Investigating the Catalytic Deactivation of a Pd Catalyst during the Continuous Hydrogenation of CO2 into Formate Using a Trickle-Bed Reactor
Abstract
1. Introduction
2. Results and Discussion
2.1. Continuous Hydrogenation of CO2 to Formate over Trickle-Bed Reactor
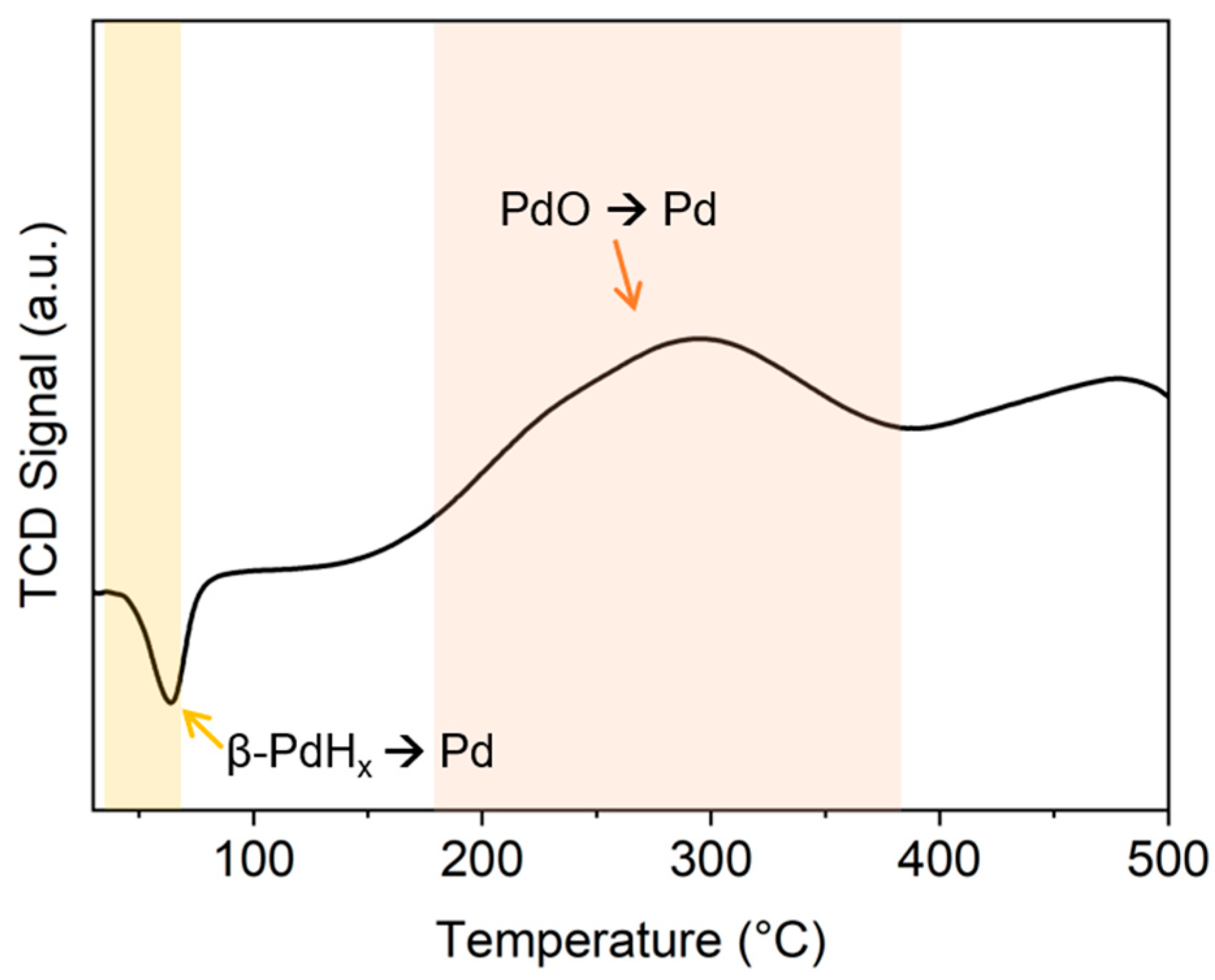
2.1.1. Preliminary Analysis on the Fresh Pd/AC Catalyst
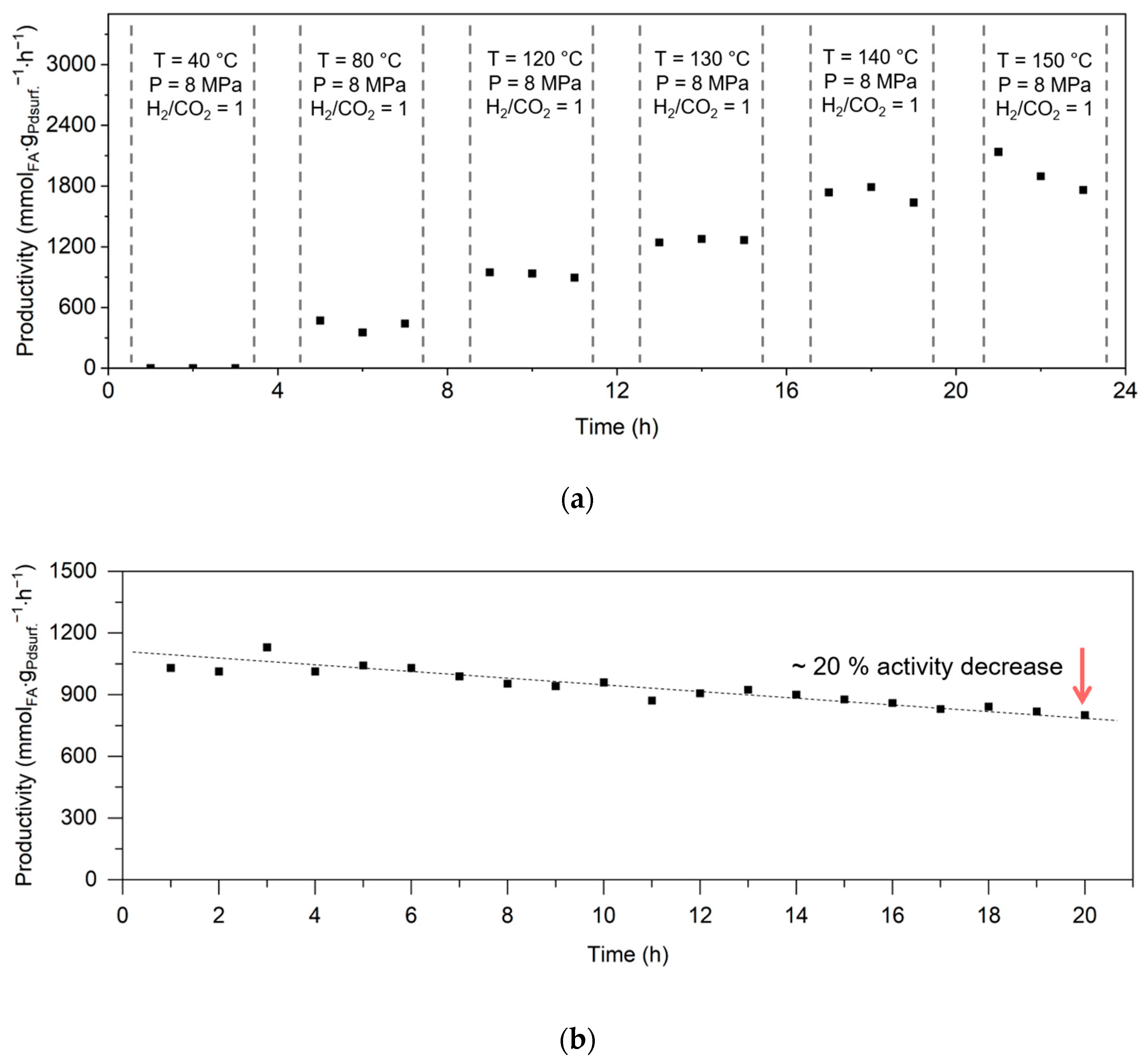
2.1.2. Dynamic Operation with a Variation of Reaction Temperature
2.2. Comprehensive Characterizations by the Comparison of Fresh and Spent Catalysts
2.2.1. N2 Adsorption and Desorption Analysis
2.2.2. Transmittance Electron Microscope Analysis
2.2.3. X-ray Diffraction Analysis
2.2.4. X-ray Photoelectron Spectroscopy Analysis
2.2.5. X-ray Absorption Spectroscopy Analysis
3. Materials and Methods
3.1. Materials
3.2. Characterization Methods
3.3. Continuous Hydrogenation of CO2 to Formate over Trickle-Bed Reactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous catalysis for sustainable hydrogen storage in formic acid and alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo-and electrochemical CO2 reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; von Langermann, J.; Schmidt, T. Carbon dioxide and formic acid—The couple for environmental-friendly hydrogen storage? Energy Environ. Sci. 2010, 3, 1207–1217. [Google Scholar] [CrossRef]
- Loges, B.; Boddien, A.; Gärtner, F.; Junge, H.; Beller, M. Catalytic generation of hydrogen from formic acid and its derivatives: Useful hydrogen storage materials. Top. Catal. 2010, 53, 902–914. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source–recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material–development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrogen Energy 2019, 44, 28533–28541. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Harrison, G.; Tzimas, E. Formic acid synthesis using CO2 as raw material: Techno-economic and environmental evaluation and market potential. Int. J. Hydrogen Energy 2016, 41, 16444–16462. [Google Scholar] [CrossRef]
- Sternberg, A.; Jens, C.M.; Bardow, A. Life cycle assessment of CO2-based C1-chemicals. Green Chem. 2017, 19, 2244–2259. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, J.; Roh, K.; Heo, S.; Lee, U.; Lee, J.H. Risk-based uncertainty assessment to identify key sustainability hurdles for emerging CO 2 utilization technologies. Green Chem. 2022, 24, 4588–4605. [Google Scholar] [CrossRef]
- Kim, C.; Lee, Y.; Kim, K.; Lee, U. Implementation of Formic Acid as a Liquid Organic Hydrogen Carrier (LOHC): Techno-Economic Analysis and Life Cycle Assessment of Formic Acid Produced via CO2 Utilization. Catalysts 2022, 12, 1113. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y.; Murphy, E.; Ly, A.; Xu, M.; Matanovic, I.; Pan, X.; Atanassov, P. Robust palladium hydride catalyst for electrocatalytic formate formation with high CO tolerance. Appl. Catal. B-Environ. 2022, 316, 121659. [Google Scholar] [CrossRef]
- Koolen, C.D.; Luo, W.; Züttel, A. From single crystal to single atom catalysts: Structural factors influencing the performance of metal catalysts for CO2 electroreduction. ACS Catal. 2022, 13, 948–973. [Google Scholar] [CrossRef]
- Zhang, J.; Pham, T.H.M.; Ko, Y.; Li, M.; Yang, S.; Koolen, C.D.; Zhong, L.; Luo, W.; Züttel, A. Tandem effect of Ag@ C@ Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Bulushev, D.A.; Ross, J.R. Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates. Catal. Rev. 2018, 60, 566–593. [Google Scholar] [CrossRef]
- Yan, N.; Philippot, K. Transformation of CO2 by using nanoscale metal catalysts: Cases studies on the formation of formic acid and dimethylether. Curr. Opin. Chem. Eng. 2018, 20, 86–92. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Park, K.; Jung, K.-D.; Yoon, S. Recent developments in the catalytic hydrogenation of CO2 to formic acid/formate using heterogeneous catalysts. Inorg. Chem. Front. 2016, 3, 882–895. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Yoon, S. Heterogenized Catalyst for the Hydrogenation of CO2 to Formic Acid or Its Derivatives. In CO2 Hydrogenation Catalysis; Wiley: Hoboken, NJ, USA, 2021; pp. 149–177. [Google Scholar]
- Sun, R.; Liao, Y.; Bai, S.-T.; Zheng, M.; Zhou, C.; Zhang, T.; Sels, B.F. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: From nanoscale to single atom. Energy Environ. Sci. 2021, 14, 1247–1285. [Google Scholar] [CrossRef]
- Verma, P.; Zhang, S.; Song, S.; Mori, K.; Kuwahara, Y.; Wen, M.; Yamashita, H.; An, T. Recent strategies for enhancing the catalytic activity of CO2 hydrogenation to formate/formic acid over Pd-based catalyst. J. CO2 Util. 2021, 54, 101765. [Google Scholar] [CrossRef]
- Ranade, V.V.; Chaudhari, R.; Gunjal, P.R. Trickle Bed Reactors: Reactor Engineering and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Kim, S.-H.; Park, H.; Kim, S.; Park, K.; Jung, K.-D.; Yoon, S. CO2 hydrogenation to formic acid over heterogenized ruthenium catalysts using a fixed bed reactor with separation units. Green Chem. 2020, 22, 1639–1649. [Google Scholar] [CrossRef]
- Ahn, S.; Park, K.; Lee, K.R.; Haider, A.; Van Nguyen, C.; Jin, H.; Yoo, S.J.; Yoon, S.; Jung, K.-D. Atomically dispersed Ru (III) on N-doped mesoporous carbon hollow spheres as catalysts for CO2 hydrogenation to formate. Chem. Eng. J. 2022, 442, 136185. [Google Scholar] [CrossRef]
- Kim, C.; Park, K.; Lee, H.; Im, J.; Usosky, D.; Tak, K.; Park, D.; Chung, W.; Han, D.; Yoon, J. Accelerating the net-zero economy with CO2-hydrogenated formic acid production: Process development and pilot plant demonstration. Joule 2024. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Benedetti, A.; Polizzi, S.; Di Mario, A.; Pinna, F.; Signoretto, M.; Pernicone, N. Structural investigation on the stoichiometry of β-PdH x in Pd/SiO2 catalysts as a function of metal dispersion. Catal. Lett. 1995, 32, 293–303. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Ogawa, N.; Sakata, K.; Takezawa, N. New catalytic functions of Pd–Zn, Pd–Ga, Pd–In, Pt–Zn, Pt–Ga and Pt–In alloys in the conversions of methanol. Catal. Lett. 1998, 54, 119–123. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, X.; Wang, Y.; Zhou, S.; Lv, Z.; Zhang, L.; Shi, W.; Li, Y.; Zhang, W.; Su, D.S. Visualizing formation of intermetallic PdZn in a palladium/zinc oxide catalyst: Interfacial fertilization by PdHx. Angew. Chem. Int. Ed. 2019, 58, 4232–4237. [Google Scholar] [CrossRef]
- Gniewek, A.; Trzeciak, A.M.; Ziółkowski, J.J.; Kępiński, L.; Wrzyszcz, J.; Tylus, W. Pd-PVP colloid as catalyst for Heck and carbonylation reactions: TEM and XPS studies. J. Catal. 2005, 229, 332–343. [Google Scholar] [CrossRef]
- Vanni, M.; Bellini, M.; Borsacchi, S.; Calucci, L.; Caporali, M.; Caporali, S.; d’Acapito, F.; Geppi, M.; Giaccherini, A.; Ienco, A. Interlayer Coordination of Pd–Pd Units in Exfoliated Black Phosphorus. J. Am. Chem. Soc. 2021, 143, 10088–10098. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaya, A.V.; Chapek, S.V.; Usoltsev, O.A.; Naranov, E.; Gorbunov, D.N.; Trigub, A.L.; Maximov, A.L.; Soldatov, A.V.; Bugaev, A.L. High-Quality In Situ X-ray Absorption Spectroscopy Monitoring of the Palladium Nucleation inside the 3D Printed Microfluidic Chip. J. Phys. Chem. C 2023, 127, 20727–20733. [Google Scholar] [CrossRef]
- Chen, B.F.; Dong, M.H.; Liu, S.L.; Xie, Z.B.; Yang, J.J.; Li, S.P.; Wang, Y.Y.; Du, J.; Liu, H.Z.; Han, B.X. CO2 Hydrogenation to Formate Catalyzed by Ru Coordinated with a N,P-Containing Polymer. ACS Catal. 2020, 10, 8557–8566. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hülsey, M.J.; Yan, N. Zirconia phase effect in Pd/ZrO2 catalyzed CO2 hydrogenation into formate. Mol. Catal. 2019, 475, 110461. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yao, S.; Song, X.; Huang, W.; Hülsey, M.J.; Yan, N. Support-dependent rate-determining step of CO2 hydrogenation to formic acid on metal oxide supported Pd catalysts. J. Catal. 2019, 376, 57–67. [Google Scholar] [CrossRef]
- Yang, G.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. PdAg alloy nanoparticles encapsulated in N-doped microporous hollow carbon spheres for hydrogenation of CO2 to formate. Appl. Catal. B-Environ. 2021, 283, 119628. [Google Scholar] [CrossRef]
- Yang, G.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. Pd-Cu Alloy Nanoparticles Confined within Mesoporous Hollow Carbon Spheres for the Hydrogenation of CO2 to Formate. J. Phys. Chem. C 2021, 125, 3961–3971. [Google Scholar] [CrossRef]
- Mori, K.; Hata, H.; Yamashita, H. Interplay of Pd ensemble sites induced by GaOx modification in boosting CO2 hydrogenation to formic acid. Appl. Catal. B-Environ. 2023, 320, 122022. [Google Scholar] [CrossRef]
- Park, K.; Lee, K.R.; Ahn, S.; Kim, S.H.; Haider, A.; Choung, S.; Han, J.W.; Jung, K.D. Structural effects of nitrogen-doped titanium oxide supports on stabilization of ruthenium active species in carbon dioxide hydrogenation to formate. Appl. Catal. B-Environ. 2023, 335, 122873. [Google Scholar] [CrossRef]
- Park, K.; Lee, K.R.; Ahn, S.; Van Nguyen, C.; Jung, K.-D. Effects of the chemical states of N sites and mesoporosity of N-doped carbon supports on single-atom Ru catalysts during CO2-to-formate conversion. Appl. Catal. B-Environ. 2024, 346, 123751. [Google Scholar] [CrossRef]
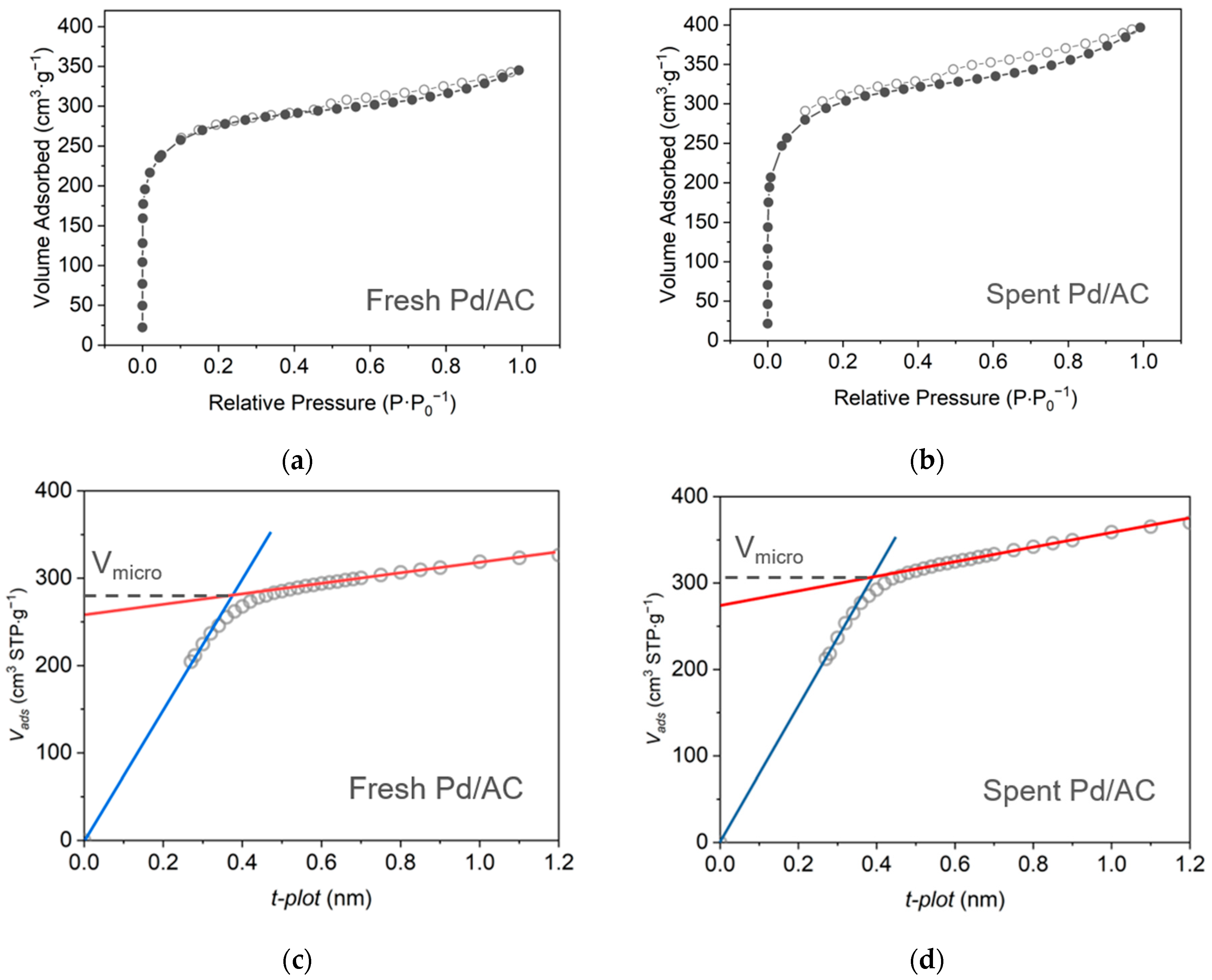
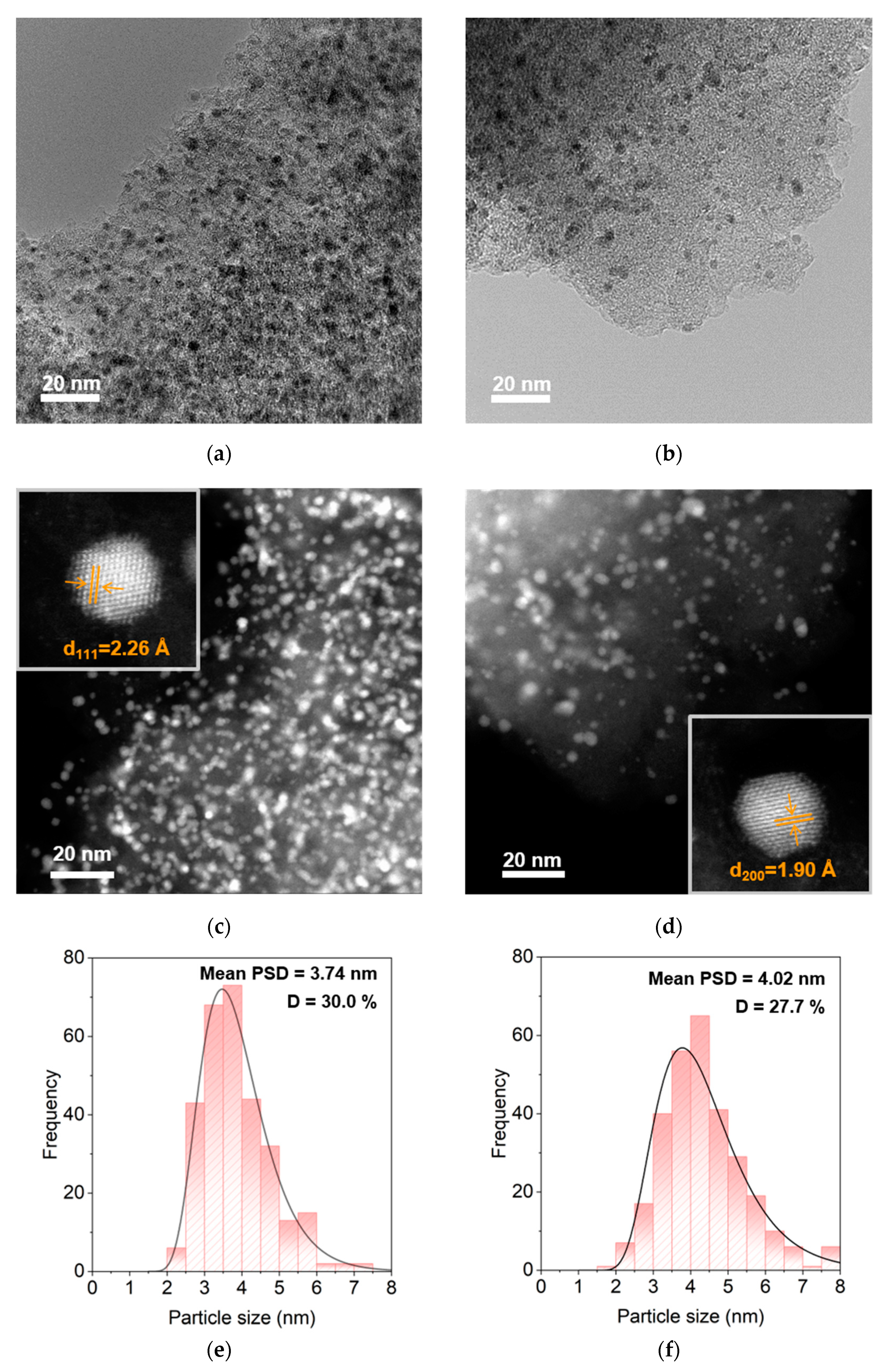
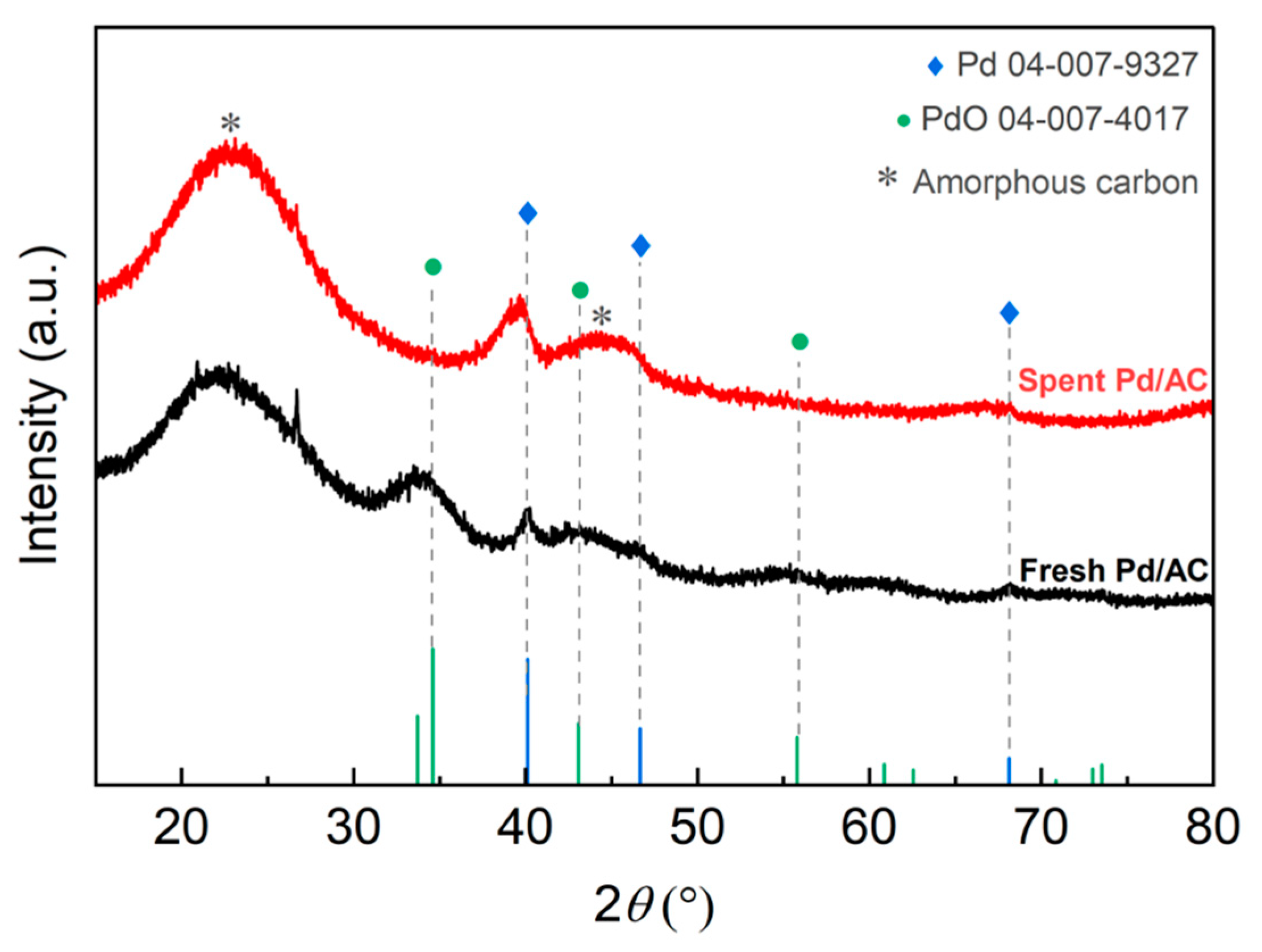
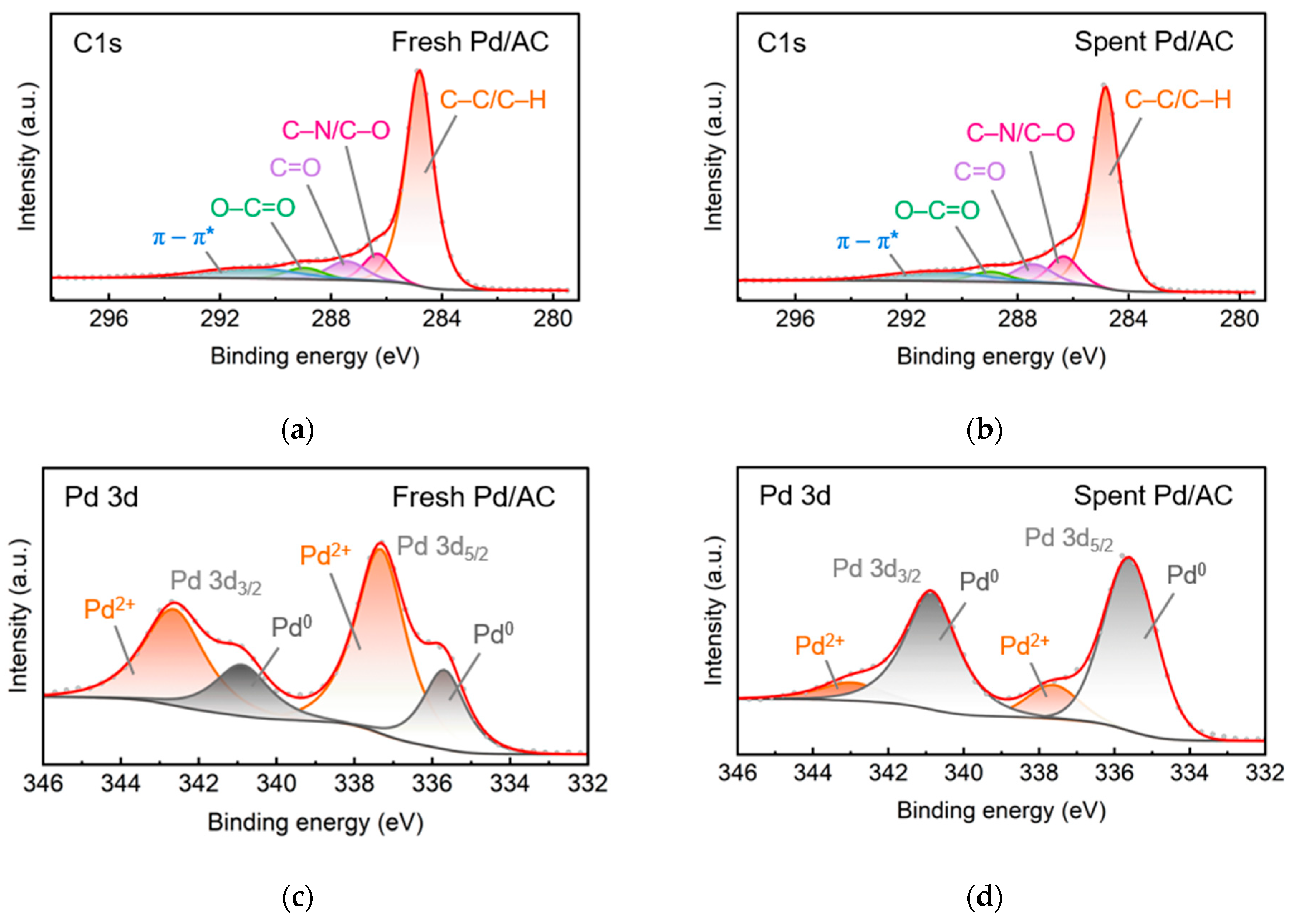
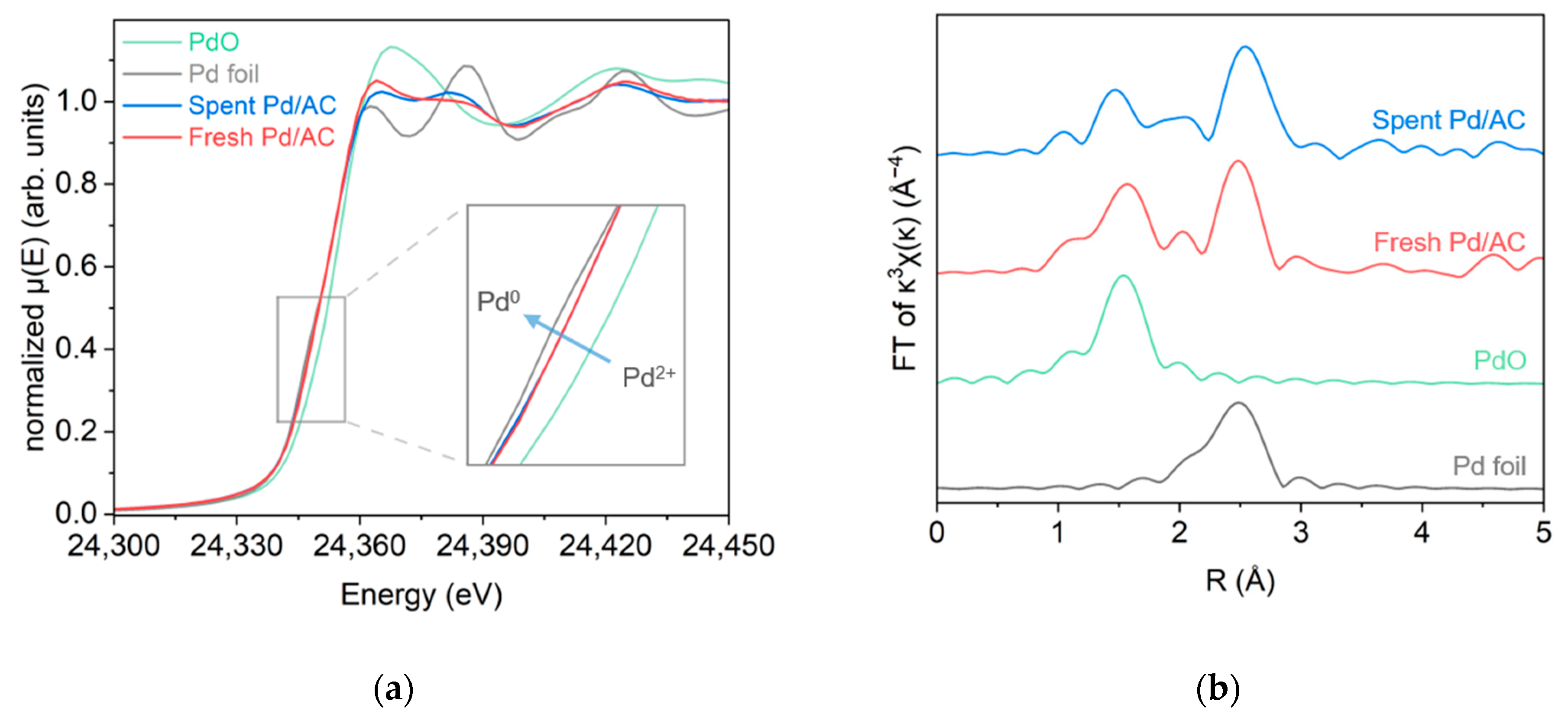
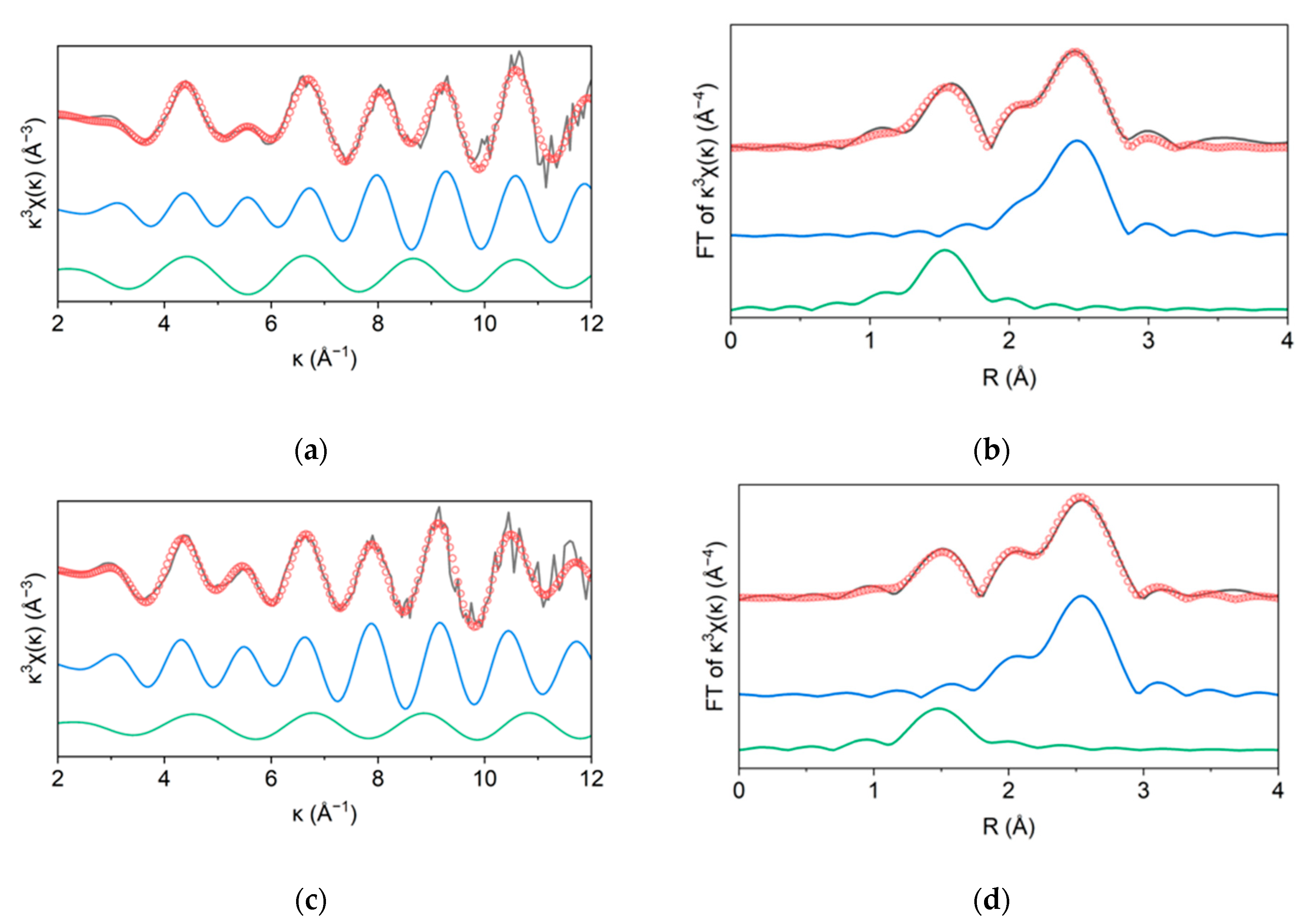
| Sample | Pd Content a (wt%) | D b (%) | d b (nm) | SABET c (m2g−1) | Vpore c (cm3g−1) | dmean c (nm) |
|---|---|---|---|---|---|---|
| Fresh Pd/AC | 4.45 | 29.4 | 3.82 | 1000 | 0.53 | 2.13 |
| Spent Pd/AC | 3.25 | 26.9 | 4.15 | 1090 | 0.61 | 2.24 |
| Sample | Bond | CN | R (Å) | σ2 (Å2) | E0 (eV) |
|---|---|---|---|---|---|
| Fresh Pd/AC | Pd-O | 1.78 | 2.01 ± 0.02 | 0.0015 | 2.737 |
| Pd-Pd | 3.83 | 2.74 ± 0.02 | 0.0071 | −2.432 | |
| Spent Pd/AC | Pd-O | 0.91 | 1.98 ± 0.02 | 0.0010 | 2.858 |
| Pd-Pd | 6.09 | 2.77 ± 0.01 | 0.0098 | −1.725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Lee, K.R.; Ahn, S.; Park, H.; Moon, S.; Yoon, S.; Jung, K.-D. Investigating the Catalytic Deactivation of a Pd Catalyst during the Continuous Hydrogenation of CO2 into Formate Using a Trickle-Bed Reactor. Catalysts 2024, 14, 187. https://doi.org/10.3390/catal14030187
Park K, Lee KR, Ahn S, Park H, Moon S, Yoon S, Jung K-D. Investigating the Catalytic Deactivation of a Pd Catalyst during the Continuous Hydrogenation of CO2 into Formate Using a Trickle-Bed Reactor. Catalysts. 2024; 14(3):187. https://doi.org/10.3390/catal14030187
Chicago/Turabian StylePark, Kwangho, Kyung Rok Lee, Sunghee Ahn, Hongjin Park, Seokyeong Moon, Sungho Yoon, and Kwang-Deog Jung. 2024. "Investigating the Catalytic Deactivation of a Pd Catalyst during the Continuous Hydrogenation of CO2 into Formate Using a Trickle-Bed Reactor" Catalysts 14, no. 3: 187. https://doi.org/10.3390/catal14030187
APA StylePark, K., Lee, K. R., Ahn, S., Park, H., Moon, S., Yoon, S., & Jung, K.-D. (2024). Investigating the Catalytic Deactivation of a Pd Catalyst during the Continuous Hydrogenation of CO2 into Formate Using a Trickle-Bed Reactor. Catalysts, 14(3), 187. https://doi.org/10.3390/catal14030187









