Abstract
Commercial non-functionalized (CNTs) and functionalized carbon nanotubes (CNT-COOH and CNT-NH2) were used as supports to synthesize vanadium-supported catalysts to be used in the gas phase partial oxidation of furfural towards maleic anhydride (MA). The CNTs and the VO2-V2O5/CNTs, so-called VO/CNT catalysts, were characterized by AAS, TGA, XRD, N2 adsorption isotherms at −196 °C, Raman, NH3-TPD and XPS. The surface area values, TGA and XRD results indicate that the larger thermal stability and larger dispersion of vanadium species is reached for the VO/CNT-NH2 catalyst. XPS indicates presence of surface VO2 and V2O5 species for the non-functionalized (CNT) and functionalized (CNT-COOH and CNT-NH2) catalysts, with a large interaction of the functional group with the surface vanadium species only for the VO/CNT-NH2 catalyst. The catalytic activity, evaluated in the range 305 °C to 350 °C, indicates that CO, CO2 and MA yield (%) and MA productivity are associated to the redox properties of the vanadium species, the oxygen exchange ability of the support and the vanadium–support interaction. For the reaction temperatures between 320 °C and 335 °C, the maximum MA yield (%) is found in the functionalized VO/CNT-COOH and VO/CNT-NH2 catalysts. This behavior is attributed to a decreased oxidation capability of the CNT with the functionalization. In addition, VO/CNT-NH2 is the more active and selective catalyst for MA productivity at 305 °C and 320 °C, which is related to the greater interaction of the surface vanadium species with the -NH2 group, which enhances the redox properties and stabilization of the VO2 and V2O5 surface active sites. Recycling at 350 °C resulted in 100% furfural conversion for all catalysts and a similar MA yield (%) compared to the fresh catalyst, indicating no loss of surface active sites.
1. Introduction
Furfural (FUR), a natural precursor of furan-based chemicals, plays an important role in the production of biofuels and biochemicals []. In particular, the development of new conversions and commercial technologies from FUR to a wider range of feedstocks supports the development of future biorefineries. Due to the presence of the aldehyde group and the conjugated double bond system in its structure, FUR is considered a platform molecule to produce by partial oxidation maleic acid or maleic anhydride (MA) []. In this context, MA is considered a bulk chemical and is one of the most important building blocks in the synthesis of various compounds such as unsaturated polyester resins, surface coatings, lubricants, additives, plasticizers, copolymers and agrochemicals [,]. Many efforts have been made to achieve active and stable catalytic systems for the conversion of FUR []. Due to the furfural conversion’s dependence on the reactor type, the main challenge is to improve the mechanical, hydrothermal and chemical stability of the catalysts [,,,]. It has been observed that in batch reactors operating under high oxygen pressure [] or using hydrogen peroxide [], leaching is the most common deactivation process [], and in fixed bed reactors the main challenge is the formation of deactivating surface resins [,].
Vanadium in combination with other elements has shown promise in both bulk and supported catalysts for the oxidation of FUR [,,,,,,]. The VIV/VV redox pair gives to vanadium unique properties in the conversion of organic molecules into oxygenated products []. This advantage is based on the ability of V to generate vacancies and reduced oxygen species of the O2− type, allowing oxidation to take place with oxygen spillover by an MVK mechanism []. Gómez-Capiro et al. and Santander et al. [,] studied the effect of the support (SiO2, γ-Al2O3, ZrO2 and TiO2) and the surface vanadium content on the partial catalytic oxidation in the gas phase of FUR to MA. They found that on ZrO2 and TiO2 a complete oxidation to CO2 and CO was obtained, which was attributed to the ability of these supports to exchange oxygen atoms. Meanwhile, on Al2O3 and SiO2, the activity is related to the dispersion of vanadium species. Alonso-Fagundez et al. [] reported that the optimum gas phase experimental conditions of O2 pressure and temperature for the FUR oxidation on vanadium oxide supported on Al2O3 were 320 °C and 5.7 kPa of O2 pressure, to obtain the high yield of MA of 73%. Alonso-Fagundez et al. [] studied the effect of temperature on the oxidation of furfural in gas phase on the V2O5/Al2O3 catalyst. The authors found that at low temperatures (240 °C), maleates and resins are deposited on the active phase of the catalyst which is rapidly and deeply deactivated. On the contrary, at high temperatures (between 255 and 315 °C), the deposition of maleates and resins decreases, and the MA yield is remarkably improved, also improved by increasing the O2/furfural mole ratio. At higher temperatures, above 315 °C, MA is overoxidized to CO2.
Carbonaceous materials exhibit interesting properties such as insolubility in aqueous media, resistance to high temperature and pressure and excellent mechanical resistance to agitation and current flow [,]. Furthermore, advances in nanocarbon technology have enabled the controllable tailoring of the pore structure and surface functionalities of porous carbon, making CNTs supported catalysts currently of major trend in the development of integrated processes for the conversion of lignocellulosic derived compounds into value added products [,,]. Liu et al. [] studied the conversion of furfural at 130 °C, 40 bar of H2 and ethanol as solvent, and reported high selectivity to tetrahydrofurfuryl alcohol (THFA) of 85% to 90% on Ni/CNTs and NiCu/CNTs catalysts. The higher conversion of furfural and improved selectivity to THFA of the Ni/CNTs and NiCu/CNTs catalysts with the counterparts supported on MgO, γ-Al2O3, TiO2 and ZrO2, respectively, are attributed to the favorable structure of CNTs, which promotes the confinement and activation of the metallic components and improves their catalytic performance. Ye et al. [] studied the effect of CNT functionalization (O-CNT) with mineral acids (HNO3 and H2SO4) on the activity of VW/Ti, VW/CNT/Ti and VW/O-CNT/Ti catalysts in NOx reduction. The authors found that functionalized CNTs (O-CNT) inhibited agglomeration, improving the nano-dispersal of active metals and surface area and pore volume, which increased the efficiency (90%) compared to those of VW/Ti (88%) and VW/CNT/Ti (76%) in NOx reduction. The activity of surface-oxidized Co/CNTs catalysts in the Fisher–Tropsch reaction was also reported []. The results showed that the high catalytic activity of Co/CNTs was attributed to a smaller particle size of Co confinement in the CNTs’ structure and to the hydrogen spillover effect by the quinone surface functional groups of the CNTs. This important result points to another advantage of CNTs in dispersing and stabilizing the active phase with prior functionalization. Ahmed et al. [] reported that the presence of the -COOH and NH2 functional groups allowed the effective immobilization of the Pt particles on the surface of the CNTs. Furthermore, Lee et al. [], reported that the amino groups of the mesoporous MCM-41 solids were then used to immobilize (VO)2+ ions which were used to study the catalytic oxidation of benzene. The anchored vanadium complexes have better catalytic activities with higher stability than the framework-substituted V-MCM-41 materials. They showed that immobilization of catalytic metal ions on a functionalized surface has the advantages of better control of available reactive sites and site isolation. Figueiredo et al. [] reported a very useful method to identify oxygenated functional groups in carbonaceous materials by XPS and temperature-programmed desorption techniques. In this sense, Xia et al. [], showed that nitric acid is effective in incorporating oxygen into the anchored functional groups, including nitrogenous functional groups [,]. The nature and concentration of surface functional groups can also be modified by oxidizing agents [,,,,].
According to the above information, considering the properties of the CNTs and the fact that there are few reports for vanadium oxides as active sites [], in this work, vanadium supported on commercially available non-functionalized (CNTs) and functionalized carbon nanotubes (CNT-COOH and CNT-NH2) were synthesized and characterized to be used in the partial oxidation of the gas phase of FUR to MA in a reaction temperature range from 305 to 350 °C. A recycle procedure was carried out at 350 °C to obtain a better catalyst.
2. Results
2.1. Thermal Properties and Chemical Composition
The thermogravimetric profiles of the commercial CNTs (CNT, CNT-COOH and CNT-NH2) shown in Figure 1 were carried out to detect differences regarding their functionalization. As expected, the lowest mass loss up to 900 °C corresponds to CNTs (26%), followed by CNT-NH2 (32%), with the highest mass loss for CNT-COOH (74%). For CNTs, the mass loss is attributed to anhydrides, lactones, hydroxides and phenol [,], and also presents in the functionalized CNT-NH2 and -COOH. The high lability per water and the decarboxylation of the -COOH groups [,] explains the larger mass loss of CNT-COOH, and the decomposition of amines and amides [] for CNT-NH2. After the addition of vanadium, higher mass loss for VO/CNT and VO/CNT-COOH compared to VO/CNT-NH2 catalyst is seen. This behavior suggests that the functional groups of CNT-NH2 anchor the vanadium species in a higher interaction compared to VO/CNT and VO/CNT-COOH catalysts. The wt% of V obtained from atomic absorption spectroscopy (AAS) on CNT, CNT-COOH and CNT-NH2 are shown Table 1, being similar for the three catalysts.
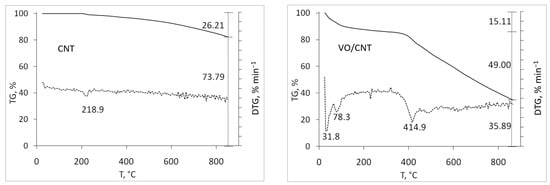
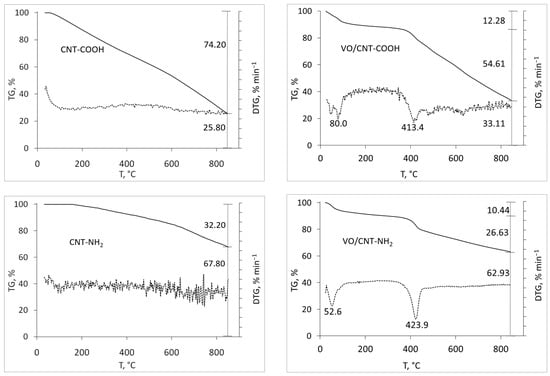
Figure 1.
Thermal TG (solid line) and DTG (dotted line) profiles for CNTs and VO/CNTs catalysts.

Table 1.
AAS vanadium content, surface area, pore volume, ID/IG ratio, Raman bands and total acidity for CNTs and VO/CNTs catalysts.
2.2. Textural Properties
The N2 adsorption isotherms (Figure S1) of the CNTs and the VO/CNTs catalysts correspond to type IV with a hysteresis loop H1 that corresponds to mesoporous solids with uniform cylindrical pores []. The pore size distributions (Figure S2) display two peaks, one in the limit zone of microporosity and the other located ~30 nm. In Table 1, it can be seen similar SBET for the CNTs, which decrease after the impregnation of vanadium. The large SBET decrease for VO/CNT-NH2 close to 47% compared to a 30% for VO/CNT and VO/CNT-COOH catalysts is attributed to the blocking of the pore structure by surface vanadium species or a change in the pore’s channels of the CNTs. Considering the previous TGA results it can be proposed a different interaction of the vanadium species with the surface -NH2 groups. This behavior agrees with previous reports for NH2 functional groups allowing an effective immobilization in Pt supported in CNTs [] and (VO)2+ in mesoporous MCM-41 [].
2.3. Structural Properties
The XRD patterns of the CNTs and the VO/CNT catalysts are shown in Figure 2. The commercial non-functionalized and functionalized CNTs do not show differences corresponding to graphene layers [] with almost no differences in the structure with the functionalization process. The most intense peak at 2θ = 26.3° and 42° corresponds to the graphite hexagonal lattice []. Regarding the VO/CNT catalysts, bulk V2O5 (ICDD ID 01-089-0612) [] is clearly detected at 2θ = 20°, for VO/CNT and VO/CNT-COOH catalysts. Unfortunately, the corresponding peak of VO2 (PDF 01-073-0514) at 2θ = 26.3° and 33.9° [,] is masked with the diffraction peaks of the CNTs. The absence of new diffraction peaks of vanadium species for VO/CNT-NH2 catalyst does not indicate absence of bulk vanadium species since it can be present in a larger dispersion degree and/or inserted in the graphene layers. In the Raman spectra (Figure S3) of the CNTs and the VO/CNT catalysts appears the characteristic D (~1350 cm−1) and G band (~1580 cm−1) [,]. The G band corresponds to the sp2 vibration in the graphitic structure and the D band, the corresponding vibration out of the plane []. An increase in the intensity of the D band indicates structural imperfection and in the G band, a more ordered graphitic structure and the ID″/IG ratio are a descriptor of crystallinity and purity, respectively []. In Table 1, the almost similar ID/IG ratios for the CNTs and the VO/CNT catalysts indicate almost no changes in the graphitic structure by the functionalization and impregnation of vanadium. Choi et al. [], report that ID″/IG values closer to 0.06 correspond to a pure and crystalline (100%) structure and ID″/IG values closer to 0.37, to a total loss of the crystalline structure. In Table 1, the ID″/IG values indicate that the crystallinity structure is maintained for the VO/CNT catalyst, whereas a decrease in the crystalline structure is obtained for the VO/CNT-COOH and VO/CNT-NH2 catalysts. This result is indicative that after the incorporation of vanadium occurs a partial destruction of the CNT structure with the formation of amorphous carbon.
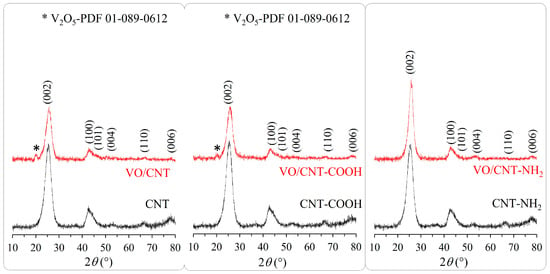
Figure 2.
XRD pattern for CNTs and VO/CNT catalysts.
2.4. Acid Properties
The NH3-TPD desorption classifies the strength of the acid sites into weak acid sites that desorb from 150 °C to 300 °C, medium sites in the range 250 to 400 °C and strong acid sites sites at temperatures >400 °C []. Figure 3 shows NH3-TPD profiles obtained and the total acidity calculated from the area under the desorption profiles is summarized in Table 1. The non-acid CNT is an expected result, and the slight increase for CNT-COOH and CNT-NH2, a consequence of their functionalization process []. The large increases in the total acidity after vanadium impregnation, with almost no dependence with the functionalized group, indicates that the vanadium species have the responsibility of the acid properties of the VO/CNTs catalysts. In this context, Shylesh et al. [] report a total acidity of 1.05 mmol NH3 g−1 for a 15% V2O5/Nd2O5 catalyst with a surface area of 7.2 m2 g−1 []. Therefore the large obtained values (>10 mmol NH3 g−1) of total acidity, shown in Table 1, indicate that the highly dispersed vanadium oxides species are responsible for the acid properties of the synthesized catalysts. However, the non-significant differences in the total acidity values between the catalysts suggest that oxidation of furfural in gas phase cannot be explained from acid properties of the catalysts [].
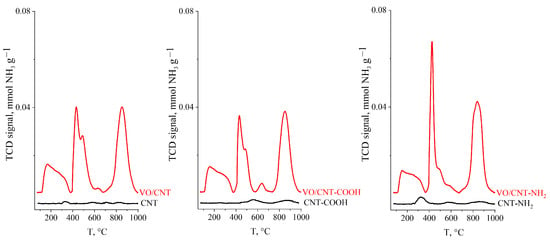
Figure 3.
NH3-TPD profiles for CNTs and VO/CNT catalysts.
2.5. XPS
The XPS measurements were carried out for CNTs and VO/CNT catalysts. The spectra for O 1s, N 1s and V 2p are presented in Figure 4, Figure 5 and Figure 6, respectively, and the calibration signals were determined with respect to C 1s at BE of 284.5 eV (Figure S4 and Table S2) []. The commercial CNTs display the six expected peaks for C 1s peaks: at 284.5 eV the C=C bond of the graphene sheets [,,], at 285.4 eV the C-C bond, at 286.9 eV the C-O, C-H and C-N bonds [,,], at 289.1 eV the C=O bond, at 290.9 eV the O-C=O bonds characteristic of carboxylic acids, esters and other organic functional groups [,,] and at 292.9 eV the π-π* interaction [,,]. The O 1s spectrum of the commercial CNTs (Figure 4) indicates the presence of two oxygen species: at 532.3 eV the O=C bond of quinones, ketones and aldehydes, and at 533.5 eV the O-C bond of ethers and phenols (Table 2) [,,]. For the VO/CNT catalysts it can be seen (Figure 4) almost no changes in the BE of the two peaks associated to the CNTs, and two new peaks appear at a lower BE, indicative of more electronic oxygen species associated to surface vanadium species. Therefore, the signal at 531.4 eV is associated with VIV and at 530.3 eV with VV []. For N 1s (Figure 5), there are two surface contributions for the CNTs at 399.0 eV to the amino (C-NH2) and at 400.9 eV to the N-C=O group amide. For the VO/CNT-NH2 catalyst appears a new N1s signal located at a high BE, indicative of a more cationic nitrogen species. The new signal at 402.8 eV has been associated with a more cationic -NH2 group [], supporting a close interaction between -NH2 groups with the surface vanadium species in the VO/CNT-NH2 catalyst. This interaction between N-groups and metallic particles has been reported by Gonçalves et al. [], demonstrating that CNTs doped with N species provided anchoring sites which favor a strongly Ni–N interaction improving the Ni sites dispersion. Lee et al. [] also report that the amino groups of the mesoporous MCM-41 allow the immobilization of (VO)2+catalytic metal ions on a functionalized surface, controlling available isolated reactive sites. For the V 2p3/2 spectra (Figure 6), two signals appear at 516.4 eV for VIV and 517.5 eV for VV species [], with almost no changes upon the functionalization of the CNTs. Table 2 also presents the V/C surface atomic ratio which slightly increases following the order: VO/CNT < VO/CNT-NH2 < VO/CNT-COOH. The increases in the surface N (%), estimated from XPS from 0.54% in CNT-NH2 to 1.7% in the VO/CNT-NH2 catalyst, is in line with the reported surface interaction of VIV and VV species with the unpaired electrons of nitrogen [,].
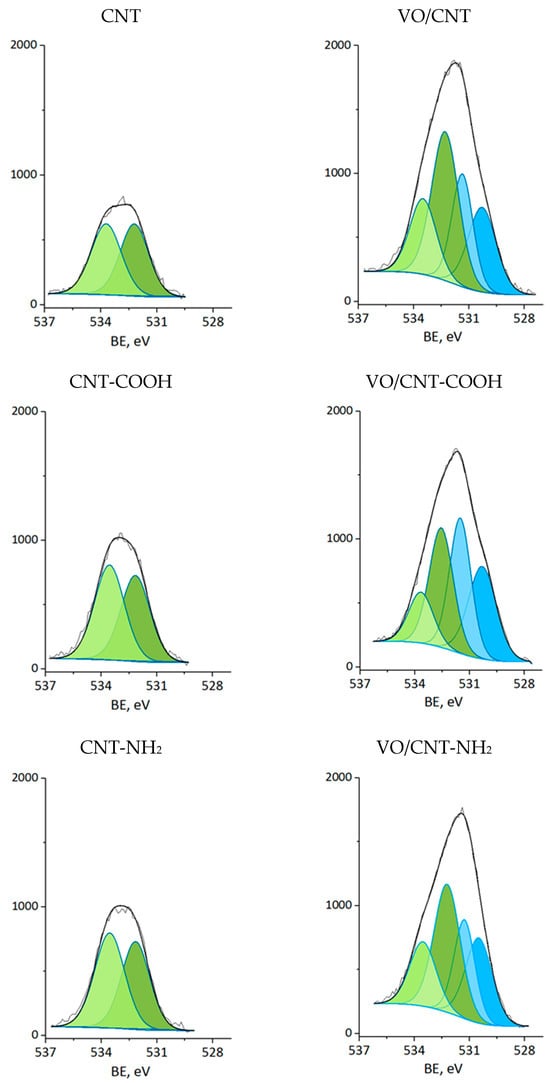
Figure 4.
XP spectra of O 1s for CNTs and VO/CNTs catalysts (light green C-O; green C=O; light blue O-VV; blue O-VIV).
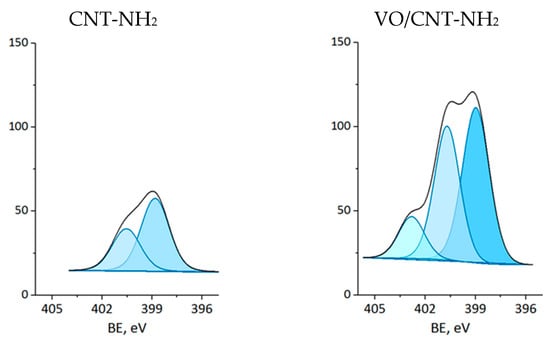
Figure 5.
XP spectra of N 1s for CNTs and VO/CNT catalysts (sky blue N-cationic, light blue N-amine and blue N-amide).
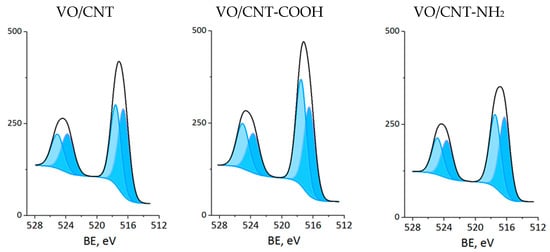
Figure 6.
XP spectra of V 2p for VO/CNT catalysts (light blue VV and blue VIV).

Table 2.
BE (eV) and surface atomic V/C ratio for CNTs and VO/CNT catalysts.
2.6. Catalytic Properties
The furfural conversion in the gas phase was evaluated in a steady state (Figure S5) established in consecutive reaction cycles. It can be seen in Table 3 that the conversion of furfural increases with temperature, reaching 100% at 335 °C and 350 °C. At 305 °C and 320 °C, the highest furfural conversion was obtained on the VO/CNT-NH2 catalyst, and the lowest values displayed on the VO/CNT-COOH catalyst. Considering the previous characterization results, the larger furfural conversion on VO/CNT-NH2 can be correlated to the larger interaction of the surface vanadium species with the -NH2 group enhancing the redox properties of vanadium and stabilization of the VO2 and V2O5 surface active sites [,,,]. On the contrary, the lability of the -COOH groups from the CNT used as a support does not favor the stability of the surface vanadium oxide species, as was observed by thermal measurements. On the other hand, in Figure 7 are shown the yields (%) to MA, CO2 and CO as functions of the reaction temperature on the VO/CNT catalysts.

Table 3.
Furfural conversion (XF) and MA Yield (%) for VO/CNT catalysts.
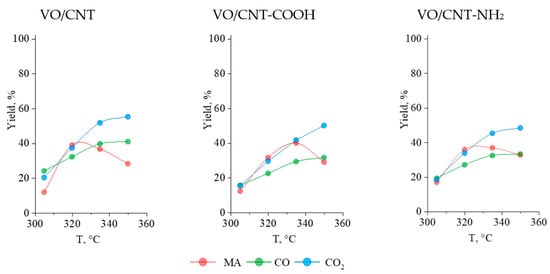
Figure 7.
Yield of MA, CO and CO2 for VO/CNT catalysts.
The complete and incomplete combustions of furfural towards CO and CO2 increase with reaction temperature on the three VO/CNT catalysts [,], whereas the yield (%) to MA differences in the functionalized catalysts are detected. At 320 °C, the maximum yield (%) to MA is achieve on the VO/CNT catalyst, at 335 °C on VO/CNT-COOH and for the VO/CNT-NH2 catalyst, the maximum MA yield (%) is obtained between 320 °C and 335 °C. Even though there are observed differences in the yield (%) of MA upon varying reaction temperatures, the maximum of the three catalysts is around 40%. This behavior indicates that the functionalized CNT with -COOH and -NH2 groups has an effect in the oxidation capability of the catalyst, decreasing the reaction rate of the further oxidation of MA towards consecutive products (CO and CO2) at larger reaction temperatures. In this line, previous reports have shown that supports such as ZrO2 and TiO2 have enough of an exchange surface O atoms ability to participate in oxidation reactions [,]. Therefore, the functionalization of a CNT with -COOH and -NH2 groups decreases the availability of the CNT to participate in the oxidation process, being detected for the functionalized VO/CNT-COOH and VO/CNT-NH2 catalysts between 320 °C and 335 °C, a more remarkable oxidation effect of the VO species.
Due to the conversion of furfural being less than 100% at 305 °C and 320 °C, the productivity towards MA can be calculated expressed as g of MA per g of vanadium and time, shown in Figure 8. It can be seen that VO/CNT-NH2 is the more active and selective catalyst to MA productivity, associated with the larger interaction of the VO2 and V2O5 surface active sites with the -NH2 group and enhancing the redox properties and stabilization of vanadium. Furthermore, because XPS indicates that the non-functionalized and functionalized vanadium catalysts display almost the same surface vanadium content, the observed differences in the productivity of MA confirm that the oxidation of furfural not only depends on the oxidizing capacity of VO, but also on the oxygen exchange ability of the support and the vanadium–support interaction.
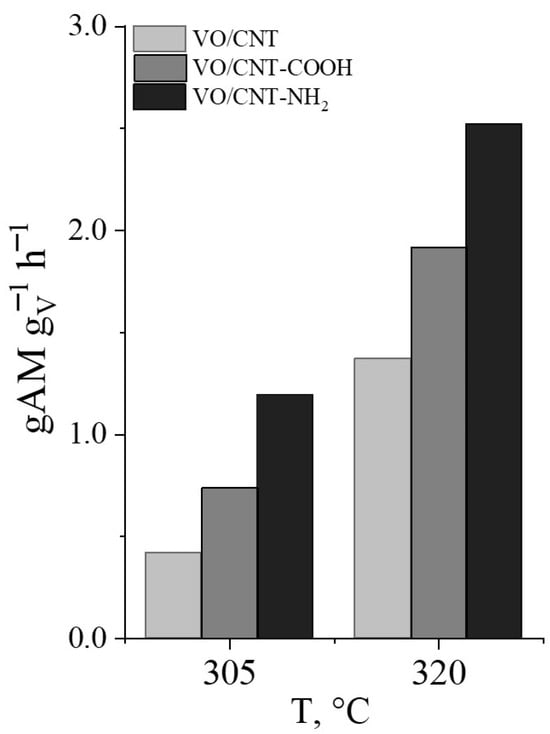
Figure 8.
Productivity of MA for VO/CNT catalysts.
Regarding the recycles, it was found that after the catalytic run, the system was brought back to 350 °C (Figure S5), recovering for the three catalysts the 100% of furfural conversion. The MA yield (%) remains practically constant in the first cycle (Table 3), indicating that the active sites are not lost after the reaction cycle [,,].
3. Materials and Methods
3.1. Synthesis
The commercial CNT, CNT-COOH and CNT-NH2 were impregnated using a solution of vanadyl sulfate (VOSO4·2H2O), Sigma-Aldrich (Concepción, Chile) (97%), to obtain a 7 wt% nominal vanadium content. VOSO4·2H2O was dissolved in water at 65 °C stirring at 500 rpm and the obtained blue dissolution was added to the commercial CNTs, CNT-COOH and CNT-NH2 (Table S1). The temperature of dispersion was 65 °C, maintained for 2 h and introduced into a vacuum oven at 60 mbar and 80 °C overnight. The resulting solids were calcined from room temperature to 300 °C at 1 °C min−1 for 7 h and cooled at 1 °C min−1 to room temperature. The remaining catalysts were labeled as VO/CNT, VO/CNT-COOH and VO/CNT-NH2.
3.2. Characterization
The bulk composition of C, H and N was determined by elemental analysis in a Fisons-EA-1108 CHNS Element Analyzer (Thermo Scientific, Concepción, Chile), and the bulk composition of vanadium was determined by AAS in a Thermo Scientific instrument model iCE Series 3000. Thermogravimetric analyses were performed in a Netzsch brand Iris TG 209F1 thermal analyzer, in N2 atmosphere (250 mL min−1) from room temperature to 850 °C (10 °C min−1). XRD patterns were recorded on a Bruker D4 Endeavor AXS diffractometer (Concepción, Chile) at 40 kV and 20 mA equipped with CuKα1 radiation (λ = 1.5418 Å). The values of 2θ were scanned from 10° to 80° at 0.02 per step. Nitrogen isotherms at −196 °C were performed in a Micromeritics TriStar II 3020 equipment using samples degassed under vacuum at 200 °C for 2 h, and the specific area was calculated using the BET equation. Acid properties were determined by NH3-TPD in a Micromeritics AutoChem II instrument (Concepción, Chile) equipped with a TCD. A sample of 50 mg was placed in a U-shaped quartz reactor and pretreated in He flow at 50 mL min−1 from room temperature to 110 °C (10 °C min−1) for 30 min. The sample was cooled to 42 °C and saturated with NH3 at a flow rate of 10 mL min−1 of for 10 min. To remove physisorbed NH3 the sample was purged with a flow rate of 50 mL min−1 of Ar at 75 °C per 1h. The amount of NH3 desorbed was calculated from the area under the curve using ammonia TPD analytical areas. The XP spectra were obtained in a SPECS brand equipment, Phoibos instrument (DLD, HSA3500, Concepción, Chile), with a step energy of 150 eV, work function (W.F.) of 4.48 and a step energy of 1.0 eV, while the spectra of the elements in the high-resolution windows were obtained with a step energy of 50 eV, using an aluminum source (1487 eV). The peaks were decomposed into several components assuming a Gaussian and Lorentzian shape. Raman spectra were performed in a HORIBA (Concepción, Chile) Scientific brand Raman spectrophotometer (laser: 633 nm); spectra were decomposed in five components assuming Lorentzian shape.
3.3. Catalytic Activity
The catalytic oxidation of furfural starts with a pretreatment of the catalyst in synthetic air (Air Liquide, Concepción, Chile, 99.999%) at a flow of 20 mL min−1 with a heating ramp of 10 °C min−1 for 30 min. The feed gases were controlled by mass flow controllers (Kofloc D8500, Concepción, Chile) and recalibrated to volumetric flow (bubble flowmeter). Furfural (Sigma-Aldrich, Concepción, Chile, 99%) was acquired in vapor-phase saturates (Teflon saturator in bath at 25 °C (XMTD-204, HH-1, Concepción, Chile), a flow of N2 (Air Liquide. 99.999%) of 19 mL min−1). The total flow consists of a stream of synthetic air (Air Liquide. 99.999%) of 4.6 mL min−1, and the stream of N2 saturated with furfural and a stream of N2 as carrier gas of 34.62 mL min−1. Once the flow is established and without leakages, the temperature of the controller is increased to 350 °C at 10 °C min−1 for 4 h, then decreased to 335 °C for 4 h, 320 °C for 4 h, 305 °C for 4 h and increased to 350 °C 10 °C min−1 for 4 h. Reagents and reaction products were analyzed in-line using a GC (SRI 8610C, Concepción, Chile) with one capillary column (30 m × 0.53 mm I.D. 1.0U MXT-WAX, Restek 70 655-273, Concepción, Chile) and three packed columns (6′ × 1/8″ molecular sieve 5A, 18″ × 1/8″ Hayesep D and 6′ × 1/8″ Hayesep D, Concepción, Chile). The system was equipped with a TCD, an FID and an FID detector equipped with a methanizer.
The furfural conversion was determined according to Equation (1).
where is the conversion of furfural. is the input flow. is output flow.
The MA yield was determined according to Equation (2).
where is the yield to a particular product. is the output flow of the product, and are stoichiometric indices of the reaction under study.
The CO2 yield was determined according to Equation (3), equivalent to the CO yield.
where is the CO2 yield. is the CO2 concentration at the reactor outlet, the concentration of maleic anhydride at the reactor outlet, the concentration of furfural at the reactor inlet and 5 the stoichiometric index of furfural in the reaction.
The selectivity to maleic anhydride was determined according to Equation (4).
where is the selectivity of MA.
The productivity towards MA was determined according to Equation (5).
where is the formation of MA in grams, is the vanadium mass per gram of catalyst and is the reaction time.
4. Conclusions
The characterization of the VO/CNT, VO/CNT-COOH and VO/CNT-NH2 catalysts shows differences in their thermal, textural, surface and structural properties. The lower weight-loss upon temperature and the larger decrease in surface area and porosity of VO/CNT-NH2 compared to the corresponding CNT-NH2 indicate a stronger interaction between the VO2 and V2O5 species with the -NH2 groups. In addition, the loss of crystallinity indicates insertion of vanadium in the graphitic structure and XPS interaction of the vanadium species with the -NH2 groups for the VO/CNT-NH2 catalyst. The catalytic activity, evaluated in the range 305 to 350 °C indicates that CO, CO2 and MA yield (%) and MA productivity are associated to the redox properties of the vanadium species, the oxygen exchange ability of the support and the vanadium–support interaction. The functionalization of the CNT with -COOH and -NH2 groups reduces the oxidation capacity, decreasing the complete oxidation to CO2 and CO at 320 °C and 335 °C for VO/CNT-COOH and VO/CNT-NH2 catalysts. The largest furfural conversion, yield (%) of MA and MA productivity for the VO/CNT-NH2 catalyst is attributed to the larger interaction of the surface vanadium species with the -NH2 group, enhancing the redox properties and stabilization of the surface VO2 and V2O5 active sites. Furthermore, at 350 °C, a similar yield to MA (%) in the recycling indicates that the active site is not lost.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14080510/s1, Figure S1: N2 adsorption–desorption isotherms for CNTs and VO/CNT catalysts; Figure S2: Pore size distribution for CNTs and VO/CNT catalysts; Figure S3: Raman spectra for CNTs and VO/CNT catalysts; Figure S4: XP spectra of C1s for CNTs and VO/CNT catalysts; Figure S5: Experimental curves of furfural conversion over time for VO/CNT catalysts; Table S1: Elemental composition of CNTs; Table S2: C1s BE (eV) and % for CNTs and VO/CNT catalysts.
Author Contributions
Conceptualization, C.S. and G.P.; methodology, C.S., P.R., C.H., J.N.D.d.L., A.K. and S.R.; validation, P.R., J.N.D.d.L. and C.P.; formal analysis, C.S., G.P. and P.R.; investigation, C.S. and G.P.; resources, C.S. and G.P.; writing—original draft preparation, P.R., C.P., C.S. and G.P.; writing—review and editing, C.S. and G.P.; funding acquisition, C.S. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondecyt 1220130 and 1210142 ANID Millennium Science Initiative NCN2021-090 for the financial support and Chile and FONDEQUIP EQM 160070.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors would like to thank David Dominguez of XPS Laboratory of Centro de Nanociencias y Nanotecnología of Universidad Nacional Autónoma de México for XPS analysis of the CNTs and VO/CNTs for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kabbour, M.; Luque, R. Chapter 10—Furfural as a platform chemical: From production to applications. In Biomass, Biofuels, Biochemicals; Saravanamurugan, S., Pandey, A., Li, H., Riisager, A., Eds.; Elsevier: Berlin/Heidelberg, Germany, 2020; pp. 283–297. [Google Scholar]
- Felthouse, T.R.; Burnett, J.C.; Horrell, B.; Mummey, M.J.; Kuo, Y.-J. Maleic Anhydride, Maleic Acid, and Fumaric Acid. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Lohbeck, K.; Haferkorn, H.; Fuhrmann, W.; Fedtke, N. Maleic and Fumaric Acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Agirre, I.; Gandarias, I.; Granados, M.L.; Arias, P.L. Process design and techno-economic analysis of gas and aqueous phase maleic anhydride production from biomass-derived furfural. Biomass Convers. Biorefin. 2020, 10, 1021–1033. [Google Scholar] [CrossRef]
- Harth, F.M.; Likozar, B.; Grilc, M. Stability of solid rhenium catalysts for liquid-phase biomass valorization–various facets of catalyst deactivation and rhenium leaching. Mater. Today Chem. 2022, 26, 101191. [Google Scholar] [CrossRef]
- Rodríguez-Montaña, A.; Brijaldo, M.H.; Rache, L.Y.; Silva, L.P.C.; Esteves, L.M. Reacciones comunes de Furfural en procesos escalables de Biomasa Residual. Cienc. Desarro. 2020, 11, 63–80. [Google Scholar] [CrossRef]
- Gómez-Cápiro, O.; Bravo, L.; Lagos, P.; Santander, P.; Pecchi, G.; Karelovic, A. Kinetic and structural understanding of bulk and supported vanadium-based catalysts for furfural oxidation to maleic anhydride. Catal. Sci. Technol. 2021, 11, 6477–6489. [Google Scholar] [CrossRef]
- Murthy, M.S.; Rajamani, K. Kinetics of vapour phase oxidation of furfural on vanadium catalyst. Chem. Eng. Sci. 1974, 29, 601–609. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Granados, M.L.; Mariscal, R.; Ojeda, M. Selective Conversion of Furfural to Maleic Anhydride and Furan with VOx/Al2O3 Catalysts. ChemSusChem 2012, 5, 1984–1990. [Google Scholar] [CrossRef]
- Li, X.; Ho, B.; Zhang, Y. Selective aerobic oxidation of furfural to maleic anhydride with heterogeneous Mo–V–O catalysts. Green Chem. 2016, 18, 2976–2980. [Google Scholar] [CrossRef]
- Santander, P.; Bravo, L.; Pecchi, G.; Karelovic, A. The consequences of support identity on the oxidative conversion of furfural to maleic anhydride on vanadia catalysts. Appl. Catal. A Gen. 2020, 595, 117513. [Google Scholar] [CrossRef]
- Rajamani, K.; Subramanian, P.; Murthy, M.S. Kinetics and Mechanism of Vapor Phase Oxidation of Furfural over Tin Vanadate Catalyst. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 232–234. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Ojeda, M.; Mariscal, R.; Fierro, J.L.G.; López Granados, M. Gas phase oxidation of furfural to maleic anhydride on V2O5/γ-Al2O3 catalysts: Reaction conditions to slow down the deactivation. J. Catal. 2017, 348, 265–275. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Piccolo, L.; Loridant, S.; Christopher, P. Supported Metal Single-Atom Thermocatalysts for Oxidation Reactions. In Supported Metal Single Atom Catalysis; Wiley: Hoboken, NJ, USA, 2022; pp. 377–423. [Google Scholar]
- Zhao, X.; Xu, J.; Wang, A.; Zhang, T. Porous carbon in catalytic transformation of cellulose. Chin. J. Catal. 2015, 36, 1419–1427. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Taghvaei, H.; Bakhtyari, A.; Reza Rahimpour, M. Carbon nanotube supported nickel catalysts for anisole and cyclohexanone conversion in the presence of hydrogen and synthesis gas: Effect of plasma, acid, and thermal functionalization. Fuel 2021, 288, 119698. [Google Scholar] [CrossRef]
- Rahzani, B.; Saidi, M.; Rahimpour, H.R.; Gates, B.C.; Rahimpour, M.R. Experimental investigation of upgrading of lignin-derived bio-oil component anisole catalyzed by carbon nanotube-supported molybdenum. RSC Adv. 2017, 7, 10545–10556. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over Ni/CNTs and bimetallic CuNi/CNTs catalysts. Int. J. Hydrogen Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S.-I.; Lee, M.; Ezazi, M.; Kim, H.-D.; Kwon, G.; Lee, D.H. Synthesis of oxygen functionalized carbon nanotubes and their application for selective catalytic reduction of NOx with NH3. RSC Adv. 2020, 10, 16700–16708. [Google Scholar] [CrossRef]
- Ghogia, A.C.; Machado, B.F.; Cayez, S.; Nzihou, A.; Serp, P.; Soulantica, K.; Pham Minh, D. Beyond confinement effects in Fischer-Tropsch Co/CNT catalysts. J. Catal. 2021, 397, 156–171. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. The Nanostructure of Nitrogen Atom Linked Carbon Nanotubes with Platinum Employed to the Electrocatalytic Oxygen Reduction. J. Nanosci. Nanotechnol. 2013, 13, 306–314. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. (VO)2+ Ions Immobilized on Functionalized Surface of Mesoporous Silica and Their Activity toward the Hydroxylation of Benzene. J. Phys. Chem. B 2003, 107, 2543–2551. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Xia, W.; Wang, Y.; Bergsträßer, R.; Kundu, S.; Muhler, M. Surface characterization of oxygen-functionalized multi-walled carbon nanotubes by high-resolution X-ray photoelectron spectroscopy and temperature-programmed desorption. Appl. Surf. Sci. 2007, 254, 247–250. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Rocha, R.P.; Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B Environ. 2011, 104, 330–336. [Google Scholar] [CrossRef]
- Toebes, M.L.; van Heeswijk, J.M.P.; Bitter, J.H.; Jos van Dillen, A.; de Jong, K.P. The influence of oxidation on the texture and the number of oxygen-containing surface groups of carbon nanofibers. Carbon 2004, 42, 307–315. [Google Scholar] [CrossRef]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Characterisation of the surface chemistry of carbon materials by temperature-programmed desorption: An assessment. Catal. Today 2023, 418, 114136. [Google Scholar] [CrossRef]
- Friedel Ortega, K.; Arrigo, R.; Frank, B.; Schlögl, R.; Trunschke, A. Acid–Base Properties of N-Doped Carbon Nanotubes: A Combined Temperature-Programmed Desorption, X-ray Photoelectron Spectroscopy, and 2-Propanol Reaction Investigation. Chem. Mater. 2016, 28, 6826–6839. [Google Scholar] [CrossRef]
- Rocha, R.P.; Silva, A.M.T.; Romero, S.M.M.; Pereira, M.F.R.; Figueiredo, J.L. The role of O- and S-containing surface groups on carbon nanotubes for the elimination of organic pollutants by catalytic wet air oxidation. Appl. Catal. B Environ. 2014, 147, 314–321. [Google Scholar] [CrossRef]
- Strong, K.L.; Anderson, D.P.; Lafdi, K.; Kuhn, J.N. Purification process for single-wall carbon nanotubes. Carbon 2003, 41, 1477–1488. [Google Scholar] [CrossRef]
- Nowicki, P.; Szymanowski, W.T.; Pietrzak, R. Textural, surface, thermal and sorption properties of the functionalized activated carbons and carbon nanotubes. Pol. J. Chem. Technol. 2015, 17, 120–127. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Güler, Ö. Mechanical and Thermal Properties of a Cu-CNT Composite with Carbon Nanotubes Synthesized by CVD Process. Mater. Test. 2014, 56, 662–666. [Google Scholar] [CrossRef]
- Shklover, V.; Haibach, T.; Ried, F.; Nesper, R.; Novák, P. Crystal Structure of the Product of Mg2+ Insertion into V2O5 Single Crystals. J. Solid State Chem. 1996, 123, 317–323. [Google Scholar] [CrossRef]
- Porwal, D.; Esther, A.C.M.; Dey, A.; Gupta, A.K.; Kumar, D.R.; Bera, P.; Barshilia, H.C.; Bhattacharya, M.; Mukhopadhyay, A.K.; Khan, K.; et al. Effect of low temperature vacuum annealing on microstructural, optical, electronic, electrical, nanomechanical properties and phase transition behavior of sputtered vanadium oxide thin films. Mater. Res. Express 2016, 3, 106407. [Google Scholar] [CrossRef]
- Roslan, M.S.; Chaudhary, K.T.; Doylend, N.; Agam, A.; Kamarulzaman, R.; Haider, Z.; Mazalan, E.; Ali, J. Growth of Wall-controlled MWCNTs by Magnetic Field Assisted Arc Discharge Plasma. J. Saudi Chem. Soc. 2019, 23, 171–181. [Google Scholar] [CrossRef]
- Choi, Y.C.; Min, K.-I.; Jeong, M.S. Novel Method of Evaluating the Purity of Multiwall Carbon Nanotubes Using Raman Spectroscopy. J. Nanomater. 2013, 2013, 615915. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Jorio, A.; Corio, P.; Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Kneipp, K. Origin of the Breit-Wigner-Fano lineshape of the tangential G-band feature of metallic carbon nanotubes. Phys. Rev. B 2001, 63, 155414. [Google Scholar] [CrossRef]
- Shylesh, S.; Radhika, T.; Rani, K.S.; Sugunan, S. Synthesis, characterization and catalytic activity of Nd2O3 supported V2O5 catalysts. J. Mol. Catal. A Chem. 2005, 236, 253–259. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Betiha, M.A.; El-Sharkawy, E.A.; Elshaarawy, R.F.M.; El-Assy, N.B.; Essawy, A.A.; Tolba, A.M.; Rabie, A.M. Highly selective epoxidation of olefins using vanadium (IV) schiff base- amine-tagged graphene oxide composite. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124520. [Google Scholar] [CrossRef]
- Gonçalves, L.P.L.; Meledina, M.; Meledin, A.; Petrovykh, D.Y.; Sousa, J.P.S.; Soares, O.S.G.P.; Kolen’ko, Y.V.; Pereira, M.F.R. Understanding the importance of N−doping for CNT-supported Ni catalysts for CO2 methanation. Carbon 2022, 195, 35–43. [Google Scholar] [CrossRef]
- Ruiz Puigdollers, A.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Kauppi, E.I.; Honkala, K.; Krause, A.O.I.; Kanervo, J.M.; Lefferts, L. ZrO2 Acting as a Redox Catalyst. Top. Catal. 2016, 59, 823–832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).