Highly Efficient PtSn/Al2O3 and PtSnZnCa/Al2O3 Catalysts for Ethane Dehydrogenation: Influence of Catalyst Pretreatment Atmosphere
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Area Studies
2.2. XRD
2.3. XPS
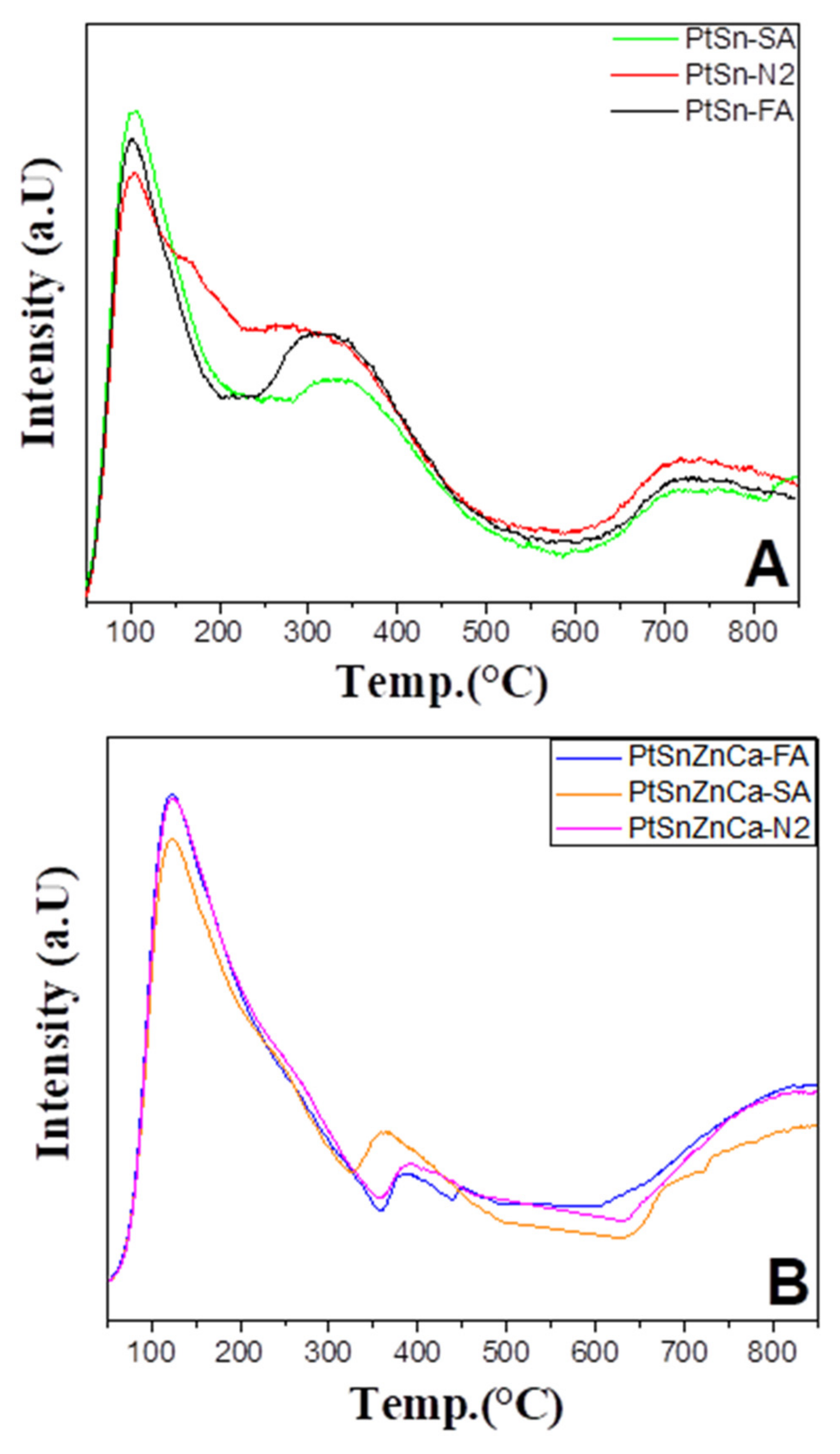
2.4. TPR
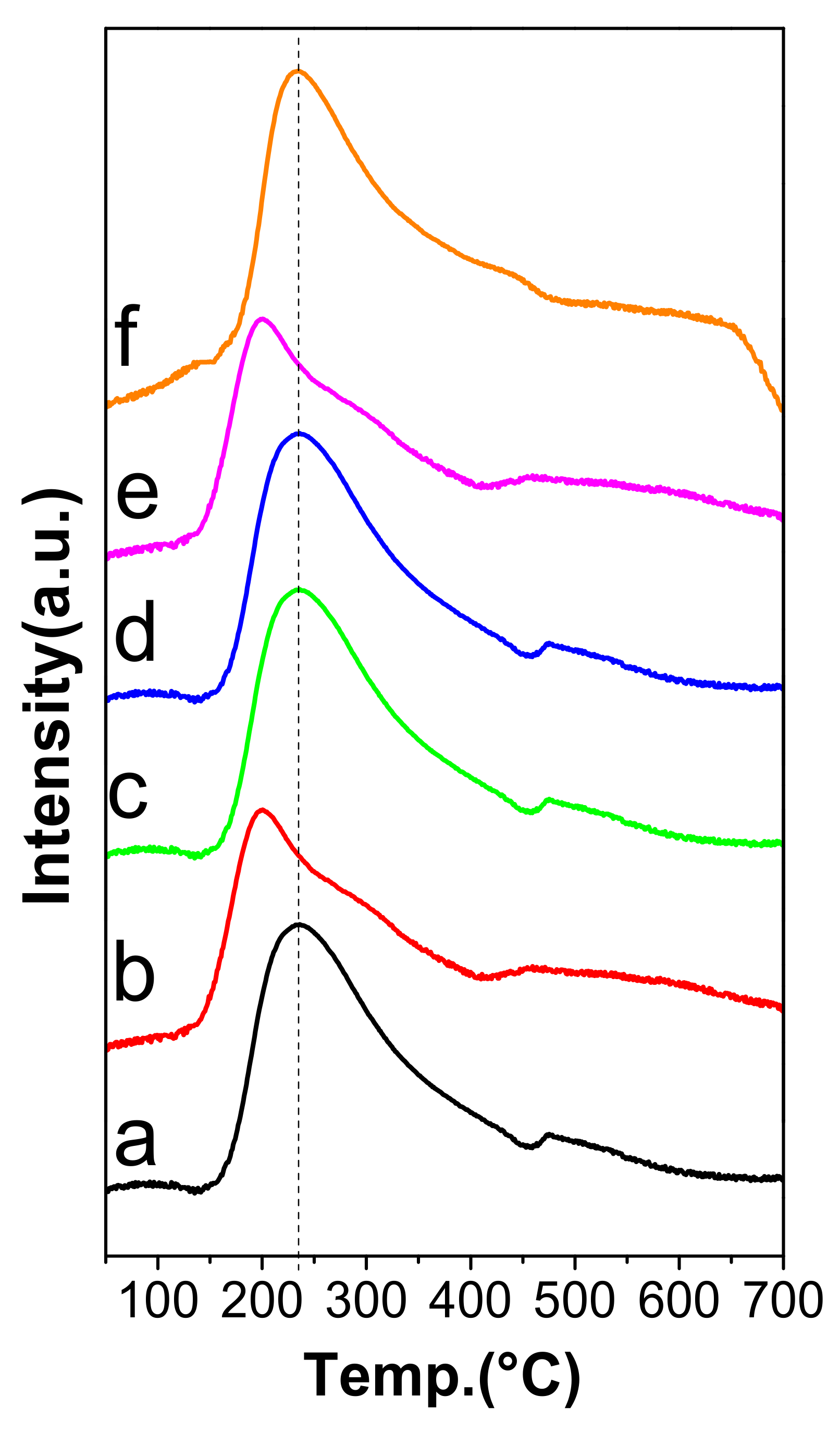
2.5. NH3-TPD
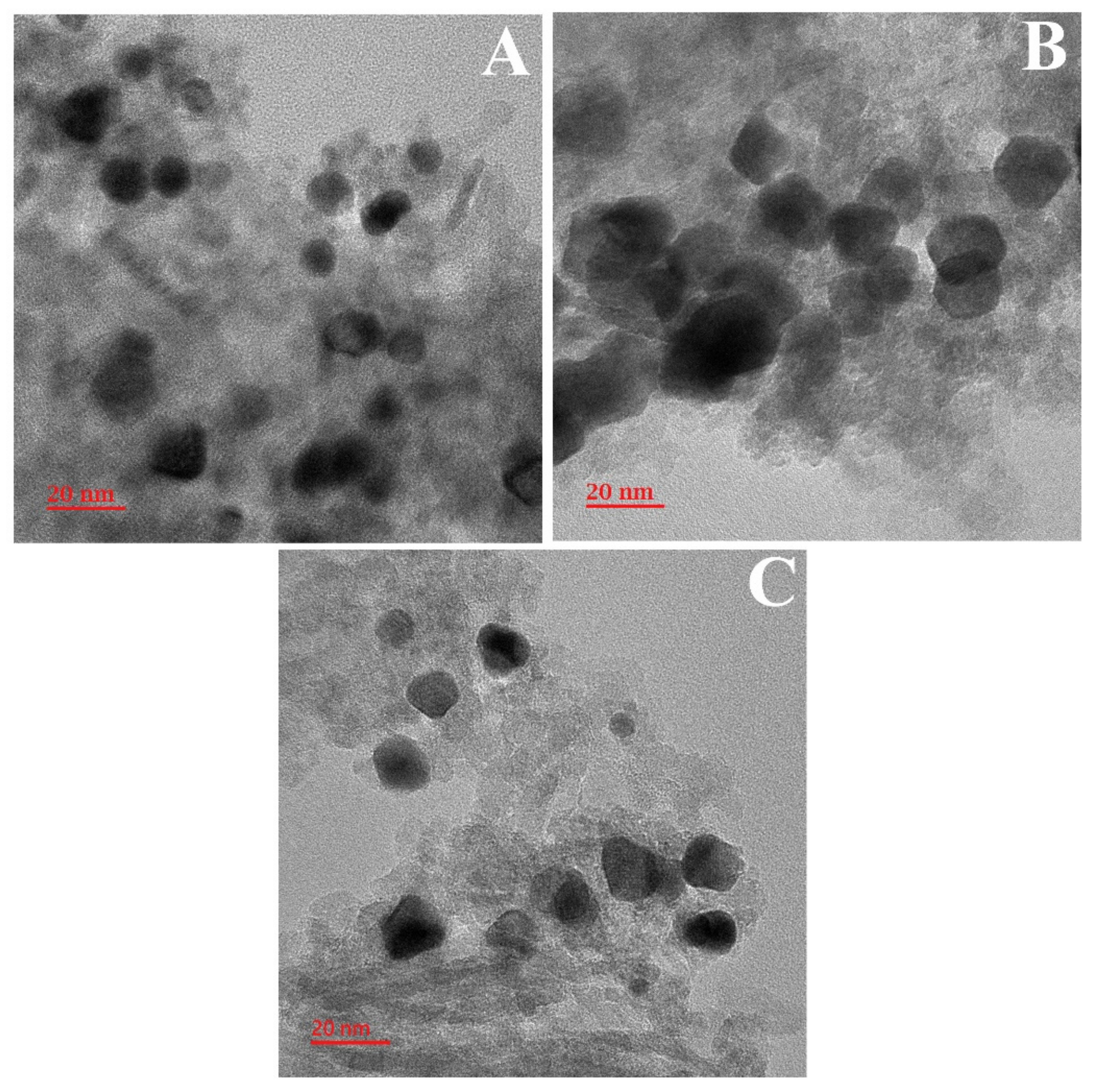
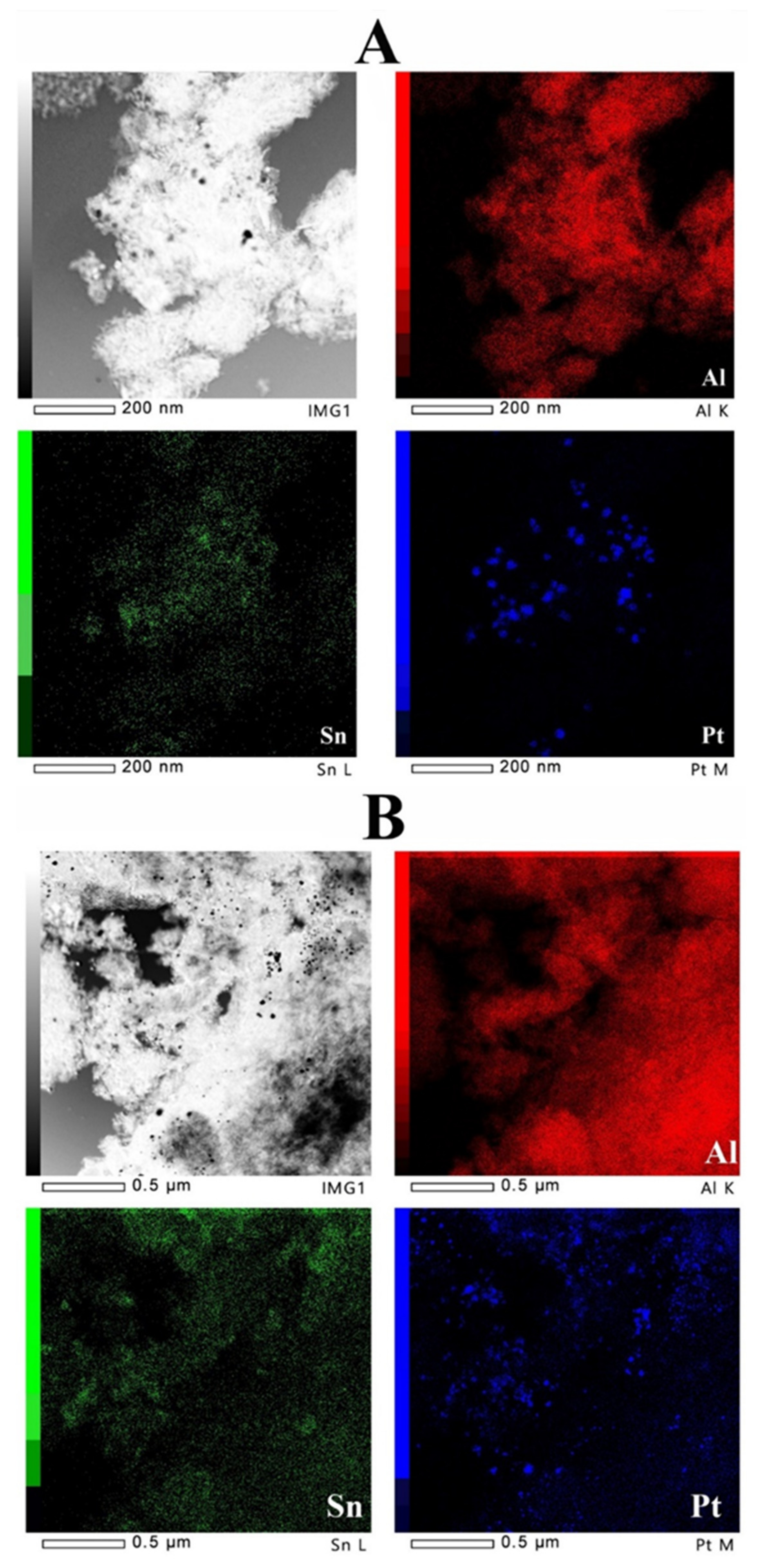
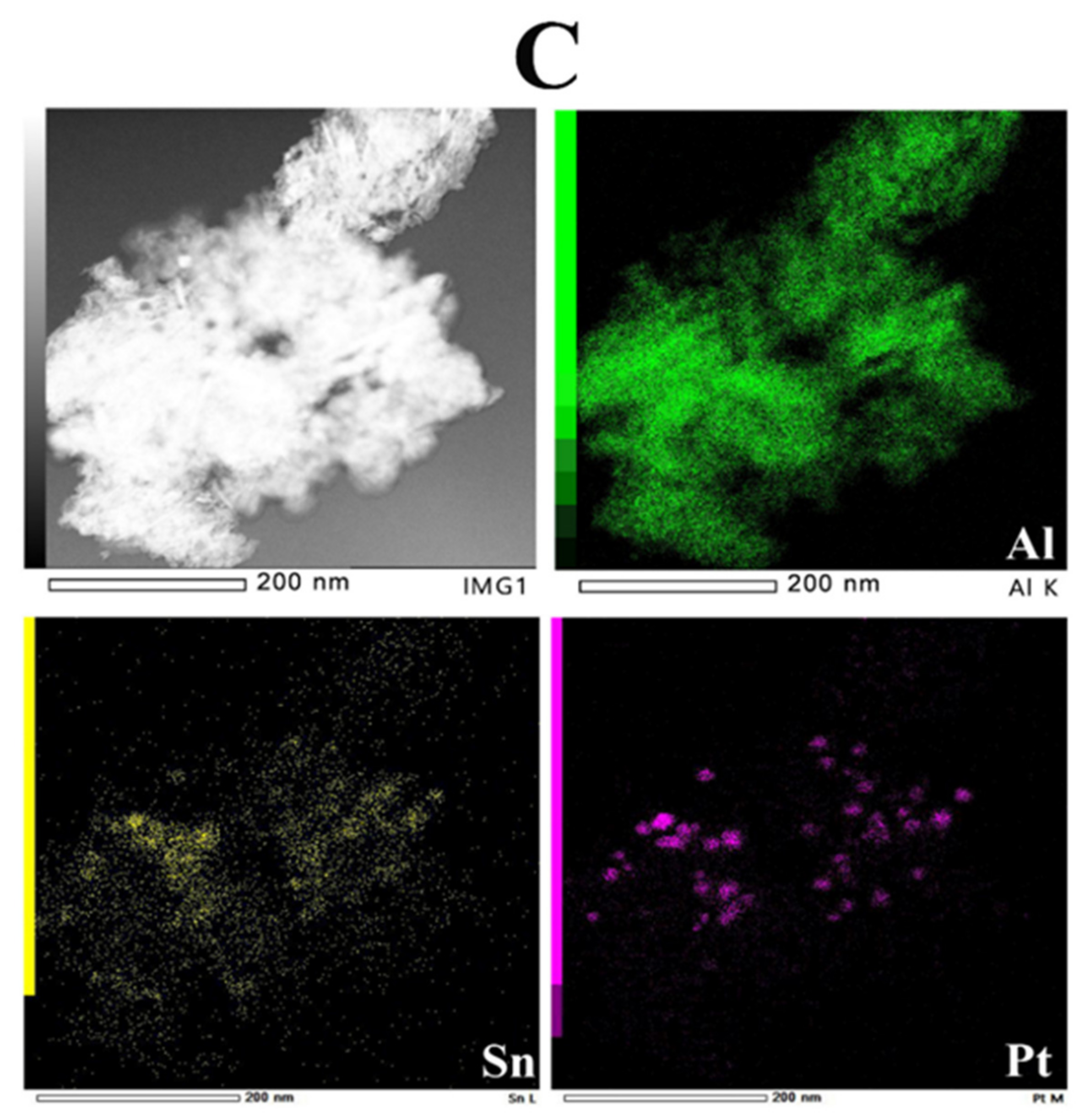
2.6. TEM Studies
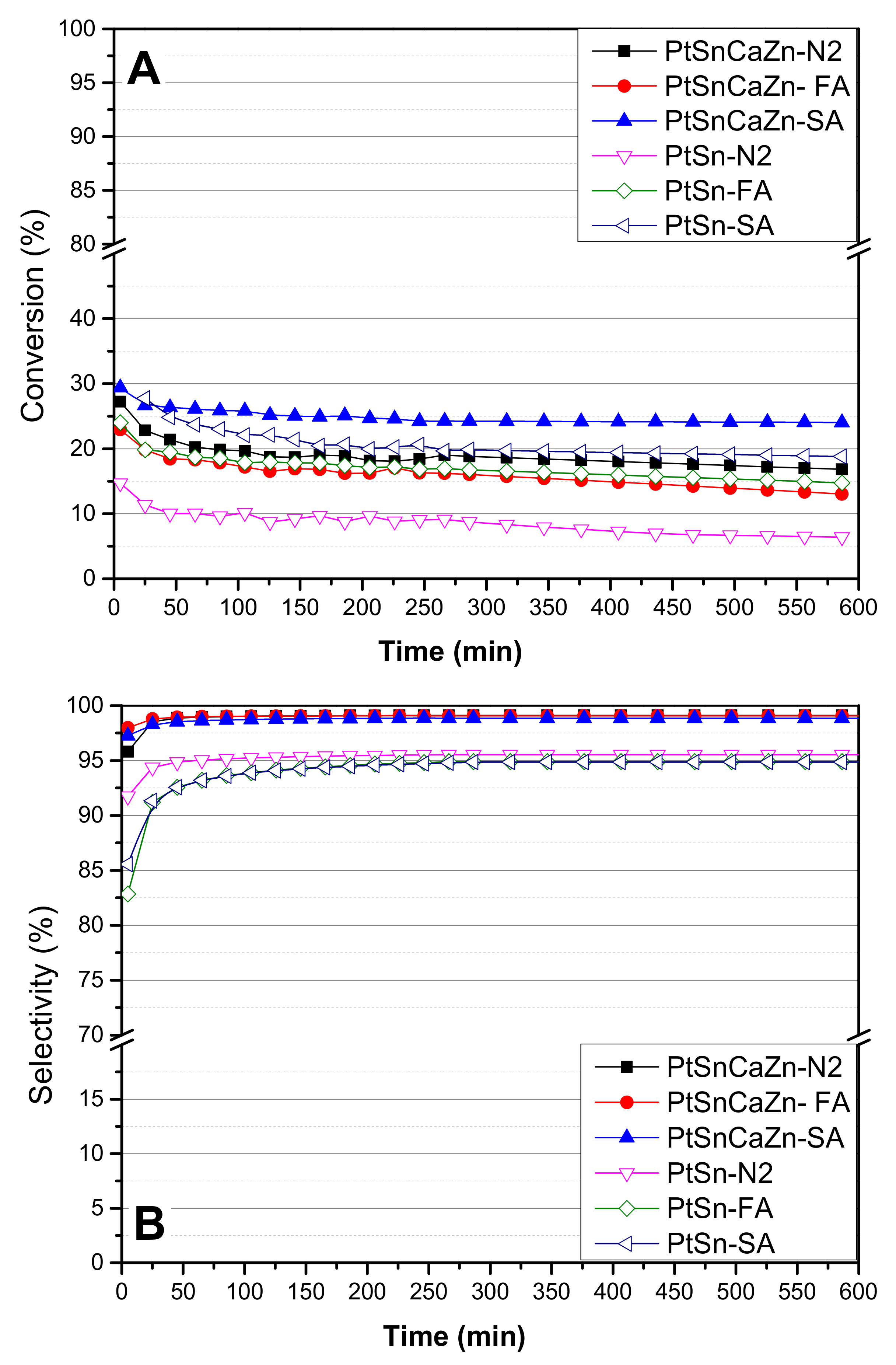
2.7. Activity
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Characterization
3.4. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zhou, Y.; Qiao, B.; Pan, X.; Wang, C.; Cao, L. Enhanced stability of Pt/Al2O3 modified by Zn promoter for catalytic dehydrogenation of ethane. J. Energy Chem. 2020, 51, 14–20. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, P.; Yang, J.; Zhu, Y.-A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peng, Z.; Bell, A.T. Effects of composition and metal particle size on ethane dehydrogenation over PtxSn100−x/Mg (Al) O (70 ≤ x ≤ 100). J. Catal. 2014, 311, 161–168. [Google Scholar] [CrossRef]
- Shee, D.; Sayari, A. Light alkane dehydrogenation over mesoporous Cr2O3/Al2O3 catalysts. Appl. Catal. A Gen. 2010, 389, 155–164. [Google Scholar] [CrossRef]
- Melnikov, D.; Novikov, A.; Glotov, A.; Reshetina, M.; Smirnova, E.; Wang, H. Dehydrogenation of Light Alkanes (A Review). Pet. Chem. 2022, 62, 1027–1046. [Google Scholar] [CrossRef]
- Wang, L.-C.; Zhang, Y.; Xu, J.; Diao, W.; Karakalos, S.; Liu, B. Non-oxidative dehydrogenation of ethane to ethylene over ZSM-5 zeolite supported iron catalysts. Appl. Catal. B Environ. 2019, 256, 117816. [Google Scholar] [CrossRef]
- Iizuka, T.; Miura, T.; Sano, M.; Hayashi, T.; Hanaya, M.; Miyake, T. Dehydrogenation of ethane to ethylene on Pt/zincosilicate. Catal. Today 2023, 410, 231–236. [Google Scholar] [CrossRef]
- Guo, H.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Cobaltous oxide supported on MFI zeolite as an efficient ethane dehydrogenation catalyst. Microporous Mesoporous Mater. 2021, 312, 110791. [Google Scholar] [CrossRef]
- Shin, H.H.; McIntosh, S. Proton-conducting perovskites as supports for Cr catalysts in short contact time ethane dehydrogenation. ACS Catal. 2015, 5, 95–103. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Beale, A.M.; Maaijen, K.; Weng, T.C.; Glatzel, P.; Weckhuysen, B.M. A combined in situ time-resolved UV–Vis, Raman and high-energy resolution X-ray absorption spectroscopy study on the deactivation behavior of Pt and PtSn propane dehydrogenation catalysts under industrial reaction conditions. J. Catal. 2010, 276, 268–279. [Google Scholar] [CrossRef]
- Pham, H.N.; Sattler, J.J.; Weckhuysen, B.M.; Datye, A.K. Role of Sn in the regeneration of Pt/γ-Al2O3 light alkane dehydrogenation catalysts. ACS Catal. 2016, 6, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Wegener, E.C.; Wu, Z.; Tseng, H.-T.; Gallagher, J.R.; Ren, Y.; Diaz, R.E. Structure and reactivity of Pt–In intermetallic alloy nanoparticles: Highly selective catalysts for ethane dehydrogenation. Catal. Today 2018, 299, 146–153. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Zhang, S.; Liu, Q.; Chen, L.; Li, X. Ga3+-stabilized Pt in PtSn-Mg (Ga)(Al) O catalyst for promoting ethane dehydrogenation. J. Catal. 2018, 368, 79–88. [Google Scholar] [CrossRef]
- Searles, K.; Chan, K.W.; Mendes Burak, J.A.; Zemlyanov, D.; Safonova, O.; Copéret, C. Highly productive propane dehydrogenation catalyst using silica-supported Ga–Pt nanoparticles generated from single-sites. J. Am. Chem. Soc. 2018, 140, 11674–11679. [Google Scholar] [CrossRef]
- Wu, J.; Sharada, S.M.; Ho, C.; Hauser, A.W.; Head-Gordon, M.; Bell, A.T. Ethane and propane dehydrogenation over PtIr/Mg (Al) O. Appl. Catal. A Gen. 2015, 506, 25–32. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.; Wimble, J.M.; Lucci, F.R.; Lee, S. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat. Chem 2018, 10, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.-Q.; Pei, G.-X.; Ren, Y.-J.; Liu, K.-P.; Chen, Z.-Q.; Yang, J.-Y. Effect of group IB metals on the dehydrogenation of propane to propylene over anti-sintering Pt/MgAl2O4. J. Catal. 2018, 366, 115–126. [Google Scholar] [CrossRef]
- Ali, A.M.; Zahrani, A.A.; Daous, M.A.; Podila, S.; Alshehri, M.K.; Rather, S.-U. Sequential and/or Simultaneous Wet-Impregnation Impact on the Mesoporous Pt/Sn/Zn/γ-Al2O3 Catalysts for the Direct Ethane Dehydrogenation. J. Nanomater. 2022, 2022, 8739993. [Google Scholar] [CrossRef]
- Hook, A.; Massa, J.D.; Celik, F.E. Effect of tin coverage on selectivity for ethane dehydrogenation over platinum–tin alloys. J. Phys. Chem. C 2016, 120, 27307–27318. [Google Scholar] [CrossRef]
- Liu, Y.; Zong, X.; Patra, A.; Caratzoulas, S.; Vlachos, D.G. Propane Dehydrogenation on PtxSny (x, y ≤ 4) Clusters on Al2O3(110). ACS Catal. 2023, 13, 2802–2812. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, Y.; Liu, Y.; Zhang, Y. The state of Pt active phase and its surrounding environment during dehydrogenation of ethane to ethylene. Appl. Surf. Sci. 2021, 554, 149611. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.-W.; Kim, D.H.; Peden, C.H. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef]
- Naseri, M.; Tahriri Zangeneh, F.; Taeb, A. Effects of Mg, Ca, and K Addition on Pt-Sn/γ-Al2O3 for Propane Dehydrogenation. Iran. J. Chem. Chem. Eng. 2022, 41, 1921–1931. [Google Scholar]
- Siri, G.J.; Bertolini, G.R.; Casella, M.L.; Ferretti, O.A. PtSn/γ-Al2O3 isobutane dehydrogenation catalysts: The effect of alkaline metals addition. Mater. Lett. 2005, 59, 2319–2324. [Google Scholar] [CrossRef]
- Long, L.-L.; Lang, W.-Z.; Liu, X.; Hu, C.-L.; Chu, L.-F.; Guo, Y.-J. Improved catalytic stability of PtSnIn/xCa–Al catalysts for propane dehydrogenation to propylene. Chem. Eng. J. 2014, 257, 209–217. [Google Scholar] [CrossRef]
- Huizinga, T.; Blik, H.v.T.; Vis, J.; Prins, R. XPS investigations of Pt and Rh supported on γ-Al2O3 and TiO2. Surf Sci. 1983, 135, 580–596. [Google Scholar] [CrossRef]
- Rimaz, S.; Chen, L.; Kawi, S.; Borgna, A. Promoting effect of Ge on Pt-based catalysts for dehydrogenation of propane to propylene. Appl. Catal. A Gen. 2019, 588, 117266. [Google Scholar] [CrossRef]
- Virnovskaia, A.; Jørgensen, S.; Hafizovic, J.; Prytz, Ø.; Kleimenov, E.; Hävecker, M. In situ XPS investigation of Pt (Sn)/Mg (Al) O catalysts during ethane dehydrogenation experiments. Surf. Sci. 2007, 601, 30–43. [Google Scholar] [CrossRef]
- Seo, H.; Lee, J.K.; Hong, U.G.; Park, G.; Yoo, Y.; Lee, J. Direct dehydrogenation of n-butane over Pt/Sn/M/γ-Al2O3 catalysts: Effect of third metal (M) addition. Catal. Commun. 2014, 47, 22–27. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, Y.; Zhang, Y.; Liu, H.; Sheng, X.; Duan, Y. Effect of calcination atmosphere on the catalytic properties of PtSnNaMg/ZSM-5 for propane dehydrogenation. Catal. Commun. 2009, 10, 2013–2017. [Google Scholar] [CrossRef]
- Bocanegra, S.A.; Zgolicz, P.D.; Scelza, O.A.; de Miguel, S.R. New trimetallic catalysts supported on coprecipitated MgAl2O4 for n-paraffins selective dehydrogenation processes. Catal. Commun. 2009, 10, 1463–1466. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, J.; Jeong, H.Y.; Kwak, J.H. Controlling the acid-base properties of alumina for stable PtSn-based propane dehydrogenation catalysts. Appl. Catal. A Gen. 2019, 572, 1–8. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, K.; Wang, C.; Ma, L.; Zhang, Q.; Liu, Q. The properties and catalytic performance of PtSn/Mg (x-Ga) AlO catalysts for ethane dehydrogenation. RSC Adv. 2017, 7, 22836–22844. [Google Scholar] [CrossRef]
- Sattler, J.J.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Garidzirai, R.; Modisha, P.; Shuro, I.; Visagie, J.; van Helden, P.; Bessarabov, D. The effect of Mg and Zn dopants on Pt/Al2O3 for the dehydrogenation of perhydrodibenzyltoluene. Catalysts 2021, 11, 490. [Google Scholar] [CrossRef]
- Housmans, T.H.M.; Wonders, A.H.; Koper, M.T.M. Structure Sensitivity of Methanol Electrooxidation Pathways on Platinum: An On-Line Electrochemical Mass Spectrometry Study. J. Phys. Chem. B 2006, 110, 10021–10031. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Meng, Y. The Fabrication of Pt/Co Nanocomposite Supported on Reduced Graphene Oxide for Methanol Oxidation. Int. J. Electrochem. Sci. 2019, 14, 6826–6839. [Google Scholar] [CrossRef]
- Lee, M.-H.; Nagaraja, B.M.; Lee, K.Y.; Jung, K.-D. Dehydrogenation of alkane to light olefin over PtSn/θ-Al2O3 catalyst: Effects of Sn loading. Catal. Today 2014, 232, 53–62. [Google Scholar] [CrossRef]
- Galvita, V.; Siddiqi, G.; Sun, P.; Bell, A.T. Ethane dehydrogenation on Pt/Mg (Al) O and PtSn/Mg (Al) O catalysts. J. Catal. 2010, 271, 209–219. [Google Scholar] [CrossRef]
- Lin, L.; Yang, W.; Jia, J.; Xu, Z.; Zhang, T.; Fan, Y. Surface structure and reaction performances of highly dispersed and supported bimetallic catalysts. Sci. China Ser. B Chem. 1999, 42, 571–580. [Google Scholar] [CrossRef][Green Version]
- Borges, L.R.; Lopez-Castillo, A.; Meira, D.M.; Gallo, J.M.R.; Zanchet, D.; Bueno, J.M.C. Effect of the Pt precursor and loading on the structural parameters and catalytic properties of Pt/Al2O3. ChemCatChem 2019, 11, 3064–3074. [Google Scholar] [CrossRef]
- Marceau, E.; Lauron-Pernot, H.; Che, M. Influence of the metallic precursor and of the catalytic reaction on the activity and evolution of Pt (Cl)/δ-Al2O3 catalysts in the total oxidation of methane. J. Catal. 2001, 197, 394–405. [Google Scholar] [CrossRef]
- Borg, Ø.; Blekkan, E.A.; Eri, S.; Akporiaye, D.; Vigerust, B.; Rytter, E. Effect of calcination atmosphere and temperature on γ-Al2O3 supported cobalt Fischer-Tropsch catalysts. Top. Catal. 2007, 45, 39–43. [Google Scholar] [CrossRef]
- Cybulskis, V.J.; Bukowski, B.C.; Tseng, H.-T.; Gallagher, J.R.; Wu, Z.; Wegener, E. Zinc promotion of platinum for catalytic light alkane dehydrogenation: Insights into geometric and electronic effects. ACS Catal. 2017, 7, 4173–4181. [Google Scholar] [CrossRef]
- Siddiqi, G.; Sun, P.; Galvita, V.; Bell, A.T. Catalyst performance of novel Pt/Mg (Ga)(Al) O catalysts for alkane dehydrogenation. J. Catal. 2010, 274, 200–206. [Google Scholar] [CrossRef]
- Wu, Z.; Wegener, E.C.; Tseng, H.-T.; Gallagher, J.R.; Harris, J.W.; Diaz, R.E. Pd–In intermetallic alloy nanoparticles: Highly selective ethane dehydrogenation catalysts. Catal. Sci. Technol. 2016, 6, 6965–6976. [Google Scholar] [CrossRef]
- Ji, Z.; Lv, H.; Pan, X.; Bao, X. Enhanced ethylene selectivity and stability of Mo/ZSM5 upon modification with phosphorus in ethane dehydrogenation. J. Catal. 2018, 361, 94–104. [Google Scholar] [CrossRef]
- Rioux, R.; Song, H.; Hoefelmeyer, J.; Yang, P.; Somorjai, G. High-surface-area catalyst design: Synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J. Phys. Chem. B 2005, 109, 2192–2202. [Google Scholar] [CrossRef]
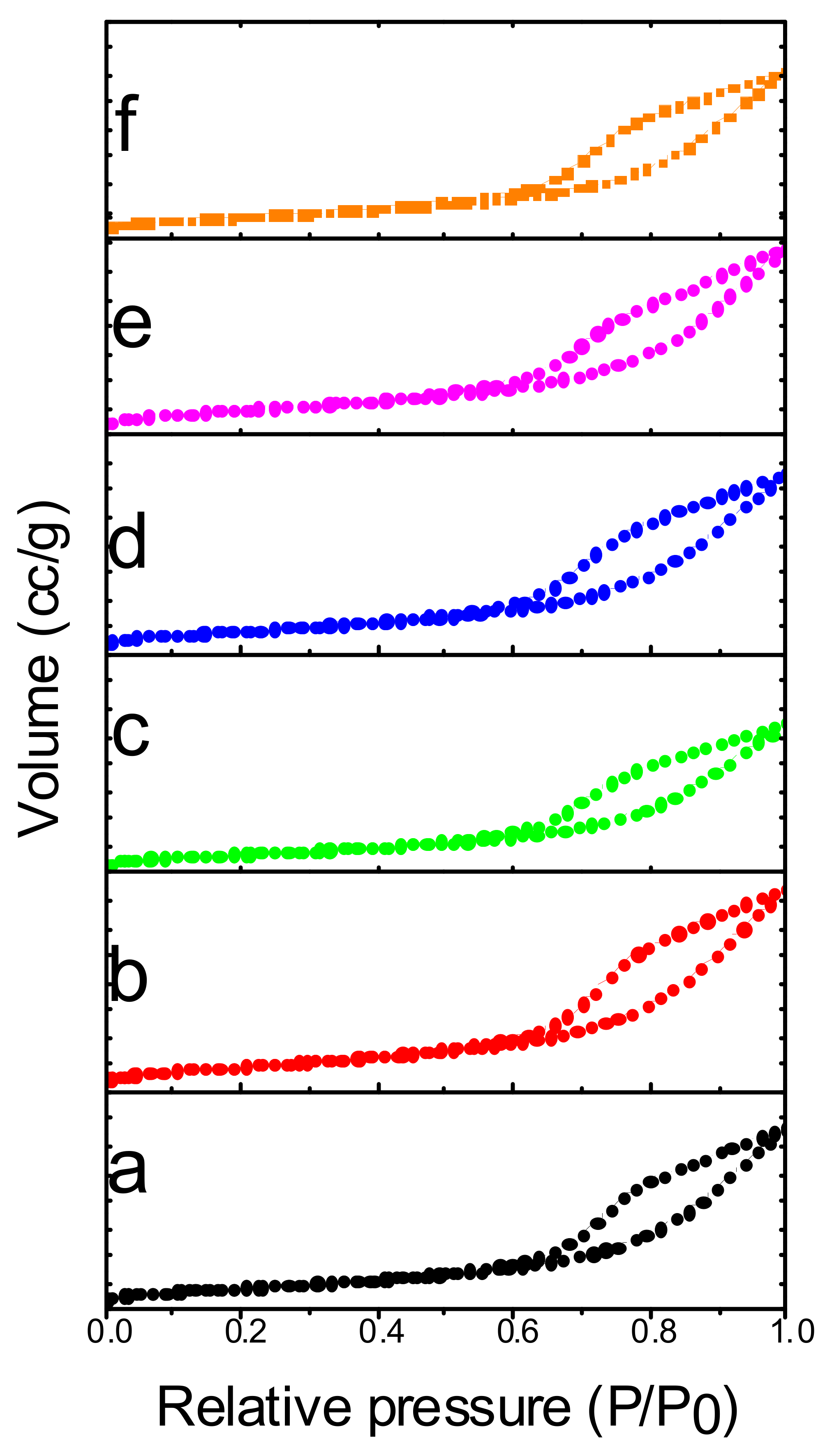
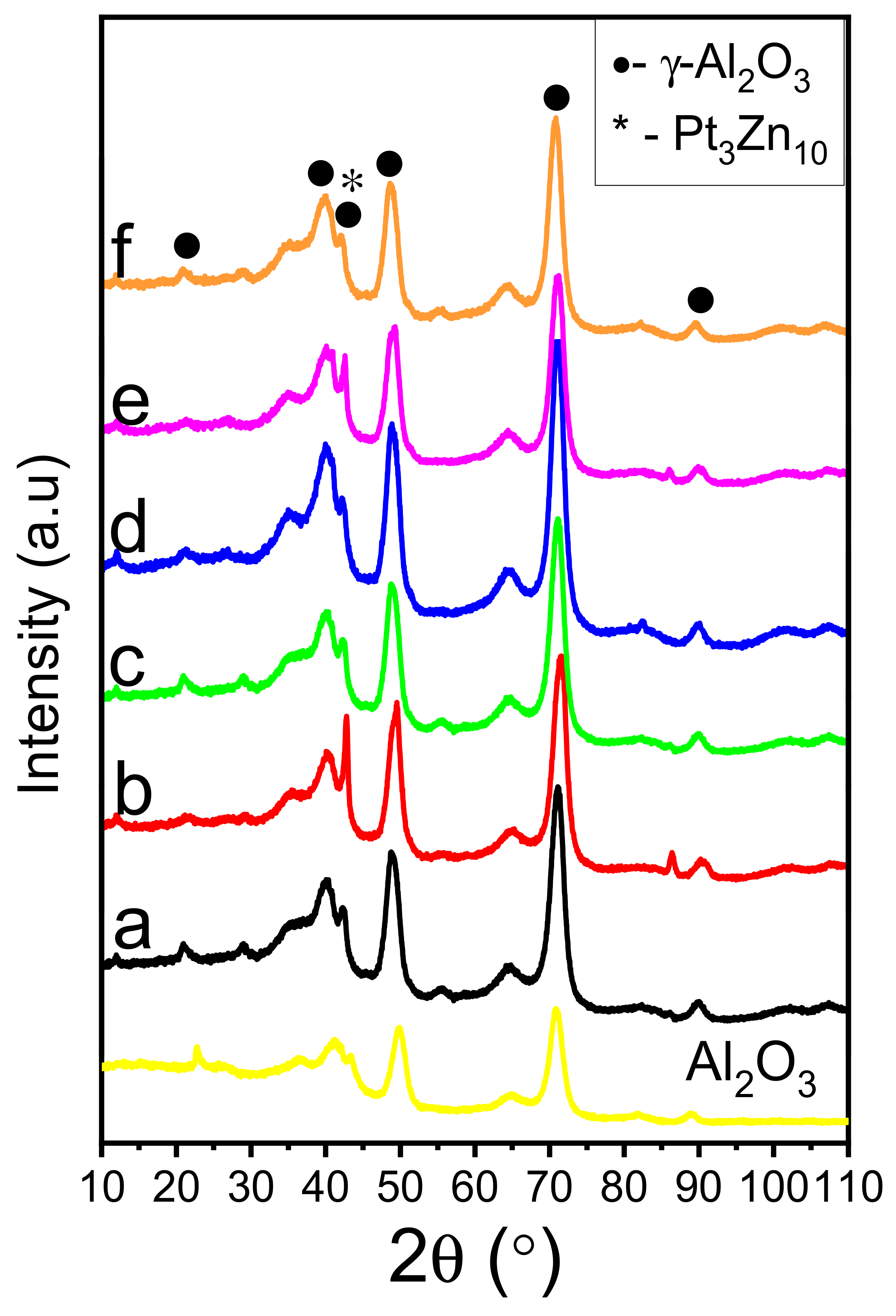
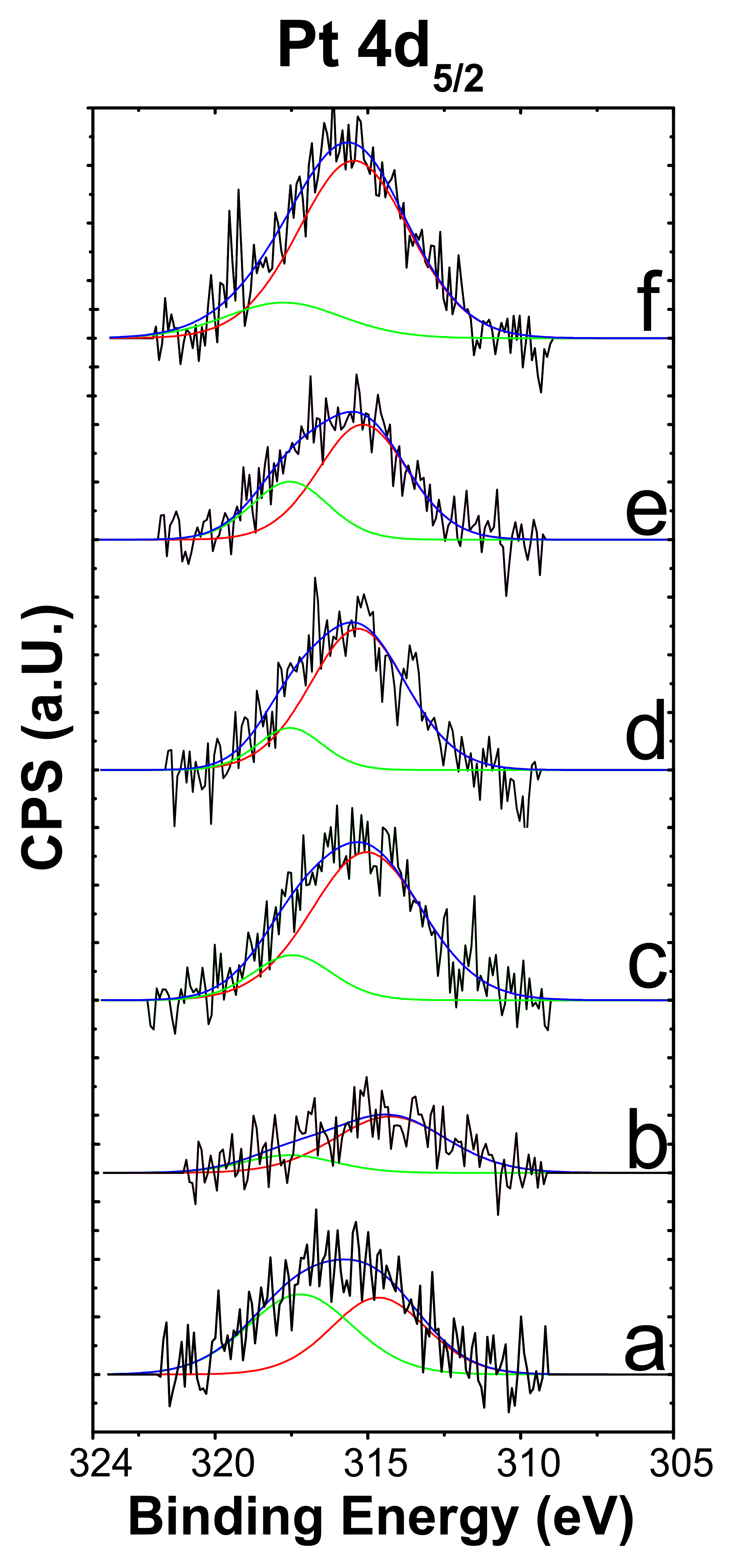
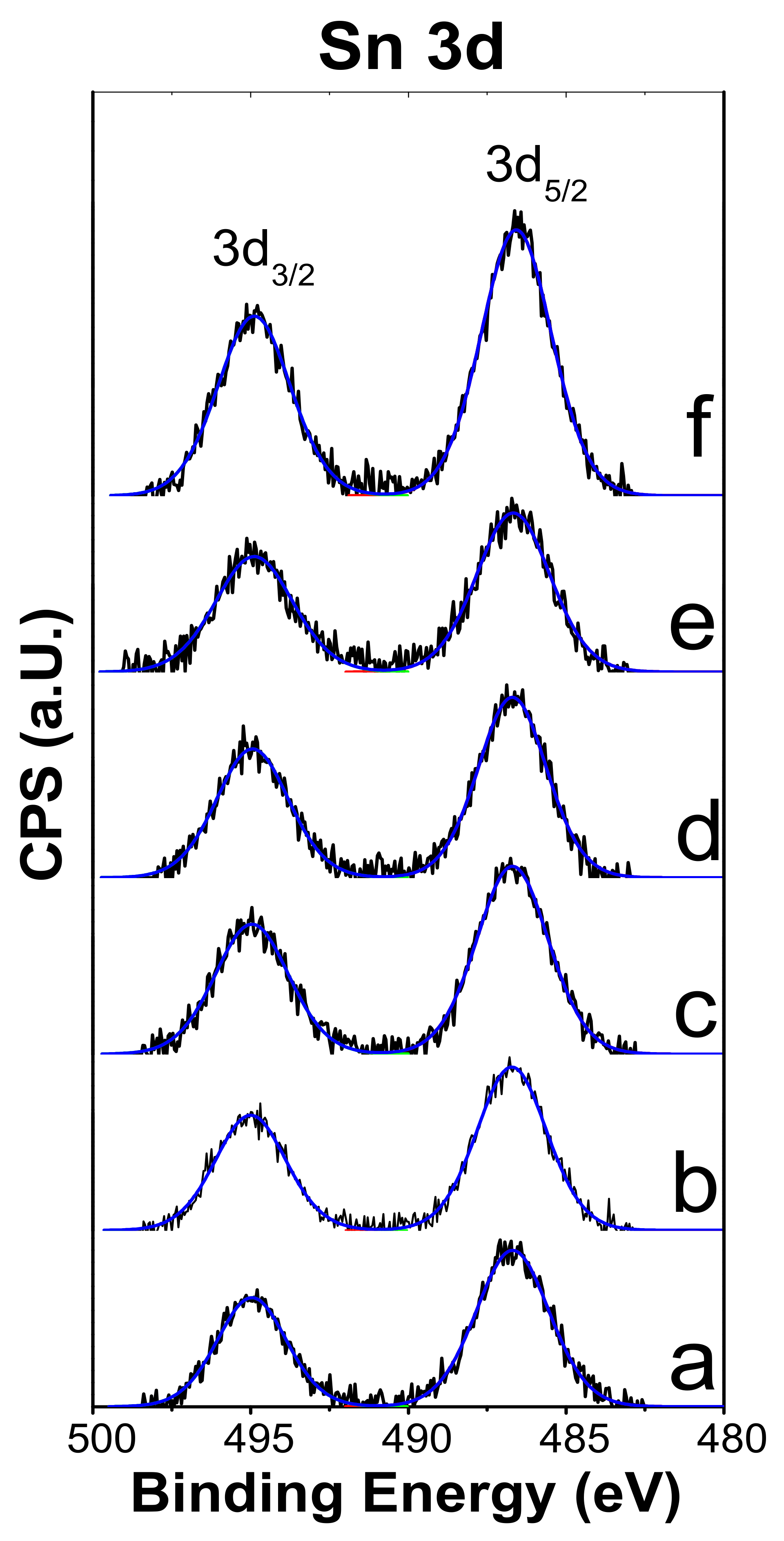


| Catalyst | Surface Area (m2/g) | H2 Consumption (µmol g−1) a | Total Acidity (µmol g−1) | |
|---|---|---|---|---|
| <125 °C | >340 °C | |||
| Al2O3 | 186.7 | - | 300.1 | 228.8 |
| PtSn-N2 | 179.8 | 18.7 | 159.8 | 78.4 |
| PtSn-SA | 134.7 | 29.9 | 137.7 | 67.1 |
| PtSn-FA | 159.5 | 25.4 | 160.5 | 99.6 |
| PtSnZnCa-N2 | 178.4 | 17.8 | 195.1 | 47.0 |
| PtSnZnCa-SA | 151.9 | 29.2 | 176.8 | 28.1 |
| PtSnZnCa-FA | 165.0 | 24.1 | 200.6 | 35.2 |
| Catalyst | Al 2s | Pt 4d5/2 | Sn 3d5/2 | O 1s | Cl 2p | Ca 2p | Zn 2p3/2 |
|---|---|---|---|---|---|---|---|
| PtSn-FA | 29.31 | 0.051 | 0.285 | 69.597 | 0.757 | - | - |
| PtSn-N2 | 30.26 | 0.023 | 0.254 | 68.726 | 0.734 | - | - |
| PtSn-SA | 29.96 | 0.057 | 0.297 | 68.872 | 0.812 | - | - |
| PtSnZnCa-FA | 29.54 | 0.049 | 0.295 | 67.992 | 1.24 | 0.631 | 0.259 |
| PtSnZnCa-N2 | 28.48 | 0.049 | 0.298 | 69.181 | 1.11 | 0.578 | 0.306 |
| PtSnZnCa-SA | 30.20 | 0.062 | 0.383 | 67.609 | 0.888 | 0.593 | 0.262 |
| Catalyst | Metal Loading (wt%) | Temp. (°C) | WHSV a (h−1) | Conver. (%) | Select. (%) | kd (h−1) | Reaction Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PtSn-N2 | 1 | 625 | 0.8 | 6.4 | 95.5 | 0.033 | 10 | This study |
| PtSn-SA | 1 | 625 | 0.8 | 19.0 | 95.0 | 0.02 | 10 | This study |
| PtSn-FA | 1 | 625 | 0.8 | 15.0 | 95.0 | 0.023 | 10 | This study |
| PtSnZnCa-N2 | 1 | 625 | 0.8 | 17.0 | 99.1 | 0.013 | 10 | This study |
| PtSnZnCa-SA | 1 | 625 | 0.8 | 24.0 | 99.0 | 0.006 | 10 | This study |
| PtSnZnCa-FA | 1 | 625 | 0.8 | 13.0 | 99.1 | 0.028 | 10 | This study |
| PtZn2/Al2O3 | 0.2 | 600 | 1.2 | 19.0 | 98.0 | 0.003 | 70 | [1] |
| PtZn/SiO2 | 9.7 | 600 | - | - | 100.0 | 0.085 | 18 | [44] |
| PtSn/Mg(Ga)(Al)O | 0.5 | 550 | - | 25.0 | 100.0 | 0.144 | 2.2 | [13] |
| Pt3Ir/Mg(Al)O | 1.9 | 600 | 3.4 | 15.0 | 95.0 | 0.649 | 0.5 | [15] |
| PtSn/Mg(Al)O | 0.8 | 600 | 12.5 | 20.0 | 99.0 | 0.862 | 1.7 | [39] |
| Pt/Mg(Ga)(Al)O | 1 | 600 | 39 | 8.0 | 100.0 | 0.013 | 2 | [45] |
| Pt/Zinc silicate | 1 | 550 | 9.6 | 14.0 | 100.0 | 0.00 | 3 | [7] |
| PdIn/SiO2 | 2 Pd | 600 | 0.2 | 15.0 | 100.0 | 0.193 | 5.8 | [46] |
| P-Mo/ZSM5 | 4.7 Mo | 600 | 0.67 | 20.0 | 70.0 | 0.876 | 4.3 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podila, S.; Al-Zahrani, A.A.; Daous, M.A.; Alhumade, H. Highly Efficient PtSn/Al2O3 and PtSnZnCa/Al2O3 Catalysts for Ethane Dehydrogenation: Influence of Catalyst Pretreatment Atmosphere. Catalysts 2024, 14, 312. https://doi.org/10.3390/catal14050312
Podila S, Al-Zahrani AA, Daous MA, Alhumade H. Highly Efficient PtSn/Al2O3 and PtSnZnCa/Al2O3 Catalysts for Ethane Dehydrogenation: Influence of Catalyst Pretreatment Atmosphere. Catalysts. 2024; 14(5):312. https://doi.org/10.3390/catal14050312
Chicago/Turabian StylePodila, Seetharamulu, Abdulrahim A. Al-Zahrani, Muhammad A. Daous, and Hesham Alhumade. 2024. "Highly Efficient PtSn/Al2O3 and PtSnZnCa/Al2O3 Catalysts for Ethane Dehydrogenation: Influence of Catalyst Pretreatment Atmosphere" Catalysts 14, no. 5: 312. https://doi.org/10.3390/catal14050312
APA StylePodila, S., Al-Zahrani, A. A., Daous, M. A., & Alhumade, H. (2024). Highly Efficient PtSn/Al2O3 and PtSnZnCa/Al2O3 Catalysts for Ethane Dehydrogenation: Influence of Catalyst Pretreatment Atmosphere. Catalysts, 14(5), 312. https://doi.org/10.3390/catal14050312







