, partially purified enzymes are used to generate effective biocatalysts at a relatively low operation cost without the need for supporting materials [14]. In addition, this simple approach allows for combining the aggregation and cross-linking of the enzyme in a single step [15]. The cross-linking method is performed by physically aggregating the partially purified enzyme with a precipitant, followed by cross-linking the aggregated enzyme with a bifunctional reagent [16].
Glutaraldehyde (GA) has been employed to cross-link several enzymes due to its low cost, availability in commercial quantities, and ability to form covalent bonds with the enzyme [17]. However, GA cannot be utilized to cross-link all enzymes, especially enzymes with low external lysine residues [17,18]. The lower content of amino groups on the enzyme surface would decrease the number of the covalent interactions, thus affecting cross-linking efficiency and activity recovery [19]. Moreover, the low molecular weight of GA could lead to the penetration of the inner part of the enzyme and interaction with catalytic amino acids causing enzyme inactivation [20]. Adjacent to all of these limitations, the risk of GA leaching out from CLEAs into the reaction media constricts its utilization in food and environmental applications, owing to its negative impact on human health [21].
Alternatively, biodegradable, eco-friendly, and polysaccharide-based cross-linkers have been considered ideal linking agents that can be applied to substitute GA. The large macromolecular size of the polysaccharides would facilitate the formation of flexible and porous CLEAs, hence improving substrate accessibility and preventing enzyme deactivation [22,23]. Furthermore, the long chain of the macromolecular cross-linkers would increase the quantities of the formed linkages and decrease the chance of penetration of the vital amino acids associated with the catalytic activity, thus enhancing the recovery activity and efficiency of CLEAs [23,24,25]. Limited studies have exploited the use of macromolecular cross-linkers and reported that chitosan to develop maltogenic amylase-CLEA [1], pectin to develop glucoamylase-CLEAs [26], and polyaldehyde dextran to develop alcohol dehydrogenase-CLEAs [20] exhibited better activity compared to CLEAs prepared with GA.
Interestingly, using various macromolecular cross-linkers to develop CLEAs of the same enzyme results in different levels of activity recovery [22,24]. The nature and structural characteristics of the macromolecular cross-linkers form specific and different intermolecular interactions with the same enzyme, thus affecting the catalytic activity [22]. Therefore, in our previous work [27], homology modeling and molecular docking were integrated with experimental screening to find a suitable macromolecular cross-linker for rlevblg1. Among seven cross-linkers (cellulose, chitosan, dialdehyde-starch (DAS), dextran, pectin, polyethylene glycol 8000 (PEG8000), and sodium alginate), in silico and experimental results revealed that dextran offered the lowest binding energy and highest activity recovery [27].
Herein, to the best of our knowledge, this is the first study that exploits the findings of computational and experimental screening to develop CLEAs of rlevblg1 using dextran (Scheme 1) for L-FOS production. The rlevblg1-dex-CLEAs were optimized by analyzing several parameters, including concentration of the enzyme, concentration of cross-linking agent, pH buffer, and cross-linking time. The thermal and pH stability, kinetic performance, morphology, functional group analysis, and L-FOS production of CLEAs were characterized. The generated rlevblg1-dex-CLEAs showed a substantially high catalytic activity with improved operational stability and selectivity. The rlevblg1-dex-CLEAs hydrolyzed levan and produced a sufficient yield of longer L-FOS; hence, they are a promising biocatalyst for industrial application.
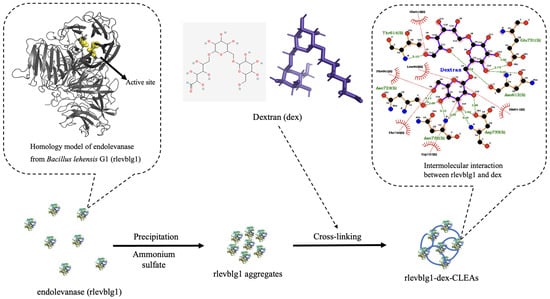
Scheme 1.
Preparation process and formation principle of cross-linked endolevanase aggregates (rlevblg1-dex-CLEAs) using macromolecule dextran as a cross-linker. The homologous 3D rlevblg1 structure and 2D schematic diagram interactions were obtained from [27], while the 2D and 3D structures of dextran were obtained from the PubChem database.
2. Results and Discussion
2.1. Optimization and Development of rlevblg1-dex-CLEAs
As the suitability of dextran as a macromolecular cross-linker for rlevblg1 was previously proven in silico and experimentally, the influence of several parameters was further optimized using the one factor at one time (OFAT) technique. The tested parameters included rlevblg1 concentration, dextran concentration, pH, and cross-linking time.
Enzyme loading is a crucial parameter that significantly affects the development of rlevblg1-dex-CLEAs. Therefore, the influence of enzyme concentration was investigated using various concentrations of rlevblg1 (0.2, 0.4, 0.6, 0.8 and, 1.0 mg/mL). As displayed in Figure 1A, the highest activity recovery of the rlevblg1-dex-CLEAs (36.8%) was obtained when 0.6 (mg/mL) of the enzyme was applied. However, the activity recovery decreased when a higher concentration of the enzyme was used. Higher enzyme loading could increase the compactness of CLEAs, thus introducing substrate diffusion limitation that causes a decrease in the recovered activity [28]. Moreover, applying less than 0.6 (mg/mL) exhibited relatively low activity recovery, which could be due to the use of an insufficient amount of the enzyme. Thus, the utilization of an exceeding or a reduced amount of the enzyme would negatively affect the formation of CLEAs and decrease the activity recovery [29,30].
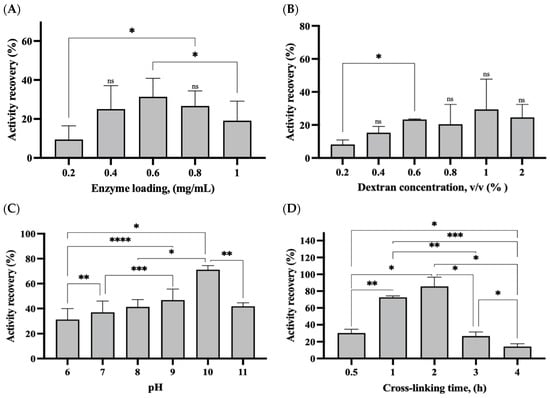
Figure 1.
Optimization in the development of rlevblg1-dex-CLEAs. The influence of (A) endolevanase concentration, (B) dextran concentration, (C) pH, and (D) cross-linking time on the activity recovery of rlevblg1-dex-CLEAs. The experiments were conducted in triplicate and error bars represent standard deviations. Average values showing significant differences are marked with varying numbers of asterisks (*) following the results of an ordinary one-way ANOVA Tukey’s multiple comparison test with a 95% confidence interval: p < 0.05 (*) shows significance; p < 0.01 (**) indicates a more significant difference; p < 0.001 (***) signifies high significance; p < 0.0001 (****) points to an extremely significant difference; (ns) denotes a non-significant result.
Cross-linker (dextran) concentration is another important factor that impacted the formation of efficient CLEAs. The recovered activity of the immobilized enzyme was increasing gradually as the concentration of dextran used increased from 0.2% to 1.0%. The highest activity (39.6%) was retained at 1% (v/v) of dextran, as shown in Figure 1B. However, the activity recovery decreased beyond the optimal concentration down to 28.2%. This reduction in the activity recovery at a high concentration of dextran could be due to the excessive cross-linking process, which caused diffusional limitation and a loss of enzyme flexibility, leading to less substrate accessibility [31,32].
In addition, the influence of pH value (6, 7, 8, 9, 10, and 11) on the formation of the rlevblg1-dex-CLEAs was further determined (Figure 1C). The activity recovery of immobilized rlevblg1 was shown to continuously increase as the pH level increased, hence the obtained activity of the rlevblg1-dex-CLEAs was higher at the alkaline environment compared to the acidic condition. The highest activity recovery of the rlevblg1-dex-CLEAs was retained with the yield of 72.1% at pH 10. The high pH preference of the rlevblg1-dex-CLEAs could be due to the coverage of the reactive groups and amino acids on the protein structure by the abundant negatively charged hydroxyl group in dextran during the cross-linking process. Thus, the enzyme surface was conferred with a negative charge and protected from any disturbances on its structure under higher pH values [25,33].
The effect of cross-linking time on the rlevblg1-dex-CLEAs was also observed using different durations ranging from 0.5 to 4 h, as demonstrated in Figure 1D. Remarkably, the highest activity recovery of 90.5% was obtained after 2 h of cross-linking time. However, the activity recovery drastically decreased when the cross-linking time was further elongated to 3 and 4 h, with yields of 27.5% and 15.3%, respectively. This indicates that exceeding 2 h on rlevblg1-dex-CLEA formation could lead to excessive cross-linking, resulting in partial deactivation of the rlevblg1-dex-CLEAs and substrate diffusional limitations [31,34]. Therefore, the optimal cross-linking time was adjusted to 2 h, while 0.6 mg/mL of enzyme was cross-linked with 1% dextran at pH 10 for the formation of efficient CLEAs. At the optimized condition, the immobilized rlevbg1-dex-CLEAs maintained a comparable specific activity (5.1 ± 0.15 U/mg) to that of the free enzyme (5.7 ± 0.59 U/mg) as well as a high recovered catalytic activity of 90.5%, which is advantageous for industrial-scale production [14].
The high activity recovery of the rlevblg1-dex-CLEAs after optimization was also predicted via the low binding affinity of dextran toward rlevblg1. From the previous study [27], we employed computational screening with different types of cross-linkers against rlevblg1. The structure of rlevbg1 was homology modeled using Modeller 9.13 software, and a docking analysis was performed to study the interaction and binding affinity of the different types of cross-linkers. Among the six other cross-linkers, dextran, which is a macromolecule with abundant hydroxyl groups, was shown to interact with the highest surface amino acid residues of rlevblg1. Additionally, the interaction of dextran displayed the farthest distance from the rlevbg1 catalytic site amino acid residues (Figure 2A), which are vital for its enzymatic activity [27]. Aligned with the computational analysis performed in our previous study, rlevblg1 cross-linked with dextran appeared to be stable, flexible, and promoted high accessibility to the active site (Figure 2C), which increased the enzymatic activity [22]. A study conducted by Jailani et al. [24] showed that among seven cross-linkers tested for CGTase-CLEA development, chitosan appeared to show the highest binding affinity in silico and corroborated the experimental result which supported our results herein. On the other hand, Abd Rahman et al. [35] reported that the use of DAS to cross-link rlevblg1 retained 67.6% activity recovery after optimization. The same study also tested only one macromolecular cross-linker (DAS) while, according to our computational analysis, DAS demonstrated possible penetration of the active site residues (Figure 2B) into the rlevblg1 structure [27], which justifies the low activity recovery. Thus, the prediction of the binding affinity and the cross-linking site through docking analysis show a crucial impact on the catalytic activity of CLEAs, as validated in this study.
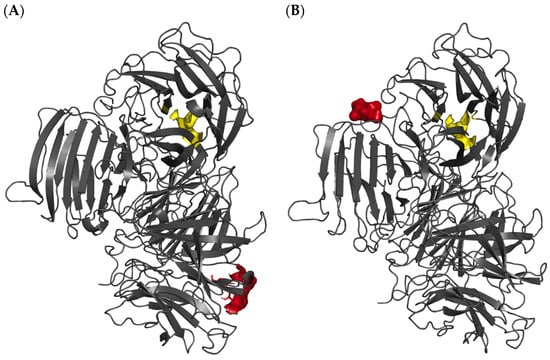
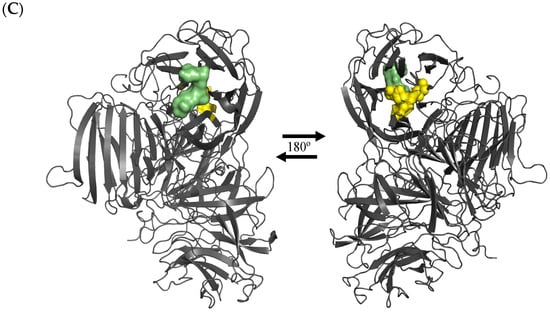
Figure 2.
Three-dimensional (3-D) structural diagram of rlevblg1 interacting with (A) dextran as a cross-linker represented in red, (B) DAS as a cross-linker represented in red, and (C) levan as a substrate represented in green. The active site of rlevblg1 is shown in yellow. The dextran, DAS, and levan structures were obtained from PubChem database, while the binding site of the ligands into rlevblg1 was simulated using Autodock Vina (1.5.6, 2014) software [27].
2.2. Characterizations of rlevblg1-dex-CLEAs
2.2.1. Optimum pH and pH Stability
From 6 to 11 on the pH scale, the influence of pH on the activity recovery of the free and immobilized rlevblg1 was determined (Figure 3A). The optimum pH for both free rlevblg1 and rlevblg1-dex-CLEAs was observed at pH 8, indicating that rlevblg1-dex-CLEA formation had no significant effect on the optimal pH value of enzyme activity. The same optimum pH for free enzyme and CLEAs has been previously reported; free halohydrin dehalogenase (HHeC) and HheC-CLEAs had pH 8 [34], whereas free lipase and p-CLEAs lipases had pH 7 as their optimum pH [36].
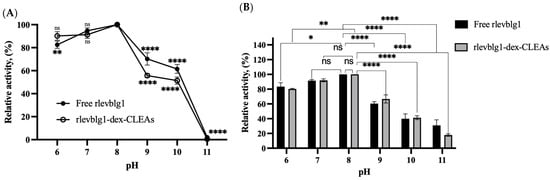
Figure 3.
(A) pH optimum of free rlevblg1 and rlevblg1-dex-CLEAs. The activity of free rlevblg1 and rlevblg1-dex-CLEAs at the optimum pH was defined as 100%. (B) pH stability of free rlevblg1 and rlevblg1-dex-CLEAs. The highest activity of free rlevblg1 and rlevblg1-dex-CLEAs was defined as 100% of relative activity. All experiments were performed in triplicate; the error bars represent standard deviations. Average values showing significant differences are marked with varying numbers of asterisks (*) following the results of an ordinary one-way ANOVA Tukey’s multiple comparison test with a 95% confidence interval, and the highest relative activity recovery was set as a control: p < 0.05 (*) shows significance; p < 0.01 (**) indicates a more significant difference; p < 0.0001 (****) points to an extremely significant difference; (ns) denotes a non-significant result.
Moreover, pH stability was evaluated using the same range of pH (6–11). As demonstrated in Figure 3B, the maximum activity of the free enzyme and rlevblg1-dex-CLEAs was detected at pH 8. Remarkably, the rlevblg1-dex-CLEAs were more stable at pH 9 and 10 compared to the free enzyme. The higher stability of the rlevblg1-dex-CLEAs in the alkaline conditions is consistent with the pH of cross-linking, as shown in Figure 1C. The cross-linking of rlevblg1 with dextran yielded increased activity recovery at higher pHs. This is because the cross-linking process stabilizes the enzyme structure of rlevblg1 as a result of efficient cross-linking, thus protecting the enzyme subunit from dissociation at higher pHs [28]. However, at pH 11, the stability of the cross-linked enzyme was lower than the free form. The exposure to the extreme alkaline environment caused the fracture of the covalent bonds that connect rlevblg1 with dextran, resulting in the decrease in the catalytic activity [37].
2.2.2. Optimum Temperature and Thermal Stability
The optimum temperature of free and immobilized rlevblg1 was determined by measuring the activity recovery across particular temperatures 20, 30, 35, 40, 45, and 50 °C, as shown in Figure 4A. The optimum temperature of free rlevblg1 was observed at 30 °C, whereas for rlevblg1-dex-CLEAs this was observed at 35 °C. Furthermore, the rlevblg1-dex-CLEAs retained a higher relative activity beyond their optimum temperature than the free enzyme. At 50 °C, the rlevblg1-dex-CLEAs exhibited 1.5-fold higher activity than their free form. The shift in the optimal temperature of the immobilized rlevblg1 might be caused by the cross-linking formation, resulting in the decrease in the enzyme flexibility and an increase in thermal tolerance [1,38].
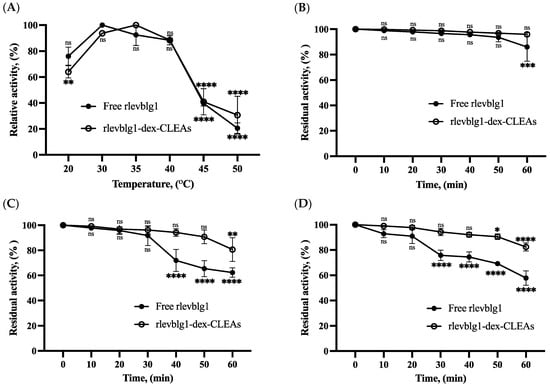
Figure 4.
Optimum temperature of (A) free rlevblg1 and rlevblg1-dex-CLEAs; the optimum temperature was adjusted to 100%. Thermal stability of free rlevblg1 and rlevblg1-dex-CLEAs at (B) 25 °C, (C) 30 °C and (D) 35 °C. The initial activity was adjusted to 100%. The experiments were conducted in triplicate and error bars represent standard deviations. Average values showing significant differences are marked with varying numbers of asterisks (*) following the results of an ordinary one-way ANOVA Tukey’s multiple comparison test with a 95% confidence interval. The highest relative activity of optimum temperature and initial activity of thermal stability was set as a control: p < 0.05 (*) shows significance; p < 0.01 (**) indicates a more significant difference; p < 0.001 (***) signifies high significance; p < 0.0001 (****) points to an extremely significant difference; ‘ns’ denotes a non-significant result.
Further, the thermal stability of free and immobilized rlevblg1 was investigated at different temperatures (25, 30, and 35 °C) for 1 h of incubation with 10 min intervals, as presented in Figure 4B–D. The residual activity of free rlevblg1 was shown to decline at a faster rate than the rlevblg1-dex-CLEAs as the incubation period and temperature increased. After 1 h of incubation, free rlevblg1 retained 86% of its initial activity at 25 °C and continued to decrease until the residual activity reached 62.3% and 57.7% at 30 °C and 35 °C, respectively. On the other hand, the rlevblg1-dex-CLEAs demonstrated higher heat resistance and retained almost 100% of their initial activity at 25 °C and more than 80% at 30 °C and 35 °C after 1 h incubation. These results have confirmed that the formed rlevblg1-dex-CLEAs had better thermal stability compared to free rlevblg1, which could be attributed to the multiple and strong attachments between the enzyme and dextran, which increased the rigidity and thermal resistance of the cross-linked rlevblg1 [39], allowing it to withstand harsh industrial processes.
2.2.3. Kinetic Analysis
The kinetic parameters of free and immobilized rlevblg1 were determined by varying the levan concentration at standard conditions and were calculated based on Lineweaver–Burk plots. The maximum reaction rate (Vmax) of the rlevblg1-dex-CLEAs (8.38 mM·min−1) was lower than that of the free enzyme (10.26 mM·min−1), as shown in Table 1. Even though the rlevblg1-dex-CLEAs possessed a slightly lower Vmax compared to the free enzyme due to its activity recovery (90.5%), the CLEAs showed higher substrate binding (Km). The Km value of the rlevblg1-dex-CLEAs (13.79 mM) was lower than that of the free rlevblg1 (14.80 mM). Since the interaction of the enzyme with the substrate was particularly measured via the Km value [40], this result confirms the improvement in the substrate affinity and internal mass transfer diffusion of the rlevblg1-dex-CLEAs. This is possibly due to the enlargement in the rlevblg1 structure after cross-linking with dextran (the macromolecular cross-linker), hence the accessibility and interaction of the substrate with the immobilized enzyme were facilitated [26]. Therefore, the overall catalytic efficiency of the rlevblg1-dex-CLEAs was about the same as the free enzyme.

Table 1.
Kinetic parameters of free rlevblg1 and rlevblg1-dex-CLEAs.
2.2.4. Effectiveness Factors of rlevblg1-dex-CLEAs
The effectiveness factor (ƞ) was determined by varying levan concentrations ranging from 1.0 to 25.0 mg/mL to investigate substrate diffusion limitations in the immobilized enzyme reaction [41]. The effectiveness factor (ƞ) of the rlevblg1-dex-CLEAs was calculated relative to the free enzyme, whereby the ƞ of the free enzyme was set as 1 at all the levan concentrations tested. The effectiveness factor of free and rlevbg1-dex-CLEA is tabulated in Table 2. The ƞ of the free enzyme increased as the levan concentration increased from 1 mg/mL to 20 mg/mL. However, a decrease in ƞ was shown by the free enzyme against the highest levan concentration (25 mg/mL) tested due to saturation of the active site cleft of the free levanase. A similar trend for rlevbg1-dex-CLEA was shown, where at increasing levan concentrations the ƞ was close to that of the free enzyme (ƞ = 1). The stability in the effectiveness factors of the rlevblg1-dex-CLEAs could be due to the increase in the flexibility and porosity that the large size of dextran provided to the immobilized enzyme structure, hence facilitating substrate accessibility [22]. Moreover, based on the docking analysis, dextran was attached to amino acids that were far from the active site [27], and its penetration to the essential amino acid residues of rlevblg1 was prevented. Therefore, the occurrence of substrate diffusional limitation was avoided, which thus confirmed that the rlevblg1-dex-CLEAs maintained similar catalytic performance to the free enzyme (Table 1).

Table 2.
Effectiveness factors (ƞ) of free rlevblg1 and rlevblg1-dex-CLEAs at different levan concentrations.
2.2.5. Morphology and Particle Size Distribution
The morphology of dextran and the rlevblg1-dex-CLEAs was investigated using FESEM at two different magnifications (500 and 3000 K). The CLEA particles could form either a spherical and well-defined structure (type I) or a cluster with a less-defined structure (type II) [42]. As displayed in Figure 5A,C, the rlevblg1-dex-CLEAs belong to type I CLEAs, owing to their uniform appearance. Moreover, the surface of the developed rlevblg1-dex-CLEAs was rough compared to native dextran, which has a smooth surface (Figure 5B,D).
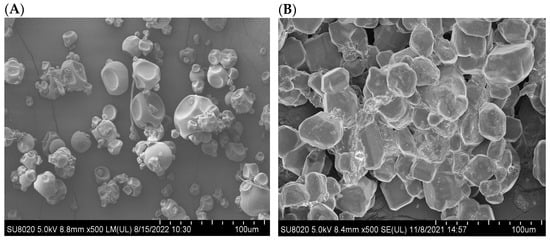
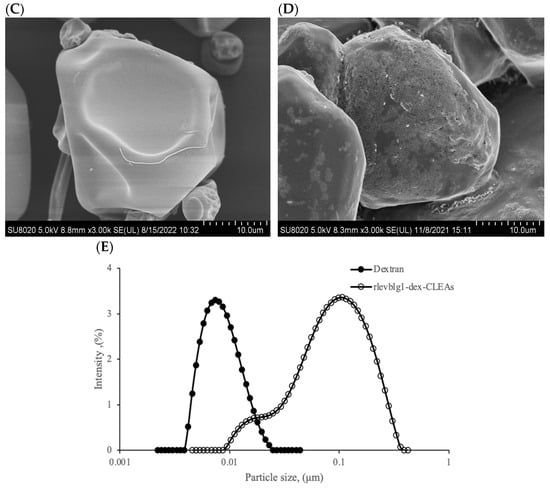
Figure 5.
FESEM images of (A) dextran, (B) rlevblg1-dex-CLEAs at 500× magnification, (C) dextran, and (D) rlevblg1-dex-CLEAs at 3000× magnification. (E) The particle size distribution of dextran and rlevblg1-dex-CLEAs using Dynamic Light Scattering (DLS).
Based on the FESEM images, the structure of the aggregates was porous with the presence of multiple cavities. However, the particle size of the rlevblg1-dex-CLEAs measured via Dynamic Light Scattering (DLS), shown in Figure 5E, was lower (0.1 μm) than that obtained from the FESEM analysis (10 μm). This could be due to the differences in sample preparation for each technique. For DLS, the samples were analyzed in a liquid environment, whereas completely dry samples were submitted for FESEM [43]. In addition, the differences in the particle size of the two methods could be due to the lack of sensitivity of DLS in analyzing complex samples [44] as well as in measuring the accumulation of structural correlation and aggregates [45], leading to misestimation of the particle size of the polydisperse samples, as is the case of the rlevblg1-dex-CLEAs in this study. Despite that, the average particle size of the rlevblg1-dex-CLEAs, using DLS, was 12.5-fold higher than that of dextran. The higher particle size [46], lower compactness [42], and presence of cavities [47] in the formed CLEAs facilitated substrate diffusion into the catalytic site and led to greater substrate affinity, which was previously confirmed by the lower Km value compared to the free enzyme (Table 1).
2.2.6. Functional Group Analysis
The chemical compositions of dextran, free rlevblg1, and the rlevblg1-dex-CLEAs were investigated using FT-IR spectroscopy (Figure 6). Based on the FT-IR spectrum of free rlevblg1, the region 3700–2900 cm−1 is associated with N–H and O–H stretching [48], and 1700 to 1600 cm−1 is assigned to the C = O stretching vibrations of amide-I bands [49]. Moreover, the area 1200–900 cm−1 is attributed to COO- [50], and the peak at 1393 cm−1 corresponds to the C-N bend of amide III [51] in rlevblg1. Compared to the free enzyme, the intensity of the O–H group (3300–2700 cm−1) in the FT-IR of the rlevblg1-dex-CLEAs was decreased, which indicates that there was intermolecular interaction between the O–H group of dextran and levanase after immobilization [52]. Interestingly, the intensity of the N-H bend at peak 1622 cm−1 significantly decreased [53] in the rlevblg1-dex-CLEAs, which indicates the interaction of dextran with the amine group in rlevblg1 to form the amide bonds [54] in CLEAs. In addition, the intensity of the 1200–900 cm−1 area and the 1393 cm−1 peak increased, which confirms the formation of carboxylate and amide linkages in the rlevblg1-dex-CLEAs, respectively.
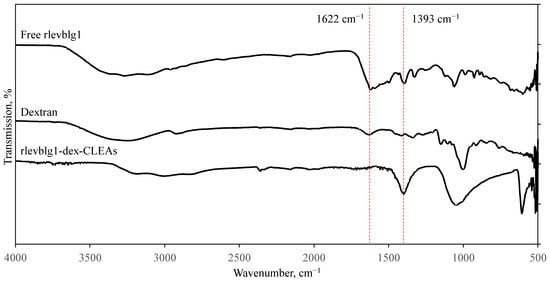
Figure 6.
Fourier transform infrared (FTIR) analysis of dextran, rlevblg1, and rlevblg1-dex-CLEAs.
2.2.7. Reusability and Storage Stability
The reusability of the enzyme is an essential feature that the CLEA technique provides during economic production in industrial applications. The reusability of the rlevblg1-dex-CLEAs was evaluated for five cycles, as illustrated in Figure 7A. Up to the third cycle, the activity of the rlevblg1-dex-CLEAs was significantly recovered and they retained 84.9% of their initial activity. In addition, almost 60% of the initial activity was retained at the fourth cycle, which is 1.2-fold higher than the residual activity of rlevblg1 cross-linked with DAS [35]. However, the rlevblg1-dex-CLEAs became unstable as the activity decreased, reaching 11.1% on the fifth cycle. This sudden reduction in the reusability could be due to the break of the covalent bonds that connect the enzyme and the cross-linker (dextran), causing enzyme leaching during the washing and separation steps after each cycle [26,55]. Leaching of the enzyme was confirmed after the separation and washing steps by measuring the protein concentration in the supernatant at the end of each cycle.
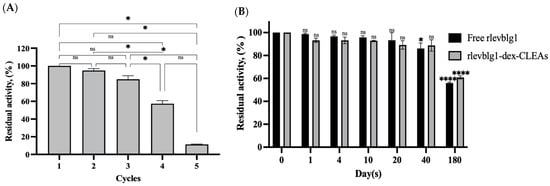
Figure 7.
(A) Reusability of rlevblg1-dex-CLEAs for 5 cycles and (B) storage stability of free rlevblg1 and rlevblg1-dex-CLEAs at 4 °C. The initial activity was adjusted to 100%. The experiments were conducted in triplicate and error bars represent standard deviations. Average values showing significant differences are marked with varying numbers of asterisks (*) following the results of an ordinary one-way ANOVA Tukey’s multiple comparison test with a 95% confidence interval, and initial activity (1st cycle and 0 days) was set as a control: p < 0.05 (*) shows significance; p < 0.0001 (****) points to an extremely significant difference; ‘ns’ denotes a non-significant result.
The storage stability of the enzyme (free and immobilized) is another crucial factor when evaluating the potential application of the enzyme in industrial processes. The storage stability of free rlevblg1 and rlevblg1-dex-CLEAs was monitored at 4 °C over 180 days by measuring the residual activity. Free and immobilized enzymes retained superior catalytic activity for over 180 days of storage (Figure 7B). After 180 days of incubation, the free rlevblg1 and rlevblg1-dex-CLEAs retained 55.9% and 60.9% of their initial activity, respectively. Moreover, the rlevblg1-dex-CLEAs exhibited significantly higher (6-fold higher) residual activity than the lipase-CLEAs, which retained only 10% of their initial activity after 180 days of incubation [36]. The enhancement in the storage stability of the rlevblg1-dex-CLEAs could be due to the strong linkages between dextran and rlevblg1 that increased the rigidity of the enzyme [39], thus extending their life span. Therefore, the properties of the rlevblg1-dex-CLEAs’ storage for a long-term duration, easy recovery, and multiple reuses would effectively reduce the overall cost of biocatalysts, thus offering an advantage for usage in industry applications.
2.3. L-FOS Production
The obtained rlevblg1-dex-CLEAs were further investigated for their hydrolysis of levan to produce L-FOS under standard reaction conditions at different reaction times (1, 2, and 3 h). Clearly, the rlevblg1-dex-CLEAs were able to hydrolyze levan, producing levanpentaose (DP5) as the major product, followed by levanbiose (DP2) and levantriose (DP3) at all reaction times (Table 3). However, levantetraose (DP4) was not detected in the final product. The degradation of DP4 has been reported previously, as endolevanase from Bacteroides thetaiotaomicron was used to hydrolyze levan [56], which supports our findings. In fact, intermediate hydrolysis of the final L-FOS profile depends on endolevanase specificity as well as reaction time [57]. Therefore, DP4 was selectively hydrolyzed with rlevblg1, even with a short time of reaction (1 h), yet not DP2, DP3, nor DP5. However, as the reaction time was extended from 2 to 3 h, a slight degradation of DP2 to fructose as well as accumulation of DP3 and DP5 were observed. Presumably, these accumulations would be re-hydrolyzed as the reaction time further elongated.

Table 3.
L-FOS production by rlevblg1-dex-CLEAs at 1, 2, and 3 h of reactions. Reactions were performed at 30 °C with 0.5% (w/v) soluble levan.
Furthermore, previous studies have reported the influence of the microbial source of the enzyme on the main product of L-FOS. Endolevanase isolated from Bacillus licheniformis [7], Bacillus sp. L7 [58], and Bacillus sp. 71 [59] produced DP2, DP3, and DP5 as the main products from levan, respectively. In the case of endolevanase from Bacillus lehensis G1 (rlevblg1), DP2 was the main product of the free enzyme and CLEAs prepared with GA [37]. Interestingly, DP3 was the main product of rlevblg1 cross-linked with DAS [35]. In this study, rlevblg1-dex-CLEAs (developed using dextran) produced DP5 as the main product. Thus, the hydrolytic product of endolevanase is not only determined by the microbial sources of the enzyme [9] but also presumably by the type of cross-linker, which has an equal impact on the formation of the final L-FOS product.
During the cross-linking process, different cross-linkers would alter the conformation of the enzyme to different extents, thus changing its active site and substrate specificity, which could potentially influence the DP of the hydrolysis products. This is consistent with our previous results, where dextran improved the effectiveness factor, morphology, size, and kinetics of immobilized rlevblg1. Therefore, the total yield of levan bioconversion by rlevblg1-dex-CLEAs increased gradually as the reaction time increased. At 3 h of reaction, 99.1% of levan (w/v) was hydrolyzed to L-FOS by the rlevblg1-dex-CLEAs, whereas the free rlevblg1, CLEA-GA, and CLEA-DAS produced 51.2% (w/v), 35.6% (w/v) [37], and 63.4% (w/v) [35] L-FOS, respectively. This significant improvement in the production of L-FOS is due to the better substrate accessibility after cross-linking rlevblg1 with dextran. In addition, the product ratio of rlevblg1-dex-CLEAs was almost the same at all reaction times, which justifies the slow rate of re-hydrolysis and the longer accumulation of L-FOS (DP5) in the final product. Evidentially, these findings emphasized the potential of rlevblg1-dex-CLEAs to produce sufficient and longer degrees of L-FOS polymerization, which is more desirable for industrial applications.
3. Methodology
3.1. Materials
All standard laboratory-grade chemicals and reagents used in this study were purchased from Thermo Fisher scientific (Waltham, MA, USA), Sigma-Aldrich (Burlington, MA, USA), and Merck (Darmstadt, Germany), unless stated otherwise.
3.2. Preparation and Optimization of Cross-Linked rlevblg1 Aggregates
Enzyme (rlevblg1) expression was performed by adding 2.0% of the grown culture (E. coli BL21 hosting the pDEST17-endolevanase plasmid) to a 1.5 L autoinduction medium incorporated with 100 μg/mL of ampicillin, at 25 °C with an agitation of 200 rpm for 24 h. Protein purification was conducted through AKTAprime plus purification system (GE Healthcare, Chicago, IL, USA) with a HisTrap HP column (GE Healthcare, Chicago, IL, USA).
The development of CLEAs was performed according to Abd Rahman et al. [35], with some modifications. For enzyme precipitation, 60% ammonium sulfate was added to 0.4 mg/mL of the purified enzyme, with 200 rpm orbital stirring at 4 °C for 1 h. The aggregated enzyme was then cross-linked by the addition of 0.8% (v/v) dextran in a total volume of 1 mL, with constant agitation of 200 rpm at 4 °C for 1 h. The formed particles were centrifuged at 10,000× g for 4 min at room temperature. The pellet was washed using glycine–NaOH buffer (50 mM, pH 8) until no activity was observed in the supernatant. Afterwards, the CLEAs were optimized by using the one factor at one time (OFAT) approach to manipulate various parameters, such as enzyme concentration (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL), concentration of cross-linker (0.2%, 0.4%, 0.6%, 0.8%, 1.0%, and 2.0% (v/v)), and pH buffer (6–11) at different lengths (0.5, 1, 2, 3, and 4 h), with 200 rpm at 4 °C. The effect of the selected parameters was analyzed based on the activity recovery of rlevblg1. The enzyme assay was performed by adding 0.5% (w/v) of levan to 1 μg of rlevblg1 in a total volume of 200 μL (50 mM glycine-NaOH buffer (pH 8.0)) at 30 °C for 10 min [60]. The enzymatic activity, recovery activity, and residual activity of free and immobilized enzymes were calculated from Equations (1)–(3), respectively, as follows:
3.3. Physiochemical Properties of Free rlevblg1 and rlevblg1-dex-CLEAs
The structure and morphology of the dextran and CLEAs were analyzed using field emission scanning electron microscope (FESEM) (Hitachi SU8020, USA). FESEM images were taken with various magnifications. Fourier transform infrared (FT-IR) spectroscopy (Perkin Elmer, Akron, OH, USA) was used to investigate the functional groups present in the wavenumber range of 370–4000 cm−1 in a transmittance mode. All samples were oven dried at 85 °C for water removal purposes prior to FESEM and FT-IR analysis. The particle size of the samples was determined using Dynamic Light Scattering (DLS) analysis via Zetasizer Nano ZSP (Malvern Instrument, Malvern, UK).
3.4. Characterization of the Biocatalytic Properties and Operational Stability of Free rlevblg1 and rlevblg1-dex-CLEAs
3.4.1. Optimum Temperature and pH
The optimum temperature of free and immobilized rlevblg1 was determined at temperatures ranging from 20 °C to 50 °C for 10 min. Likewise, the optimum pH of free and immobilized rlevblg1 was examined at different pH values (pH 6 to pH 11) at 30 °C for 10 min. The experimental buffers were prepared as described by Abd Rahman et al. [35], which were 50 mM sodium acetate (pH 5.0), 50 mM potassium phosphate (pH 6.0–7.0), and 50 mM glycine-NaOH (pH 8.0–11.0). The activity of the free and immobilized rlevblg1 at different temperatures and pHs were calculated as a relative activity to the optimum condition, which was set as 100%.
3.4.2. Thermal and pH Stability
The thermal stability of the free rlevblg1 and rlevblg1-dex-CLEAs was identified by measuring the residual activity after incubation without substrate (levan) at different temperatures (25, 30, 35 °C) for 1 h in 50 mM of glycine-NaOH buffer (pH 8.0). The enzyme activity was observed after a specific interval of every 10 min. The residual rlevblg1 activity was subsequently determined by conducting an enzyme assay and was calculated relative to the initial activity prior incubation, which was set as 100%.
The pH stability was evaluated by pre-incubating the free rlevblg1 and rlevblg1-dex-CLEAs without substrate (levan) at different pH buffers ranging from pH 6 to 11 for 1 h at 4 °C, as previously performed by Abd Rahman et al. [35]. The stability of pH was determined by conducting an enzyme assay and was calculated as the relative activity to the optimum condition, which was set as 100%.
3.4.3. Kinetic Analysis of Free and Immobilized rlevblg1
The kinetic parameters of the free rlevblg1 and rlevblg1-dex-CLEAs were determined by varying levan concentrations (1.0 to 25.0% (w/v)) under standard conditions. The tested parameters, namely the maximum velocity (Vmax), Michaelis–Menten constant (Km), turnover number (kcat), and catalytic efficiency (kcat/Km), were measured from the Lineweaver–Burk plot [61].
Meanwhile, the effectiveness factor (η) of the free and immobilized rlevblg1 was calculated from the following equation:
3.4.4. Reusability and Storage Stability of rlevblg1-dex-CLEAs
The reusability of the synthesized rlevblg1-dex-CLEAs was evaluated for five cycles under standard assay conditions. At the end of each cycle, samples were recovered via centrifugation at 10,000× g and 25 °C for 2 min. The supernatant was then drained, and the pellet was cleaned using glycine-NaOH buffer (50 mM, pH 8.0) and resuspended in 0.2 mL of the freshly prepared reaction mixture. The residual activity at the end of each cycle was quantified via an enzyme assay, with the enzyme activity for the first cycle calibrated as 100%.
The storage stability of the free and immobilized rlevblg1 was determined by the Kumar et al. [62] method, with some modifications. Samples were stored in 50 mM of glycine-NaOH buffer (pH 8.0) without substrate (levan) at 4 °C for up to six months. The activity recovery of the samples was tested on day (0, 1, 4, 10, 20, 40, and 180) by centrifuging the samples at 10,000× g for 4 min at 25 °C, and the pellet was washed. The catalytic activity of the samples was measured via a DNS assay, and residual activity was determined relative to the initial activity prior incubation (day-0), which was set as 100%.
3.5. Evaluation of the Hydrolysis Reaction Efficiency of rlevblg1-dex-CLEAs
The immobilized rlevblg1 was tested for the stability and kinetics of levan-type fructooligosaccharides (L-FOS) conversion using the method established by Abd Rahman et al. [35]. L-FOSs conversion was investigated by adding 0.5% levan (1100–1600 kDa) to rlevblg1-dex-CLEAs incubated in a thermomixer (Eppendorf, Hamburg, Germany) for a period of time (1, 2, and 3 h) at 30 °C under 850 rpm. The bioconversion of levan into L-FOSs and reducing sugars was assessed and confirmed using high-performance liquid chromatography (HPLC) with a refractive index detector (Waters, Milford, MA, USA). The samples were separated using a Cosmosil Sugar-D column (4.6 mm × 250 mm), and the column temperature was set to 30 °C. Meanwhile, the sensitivity of the detector and the internal temperature were adjusted to 64 MV and 37 °C, respectively. The mobile phase used comprised of acetonitrile–water (70:30) delivered at a flow rate of 1.0 mL/min. The concentration of monosaccharides and oligosaccharides was quantitated using an equation obtained from a standard calibration curve (Supplementary data). The standards were employed to generate a calibration graph comprised of a mixture of monosaccharides (fructose) and oligosaccharides (levanbiose; DP2, levantriose; DP3, levantetraose; DP4 and levanpentaose; DP5). The standards were obtained from Sigma-aldrich, with a HPLC purity of 99%. All standards and samples were centrifuged at 10,652× g for 15 min at 25 °C and filtered using a 0.22 μm syringe filter before the (20 μL) samples were injected into the column. The fraction of bioconversion was determined from the consumption of the substrate.
3.6. Statistical Analysis
Statistical analyses of CLEA immobilization development and characterization of the rlevblg1-dex-CLEAs were performed using analysis of variance (ANOVA) and Tukey’s multiple-comparison post-test with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences between groups of analyses were defined as not significant (represented in Figure 1, Figure 3, Figure 4 and Figure 7 by ns) at a p value of >0.05. All tests were conducted in triplicate, and the level of the significance was 95%.
4. Conclusions
This study addresses the potential of using dextran to cross-link rlevblg1 in the formation of an efficacious biocatalyst. After optimization, the rlevblg1-dex-CLEAs exhibited a significant improvement of 90.5% in the activity recovery. Moreover, the rlevblg1-dex-CLEAs demonstrated better characteristic properties in terms of thermal and storage stability compared to free rlevblg1. In addition, the substrate accessibility and affinity of rlevblg1-dex-CLEAs were improved, leading to a high yield of longer levan-type FOS. Thus, immobilized rlevblg1 can be reused and can endure harsh industrial conditions, resulting in a cost-effective production of L-FOS. Further, due to its biocompatibility, biodegradability, and ability to form stable cross-linking, dextran is an attractive cross-linking agent in the development of robust CLEAs that can be applied in the food processing industry, including in L-FOS synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14090584/s1, Figure S1: The standard curve of absorbance, A540 versus fructose mass, μmol, Figure S2: HPLC standard curve for fructose, Figure S3: HPLC standard curve for glucose, Figure S4: HPLC standard curve for levanbiose (DP2), Figure S5: HPLC standard curve for levantriose (DP3), Figure S6: HPLC standard curve for levantetraose (DP4), Figure S7: HPLC standard curve for levanpentaose (DP5).
Author Contributions
H.H.M.: methodology; investigation; analysis validation; writing—original draft; writing—review and editing. N.R.J.: methodology; validation; writing—original draft; writing—review and editing. N.J.: methodology; validation; writing—original draft; writing—review and editing. A.A.A.: Writing—review and editing. Z.R.: Methodology and R.M.I.: conceptualization; funding acquisition; validation; project administration; supervision, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Higher Education of Saudi Arabia, Universiti Teknologi Malaysia, under the UTM RA ICONIC research grant (PY/2020/04436) and Prince Sattam bin Abdulaziz University, project number (PSAU/2024/R1445).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nawawi, N.N.; Hashim, Z.; Manas, N.H.A.; Azelee, N.I.W.; Illias, R.M. A porous-cross linked enzyme aggregates of maltogenic amylase from Bacillus lehensis G1: Robust biocatalyst with improved stability and substrate diffusion. Int. J. Biol. Macromol. 2020, 148, 1222–1231. [Google Scholar] [CrossRef]
- Matella, N.; Dolan, K.; Lee, Y. Comparison of galactooligosaccharide production in free-enzyme ultrafiltration and in immobilized-enzyme systems. J. Food Sci. 2006, 71, C363–C368. [Google Scholar] [CrossRef]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol. 2014, 7, 324–337. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Sangmanee, S.; Nakapong, S.; Pichyangkura, R.; Kuttiyawong, K. Levan-type fructooligosaccharide production using Bacillus licheniformis RN-01 levansucrase Y246S immobilized on chitosan beads. Songklanakarin J. Sci. Technol. 2016, 38, 295–303. [Google Scholar]
- Porras-Domínguez, J.R.; Ávila-Fernández, Á.; Rodríguez-Alegría, M.E.; Miranda-Molina, A.; Escalante, A.; González-Cervantes, R.; Olvera, C.; Munguía, A.L. Levan-type FOS production using a Bacillus licheniformis endolevanase. Process Biochem. 2014, 49, 783–790. [Google Scholar] [CrossRef]
- Marín-Navarro, J.; Talens-Perales, D.; Polaina, J. One-pot production of fructooligosaccharides by a Saccharomyces cerevisiae strain expressing an engineered invertase. Appl. Microbiol. Biotechnol. 2015, 99, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, W.; Ni, D.; Dai, Q.; Guang, C.; Zhang, T.; Mu, W. An overview of levan-degrading enzyme from microbes. Appl. Microbiol. Biotechnol. 2019, 103, 7891–7902. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [PubMed]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). Rsc Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Sheldon, R. Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.; Schoevaart, R.; Van Langen, L. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransform. 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. Rsc Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Amaral-Fonseca, M.; Kopp, W.; Giordano, R.d.L.C.; Fernández-Lafuente, R.; Tardioli, P.W. Preparation of magnetic cross-linked amyloglucosidase aggregates: Solving some activity problems. Catalysts 2018, 8, 496. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Van Langen, L.M.; Van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef]
- Arsenault, A.; Cabana, H.; Jones, J.P. Laccase-based CLEAs: Chitosan as a novel cross-linking agent. Enzym. Res. 2011, 1, 376015. [Google Scholar] [CrossRef]
- Nadar, S.S.; Muley, A.B.; Ladole, M.R.; Joshi, P.U. Macromolecular cross-linked enzyme aggregates (M-CLEAs) of α-amylase. Int. J. Biol. Macromol. 2016, 84, 69–78. [Google Scholar] [CrossRef]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- Jailani, N.; Jaafar, N.R.; Suhaimi, S.; Mackeen, M.M.; Bakar, F.D.A.; Illias, R.M. Cross-linked cyclodextrin glucanotransferase aggregates from Bacillus lehensis G1 for cyclodextrin production: Molecular modeling, developmental, physicochemical, kinetic and thermodynamic properties. Int. J. Biol. Macromol. 2022, 213, 516–533. [Google Scholar] [CrossRef]
- Valdés, E.; Soto, L.; Arcaya, G. Influence of the pH of glutaraldehyde and the use of dextran aldehyde on the preparation of cross-linked enzyme aggregates (CLEAs) of lipase from Burkholderia cepacia. Electron. J. Biotechnol. 2011, 14, 10. [Google Scholar] [CrossRef]
- Talekar, S.; Nadar, S.; Joshi, A.; Joshi, G. Pectin cross-linked enzyme aggregates (pectin-CLEAs) of glucoamylase. RSC Adv. 2014, 4, 59444–59453. [Google Scholar] [CrossRef]
- Makki, H.H.; Jaafar, N.R.; Rahman, R.A.; Rahmat, Z.; Puspaningsih, N.N.T.; Illias, R.M. Protein interaction studies of cross-linked endolevanase aggregates from Bacillus lehensis G1. J. Teknol. (Sci. Eng.) 2024, 86, 159–167. [Google Scholar] [CrossRef]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Yao, S.; Qian, J.; Guo, H.; Cai, X. Preparation of immobilized lipase on magnetic nanoparticles dialdehyde starch. Carbohydr. Polym. 2019, 218, 324–332. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef]
- Rahman, M.A.; Culsum, U.; Kumar, A.; Gao, H.; Hu, N. Immobilization of a novel cold active esterase onto Fe3O4∼ cellulose nano-composite enhances catalytic properties. Int. J. Biol. Macromol. 2016, 87, 488–497. [Google Scholar] [CrossRef]
- Liao, Q.; Du, X.; Jiang, W.; Tong, Y.; Zhao, Z.; Fang, R.; Feng, J.; Tang, L. Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1: Preparation, characterization and application as a biocatalyst. J. Biotechnol. 2018, 272, 48–55. [Google Scholar] [CrossRef]
- Abd Rahman, N.H.; Jaafar, N.R.; Annuar, N.A.S.; Rahman, R.A.; Murad, A.M.A.; El-Enshasy, H.A.; Illias, R.M. Efficient substrate accessibility of cross-linked levanase aggregates using dialdehyde starch as a macromolecular cross-linker. Carbohydr. Polym. 2021, 267, 118159. [Google Scholar] [CrossRef]
- Miao, C.; Li, H.; Zhuang, X.; Wang, Z.; Yang, L.; Lv, P.; Luo, W. Synthesis and properties of porous CLEAs lipase by the calcium carbonate template method and its application in biodiesel production. RSC Adv. 2019, 9, 29665–29675. [Google Scholar] [CrossRef]
- Abd Rahman, N.H.; Jaafar, N.R.; Murad, A.M.A.; Bakar, F.D.A.; Annuar, N.A.S.; Illias, R.M. Novel cross-linked enzyme aggregates of levanase from Bacillus lehensis G1 for short-chain fructooligosaccharides synthesis: Developmental, physicochemical, kinetic and thermodynamic properties. Int. J. Biol. Macromol. 2020, 159, 577–589. [Google Scholar] [CrossRef]
- Yakup Arica, M.; Soydogan, H.; Bayramoglu, G. Reversible immobilization of Candida rugosa lipase on fibrous polymer grafted and sulfonated p (HEMA/EGDMA) beads. Bioprocess Biosyst. Eng. 2010, 33, 227–236. [Google Scholar] [CrossRef]
- Kumar, V.V.; Sivanesan, S.; Cabana, H. Magnetic cross-linked laccase aggregates—Bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci. Total Environ. 2014, 487, 830–839. [Google Scholar] [CrossRef]
- Mortazavi, S.; Aghaei, H. Make proper surfaces for immobilization of enzymes: Immobilization of lipase and α-amylase on modified Na-sepiolite. Int. J. Biol. Macromol. 2020, 164, 1–12. [Google Scholar] [CrossRef]
- Sanjay, G.; Sugunan, S. Glucoamylase immobilized on montmorillonite: Synthesis, characterization and starch hydrolysis activity in a fixed bed reactor. Catal. Commun. 2005, 6, 525–530. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.; Golubovic, M.; Ottens, M.; Kieboom, A.; Van Rantwijk, F.; Van Der Wielen, L.; Sheldon, R. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Mikani, M. Nano-magnetic cross-linked enzyme aggregates of naringinase an efficient nanobiocatalyst for naringin hydrolysis. Int. J. Biol. Macromol. 2018, 117, 134–143. [Google Scholar] [CrossRef]
- Ramirez, L.M.F.; Rihouey, C.; Chaubet, F.; Le Cerf, D.; Picton, L. Characterization of dextran particle size: How frit-inlet asymmetrical flow field-flow fractionation (FI-AF4) coupled online with dynamic light scattering (DLS) leads to enhanced size distribution. J. Chromatogr. A 2021, 1653, 462404. [Google Scholar] [CrossRef]
- Kamal, M.A.; Brizioli, M.; Zinn, T.; Narayanan, T.; Cerbino, R.; Giavazzi, F.; Pal, A. Dynamics of anisotropic colloidal systems: What to choose, DLS, DDM or XPCS? J. Colloid Interface Sci. 2024, 660, 314–320. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghaei, H.; Soleimanian, S.; Sedaghat, M. Enzyme immobilization: Part 2: Immobilization of alkaline phosphatase on Na-bentonite and modified bentonite. Appl. Clay Sci. 2009, 43, 308–316. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- D’Souza, L.; Devi, P.; Divya Shridhar, M.; Naik, C.G. Use of Fourier Transform Infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal. Chem. Insights 2008, 3, 117739010800300001. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef]
- Lv, B.-H.; Tan, W.; Zhu, C.-C.; Shang, X.; Zhang, L. Properties of a stable and sustained-release formulation of recombinant human parathyroid hormone (rhPTH) with chitosan and silk fibroin microparticles. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7532. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. FTIR spectral band shifts explained by OM–cation interactions. J. Plant Nutr. Soil Sci. 2021, 184, 388–397. [Google Scholar] [CrossRef]
- Steen Redeker, E.; Ta, D.T.; Cortens, D.; Billen, B.; Guedens, W.; Adriaensens, P. Protein engineering for directed immobilization. Bioconjugate Chem. 2013, 24, 1761–1777. [Google Scholar] [CrossRef]
- Ottone, C.; Romero, O.; Urrutia, P.; Bernal, C.; Illanes, A.; Wilson, L. Enzyme biocatalysis and sustainability. In Nanostructured Catalysts for Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 383–413. [Google Scholar]
- Ernits, K.; Eek, P.; Lukk, T.; Visnapuu, T.; Alamäe, T. First crystal structure of an endo-levanase–the BT1760 from a human gut commensal Bacteroides thetaiotaomicron. Sci. Rep. 2019, 9, 8443. [Google Scholar]
- Ávila-Fernández, Á.; Montiel, S.; Rodríguez-Alegría, M.E.; Caspeta, L.; López Munguía, A. Simultaneous enzyme production, Levan-type FOS synthesis and sugar by-products elimination using a recombinant Pichia pastoris strain expressing a levansucrase-endolevanase fusion enzyme. Microb. Cell Factories 2023, 22, 18. [Google Scholar] [CrossRef]
- Miasnikov, A.N. Characterization of a novel endo-levanase and its gene from Bacillus sp. L7. FEMS Microbiol. Lett. 1997, 154, 23–28. [Google Scholar] [CrossRef]
- Murakami, H.; Kuramoto, T.; Mizutani, K.; Nakano, H.; Kitahata, S. Purification and some properties of a new levanase from Bacillus sp. No. 71. Biosci. Biotechnol. Biochem. 1992, 56, 608–613. [Google Scholar] [CrossRef][Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Kumar, V.V.; Kumar, M.P.; Thiruvenkadaravi, K.; Baskaralingam, P.; Kumar, P.S.; Sivanesan, S. Preparation and characterization of porous cross linked laccase aggregates for the decolorization of triphenyl methane and reactive dyes. Bioresour. Technol. 2012, 119, 28–34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).