Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies
Abstract
1. Introduction
2. Results and Discussion
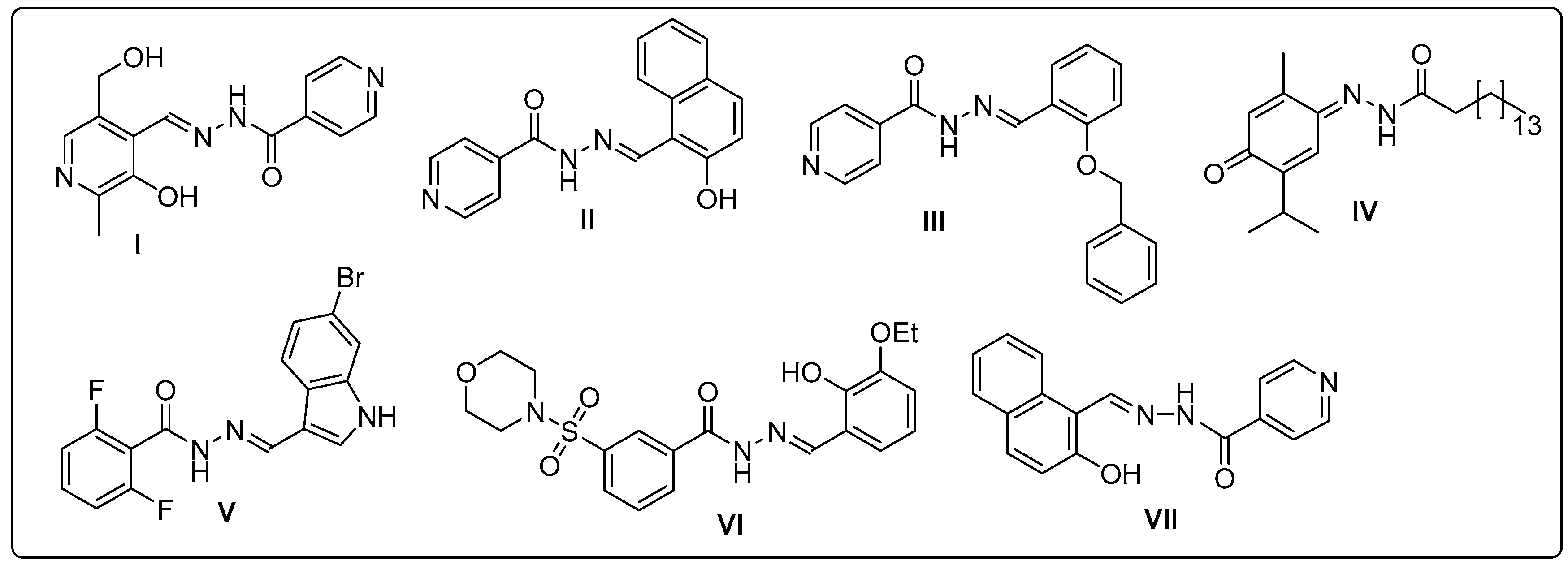
2.1. Antitumor Study
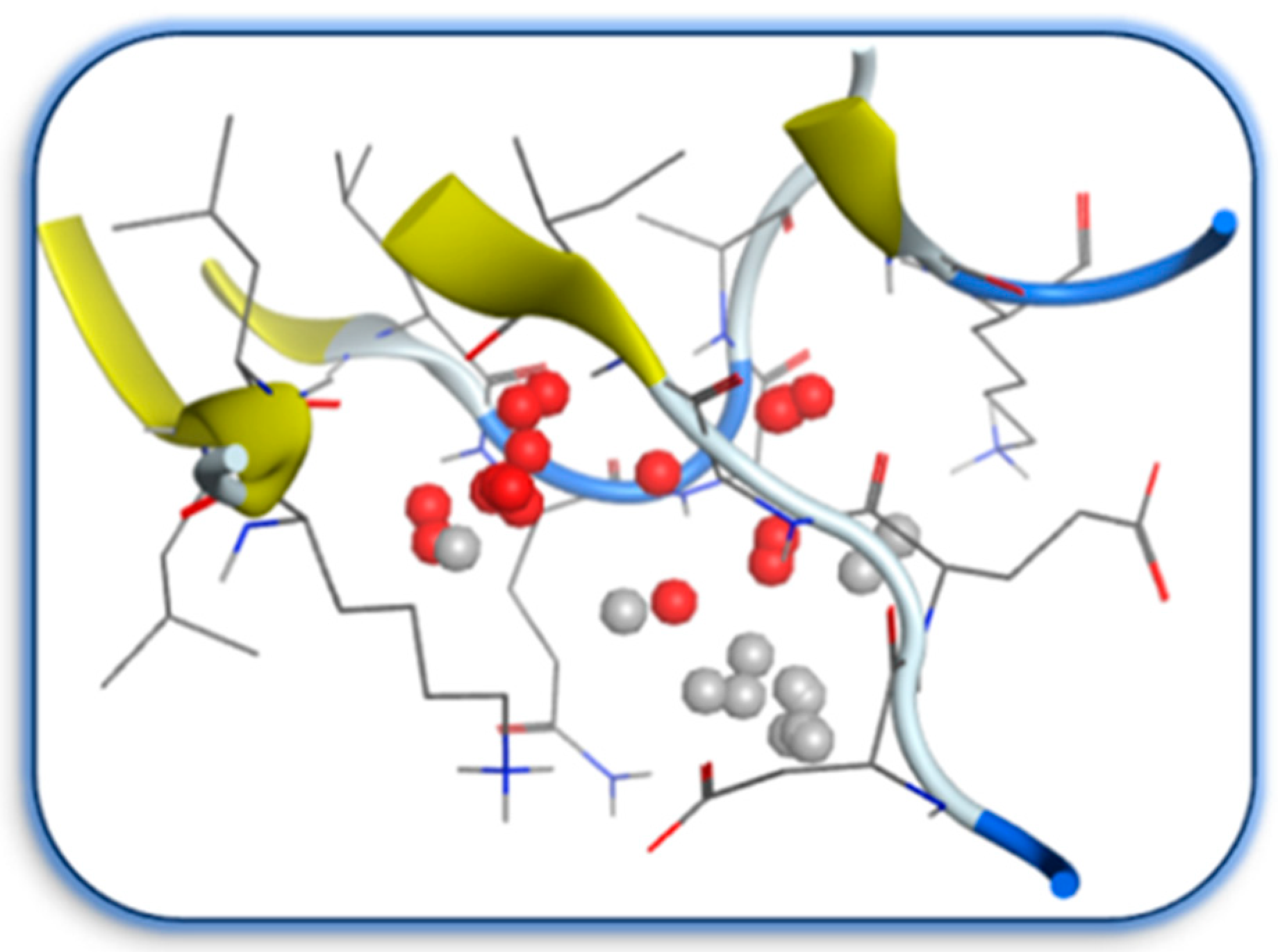
2.2. Molecular Docking Study
2.3. In Silico Pharmacokinetic Profile (ADMET)
3. Experimental
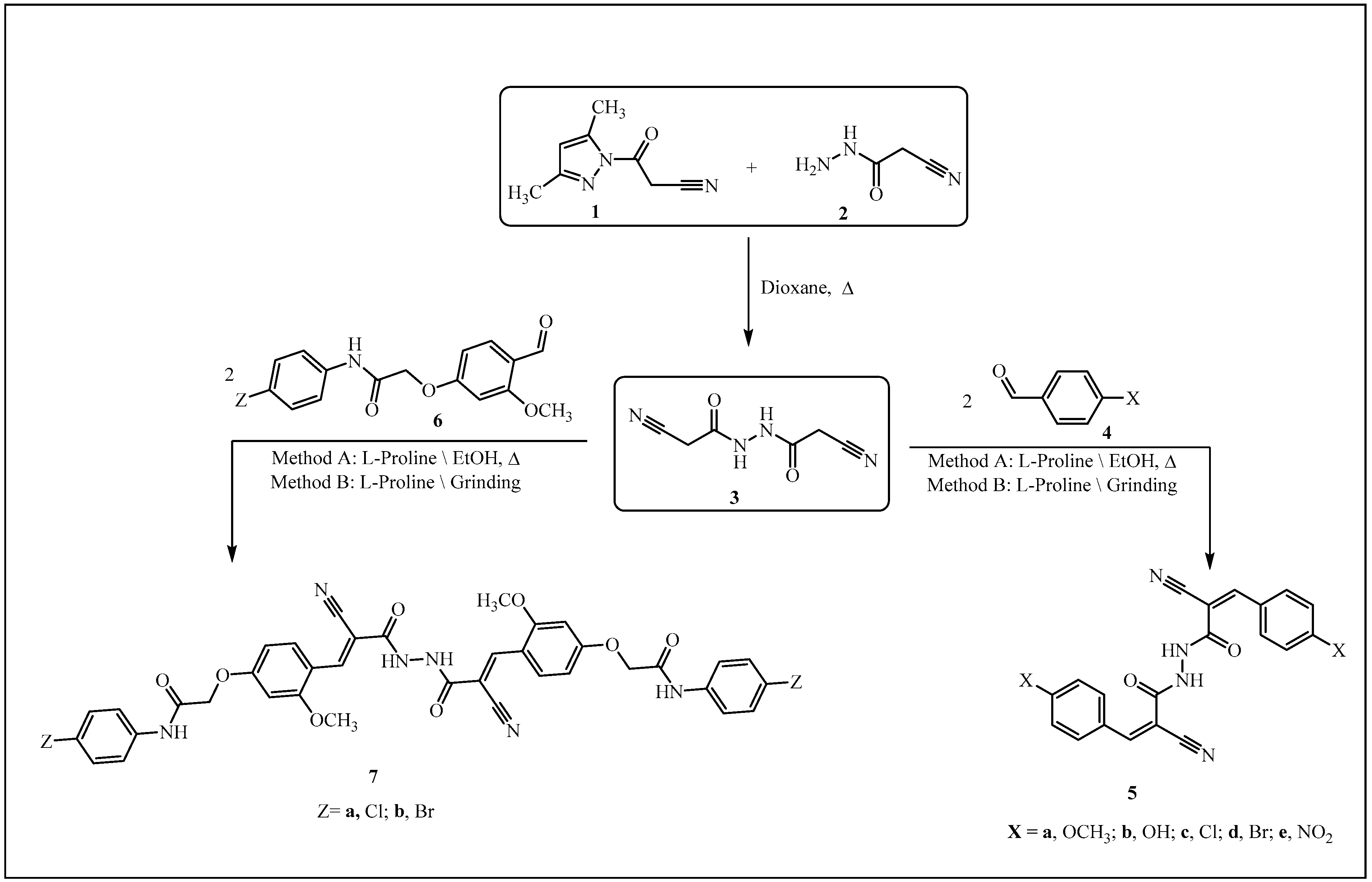
3.1. Synthesis of 2-cyano-N’-(2-cyanoacetyl)acetohydrazide (3)
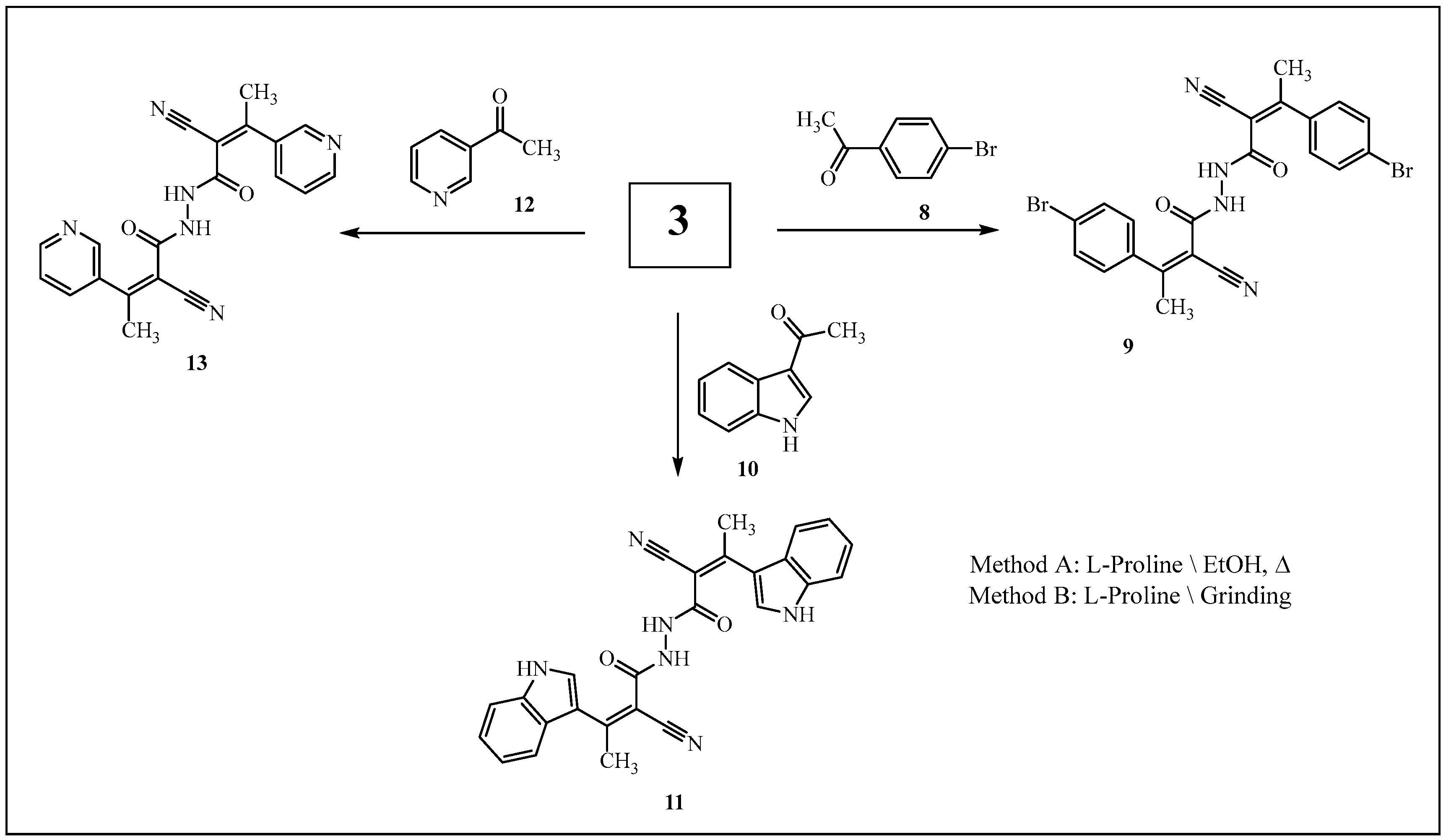
3.2. Reaction of 3 with Aromatic (or Heteroarmatic) Carbonyl Compounds 4a–e, 6a,b, 8, 10, and 12
3.2.1. Method A
3.2.2. Method B
3.3. Cytotoxicity Assay
3.4. Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, Z.; Qu, W.; Yang, R.; Lin, X.; Yan, S.; Lin, J. An environmentally benign, mild, and catalyst-free reaction of quinones with heterocyclic ketene aminals in ethanol: Site-selective synthesis of rarely fused [1,2-a]indolone derivatives via an unexpected anti-Nenitzescu strategy. Green Chem. 2014, 16, 4359–4370. [Google Scholar] [CrossRef]
- Kiyani, H.; Ghorbani, F. Expeditious Green Synthesis of 3,4-disubstituted isoxazole-5(4H)-ones Catalyzed by Nano-MgO. Res. Chem. Intermed. 2016, 42, 6831–6844. [Google Scholar] [CrossRef]
- Kiyani, H.; Darbandi, H.; Mosallanezhad, A.; Ghorbani, F. 2-Hydroxy-5-sulfobenzoic acid: An efficient organocatalyst for the three-component synthesis of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones. Res. Chem. Intermed. 2015, 41, 7561–7579. [Google Scholar] [CrossRef]
- Brahmachari, G.; Banerjee, B. Facile and One-Pot Access to Diverse and Densely Functionalized 2-Amino-3-cyano-4H-pyrans and Pyran-Annulated Heterocyclic Scaffolds via an Eco-Friendly Multicomponent Reaction at Room Temperature Using Urea as a Novel Organo-Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 411–422. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelaziz, M.R.; Abdel-aziz, H.M.; Hassan, S.A. Green Synthesis and Molecular Docking of Thiazolyl-thiazole Derivatives as Potential Cytotoxic Agents. Mini-Rev. Med. Chem. 2017, 17, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Badrey, M.G.; Arafa, W.A.A. DABCO-catalyzed green synthesis of thiazole and 1,3-thiazine derivatives linked to benzofuran. Heterocycles 2016, 92, 1450–1461. [Google Scholar]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, H.M.; Matar, I.K.; El-Sayed, A.A. Green synthesis, molecular docking and anticancer activity of novel 1,4-dihydropyridine-3,5-dicarbohydrazones under grind-stone chemistry. Green Chem. Lett. Rev. 2020, 13, 6–17. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D.; El-Zanate, A.M.; Riyadh, S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 2013, 87, 1109–1120. [Google Scholar]
- Kumar, S.; Sharma, M. A grinding-induced catalyst- and solvent-free synthesis of highly functionalized 1,4-dihydropyridines via a domino multicomponent reaction. Green Chem. 2011, 13, 2017–2020. [Google Scholar] [CrossRef]
- Pasha, M.A.; Nizam, A. Amberlite IR-120 catalyzed, microwave-assisted rapid synthesis of 2-aryl-benzimidazoles. J. Saudi Chem. Soc. 2012, 16, 237–240. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Padmini, N.; Bhuvanesh, N.S.P. Chemodivergent, One-Pot, Multi-Component Synthesis of Pyrroles and Tetrahydropyridines under Solvent- and Catalyst-Free Conditions Using the Grinding Method. ACS Comb. Sci. 2016, 18, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1047. [Google Scholar] [CrossRef] [PubMed]
- Dolotko, J.W.; Wiench, K.W.; Dennis, K.W.; Pecharsky, V.K.; Balema, V.P. Mechanically induced reactions in organic solids: Liquid eutectics or solid-state processes? New J. Chem. 2010, 34, 25–28. [Google Scholar] [CrossRef]
- Brahmachari, G.; Das, S. L-Proline catalyzed multicomponent one-pot synthesis of gem-diheteroarylmethane derivatives using facile grinding operation under solvent-free conditions at room temperature. RSC Adv. 2014, 4, 7380. [Google Scholar] [CrossRef]
- Edrees, M.M.; Abu-Melha, S.A.; Saad, M.; Kheder, N.A.; Gomha, S.M.; Muhammad, Z.A. Eco-Friendly synthesis, characterization and biological evaluation of some novel pyrazolines containing thiazole moiety as potential anticancer and antimicrobial agents. Molecules 2018, 23, 2970. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, H.R.M.; Gomha, S.M.; El-Gendey, M.S.; El-Hashash, M.A.; Soliman, A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo[1,2-b]phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018, 11, 264–274. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-aziz, H.M.; Abolibda, T.Z.; Hassan, S.A.; Abdalla, M.M. Green synthesis, molecular docking and pharmacological evaluation of new triazolo-thiadiazepinylcoumarine derivatives as sedative-hypnotic scaffold. J. Heterocycl. Chem. 2020, 57, 1034–1043. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304. [Google Scholar] [CrossRef]
- Huang, W.; Anwar, S.; Chen, K. Morita–Baylis–Hillman (MBH) reaction derived nitroallylic alcohols, acetates and amines as synthons in organocatalysis and heterocycle synthesis. Chem. Rec. 2017, 17, 363–381. [Google Scholar] [CrossRef]
- Amarante, G.W.; Coelho, F. Quim. p-Sulfonic acid calix[4]arene as a new reusable organocatalyst for the transesterification of vegetable oil. Nova 2009, 32, 469. [Google Scholar]
- Stowe, G.N.; Janda, K.D. Diels-Alder reaction conducted within the parameters of aqueous organocatalysis: Still just smoke and mirrors. Tetrahedron Lett. 2011, 52, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Mecadon, H.; Rumum, M.; Kharbangar, I.; Laloo, B.M.; Kharkongor, I.; Rajbangshi, M.; Myrboh, B. L-Proline as an efficicent catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2, 4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in water. Tetrahedron Lett. 2011, 52, 3228. [Google Scholar] [CrossRef]
- Hernández, J.G.; García-López, V.; Juaristi, E. Solvent-free asymmetric aldol reaction organocatalyzed by (S)-proline-containing thiodipeptides under ball-milling conditions. Tetrahedron 2012, 68, 92–97. [Google Scholar] [CrossRef]
- Yarhosseini, M.; Javanshir, S.; Dekamin, M.G.; Farhadnia, M. Tetraethylammonium 2-(carbamoyl)benzoate as a bifunctional organocatalyst for one-pot synthesis of Hantzsch 1,4-dihydropyridine and polyhydroquinoline derivatives. Monatsh. Chem. 2016, 147, 1779–1787. [Google Scholar] [CrossRef]
- Schettini, R.; De Riccardis, F.; Sala, G.D.; Izzo, I. Enantioselective Alkylation of Amino Acid Derivatives Promoted by Cyclic Peptoids under Phase-Transfer Conditions. J. Org. Chem. 2016, 81, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Harakeh, S.M. Bioactive 2(1H)-Pyrazinones and Diketopiperazine Alkaloids from a Tunicate-Derived Actinomycete Streptomyces sp. Molecules 2016, 21, 1116. [Google Scholar] [CrossRef] [PubMed]
- Schettini, R.; Costabile, C.; Della Sala, G.; Buirey, J.; Tosolini, M.; Tecilla, P.; Vaccaro, M.C.; Bruno, I.; De Riccardis, F.; Izzo, I. Tuning the biomimetic performances of 4-hydroxyproline-containing cyclic peptoids. Org. Biomol. Chem. 2018, 16, 6708–6717. [Google Scholar] [CrossRef]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Francies, F.Z.; Oyomno, M.; Dlamini, Z. Colorectal cancer genetics, incidence and risk factors: In search for targeted therapies. Cancer Manag. Res. 2020, 12, 9869–9882. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Rejhova, A.; Opattova, A.; Cumova, A.; Slíva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Alam, W.; Bouferraa, Y.; Haibe, Y.; Mukherji, D.; Shamseddine, A. Management of colorectal cancer in the era of COVID-19: Challenges and suggestions. Sci. Prog. 2021, 104, 00368504211010626. [Google Scholar] [CrossRef]
- Fang, F.Q.; Guo, H.S.; Zhang, J.; Ban, L.Y.; Liu, J.W.; Yu, P.Y. Anti-cancer effects of 2-oxoquinoline derivatives on the HCT116 and LoVo human colon cancer cell lines. Mol. Med. Rep. 2015, 12, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Bornstein, J.C.; Nurgali, K. Anti-colorectal cancer chemotherapy-induced diarrhoea: Current treatments and side effects. Int. J. Clin. Med. 2014, 5, 393–406. [Google Scholar] [CrossRef]
- Ismail, T.; Donati-Zeppa, S.; Akhtar, S.; Turrini, E.; Layla, A.; Sestili, P.; Fimognari, C. Coffee in cancer chemoprevention: An updated review. Expert Opin. Drug Metab. Toxicol. 2020, 17, 69–85. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, Ş.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Raghav, N. Biological activities of hydrazones: A review. Int. J. Pharm. Pharm. Sci. 2011, 3, 26–32. [Google Scholar]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Rahmat Ali, M.; Mumtaz Alam, M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar]
- Corcé, V.; Gouin, S.G.; Renaud, S.; Gaboriau, F.; Deniaud, D. Recent advances in cancer treatment by iron chelators. Bioorg. Med. Chem. Lett. 2016, 26, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.S.; Parumasivam, T.; Jumaat, F.; Ibrahim, P.; Asmawi, M.Z.; Sadikun, A. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med. Chem. Res. 2014, 23, 269–279. [Google Scholar] [CrossRef]
- Wirries, A.; Breyer, A.; Quint, K.; Schobert, R.; Ocker, M. Thymoquinone hydrazone derivatives cause cell cycle arrest in p53-competent colorectal cancer cells. Exp. Ther. Med. 2010, 1, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing powerhouse in colon cancer cells by hydrazide−hydrazone-based small molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gutierrez, E.; Kovacevic, E.; Saletta, F.; Obeidy, P.; Suryo Rahmanto, Y.; Richardson, D.R. Iron chelators for the treatment of cancer. Curr. Med. Chem. 2012, 19, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Peng, J.-D.; Zhou, W.; Qiao, H.; Deng, X.; Li, Z.-H.; Li, J.-D.; Fu, Y.-D.; Li, S.; Sun, K.; et al. Potent hydrazone derivatives targeting esophageal cancer cells. Eur. J. Med. Chem. 2018, 148, 359–371. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Jang, G.R.; Harris, R.Z.; Lau, D.T. Pharmacokinetics and its role in small molecule drug discovery research. Med. Res. Rev. 2001, 21, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Eddershaw, P.J.; Beresford, A.P.; Bayliss, M.K. ADME/PK as part of a rational approach to drug discovery. Drug Discov. Today 2000, 5, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 2001, 6, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdalla, M.A.; Abdelaziz, M.; Serag, N. Eco-friendly one-pot synthesis and antiviral evaluation of pyrazolyl pyrazolines of medicinal interest. Turk. J. Chem. 2016, 40, 484–498. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Farag, B.; Al-Hussain, S.A.; Zaki, M.E.A.; Mohamed, M.A. Green Synthesis of Hydrazono-Thiazolones Using Vitamin B1 and Their Antibacterial Implications. Green Chem. Lett. Rev. 2024, 17, 2380746. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Riyadh, S.M.; Abbas, I.M.; Gomha, S.M. Synthesis and biological activities of 7-arylazo-7H-pyrazolo[5,1-c][1,2,4]triazolo-6(5H)-ones and 7-arylhydrazono-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. J. Chin. Chem. Soc. 2005, 52, 987–994. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Gomha, S.M.; Shawali, A.S.; Abdelhamid, O.A. Convenient method for the synthesis of various fused heterocycles via the utility of 4-acetyl-5-methyl-1-phenyl-pyrazole as a precursor. Turk. J. Chem. 2014, 38, 865–879. [Google Scholar] [CrossRef]
- Gomha, S.M.; El-Sayed, A.A.A.; Alrehaily, A.; Elbadawy, H.M.; Farag, B.; Al-Shahri, A.A.; Alsenani, S.R.; Abdelgawad, F.E.; Zaki, M.E.A. Synthesis, molecular docking, in silico study, and evaluation of bis-thiazole-based curcumin derivatives as potential antimicrobial agents. Res. Chem. 2024, 7, 101504. [Google Scholar] [CrossRef]
- Kurdi, A.A.; Morad, M.; Abumelha, H.M.; Alkhamis, K.; Aljohani, M.M.; Mersal, G.A.M.; El-Metwaly, N. Synthesis and characterization of novel VO(II) complexes from cyanoacetohydrazide-based ligands; Hirshfeld crystals, DFT study and biodiesel catalytic synthesis from waste frying oil. J. Mol. Struct. 2021, 1240, 130603. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelaziz, M.R.; Abdel-aziz, H.M.; Hassan, S.A. Green route synthesis and molecular docking of azines using cellulose sulfuric acid under microwave irradiation. Crystals 2023, 13, 260. [Google Scholar] [CrossRef]
- Abolibda, T.Z.; Albalawi, M.; Abdelaziz, M.R.; Abdel-aziz, H.M.; Gomha, S.M. Synthesis and molecular docking of some novel 3-thiazolyl-coumarins as inhibitors of VEGFR-2 kinase. Molecules 2023, 28, 689. [Google Scholar] [CrossRef] [PubMed]
- Caria, S.; Saka, Y.; Gervasio, F.L.; Tanaka, H. Structural analysis of phosphorylation-associated interactions of human MCC with Scribble PDZ domains. FEBS J. 2019, 286, 4910–4925. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; El-Gohary, N.S.; El-Messiry, M.A.; Mohamed, N.A.; Gomha, S.M. Mechanochemical synthesis and molecular docking studies of new azines bearing indole as anticancer agents. Molecules 2023, 28, 3869. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids 2019, 152, 108485. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Moda, T.L.; Bender, A.; Williams, A.J.; Ekins, S. PK/DB: Database for pharmacokinetic properties and predictive in silico ADME models. Bioinformatics 2008, 24, 2270–2271. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Sun, X.; Zhang, L.; Miao, R.; Zhang, H.; Jiang, H.; Wang, J. ADMET evaluation in drug discovery. 11. PharmacoKinetics Knowledge Base (PKKB): A comprehensive database of pharmacokinetic and toxic properties for drugs. J. Chem. Inf. Model. 2012, 52, 1132–1137. [Google Scholar] [CrossRef]
- Cumming, J.G.; Davis, A.M.; Muresan, S.; Haeberlein, M.; Chen, H. Chemical predictive modelling to improve compound quality. Nat. Rev. Drug Discov. 2013, 12, 948–962. [Google Scholar] [CrossRef]


| Compounds | Thermal Method | Grinding Method | ||
|---|---|---|---|---|
| Time (h) | (%) Yield | Time (min) | (%) Yield | |
| 5a | 5 | 75 | 25 | 90 |
| 5b | 4 | 74 | 27 | 89 |
| 5c | 3 | 79 | 21 | 92 |
| 5d | 4 | 73 | 26 | 91 |
| 5e | 3 | 78 | 20 | 89 |
| 7a | 5 | 72 | 29 | 84 |
| 7b | 5 | 70 | 30 | 85 |
| 9 | 4 | 78 | 27 | 91 |
| 11 | 5 | 77 | 23 | 90 |
| 13 | 4 | 79 | 28 | 90 |
| Entry | Catalyst (mol%) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | 1 | 25 | 25 | 71 |
| 2 | 3 | 25 | 25 | 83 |
| 3 a | 5 | 25 | 25 | 90 |
| 4 | 10 | 25 | 25 | 90 |
| State of Catalyst | New Catalyst | Recycled Catalyst | ||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | ||
| Yield of product 5a | 90 | 87 | 81 | 78 | 74 | 49 |
| Tested Compounds | IC50 Values (μM) | Tested Compounds | IC50 Values (μM) |
|---|---|---|---|
| 5a | 3.8 ± 0.7 | 7b | 37.2 ± 4.2 |
| 5b | 3.2 ± 1.1 | 9 | 9.3 ± 1.7 |
| 5c | 9.3 ± 1.4 | 11 | 2.5 ± 0.81 |
| 5d | 8.5 ± 1.3 | 13 | 3.7 ± 1.0 |
| 5e | 27.0 ± 2.5 | Cisplatin* | 2.43 ± 1.1 |
| 7a | 35.3 ± 3.1 |
| Compounds | Docking Score (kcal/mol) | No. of Hydrogen Bonding | No. of Arene Interaction | Donor Atom | Acceptor Atom |
|---|---|---|---|---|---|
| 5a | −5.75 | 1 (Gly739) 1 (Ile740) 1 (Arg733) 1 (Gly737) | 1 (π-H) [Gly739] | - | O N |
| 5b | −5.78 | 1 (Ile740) 2 (Gly737) 1 (Gly739) | - | - | O O/N O |
| 5c | −5.61 | 1 (Ile740) | 1 (π-H) [Leu738] 1 (π-Cation) [Arg801] | N | - |
| 5d | −5.72 | 1(Gly739) | 1 (π-H) [Gly739] | - | O |
| 11 | −6.40 | 1 (Ile740) 1 (Leu738) 1 (Arg733) 1 (Arg801) | - | N - - - | - O N N |
| CCL | −4.60 | 2 (Ile740) 1 (Gly739) 1 (Leu738) 1 (Arg733) | - | N/- - - - | -/O O O O |
| Compounds | Absorption | Distribution | Metabolism | Excretion (Log mL/min/kg) | Toxicity |
|---|---|---|---|---|---|
| Intestinal Absorption (Human) Numeric (% Absorbed) | VDss (Log L/kg) | AMES Toxicity (Categorical) (Yes/No) | |||
| 5a | 74.39 | −0.5 | CYP3A4 Substrate and CYP3A4 inhibitor | 0.25 | No |
| 5b | 65.21 | −0.33 | CYP1A2 inhibitor | 0.13 | No |
| 5c | 81.9 | −0.51 | CYP3A4 substrate; CYP1A2, CYP2C19, CYP2C9 and CYP3A4 inhibitor | −0.57 | No |
| 5d | 81.37 | −0.48 | CYP3A4 substrate; CYP2C19, CYP2C9 and CYP3A4 inhibitor | −0.61 | No |
| 11 | 83.02 | −0.04 | CYP2D6, CYP3A4 substrate; CYP1A2, CYP2C19, CYP2C9, and CYP3A4 inhibitor | 0.76 | No |
| Cisplatin * | 92.60 | 0.30 | - | 1.24 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomha, S.M.; Abolibda, T.Z.; Alruwaili, A.H.; Farag, B.; Boraie, W.E.; Al-Hussain, S.A.; Zaki, M.E.A.; Hussein, A.M. Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts 2024, 14, 489. https://doi.org/10.3390/catal14080489
Gomha SM, Abolibda TZ, Alruwaili AH, Farag B, Boraie WE, Al-Hussain SA, Zaki MEA, Hussein AM. Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts. 2024; 14(8):489. https://doi.org/10.3390/catal14080489
Chicago/Turabian StyleGomha, Sobhi M., Tariq Z. Abolibda, Awatif H. Alruwaili, Basant Farag, Waleed E. Boraie, Sami A. Al-Hussain, Magdi E. A. Zaki, and Ahmed M. Hussein. 2024. "Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies" Catalysts 14, no. 8: 489. https://doi.org/10.3390/catal14080489
APA StyleGomha, S. M., Abolibda, T. Z., Alruwaili, A. H., Farag, B., Boraie, W. E., Al-Hussain, S. A., Zaki, M. E. A., & Hussein, A. M. (2024). Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts, 14(8), 489. https://doi.org/10.3390/catal14080489










