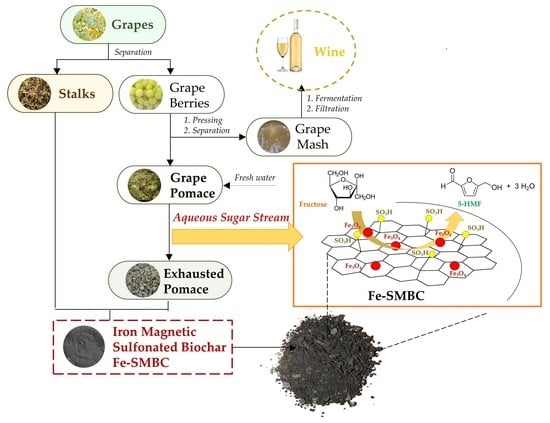
A Closed-Loop Biorefinery Approach for the Valorization of Winery Waste: The Production of Iron-Sulfonated Magnetic Biochar Catalysts and 5-Hydroxymethyl Furfural from Grape Pomace and Stalks
Abstract
1. Introduction
1.1. Global Wine Production and Waste Management
1.2. Integrated Approach to Biorefinery: Development of Heterogeneous Carbon-Based Catalysts for Industrial Processes
1.3. Dehydration of Fructose for Synthesis of 5-HMF
2. Results and Discussion
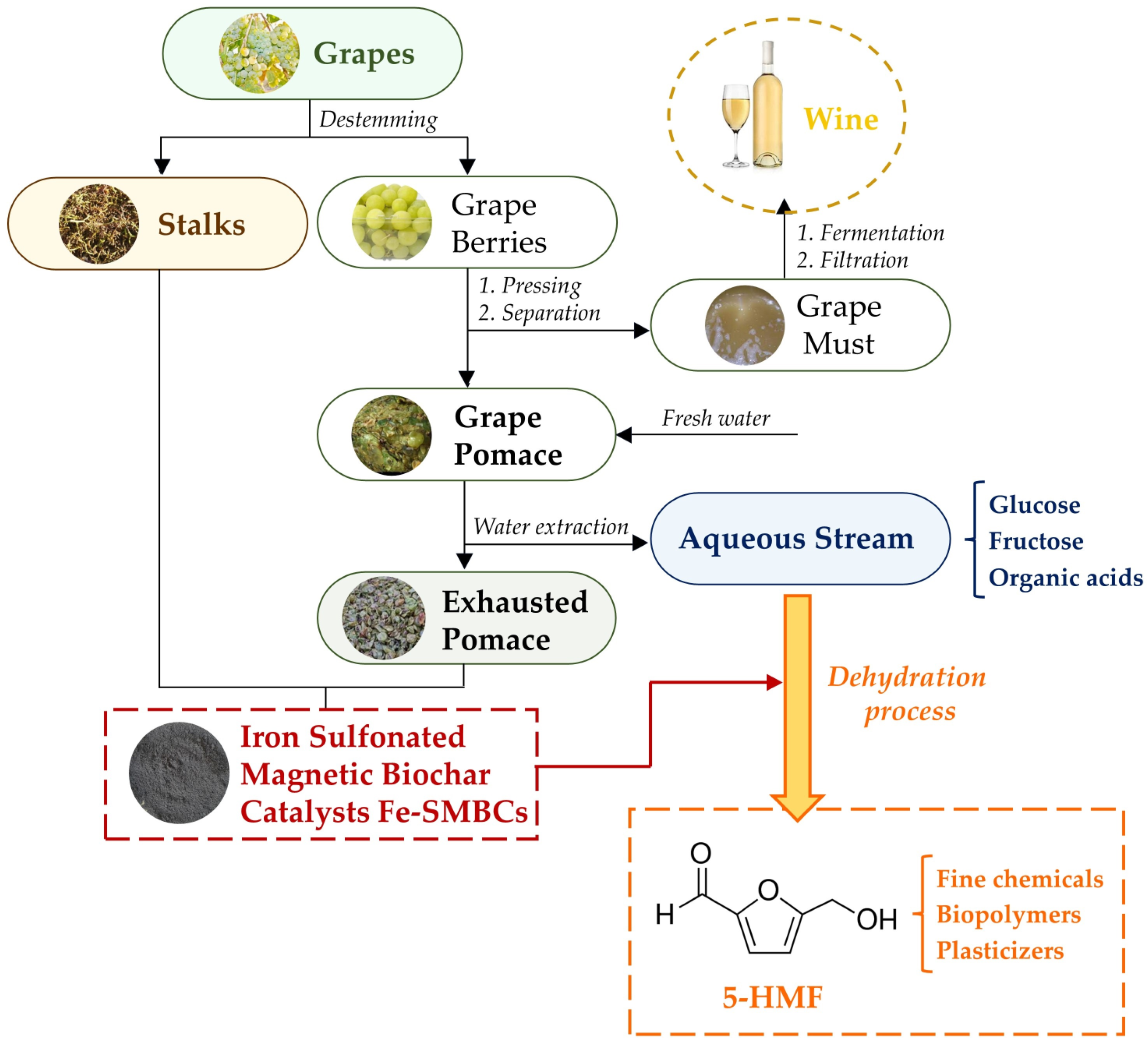
2.1. Scheme of Valorization Route: Chemical Characterization of Winery Waste
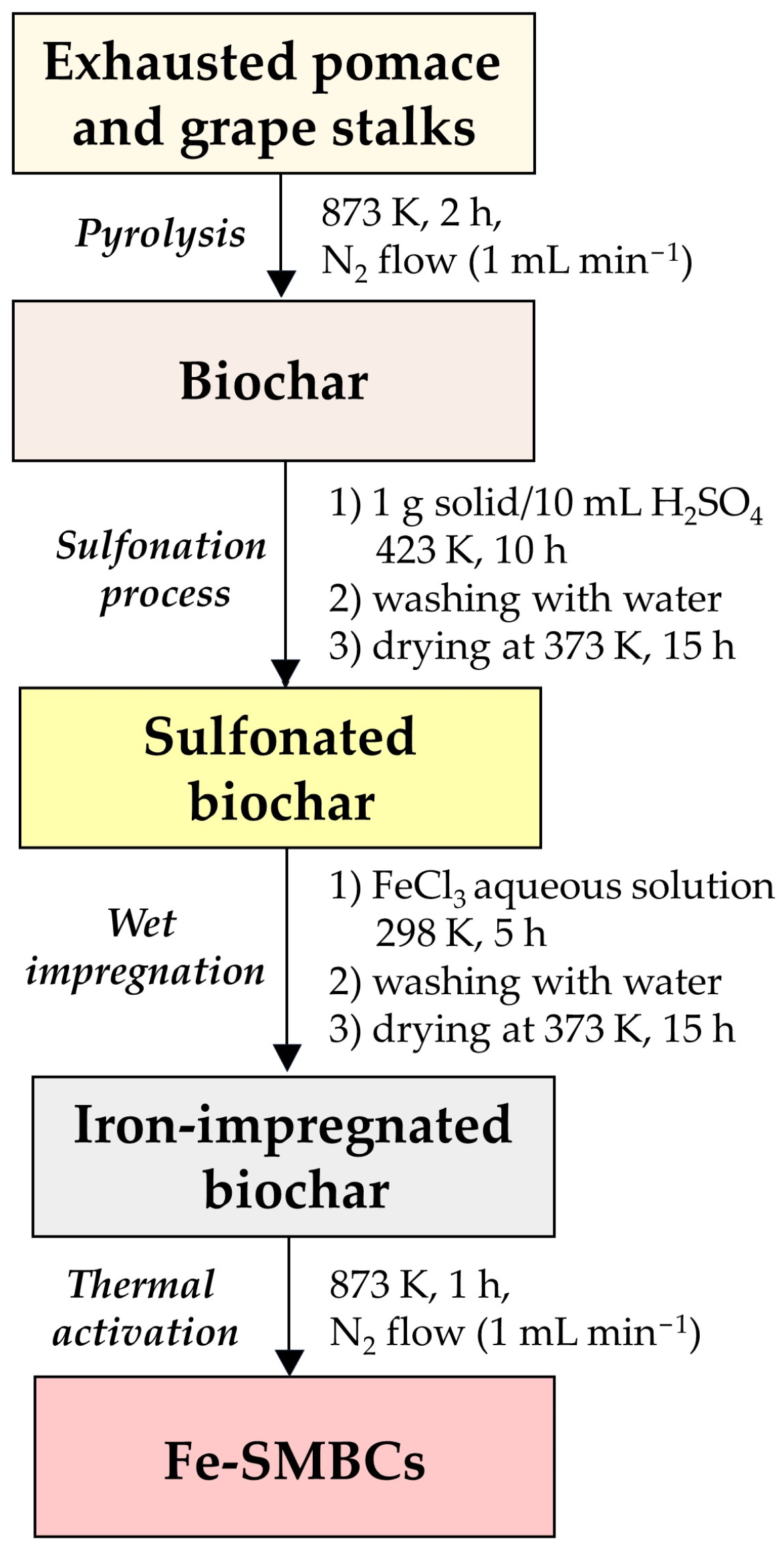
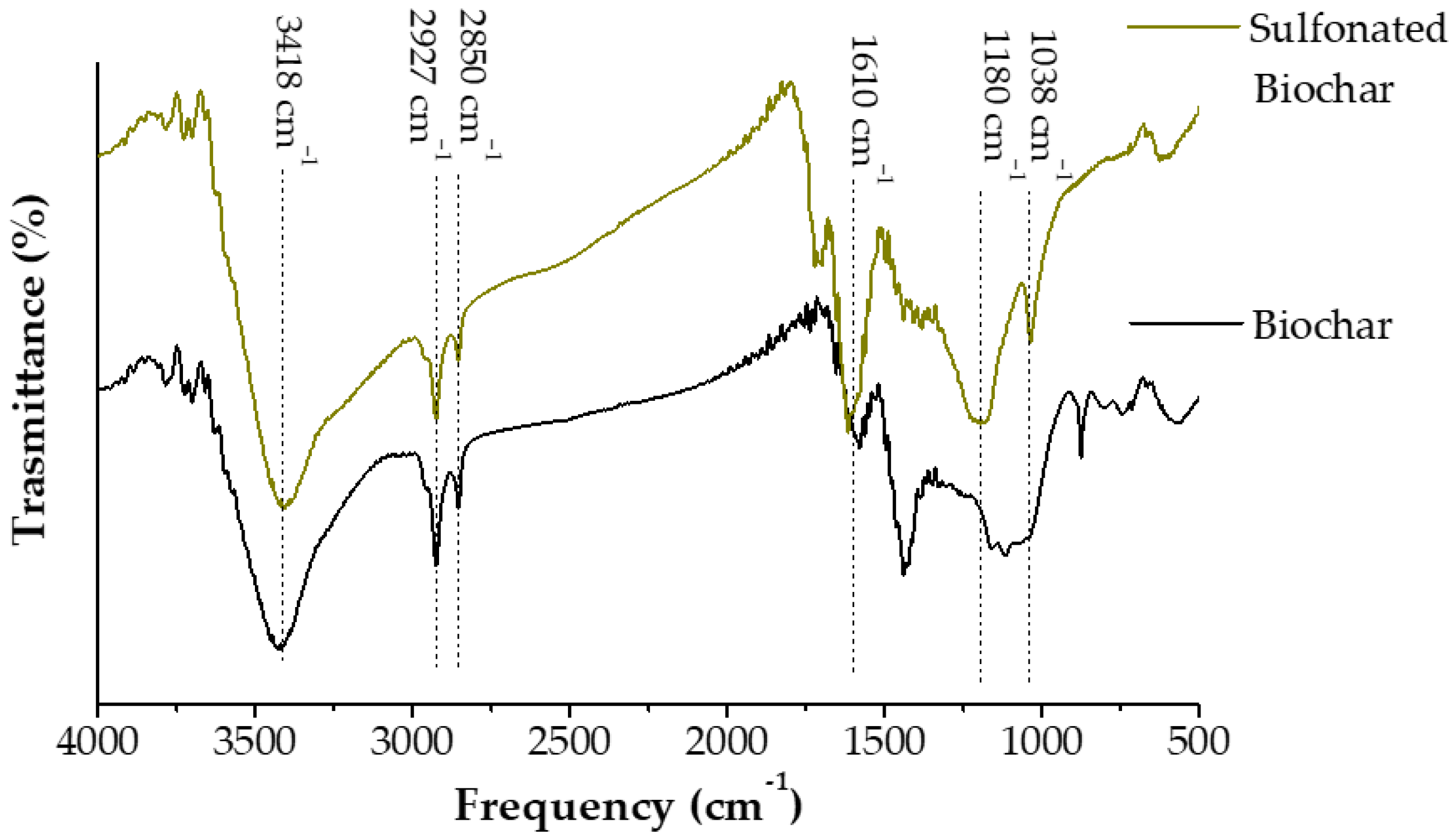
2.2. Synthesis and Characterization of Iron-Sulfonated Magnetic Biochar Catalysts (Fe-SMBCs)
2.3. Use of Fe-SMBCs in Dehydration of Fructose for the Synthesis of 5-HMF
2.4. Scaling up of Dehydration Process and Future Perspectives
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Winery Waste
3.3. Biomass Characterization
3.3.1. Determination of Total Lipids
3.3.2. Determination of Structural Carbohydrates and Lignin Content
3.3.3. Determination of Protein Content
- (i)
- Solution A: 268 mg/L sodium tartrate dihydrate (C4H4Na2O6·2H2O), 23.4 g/L sodium carbonate (Na2CO3) and 4 g/L sodium hydroxide (NaOH);
- (ii)
- Solution B: 1.56%wt copper sulfate (CuSO4);
- (iii)
- Solution C: volume ratio Solution A-to-Solution B of 100-to-1;
- (iv)
- Solution D: 50%vol Folin–Ciocalteu reagent
3.3.4. Determination of Ashes
3.4. Synthesis of Iron Sulfonated Magnetic Biochar Catalysts
Determination of Total Acid Sites
3.5. Dehydration Tests
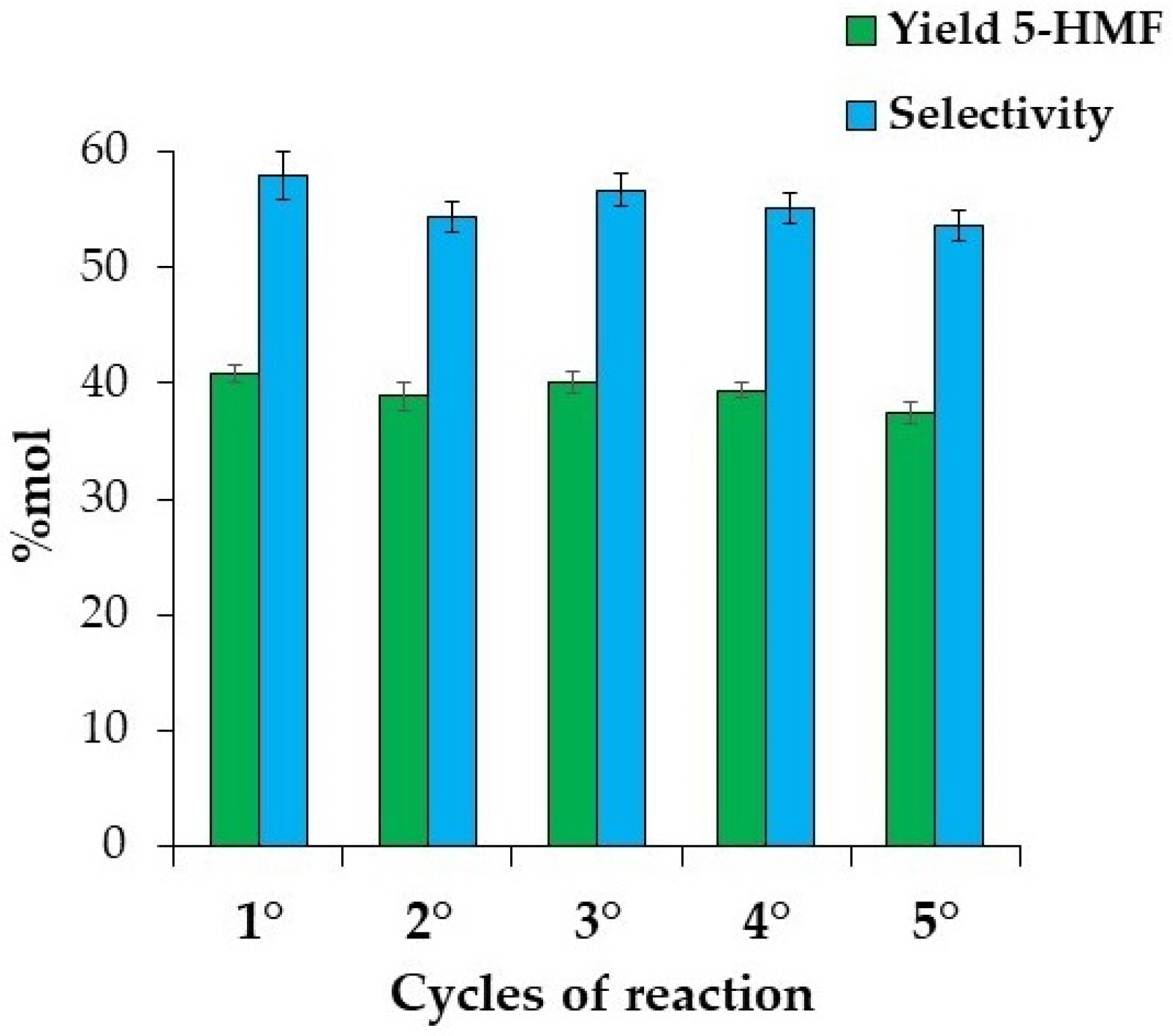
Reusability of the Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Wine Production Outlook, OIV First Estimates, 7 November 2023. Available online: https://www.oiv.int/sites/default/files/documents/OIV_World_Wine_Production_Outlook_2023.pdf (accessed on 15 January 2023).
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.S.; Muro, G.S.; García, M.O.; Motilva, M.J. Winemaking by-products as a source of phenolic compounds: Comparative study of dehydration processes. LWT 2022, 165, 113774. [Google Scholar] [CrossRef]
- Toscano, G.; Riva, G.; Duca, D.; Pedretti, E.F.; Corinaldesi, F.; Rossini, G. Analysis of the characteristics of the residues of the wine production chain finalized to their industrial and energy recovery. Biomass Bioenergy 2013, 55, 260–267. [Google Scholar] [CrossRef]
- Pinto, R.; Correia, C.; Mourão, I.; Brito, L.M. Composting Waste from the White Wine Industry. Sustainability 2023, 15, 3454. [Google Scholar] [CrossRef]
- Niculescu, V.C.; Ionete, R.E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Flores, S.S. What is sustainability in the wine world? A cross-country analysis of wine sustainability frameworks. J. Clean. Prod. 2018, 172, 2301–2312. [Google Scholar] [CrossRef]
- EUR-Lex. Access to European Union Law. Document 32008R0479. Council Regulation (EC) No 479/2008 of 29 April 2008 on the Common Organization of the Market in Wine, Amending Regulations (EC) No 1493/1999, (EC) No 1782/2003, (EC) No 1290/2005, (EC) No 3/2008 and Repealing Regulations (EEC) No 2392/86 and (EC) No 1493/1999. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008R0479 (accessed on 15 January 2023).
- Poblete, R.; Bakit, J. Technical and economical assessment of the treatment of vinasse from Pisco production using the advanced oxidation process. Environ. Sci. Pollut. Res. 2023, 30, 70213–70228. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.O.; Amaral, C.; Fernandes, J.M.; Fraga, I.; Semitela, S.; Braga, F.; Coimbra, A.M.; Dias, A.A.; Bezerra, R.M.; Sampaio, A. Hazardous impact of vinasse from distilled winemaking by-products in terrestrial plants and aquatic organisms. Ecotoxicol. Environ. Saf. 2019, 183, 109493. [Google Scholar] [CrossRef]
- Semitela, S.; Pirra, A.; Braga, F.G. Impact of mesophilic co-composting conditions on the quality of substrates produced from winery waste activated sludge and grape stalks: Lab-scale and pilot-scale studies. Bioresour. Technol. 2019, 289, 121622. [Google Scholar] [CrossRef]
- Cortés, A.; Oliveira, L.F.; Ferrari, V.; Taffarel, S.R.; Feijoo, G.; Moreira, M.T. Environmental assessment of viticulture waste valorisation through composting as a biofertilisation strategy for cereal and fruit crops. Environ. Pollut. 2020, 264, 114794. [Google Scholar] [CrossRef]
- Zacharof, M.P. Grape winery waste as feedstock for bioconversions: Applying the biorefinery concept. Waste Biomass Valoriz. 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Mandade, P.; Gnansounou, E. Potential value-added products from wineries residues. In Biomass, Biofuels, Biochemicals. Green-Economy: Systems Analysis for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–396. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing value of winery residues through integrated biorefinery processes: A review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, V.; Martinez, G.; Jones, E.; Bertin, L.; Pastore, C. Ethyl Hexanoate Rich Stream from Grape Pomace: A Viable Route to Obtain Fine Chemicals from Agro by-Products. Sep. Purif. Technol. 2023, 309, 123100. [Google Scholar] [CrossRef]
- Hayrapetyan, G.; Trchounian, K.; Buon, L.; Noret, L.; Pinel, B.; Lagrue, J.; Assifaoui, A. Sequential extraction of high-value added molecules from grape pomaces using supercritical fluids with water as a co-solvent. RSC Sustain. 2023, 1, 2014–2023. [Google Scholar] [CrossRef]
- Licursi, D.; Raspolli Galletti, A.M.; Antonetti, C.; Martinez, G.A.; Jones, E.; Bertin, L.; Di Fidio, N.; Fulignani, S.; Pasini, G.; Frigo, S. Tunable Production of Diesel Bio-Blendstock by Rhenium-Catalyzed Hydrogenation of Crude Hexanoic Acid from Grape Pomace Fermentation. Catalysts 2022, 12, 1550. [Google Scholar] [CrossRef]
- Perra, M.; Leyva-Jiménez, F.J.; Manca, M.L.; Manconi, M.; Rajha, H.N.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Application of pressurized liquid extraction to grape by-products as a circular economy model to provide phenolic compounds enriched ingredient. J. Clean. Prod. 2023, 402, 136712. [Google Scholar] [CrossRef]
- Rodrigues Machado, A.; Atatoprak, T.; Santos, J.; Alexandre, E.M.; Pintado, M.E.; Paiva, J.A.; Nunes, J. Potentialities of the Extraction Technologies and Use of Bioactive Compounds from Winery By-Products: A Review from a Circular Bioeconomy Perspective. Appl. Sci. 2023, 13, 7754. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive mill and winery wastes as viable sources of bioactive compounds: A study on polyphenols recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent advancements and challenges in emerging applications of biochar-based catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and application of biochar-based catalysts for biofuel production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A review on biochar production from different biomass wastes by recent carbonization technologies and its sustainable applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Ghesti, G.F.; Silveira, E.A.; Guimarães, M.G.; Evaristo, R.B.; Costa, M. Towards a sustainable waste-to-energy pathway to pequi biomass residues: Biochar, syngas, and biodiesel analysis. Waste Manag. 2022, 143, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Merodio-Morales, E.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Reynel-Avila, H.E.; Milella, A.; di Bitonto, L.; Pastore, C. A novel CO2 activation at room temperature to prepare an engineered lanthanum-based adsorbent for a sustainable arsenic removal from water. Chem. Eng. Res. Des. 2022, 185, 239–252. [Google Scholar] [CrossRef]
- Gasim, M.F.; Choong, Z.Y.; Koo, P.L.; Low, S.C.; Abdurahman, M.H.; Suryawan, W.K.; Ho, Y.C.; Lim, J.W.; Oh, W.D. Application of biochar as functional material for remediation of organic pollutants in water: An overview. Catalysts 2022, 12, 210. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. State-of-the-art and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Leesing, R.; Siwina, S.; Fiala, K. Yeast-based biodiesel production using sulfonated carbon-based solid acid catalyst by an integrated biorefinery of durian peel waste. Renew. Energy 2021, 171, 647–657. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Spessato, L.; Cazetta, A.L.; da Silva, C.; Almeida, V.D.C. Sulfonated carbon: Synthesis, properties and production of biodiesel. Chem. Eng. Process. Process Intensif. 2022, 170, 108668. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- di Bitonto, L.; Scelsi, E.; Errico, M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Corazza, M.L.; Kanda, L.S.R.; Hájek, M.; Stateva, R.P.; et al. A Network of Processes for Biorefining Burdock Seeds and Roots. Molecules 2024, 29, 937. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Likozar, B. A review of bio-refining process intensification in catalytic conversion reactions, separations and purifications of hydroxymethylfurfural (HMF) and furfural. Chem. Eng. J. 2022, 429, 132325. [Google Scholar] [CrossRef]
- Kong, Q.S.; Li, X.L.; Xu, H.J.; Fu, Y. Conversion of 5-hydroxymethylfurfural to chemicals: A review of catalytic routes and product applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Ji, Z.; Cheng, Y.; Zheng, L.; Wang, L.; Li, X. Experiments and Kinetic Modeling of Fructose Dehydration to 5-Hydroxymethylfurfural with Hydrochloric Acid in Acetone–Water Solvent. Ind. Eng. Chem. Res. 2022, 61, 13877–13885. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Li, Q.; Xia, H. High-Yield Synthesis of 5-Hydroxymethylfurfural from Untreated Wheat Straw Catalyzed by FePO4 and Organic Acid in a Biphasic System. Energy Fuels 2023, 37, 12953–12965. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.; Qin, H.; Qi, Z. Advances of Ionic Liquids and Deep Eutectic Solvents in Green Processes of Biomass-Derived 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202102635. [Google Scholar] [CrossRef]
- Sampath, G.; Kannan, S. Fructose dehydration to 5-hydroxymethylfurfural: Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies. Catal. Commun. 2013, 37, 41–44. [Google Scholar] [CrossRef]
- Takagaki, A. Production of 5-hydroxymethylfurfural from glucose in water by using transition metal-oxide nanosheet aggregates. Catalysts 2019, 9, 818. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Pastore, C.; di Bitonto, L.; Angelini, A.; Quaranta, E. Conversion of fructose into 5-HMF: A study on the behaviour of heterogeneous cerium-based catalysts and their stability in aqueous media under mild conditions. RSC Adv. 2015, 5, 26941–26948. [Google Scholar] [CrossRef]
- Saidi, M.; Safaripour, M.; Ameri, F.A.; Jomeh, M.E. Application of sulfonated biochar-based magnetic catalyst for biodiesel production: Sensitivity analysis and process optimization. Chem. Eng. Process.-Process Intensif. 2023, 190, 109419. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, X.; Shou, J. Synthesis of Novel Magnetic Carbon Microtube-Based Solid Acid and Its Catalytic Activities for Biodiesel Synthesis. Catalysts 2022, 12, 305. [Google Scholar] [CrossRef]
- Liu, W.J.; Tian, K.; Jiang, H.; Yu, H.Q. Facile Synthesis of Highly Efficient and Recyclable Magnetic Solid Acid from Biomass Waste. Sci. Rep. 2013, 3, 2419. [Google Scholar] [CrossRef] [PubMed]
- Licursi, D.; Galletti, A.M.R.; Bertini, B.; Ardemani, L.; Scotti, N.; Di Fidio, N.; Fulignati, S.; Antonetti, C. Design approach for the sustainable synthesis of sulfonated biomass-derived hydrochars and pyrochars for the production of 5-(hydroxymethyl) furfural. Sustain. Chem. Pharm. 2023, 35, 101216. [Google Scholar] [CrossRef]
- Xiong, X.; Iris, K.M.; Chen, S.S.; Tsang, D.C.; Cao, L.; Song, H.; Kwon, E.E.; Ok, Y.S.; Zhang, S.; Poon, C.S. Sulfonated biochar as acid catalyst for sugar hydrolysis and dehydration. Catal. Today 2018, 314, 52–61. [Google Scholar] [CrossRef]
- Gao, W.; Wan, Y.; Dou, Y.; Zhao, D. Synthesis of partially graphitic ordered mesoporous carbons with high surface areas. Adv. Energy Mat. 2011, 1, 115–123. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. C 2020, 6, 21. [Google Scholar] [CrossRef]
- da Luz Corrêa, A.P.; Bastos, R.R.C.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef] [PubMed]
- Ngaosuwan, K.; Goodwin, J.G., Jr.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Zhao, S.; Chen, W.; Chen, Z.; Chang, C.; Wu, H. Efficient conversion of fructose to produce high-purity 5-hydroxymethylfurfural under low temperature. Biomass Convers. Biorefin. 2023, 1–9. [Google Scholar] [CrossRef]
- Yan, P.; Xia, M.; Chen, S.; Han, W.; Wang, H.; Zhu, W. Unlocking biomass energy: Continuous high-yield production of 5-hydroxymethylfurfural in water. Green Chem. 2020, 22, 5274–5284. [Google Scholar] [CrossRef]
- Belle-Oudry, D. Quantitative analysis of sulfate in water by indirect EDTA titration. J. Chem. Edu. 2008, 85, 1269. [Google Scholar] [CrossRef]
- Metkar, P.S.; Till, E.J.; Corbin, D.R.; Pereira, C.J.; Hutchenson, K.W.; Sengupta, S.K. Reactive distillation process for the production of furfural using solid acid catalysts. Green Chem. 2015, 17, 1453–1466. [Google Scholar] [CrossRef]
- Rachamontree, P.; Douzou, T.; Cheenkachorn, K.; Sriariyanun, M.; Rattanaporn, K. Furfural: A sustainable platform chemical and fuel. Appl. Sci. Eng. Prog. 2020, 13, 3–10. [Google Scholar] [CrossRef]
- Zheng, J.; Pan, B.; Xiao, J.; He, X.; Chen, Z.; Huang, Q.; Lin, X. Experimental and mathematical simulation of noncompetitive and competitive adsorption dynamic of formic acid–Levulinic acid–5-Hydroxymethylfurfural from single, binary, and ternary systems in a fixed-bed column of SY-01 resin. Ind. Eng. Chem. Res. 2018, 57, 8518–8528. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Luo, Q.X.; Lu, M.H.; Luo, D.; Liu, Z.W.; Liu, Z.T. Controllable and scalable synthesis of hollow-structured porous aromatic polymer for selective adsorption and separation of HMF from reaction mixture of fructose dehydration. Chem. Eng. J. 2019, 358, 467–479. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Y.; Hu, D.; Guo, R.; Yan, K.; Luque, R. Chemical transformations of 5-hydroxymethylfurfural into highly added value products: Present and future. Green Chem. 2023, 25, 871–892. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward biomass-derived renewable plastics: Production of 2, 5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, eaap9722. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Wang, J.; Zhang, X.; Jia, Z.; Jiang, Y.; Liu, X. Recent progress on bio-based polyesters derived from 2, 5-furandicarbonxylic acid (FDCA). Polymers 2022, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yang, D.; Cao, Q.; Dai, J.; Yang, R.; Gu, X.; Li, F. Recent advances in catalytic synthesis of 2, 5-furandimethanol from 5-hydroxymethylfurfural and carbohydrates. Bioresour. Bioproc. 2023, 10, 52. [Google Scholar] [CrossRef]
- Castillo, C.; Brijaldo, M.H.; Silva, L.P.; Passos, F.B. Recent Advances in Catalytic Conversion of 5-Hydroxymethylfurfural (5-HMF) to 2,5-Dimethylfuran (2,5-DMF): Mechanistic Insights and Optimization Strategies. ChemCatChem 2023, 15, e202300480. [Google Scholar] [CrossRef]
- Iriondo, A.; Mendiguren, A.; Güemez, M.B.; Requies, J.; Cambra, J.F. 2,5-DMF production through hydrogenation of real and synthetic 5-HMF over transition metal catalysts supported on carriers with different nature. Catal. Today 2017, 279, 286–295. [Google Scholar] [CrossRef]
- di Bitonto, L.; Scelsi, E.; Locaputo, V.; Mustafa, A.; Pastore, C. Enhancing biodiesel production from urban sewage sludge: A novel industrial configuration and optimization model. Sustain. Energy Technol. Assess. 2023, 60, 103567. [Google Scholar] [CrossRef]
- di Bitonto, L.; D’Ambrosio, V.; Pastore, C. A novel and efficient method for the synthesis of methyl (R)-10-hydroxystearate and FAMEs from sewage scum. Catalysts 2021, 11, 663. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Springer Protocols Handbooks; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Landin-Rodriguez, L.E.; Leyva-Ramos, S.; Medellin-Castillo, N.A. Modification of corncob with citric acid to enhance its capacity for adsorbing cadmium (II) from water solution. Chem. Eng. J. 2012, 180, 113–120. [Google Scholar] [CrossRef]

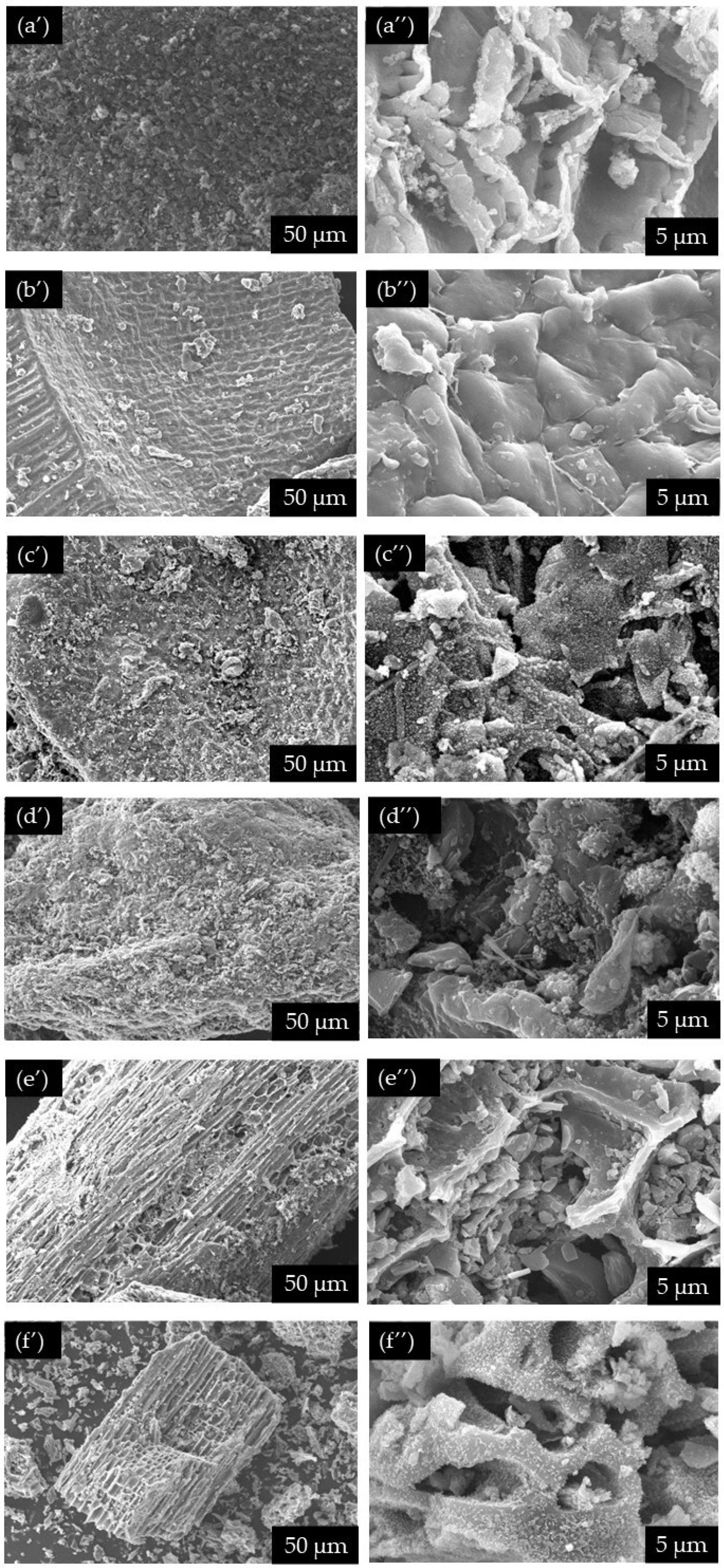
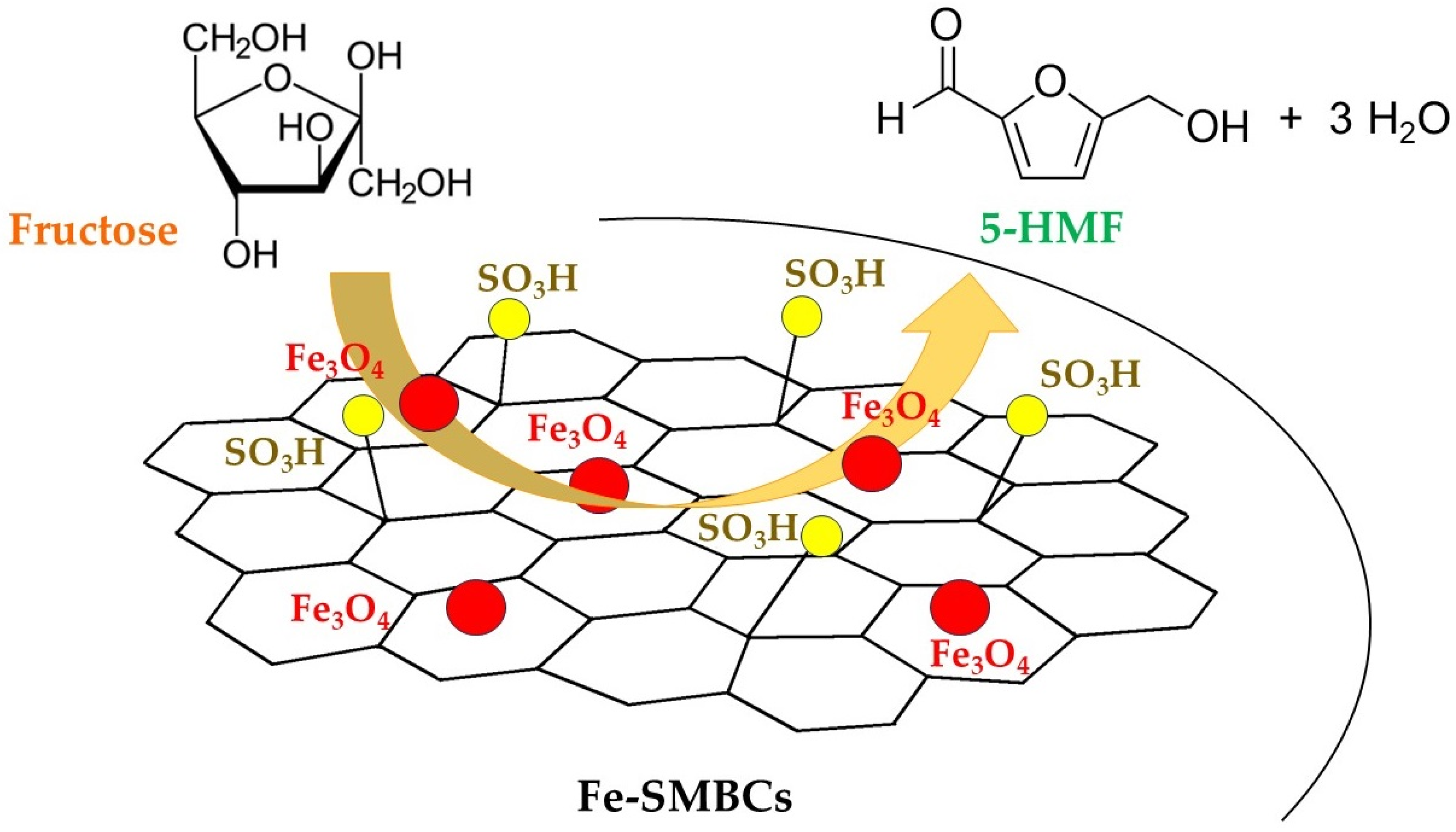
| Winery Waste | Grape Pomace | Exhausted Pomace | Grape Stalks |
|---|---|---|---|
| Total Solids (TS, %wt) | 67.9 ± 2.2 | 44.0 ± 1.4 | 91.8 ± 2.7 |
| TS composition (mg/gTS) | |||
| Total Lipids | 118.8 ± 3.6 | 86.7 ± 2.7 | 19.0 ± 0.3 |
| Free simple sugars Glucose | 30.2 ± 0.6 | 2.3 ± 0.1 | - |
| Fructose | 80.6 ± 1.4 | 5.4 ± 0.2 | - |
| Total | 110.8 ± 2.0 | 7.7 ± 0.3 | - |
| Easy Hydrolysable Sugars (EHSs) Arabinose | 5.9 ± 0.2 | 8.4 ± 0.2 | 1.1 ± 0.3 |
| Glucosamine | 10.6 ± 0.3 | 15.3 ± 0.4 | - |
| Galactose | 18.9 ± 0.4 | 25.9 ± 0.7 | 16.1 ± 0.6 |
| Glucose | 27.2 ± 1.1 | 34.7 ± 0.5 | 61.0 ± 1.8 |
| Xylose | 10.0 ± 0.4 | 13.1 ± 0.2 | 77.9 ± 2.6 |
| Total | 72.6 ± 2.4 | 97.4 ± 2.0 | 156.1 ± 5.3 |
| Cellulose Glucose | 162.9 ± 2.7 | 251.7 ± 3.5 | 313.6 ± 4.2 |
| Xylose | 25.1 ± 0.8 | 41.6 ± 0.7 | 31.4 ± 0.8 |
| Total | 188.0 ± 3.5 | 293.3 ± 4.2 | 345.8 ± 5.0 |
| Lignin | 248.3 ± 6.5 | 277.8 ± 4.1 | 310.4 ± 5.1 |
| Proteins | 96.3 ± 2.1 | 84.9 ± 2.6 | 35.3 ± 1.2 |
| Ashes | 57.2 ± 1.4 | 69.9 ± 1.4 | 65.3 ± 2.1 |
| Chemical Composition | Concentration (mmol/L) |
|---|---|
| Glucose | 120 ± 0.9 |
| Fructose | 320 ± 1.6 |
| Tartaric acid | 14 ± 0.4 |
| Malic acid | 260 ± 1.4 |
| Succinic acid | 26 ± 1.0 |
| Acetic acid | 24 ± 0.7 |
| Samples | Elemental Composition (%wt) | ||||
|---|---|---|---|---|---|
| C | O | Ca | S | Fe | |
| Exhausted pomace | |||||
| Biochar | 62.6 ± 2.3 | 18.6 ± 2.1 | 0.5 | - | - |
| Sulfonated biochar | 63.3 ± 3.6 | 28.9 ± 2.6 | 0.7 | 5.8 ± 0.7 | - |
| Fe-SMBCs | 51.4 ± 9.4 | 15.4 ± 2.5 | 0.5 | 4.0 ± 0.4 | 18.6 ± 3.9 |
| Grape stalks | |||||
| Biochar | 75.5 ± 2.3 | 23.1 ± 3.3 | 0.5 | - | - |
| Sulfonated biochar | 61.3 ± 1.7 | 29.8 ± 1.2 | 0.9 | 6.8 ± 0.4 | - |
| Fe-SMBCs | 46.1 ± 1.9 | 15.8 ± 1.8 | 0.8 | 4.3 | 21.1 ± 0.4 |
| Samples | Total Surface Area (m2/g) | Total Pore Volume (cm3/g) | Mesoroporous Volume (cm3/g) | Total Acid Density (mmolSO3H/g) |
|---|---|---|---|---|
| Exhausted pomace | ||||
| Biochar | 6.7 | 0.039 | 0.023 | - |
| Sulfonated biochar | 2.5 | 0.006 | 0.005 | 0.86 ± 0.01 |
| Fe-SMBCs | 4.1 | 0.013 | 0.010 | 0.91 ± 0.03 |
| Grape stalks | ||||
| Biochar | 17.0 | 0.029 | 0.024 | - |
| Sulfonated biochar | 10.4 | 0.019 | - | 0.85 ± 0.01 |
| Fe-SMBCs | 6.2 | 0.019 | 0.015 | 0.92 ± 0.02 |
| E | Solvent | Catalyst | Time (h) | Fructose Conversion (%mol) | 5-HMF | Formic Acid | Levulinic Acid | ||
|---|---|---|---|---|---|---|---|---|---|
| Yield (%mol) | Selectivity (%mol) | R | Yield (%mol) | Yield (%mol) | |||||
| 1 | MIBK | No catalyst | 3 | 30.4 ± 0.6 | 4.0 ± 0.2 | 13.2 ± 0.9 | 2.0 | - | - |
| 2 | 6 | 34.7 ± 1.2 | 8.1 ± 0.3 | 23.3 ± 1.7 | 2.2 | - | - | ||
| 4 | Amberlyst-15 | 3 | 61.3 ± 2.3 | 8.2 ± 0.3 | 13.4 ± 1.0 | 2.2 | 14.2 ± 0.5 | 10.4 ± 0.4 | |
| 5 | 6 | 91.8 ± 1.4 | 14.5 ± 0.5 | 15.8 ± 0.8 | 2.4 | 32.3 ± 1.6 | 28.6 ± 1.4 | ||
| 6 | Fe-SMBCs (EX) | 3 | 45.8 ± 0.8 | 5.2 ± 0.2 | 11.4 ± 0.6 | 2.0 | - | - | |
| 7 | 6 | 61.2 ± 1.2 | 23.4 ± 0.7 | 38.2 ± 1.9 | 2.4 | - | - | ||
| 8 | Fe-SMBCs (GSs) | 6 | 60.1 ± 1.0 | 22.1 ± 0.5 | 36.8 ± 1.4 | 2.7 | - | - | |
| 9 | GVL | No catalyst | 6 | 33.0 ± 0.7 | 5.6 ± 0.2 | 17.0 ± 0.9 | |||
| 10 | Fe-SMBCs (EX *) | 3 | 50.7 ± 1.2 | 20.1 ± 0.6 | 39.6 ± 2.1 | - | - | - | |
| 11 | 6 | 72.5 ± 1.1 | 41.8 ± 0.8 | 57.7 ± 1.9 | - | - | - | ||
| 12 | Fe-SMBCs (GSs *) | 6 | 70.5 ± 1.2 | 40.8 ± 0.7 | 57.9 ± 2.1 | - | - | - | |
| 6 | 8.1 ± 0.4 ** | 2.1 ± 0.1 | 25.9 ± 0.7 | ||||||
| E | Solvent | Catalyst | Sugar Conversion (%mol) | 5-HMF | |
|---|---|---|---|---|---|
| Yield (%mol) | Selectivity (%mol) | ||||
| 1 | GVL | No catalyst | 26.9 ± 0.8 | 5.6 ± 0.2 | 20.8 ± 1.3 |
| 2 | Fe-SMBCs (EX *) | 69.5 ± 1.4 | 40.7 ± 1.2 | 58.6 ± 2.8 | |
| 3 | Fe-SMBCs (GSs *) | 68.4 ± 1.3 | 40.9 ± 1.1 | 59.8 ± 2.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Bitonto, L.; Scelsi, E.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Hájek, M.; Mustafa, A.; Pastore, C. A Closed-Loop Biorefinery Approach for the Valorization of Winery Waste: The Production of Iron-Sulfonated Magnetic Biochar Catalysts and 5-Hydroxymethyl Furfural from Grape Pomace and Stalks. Catalysts 2024, 14, 185. https://doi.org/10.3390/catal14030185
di Bitonto L, Scelsi E, Reynel-Ávila HE, Mendoza-Castillo DI, Bonilla-Petriciolet A, Hájek M, Mustafa A, Pastore C. A Closed-Loop Biorefinery Approach for the Valorization of Winery Waste: The Production of Iron-Sulfonated Magnetic Biochar Catalysts and 5-Hydroxymethyl Furfural from Grape Pomace and Stalks. Catalysts. 2024; 14(3):185. https://doi.org/10.3390/catal14030185
Chicago/Turabian Styledi Bitonto, Luigi, Enrico Scelsi, Hilda Elizabeth Reynel-Ávila, Didilia Ileana Mendoza-Castillo, Adrián Bonilla-Petriciolet, Martin Hájek, Ahmad Mustafa, and Carlo Pastore. 2024. "A Closed-Loop Biorefinery Approach for the Valorization of Winery Waste: The Production of Iron-Sulfonated Magnetic Biochar Catalysts and 5-Hydroxymethyl Furfural from Grape Pomace and Stalks" Catalysts 14, no. 3: 185. https://doi.org/10.3390/catal14030185
APA Styledi Bitonto, L., Scelsi, E., Reynel-Ávila, H. E., Mendoza-Castillo, D. I., Bonilla-Petriciolet, A., Hájek, M., Mustafa, A., & Pastore, C. (2024). A Closed-Loop Biorefinery Approach for the Valorization of Winery Waste: The Production of Iron-Sulfonated Magnetic Biochar Catalysts and 5-Hydroxymethyl Furfural from Grape Pomace and Stalks. Catalysts, 14(3), 185. https://doi.org/10.3390/catal14030185