Photoinduced Mechanisms of C–S Borylation of Methyl(p-tolyl)Sulfane with Bis(Pinacolato)diboron: A Density Functional Theory Investigation
Abstract
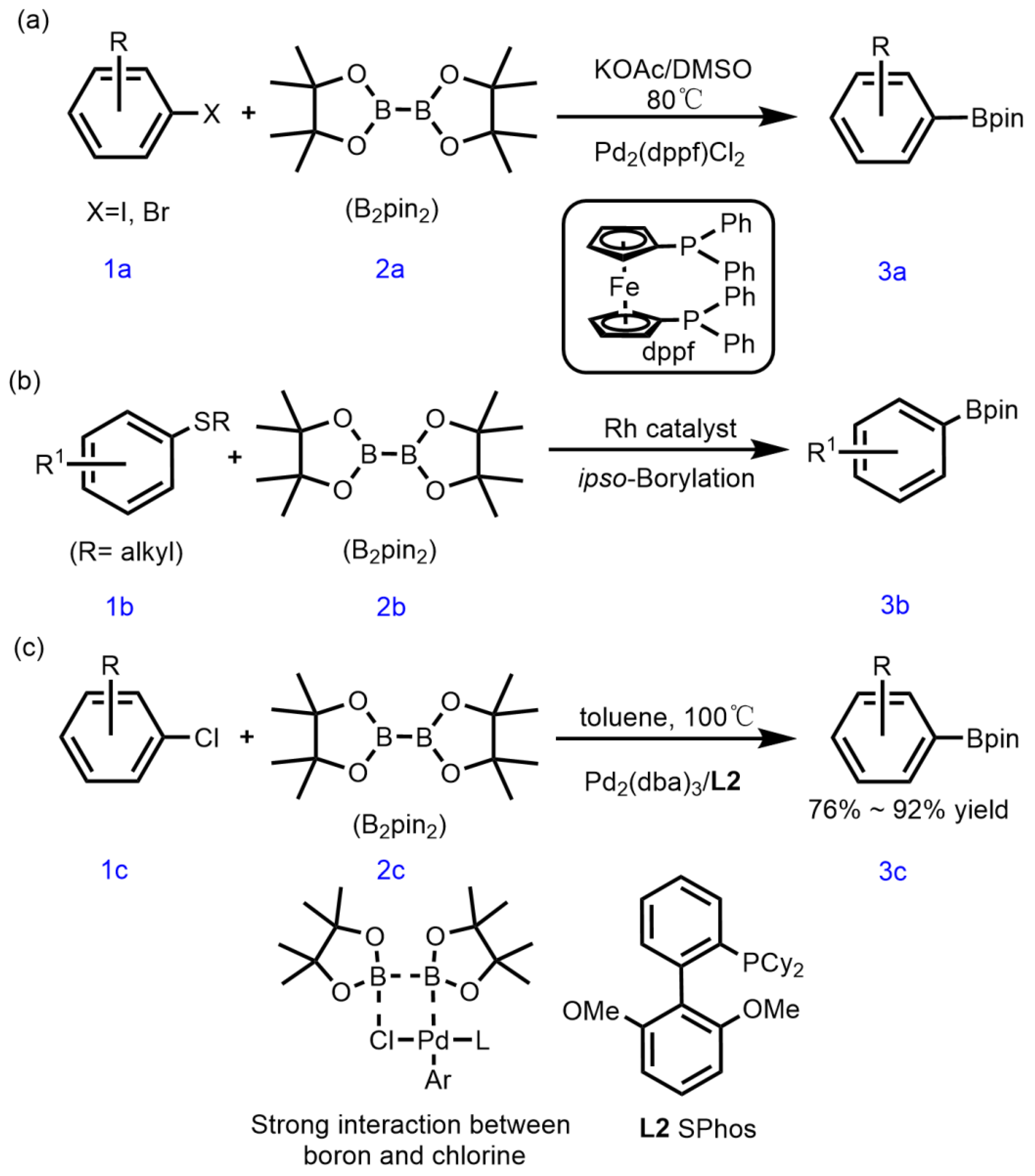
1. Introduction
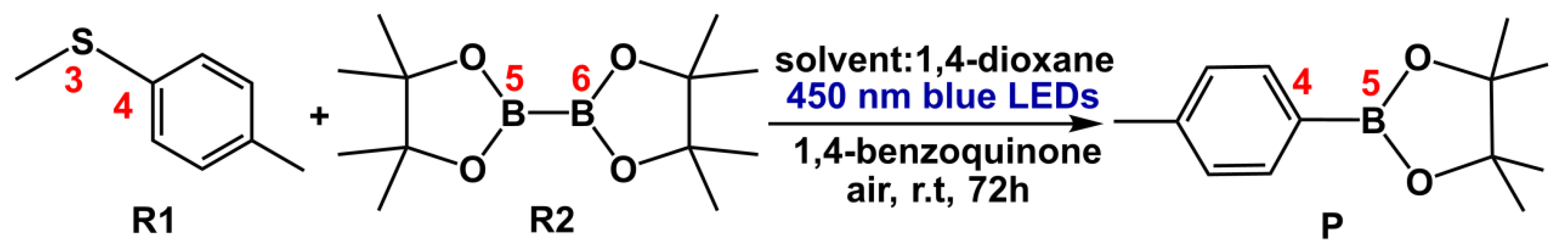
2. Results and Discussion
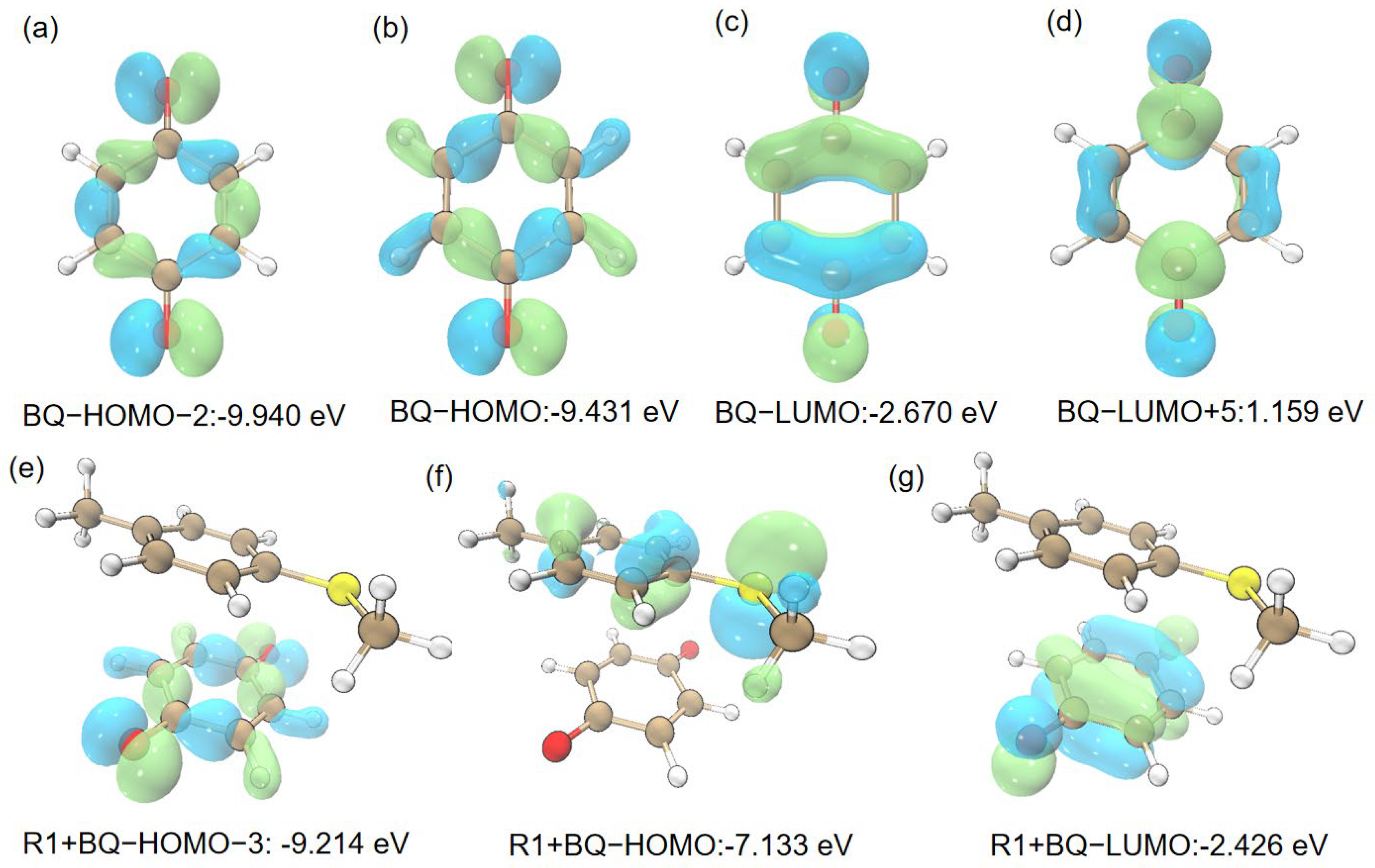
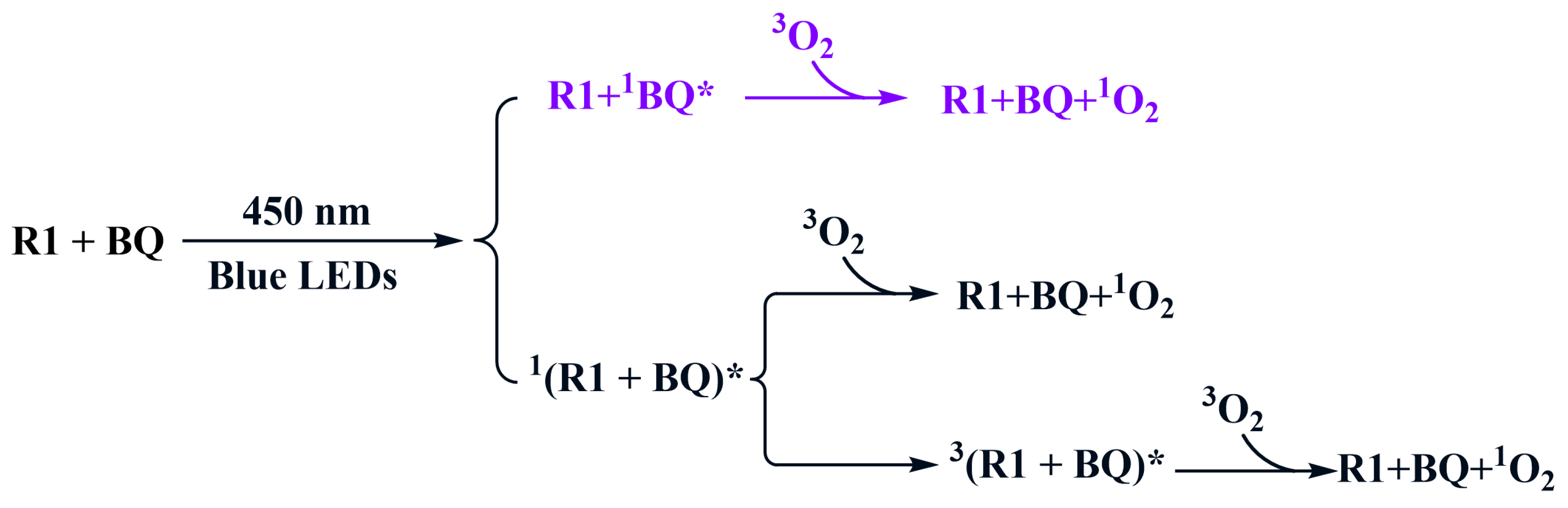
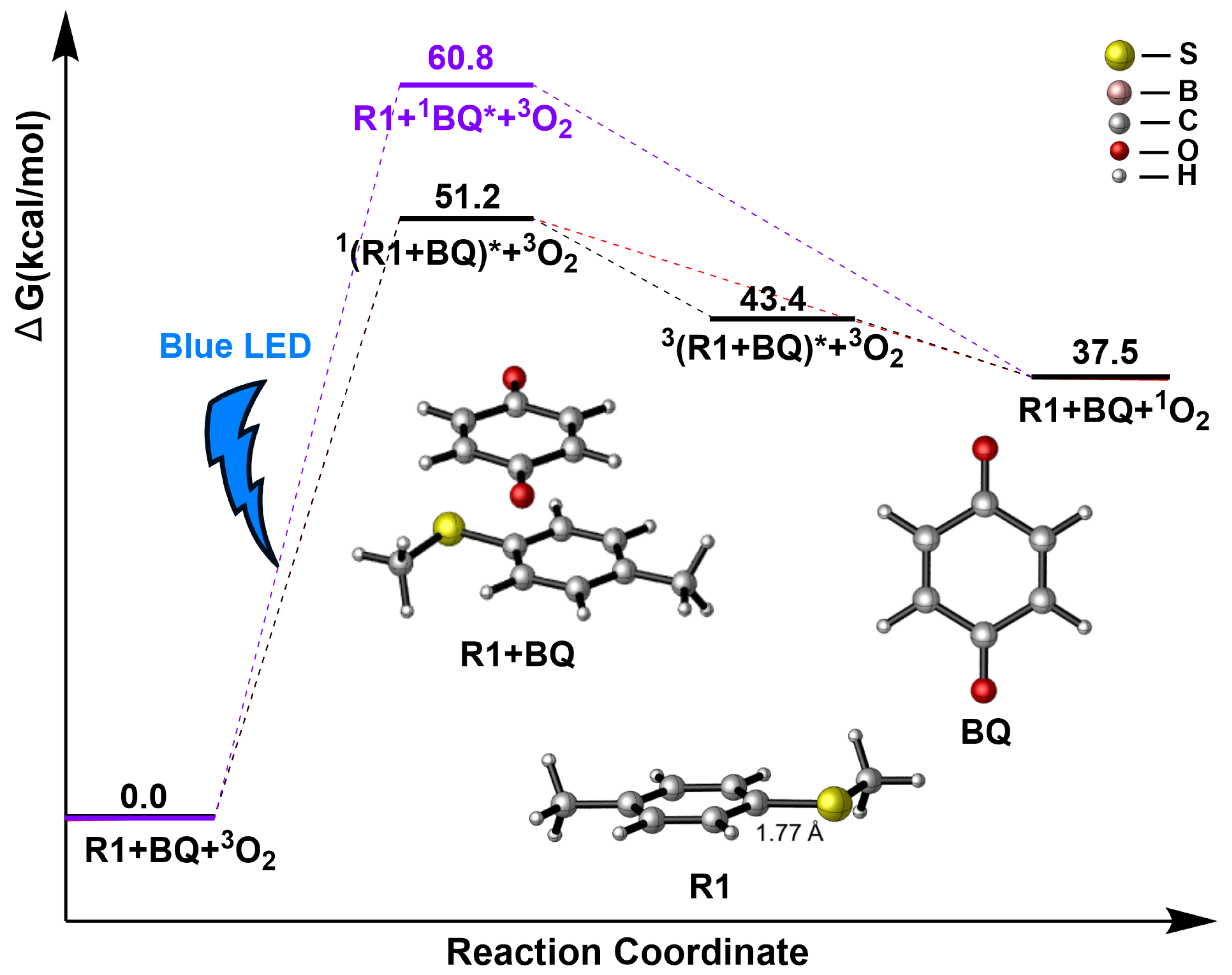
2.1. Photocatalysis Process
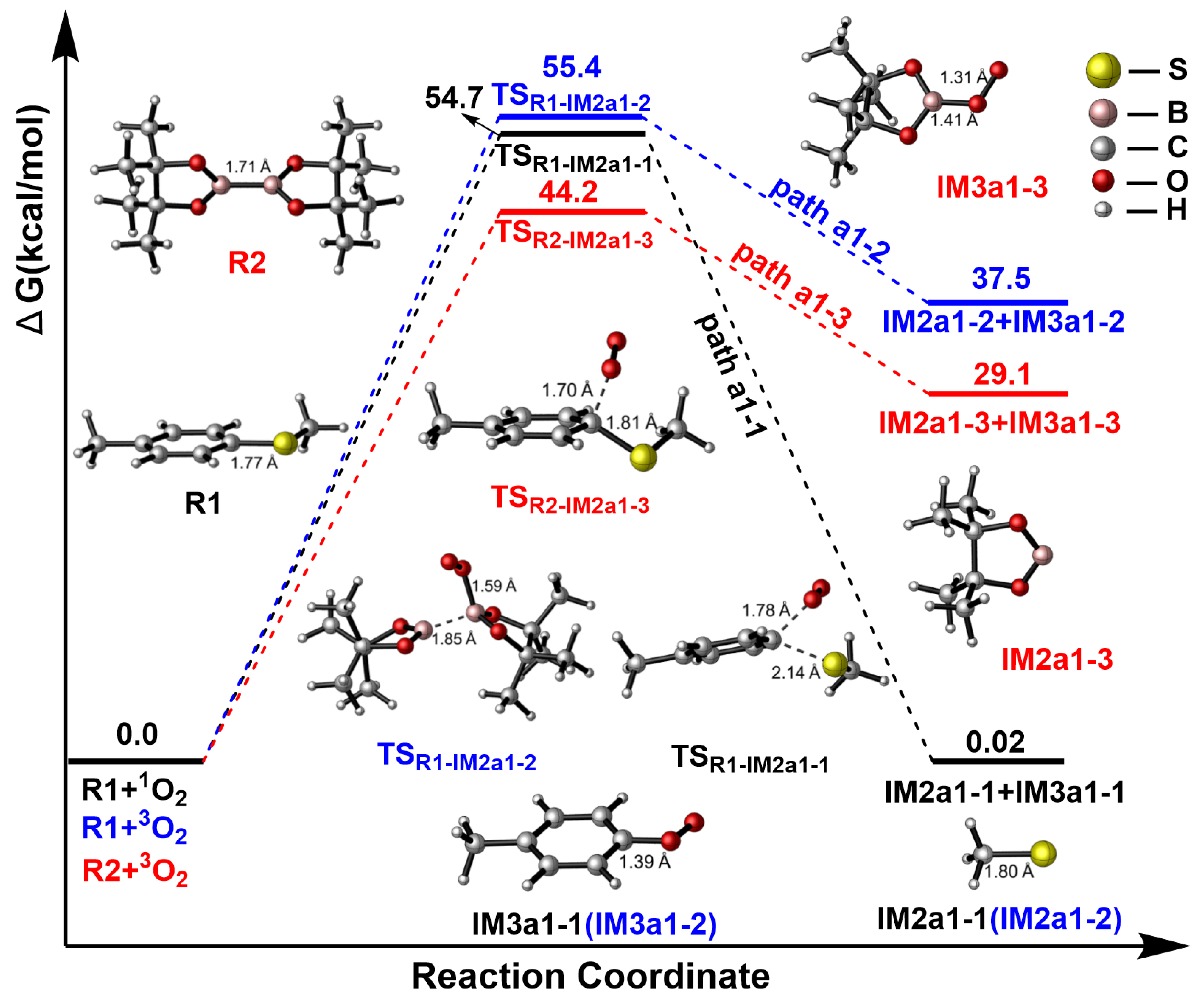
2.2. R1 + BQ→IM1(1O2)
2.3. IM1(1O2)→P
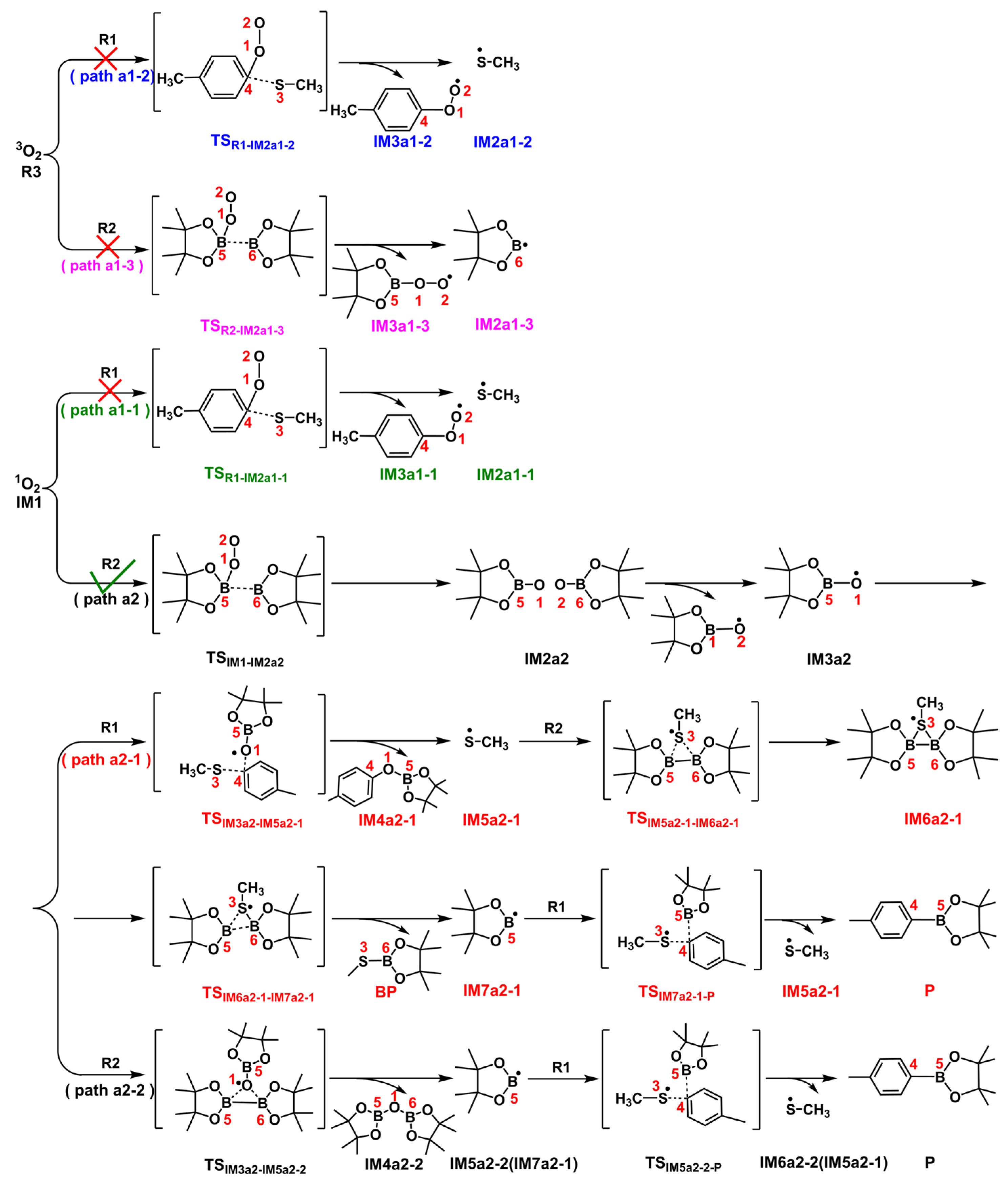
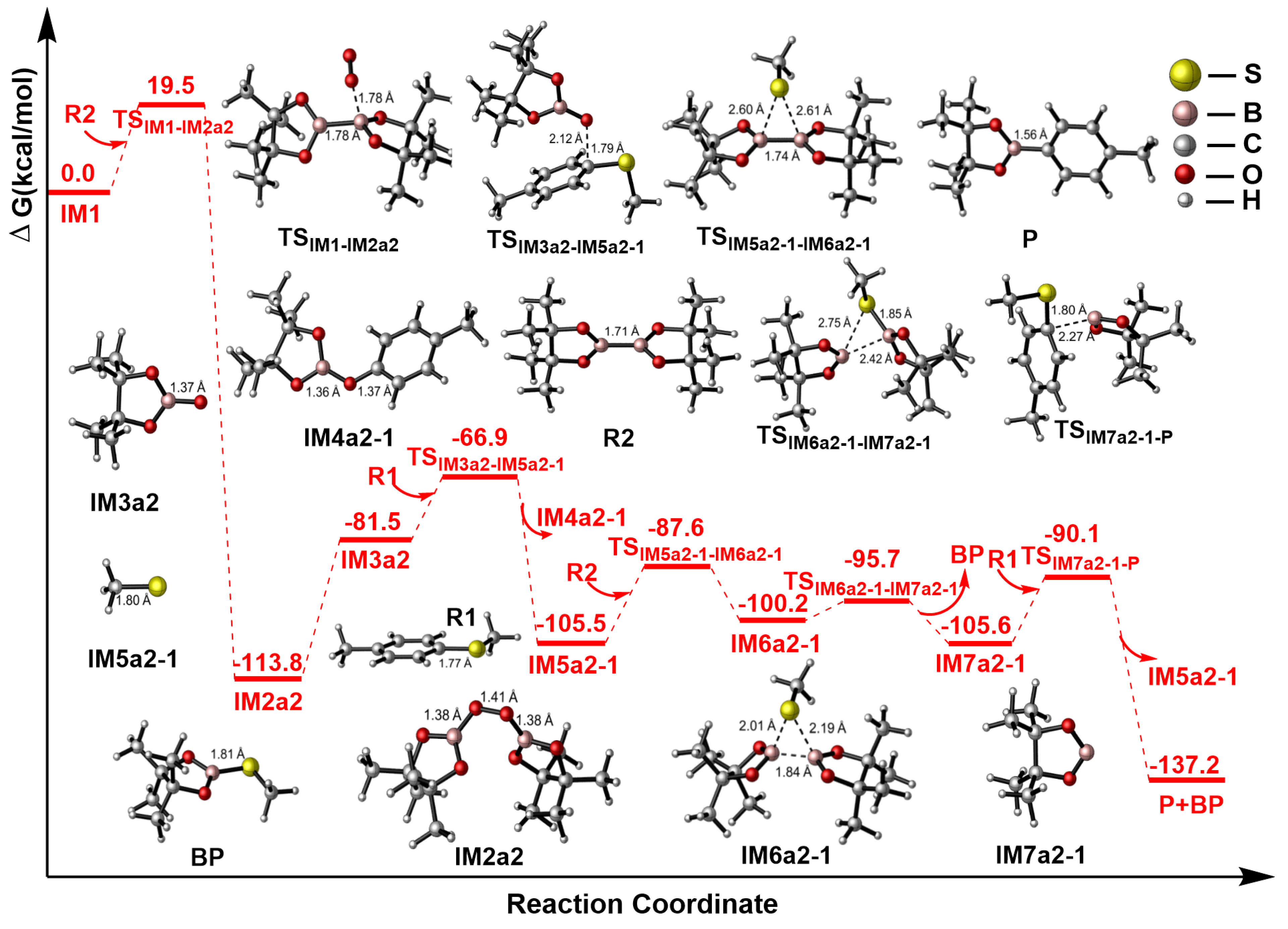
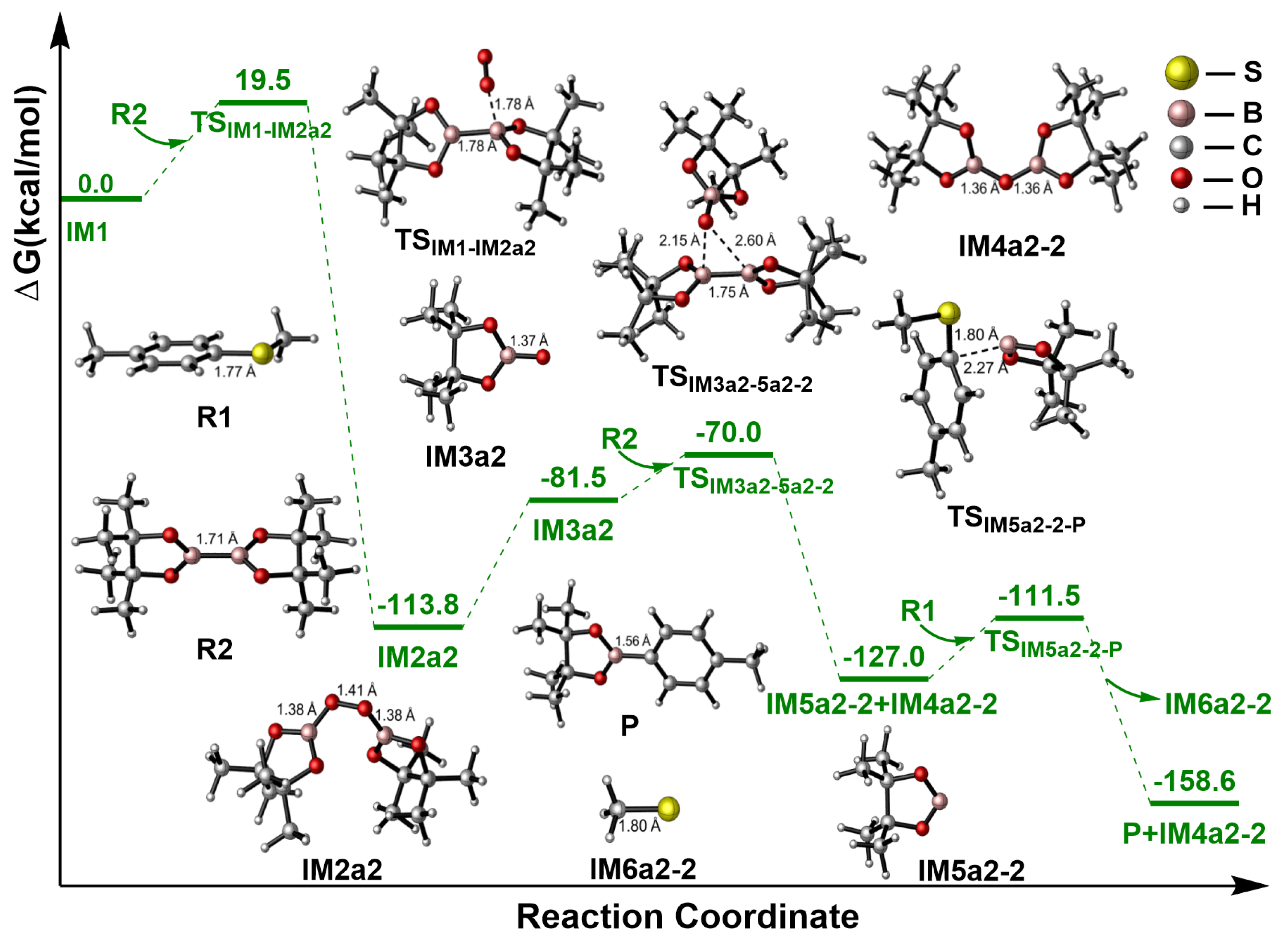
2.3.1. Path a1
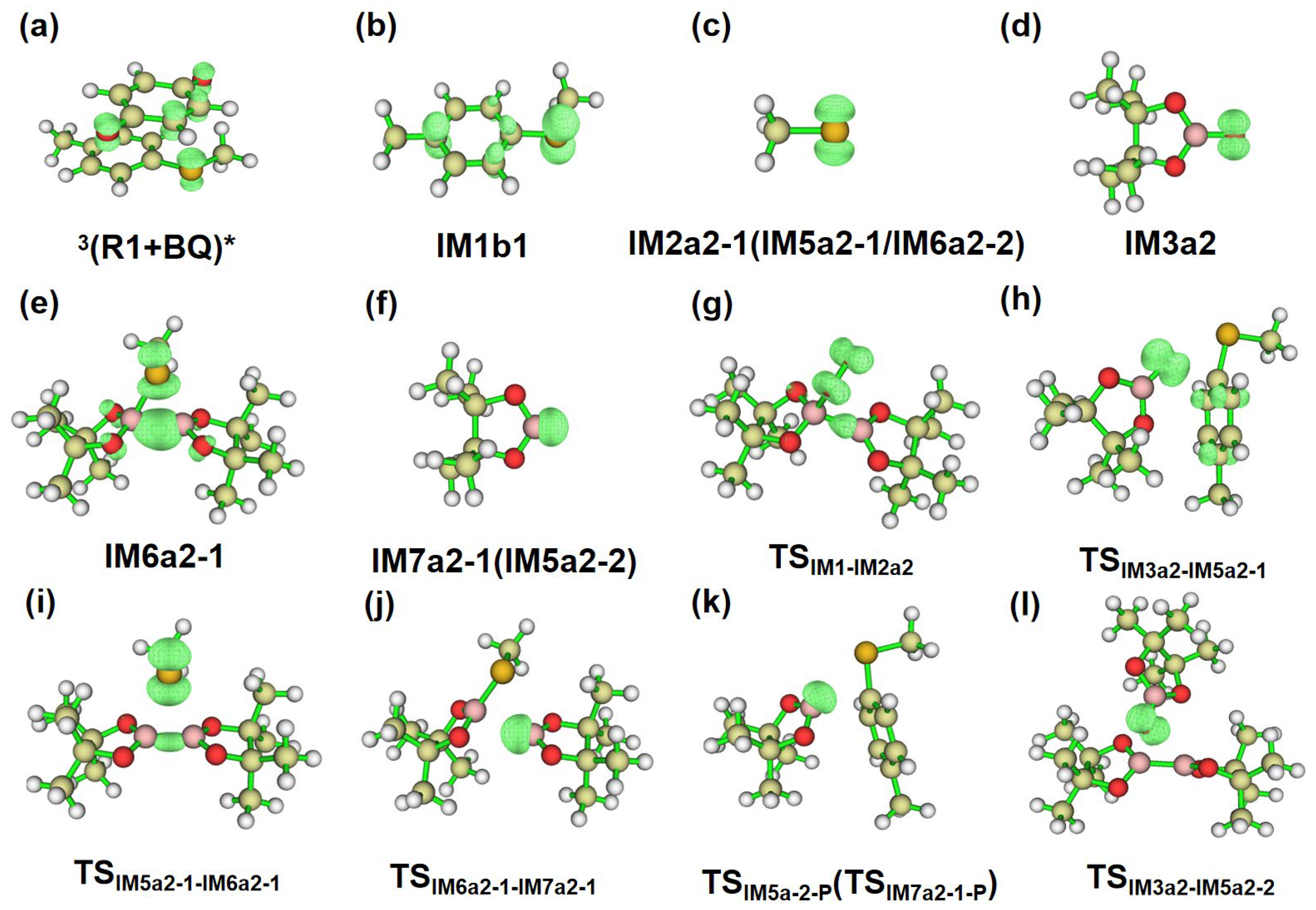
2.3.2. Path a2
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lou, J.; Wang, Q.; Wu, P.; Wang, H.; Zhou, Y.G.; Yu, Z. Transition-metal mediated carbon–sulfur bond activation and transformations: An update. Chem. Soc. Rev. 2020, 49, 4307–4359. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.L.; Zhu, Q.L.; Dong, Z.B.; Chen, J.Q.; Shi, Z. Synthesis of Aryl Dithiocarbamates from Tetramethylthiuram Monosulfide (TMTM) and Aryl Boronic Acids: Copper-Catalyzed Construction of C(sp2)–S Bonds. Synthesis 2021, 54, 475–482. [Google Scholar] [CrossRef]
- Delcaillau, T.; Boehm, P.; Morandi, B. Nickel-Catalyzed Reversible Functional Group Metathesis between Aryl Nitriles and Aryl Thioethers. J. Am. Chem. Soc. 2021, 143, 3723–3728. [Google Scholar] [CrossRef]
- Boehm, P.; Müller, P.; Finkelstein, P.; Rivero-Crespo, M.A.; Ebert, M.O.; Trapp, N.; Morandi, B. Mechanistic Investigation of the Nickel-Catalyzed Metathesis between Aryl Thioethers and Aryl Nitriles. J. Am. Chem. Soc. 2022, 144, 13096–13108. [Google Scholar] [CrossRef] [PubMed]
- Delcaillau, T.; Woenckhaus-Alvarez, A.; Morandi, B. Nickel-Catalyzed Cyanation of Aryl Thioethers. Org. Lett. 2021, 23, 7018–7022. [Google Scholar] [CrossRef]
- Delcaillau, T.; Morandi, B. Nickel-Catalyzed Thiolation of Aryl Nitriles. Chem. Eur. J. 2021, 27, 11823. [Google Scholar] [CrossRef]
- Yorimitsu, H. Catalytic Transformations of Sulfonium Salts via C–S Bond Activation. Chem. Rec. 2021, 21, 3356–3369. [Google Scholar] [CrossRef]
- Fan, R.; Tan, C.; Liu, Y.; Wei, Y.; Zhao, X.; Liu, X.; Tan, J.; Yoshida, H. A leap forward in sulfonium salt and sulfur ylide chemistry. Chin. Chem. Lett. 2020, 32, 299–312. [Google Scholar] [CrossRef]
- Ma, N.N.; Ren, J.A.; Liu, X.; Chu, X.Q.; Rao, W.; Shen, Z.L. Nickel-Catalyzed Direct Cross-Coupling of Aryl Sulfonium Salt with Aryl Bromide. Org. Lett. 2022, 24, 1953–1957. [Google Scholar] [CrossRef]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals To Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2013, 57, 2832–2842. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Pattison, G. Fluorination of organoboron compounds. Org. Biomol. Chem. 2019, 17, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.N.; Sakurai, H. Oxidative Coupling of Organoboron Compounds. Asian, J. Org. Chem. 2014, 3, 668–684. [Google Scholar] [CrossRef]
- Wang, J. When diazo compounds meet with organoboron compounds. Pure Appl. Chem. 2017, 90, 617–623. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Z. Methodologies and Strategies for Selective Borylation of C–Het and C–C Bonds. Chem. Rev. 2020, 120, 7348–7398. [Google Scholar] [CrossRef]
- Rout, L.; Punniyamurthy, T. Recent advances in transition-metal-mediated Csp2-B and Csp2-P cross-coupling reactions. Co-ord. Chem. Rev. 2020, 431, 213675. [Google Scholar] [CrossRef]
- Gao, M.Y.; Gosmini, C. Cobalt-Catalyzed Reductive Cross-Coupling to Construct Csp3–Csp3 Bonds via Csp3–S and Csp3–X Bonds Activation. Org. Lett. 2023, 25, 7689–7693. [Google Scholar] [CrossRef]
- Bhanuchandra, M.; Baralle, A.; Otsuka, S.; Nogi, K.; Yorimitsu, H.; Osuka, A. Palladium-Catalyzed ipso-Borylation of Aryl Sulfides with Diborons. Org. Lett. 2016, 18, 2966–2969. [Google Scholar] [CrossRef] [PubMed]
- Uetake, Y.; Niwa, T.; Hosoya, T. Rhodium-Catalyzed ipso-Borylation of Alkylthioarenes via C–S Bond Cleavage. Org. Lett. 2016, 18, 2758–2761. [Google Scholar] [CrossRef]
- Minami, H.; Otsuka, S.; Nogi, K.; Yorimitsu, H. Palladium-Catalyzed Borylation of Aryl Sulfoniums with Diborons. ACS Catal. 2017, 8, 579–583. [Google Scholar] [CrossRef]
- Huang, C.; Feng, J.; Ma, R.; Fang, S.; Lu, T.; Tang, W.; Du, D.; Gao, J. Redox-Neutral Borylation of Aryl Sulfonium Salts via C–S Activation Enabled by Light. Org. Lett. 2019, 21, 9688–9692. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, H. Research Progress towards Synthesis of Aryl Boronic Acid Compounds. Chin. J. Org. Chem. 2018, 38, 738–751. [Google Scholar] [CrossRef]
- Fang, H.P.; Fu, C.C.; Tai, C.K.; Chang, K.H.; Yang, R.H.; Wu, M.J.; Chen, H.C.; Li, C.J.; Huang, S.Q.; Lien, W.H.; et al. Synthesis and stability study of isocyano aryl boronate esters and their synthetic applications. RSC Adv. 2016, 6, 30362–30371. [Google Scholar] [CrossRef]
- Zhou, J.; Berthel, J.H.J.; Kuntze Fechner, M.W.; Friedrich, A.; Marder, T.B.; Radius, U. NHC Nickel-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions of Aryl Boronate Esters with Perfluorobenzenes. J. Org. Chem. 2016, 81, 5789–5794. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.K. Green Chemistry: An Introductory Text. Green Chem. Lett. Rev. 2017, 10, 30–31. [Google Scholar] [CrossRef]
- Al-Shatti, B.J.; Alsairafi, Z.; Al-Tannak, N.F. Green chemistry and its implementation in pharmaceutical analysis. Rev. Anal. Chem. 2023, 42, 20230069. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2021, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, Z.; Hu, X.; Zhang, H. Photoinduced aerobic C–S borylation of aryl sulfides. Org. Chem. Front. 2022, 9, 3034–3038. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, D.G. Dft Investigation for the Mechanisms of γ-Butyrolactones from Styrene and Acrylic Acid, Catalyzed by Nma*Bf 4 and Phssph. Comput. Theor. Chem. 2024, 1237, 114654. [Google Scholar] [CrossRef]
- Zheng, X.F.; Zhou, D.G.; Yang, L.J. DFT investigation of the DDQ-catalytic mechanism for constructing C–O bonds. Org. Biomol. Chem. 2024, 22, 3693–3707. [Google Scholar] [CrossRef]
- Belguidoum, K.; Boulmokh, Y.; Hamamdia, F.Z.; Madi, F.; Nouar, L.; Amira Guebailia, H. Tetradentate square-planar acety-lumbelliferone–nickel (II) complex formation: A DFT and TD-DFT study. Theor. Chem. Acc. 2022, 141, 48. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. Gaussian 09, R.E. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Saheb, V.; Bahadori, A. Theoretical studies on the kinetics of the hydrogen-abstraction reactions from 1,3,5-trioxane and 1,4-dioxane by OH radicals. Prog. React. Kinet. Mech. 2020, 45, 1–13. [Google Scholar] [CrossRef]
- Cheng, X. Computational insights into the coupling mechanism of benzoic acid, phenoxy acetylene and dihydroisoquinoline catalyzed by silver ion as polarizer and stabilizer. Appl. Organomet. Chem. 2020, 34, e5903. [Google Scholar] [CrossRef]
- Kenichi, F. The Path of Chemical Reactions—The Irc Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Feng, T.T.; Lin, Y.; Chen, B.; Zhou, D.G.; Li, R. Alkali metal hydroxide-catalyzed mechanisms of Csp–H silylation of alkynes: A DFT investigation. Org. Biomol. Chem. 2024, 22, 6352–6361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.G.; Wang, P. Mechanisms of the reaction between benzonitrile and 4-octyne catalyzed by Ni(PMe3)2: A theoretical investigation. J. Phys. Org. Chemistry. 2019, 32, 3932. [Google Scholar] [CrossRef]
- Zhou, D.G.; Li, Y.Q. Mechanistic Study of 1,4-Benzodiazepine-2,5-diones from Diphenylamine and Diethyl 2-Phenylmalonate by Density Functional Theory. J. Phys. Chem. A 2019, 124, 395–408. [Google Scholar] [CrossRef]
- Zhou, D.G.; Zhou, P.P.; Jing, H.W. Mechanisms of Csp3–H functionalization of ethyl 2-(methyl(p-tolyl)amino)acetate: A theoretical investigation. Comput. Theor. Chem. 2017, 1118, 144–152. [Google Scholar] [CrossRef]
- Zhou, D.G. DFT investigation on the mechanism of catalytic reaction between 3-diazoindolin-2-imines and N-ethylaniline catalyzed by Rh2(Oct)4. Chem. Phys. 2019, 531, 110661. [Google Scholar] [CrossRef]
- Zheng, X.F.; Zhou, D.G. Mechanisms of asymmetric sulfa-Michael additions between phenylacetylene and thiolacetic acid: A DFT investigation. Comput. Theor. Chem. 2021, 1207, 113523. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Li, S.J.; Wang, X.H.; Shi, Q.Q.; Li, X.; Qu, L.B.; Wei, D.H.; Lan, Y. Multiple Functional Organocata-lyst-Promoted Inert C-C Activation: Mechanism and Origin of Selectivities. ACS Catal. 2021, 11, 3443–3454. [Google Scholar] [CrossRef]
- Zhou, D.G. Mechanisms of Csp3-H functionalization of acetonitrile or acetone with coumarins: A DFT investigation. Mol. Catal. 2020, 498, 111246. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.G.; Yang, L.J. Reaction mechanism of acetonitrile, olefins, and amines catalyzed by Ag2CO3: A DFT inves-tigation. J. Phys. Org. Chem. 2023, 37, e4594. [Google Scholar] [CrossRef]
- Budyka, M.F. Density functional theory study of the styrylbenzoquinoline dyad and the related dibenzoquinolylcyclobutane formed in the [2 + 2] photocycloaddition reaction. Int. J. Quantum Chem. 2023, 124, e27264. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Cui, J.; Li, W.; Fang, C.; Su, S.; Luan, J.; Gao, T.; Hu, L.; Lu, Y.; Chen, G. AdaBoost Ensemble Correction Models for TDDFT Calculated Absorption Energies. IEEE Access 2019, 7, 38397–38406. [Google Scholar] [CrossRef]
- Lyon, K.; Preciado-Rivas, M.R.; Zamora-Ledezma, C.; Despoja, V.; Mowbray, D.J. LCAO-TDDFT-k-ω: Spectroscopy in the optical limit. J. Phys. Condens. Matter. 2020, 32, 415901. [Google Scholar] [CrossRef]
- Tiwary, A.S. Prediction of Charge Transfer Transition Energies of the Molecular Complexes of Pmda with a Series of Methylbenzenes by Tddft. J. Indian Chem. Soc. 2019, 96, 659–665. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Legault, C.Y. CYLview, version 1.0b; Université de Sherbrooke: Quebec, QC, Canada, 2009.

| State | E (kcal/mol) | Λ (nm) | fOS | Orbital (Coefficient) | |
|---|---|---|---|---|---|
| BQ | S1 | 265.6 | 450.2 | 0.0000 | H > L (91.6%) |
| S2 | 295.1 | 405.2 | 0.0000 | H-2 > L (89.0%) | |
| S3 | 417.7 | 286.3 | 0.0000 | H-1 > L (99.9%) | |
| BQ + R1 | S1 | 266.0 | 449.6 | 0.0033 | H-3 > L (37.5%) |
| H > L (57.5%) | |||||
| S2 | 274.0 | 436.4 | 0.0029 | H-3 > L (51.5%) | |
| H > L (42.0%) | |||||
| S3 | 299.9 | 398.7 | 0.0000 | H-5 > L (79.4%) | |
| H-4 > L (9.0%) |
| Atom | q(N) | q(N+1) | q(N−1) | f− | f+ | CDD | |
|---|---|---|---|---|---|---|---|
| R1 | S3 | −0.0355 | −0.1054 | 0.2834 | 0.3189 | 0.0699 | −0.2491 |
| C4 | −0.0227 | −0.0726 | 0.0246 | 0.0473 | 0.0499 | 0.0025 | |
| R2 | B5 | 0.1650 | 0.0470 | 0.2085 | 0.0435 | 0.1181 | 0.0746 |
| B6 | 0.1650 | 0.0470 | 0.2085 | 0.0435 | 0.1181 | 0.0746 | |
| IM3a2 | O1 | −0.1258 | −0.1258 | −0.0759 | 0.0499 | 0.0176 | −0.0323 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, Y.; Feng, T.; Chen, B.; Zhou, D. Photoinduced Mechanisms of C–S Borylation of Methyl(p-tolyl)Sulfane with Bis(Pinacolato)diboron: A Density Functional Theory Investigation. Catalysts 2024, 14, 550. https://doi.org/10.3390/catal14080550
Ming Y, Feng T, Chen B, Zhou D. Photoinduced Mechanisms of C–S Borylation of Methyl(p-tolyl)Sulfane with Bis(Pinacolato)diboron: A Density Functional Theory Investigation. Catalysts. 2024; 14(8):550. https://doi.org/10.3390/catal14080550
Chicago/Turabian StyleMing, Yuxiao, Tiantian Feng, Bin Chen, and Dagang Zhou. 2024. "Photoinduced Mechanisms of C–S Borylation of Methyl(p-tolyl)Sulfane with Bis(Pinacolato)diboron: A Density Functional Theory Investigation" Catalysts 14, no. 8: 550. https://doi.org/10.3390/catal14080550
APA StyleMing, Y., Feng, T., Chen, B., & Zhou, D. (2024). Photoinduced Mechanisms of C–S Borylation of Methyl(p-tolyl)Sulfane with Bis(Pinacolato)diboron: A Density Functional Theory Investigation. Catalysts, 14(8), 550. https://doi.org/10.3390/catal14080550







