Kinetics of Electrocatalytic Oxygen Reduction Reaction over an Activated Glassy Carbon Electrode in an Alkaline Medium
Abstract
1. Introduction
2. Results and Discussion
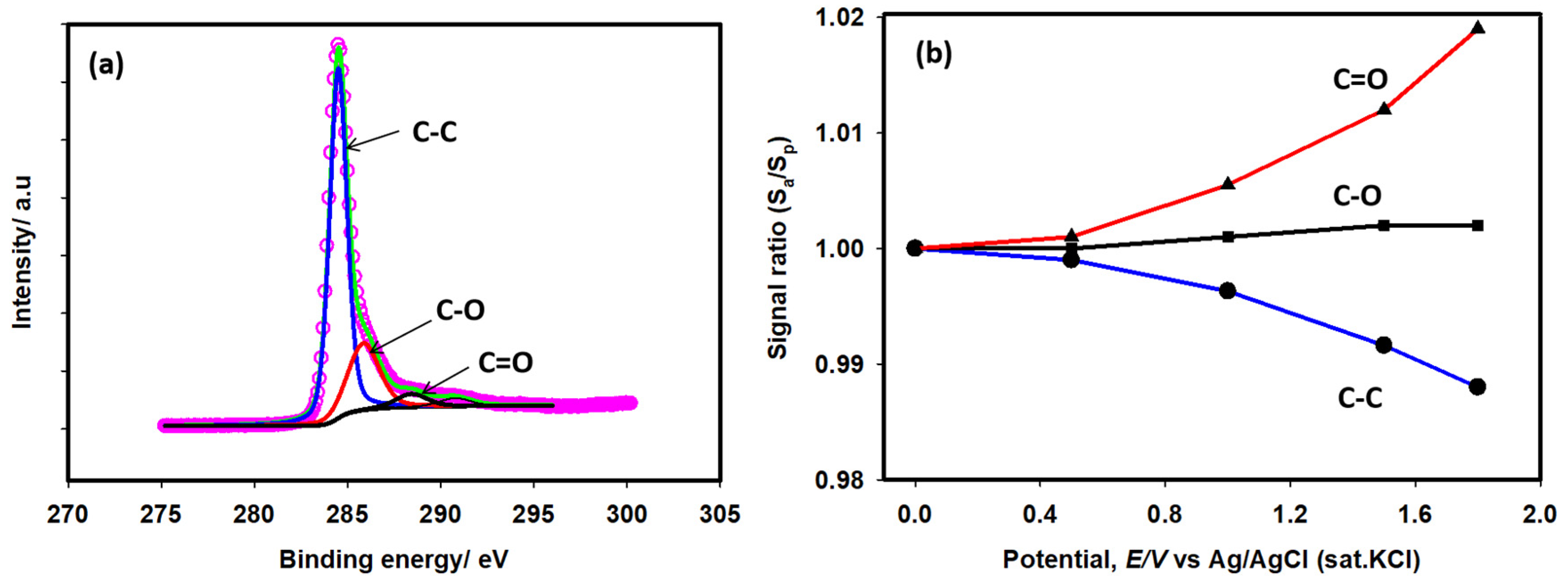
2.1. Spectral Characterization
2.2. OCP and EIS Analyses
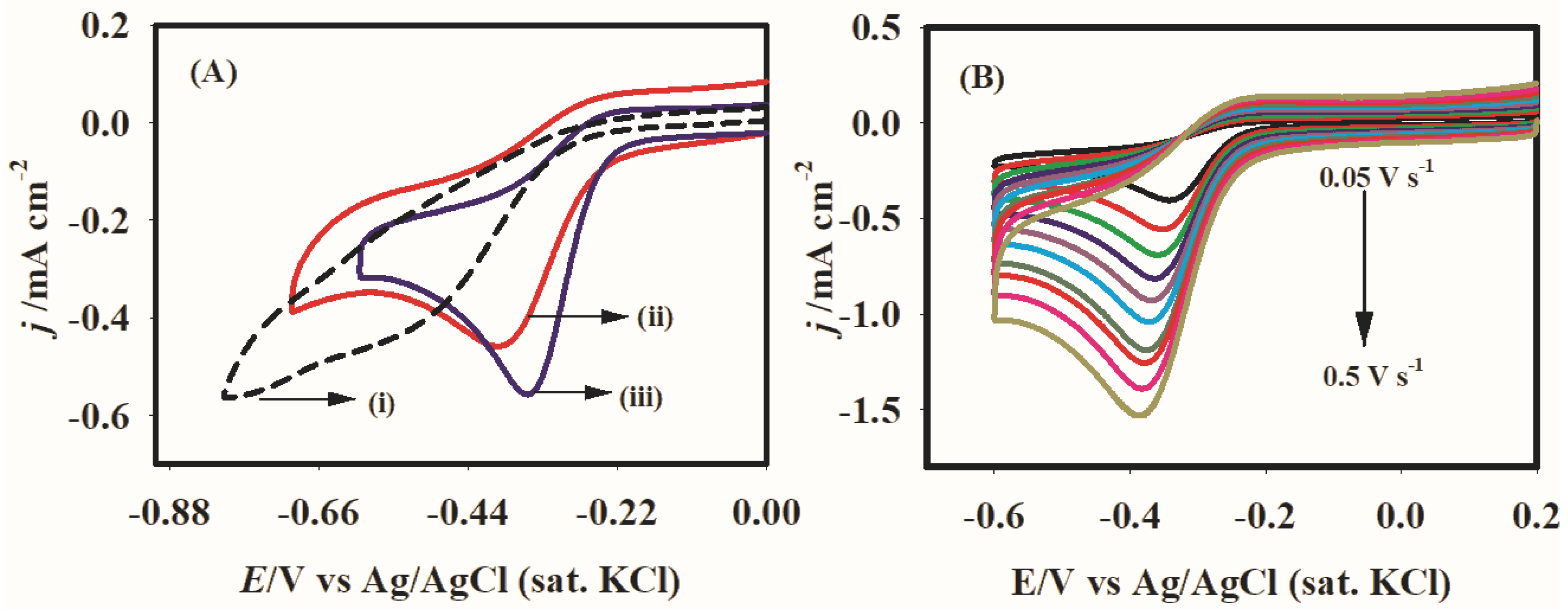
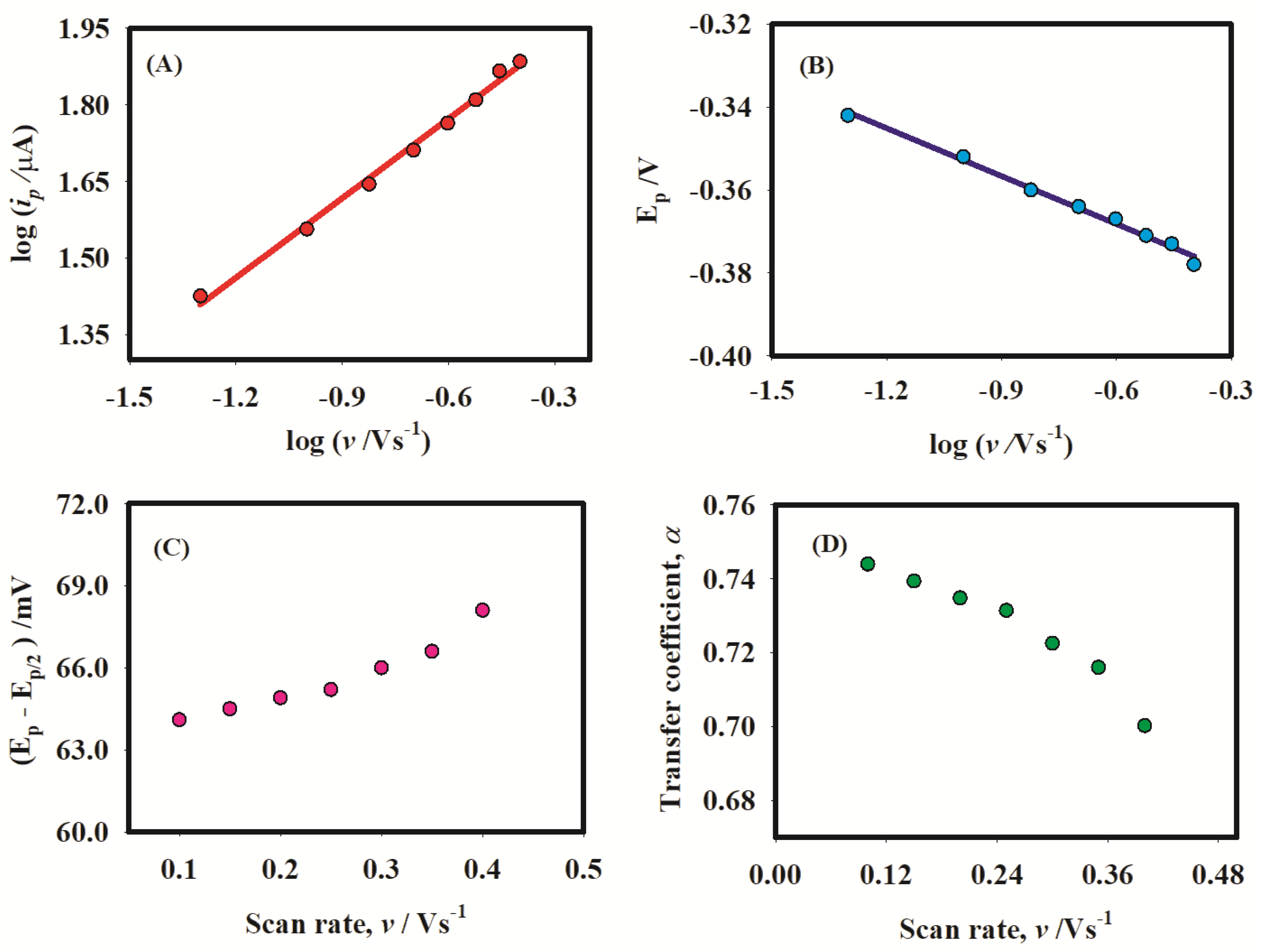
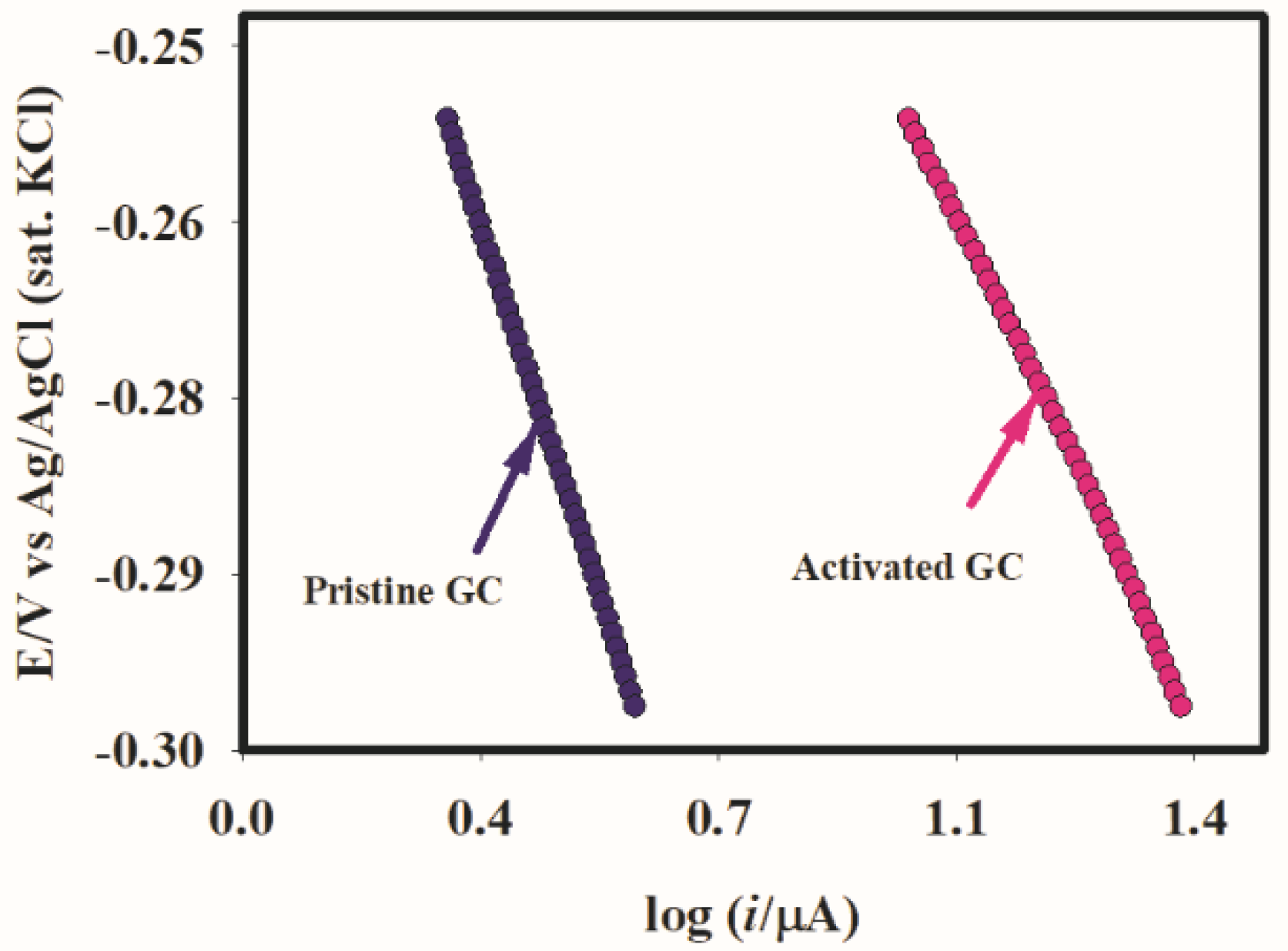
2.3. Catalysis
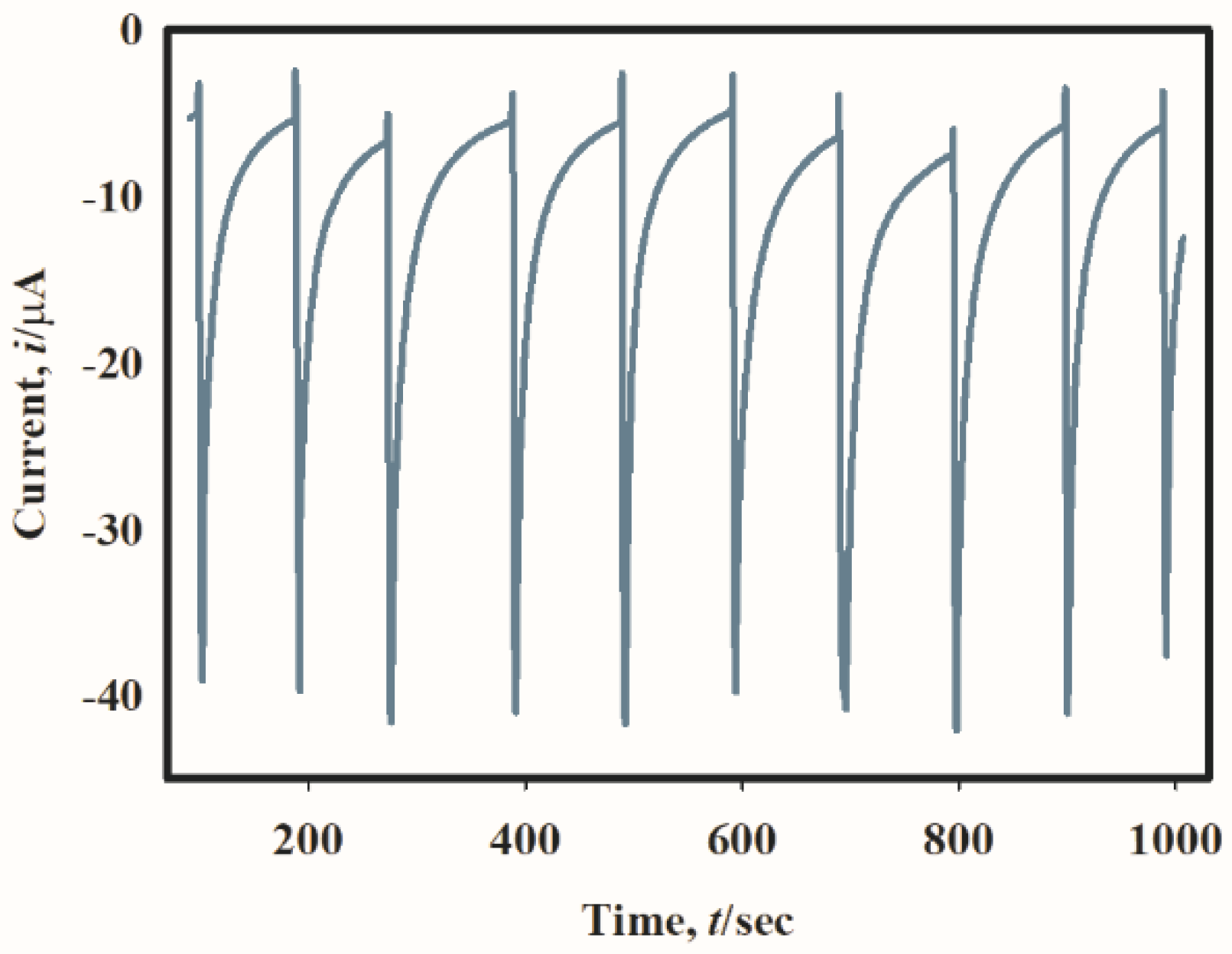
2.4. Hydrodynamic Voltammetry
2.5. Stability
3. Materials and Methods
3.1. Chemicals
3.2. Electrode Activation and Electrochemical Measurements
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiang, Z.; Chang, J.H.; Huang, C.P. Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions. Water Res. 2002, 36, 85–94. [Google Scholar] [CrossRef]
- Feng, L.; Yang, L.; Huang, Z.; Luo, J.; Li, M.; Wang, D.; Chen, Y. Enhancing electrocatalytic oxygen reduction on nitrogen-doped graphene by active sites implantation. Sci. Rep. 2013, 3, 3306. [Google Scholar] [CrossRef]
- Raj, C.; Retna, C.; Samanta, A.; Hyo Noh, S.; Mondal, S.; Okajima, T.; Ohsaka, T. Emerging new generation electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2016, 29, 11156–11178. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental understanding and application of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite in energy storage and conversion: Past, present, and future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.T.; Hor, T.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two-Electron Oxygen Reduction Reaction. Adv.Sci. 2021, 8, 2100076. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, C.; Cheng, Y.; Min, X.; Jiang, S.P.; Wang, S. Bifunctional Catalysts for Reversible Oxygen Evolution Reaction and Oxygen Reduction Reaction. Chem. Eur. J. 2020, 26, 3906–3929. [Google Scholar] [CrossRef] [PubMed]
- Risch, M. Perovskite electrocatalysts for the Oxygen reduction reaction in alkaline media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef]
- Lu, X.F.; Xia, B.Y.; Zang, S.Q.; Lou, X.W. Metal–Organic Frameworks Based Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 4634–4650. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Nia, P.M.; Etesami, M.; Abdullah, E.C.; Abdullah, L.C. Electrocatalytic activity of starch/Fe3O4/zeolite bionanocomposite for oxygen reduction reaction. Arab. J. Chem. 2020, 13, 1297–1308. [Google Scholar] [CrossRef]
- Feng, J.; Cai, R.; Magliocca, E.; Luo, H.; Higgins, L.; Romario, G.L.F.; Liang, X.; Pedersen, A.; Xu, Z.; Guo, Z.; et al. Iron, Nitrogen Co-Doped Carbon Spheres as Low Cost, Scalable Electrocatalysts for the Oxygen Reduction Reaction. Adv. Funct. Mater. 2021, 31, 2902974. [Google Scholar] [CrossRef]
- Shahid, M.M.; Zhan, Y.; Alizadeh, M.; Sagadevan, S.; Paiman, S.; Oh, W.C. A glassy carbon electrode modified with tailored nanostructures of cobalt oxide for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 18850–18858. [Google Scholar] [CrossRef]
- Islam, N.; Abir, A.Y.; Ahmed, J.; Faisal, M.; Algethami, J.S.; Harraz, F.A.; Hasnat, M.A. Electrocatalytic oxygen reduction reaction at FeS2-CNT/GCE surface in alkaline medium. J. Electroanal. Chem. 2023, 941, 117568. [Google Scholar] [CrossRef]
- Nyoni, S.; Nyokong, T. Development of graphene/CdSe quantum dots-co phthalocyanine nanocomposite for oxygen reduction reaction. Electroanalysis 2014, 26, 2261–2272. [Google Scholar] [CrossRef]
- Pickrahn, K.L.; Park, S.W.; Gorlin, Y.; Lee, H.B.R.; Jaramillo, T.F.; Bent, S.F. Active MnO x electrocatalysts prepared by atomic layer deposition for oxygen evolution and oxygen reduction reactions. Adv. Energy Mater. 2012, 2, 1269–1277. [Google Scholar] [CrossRef]
- Calfumán, K.; Quezada, D.; Isaacs, M.; Bollo, S. Enhanced Hydrogen Peroxide Sensing Based on Tetraruthenated Porphyrins/Nafion/Glassy Carbon-modified Electrodes via Incorporating of Carbon Nanotubes. Electroanalysis 2015, 27, 2778–2784. [Google Scholar] [CrossRef]
- Çakar, I.; Özdokur, K.V.; Demir, B.; Yavuz, E.; Demirkol, D.O.; Koçak, S.; Timur, S.; Ertaş, F.N. Molybdenum oxide/platinum modified glassy carbon electrode: A novel electrocatalytic platform for the monitoring of electrochemical reduction of oxygen and its biosensing applications. Sens. Actuators B Chem. 2013, 185, 331–336. [Google Scholar] [CrossRef]
- Seinberg, J.M.; Kullapere, M.; Mäeorg, U.; Maschion, F.C.; Maia, G.; Schiffrin, D.J.; Tammeveski, K. Spontaneous modification of glassy carbon surface with anthraquinone from the solutions of its diazonium derivative: An oxygen reduction study. J. Electroanal. Chem. 2008, 624, 151–160. [Google Scholar] [CrossRef]
- Krivenko, A.G.; Manzhos, R.A.; Protasova, S.G. Effect of impulse high voltage anodic and cathodic electrochemical treatment of a glassy carbon electrode on the oxygen reduction reaction in alkaline media. Electrochem. Commun. 2018, 96, 57–60. [Google Scholar] [CrossRef]
- Sharma, R.K.; Müller, V.; Chatenet, M.; Djurado, E. Oxygen reduction reaction electrocatalysis in alkaline electrolyte on glassy-carbon-supported nanostructured Pr6O11 thin-films. Catalysts 2018, 8, 461. [Google Scholar] [CrossRef]
- Machhindra, L.A.; Yen, Y.K. A Highly Sensitive Electrochemical Sensor for Cd2+ Detection Based on Prussian Blue-PEDOT-Loaded Laser-Scribed Graphene-Modified Glassy Carbon Electrode. Chemosensors 2022, 10, 209. [Google Scholar] [CrossRef]
- Ntsendwana, B.; Mamba, B.B.; Sampath, S.; Arotiba, O.A. Electrochemical detection of bisphenol a using graphene-modified glassy carbon electrode. Int. J. Electrochem. Sci. 2012, 7, 3501–3512. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.M. The electrochemical properties of acetaminophen on bare glassy carbon electrode. Int. J. Electrochem. Sci. 2012, 7, 2175–2187. [Google Scholar] [CrossRef]
- Tsuda, T.; Kurihara, T.; Hoshino, Y.; Kiyama, T.; Okazaki, K.I.; Torimoto, T.; Kuwabata, S. Electrocatalytic activity of platinum nanoparticles synthesized by room-temperature ionic liquid-sputtering method. Electrochemistry 2009, 77, 693–695. [Google Scholar] [CrossRef][Green Version]
- Cogal, S. Electrochemical Determination of Dopamine Using a Poly(3,4-Ethylenedioxythiophene)-Reduced Graphene Oxide-Modified Glassy Carbon Electrode. Anal. Lett. 2018, 51, 1666–1679. [Google Scholar] [CrossRef]
- Chetankumar, K.; Kumara, B.E.S. Electrochemically nitric acid pre-treated glassy carbon electrode sensor for catechol and hydroquinone: A voltammetric study. Sens. Int. 2020, 1, 10001. [Google Scholar] [CrossRef]
- Kairy, P.; Hossain, M.; Khan, A.; Almahri, A.; Raham, M.M.; Hasnat, M.A. Electrocatalytic oxidation of ascorbic acid in the basic medium over electrochemically functionalized glassy carbon surface. Surf. Interfaces 2022, 33, 102200. [Google Scholar] [CrossRef]
- Siddika, M.; Ahmed, J.; Aoki, K.; Faisal, M.; Algethami, J.S.; Harraz, F.A.; Nagao, Y.; Hasnat, M.A. Kinetics of Electrocatalytic Oxidation of Gallic Acid by Activated Glassy Carbon Electrode in Acidic Medium. ChemistrySelect 2023, 8, e202302074. [Google Scholar] [CrossRef]
- Hossain, M.I.; Dutta, A.; Ahmed, J.; Aoki, K.; Faisal, M.; Algethami, J.S.; Harraz, F.A.; Nagao, Y.; Hasnat, M.A. Electroless Deposition of Gold on a Glassy Carbon Electrode (GCE) Surface for Selective Detection of Hydrogen Peroxide. ChemistrySelect 2024, 8, e202301638. [Google Scholar] [CrossRef]
- Ilangovan, G.; Pillai, K.C. Electrochemical and XPS characterization of glassy carbon electrode surface effects on the preparation of a monomeric molybdate(VI)-modified electrode. Langmuir 1997, 13, 566–575. [Google Scholar] [CrossRef]
- Mondal, S.K.; Raman, R.K.; Shukla, A.K.; Munichandraiah, N. Electrooxidation of ascorbic acid on polyaniline and its implications to fuel cells. J. Power Sources 2005, 145, 16–20. [Google Scholar] [CrossRef][Green Version]
- Hur, J.; Lee, B.M.; Lee, T.H.; Park, D.H. Estimation of biological oxygen demand and chemical oxygen demand for combined sewer systems using synchronous fluorescence spectra. Sensors 2010, 10, 2460–2471. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Yasukawa, T.; Shiku, H.; Matsue, T. Fabrication of miniature Clark oxygen sensor integrated with microstructure. Sens. Actuators B Chem. 2005, 110, 342–349. [Google Scholar] [CrossRef]
- Wang, Y.; Hosono, T.; Hasebe, Y. Hemin-adsorbed carbon felt for sensitive and rapid flow-amperometric detection of dissolved oxygen. Microchim. Acta. 2013, 180, 1295–1302. [Google Scholar] [CrossRef]
- Choi, K.; Kim, S. Theoretical Study of Oxygen Reduction Reaction Mechanism in Metal-Free Carbon Materials: Defects, Structural Flexibility, and Chemical Reaction. ACS Nano 2022, 16, 16394–16401. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.S.F.; Fortunato, G.V.; Maia, G. Use of Rotating Ring-Disk Electrodes to Investigate Graphene Nanoribbon Loadings for the Oxygen Reduction Reaction in Alkaline Medium. ChemElectroChem 2018, 5, 1691–1701. [Google Scholar] [CrossRef]
- Kruusenberg, I.; Matisen, L.; Jiang, H.; Huuppola, M.; Kontturi, K.; Tammeveski, K. Electrochemical reduction of oxygen on double-walled carbon nanotube modified glassy carbon electrodes in acid and alkaline solutions. Electrochem. Commun. 2010, 12, 920–923. [Google Scholar] [CrossRef]
- Golubović, J.; Srejić, I.; Štrbac, S. Oxygen Reduction on Glassy Carbon-Supported PdAu Nanoparticles in Perchloric Acid Solution. Int. J. Electrochem. Sci. 2021, 16, 210818. [Google Scholar] [CrossRef]
- Seyyedi, B.; Ahmadi, V.B.; Habibi, E. Bio-inspired iron/sulfur/graphene nanocomposite and its use in the catalysis of the oxygen reduction reaction at room temperature in alkaline media on a glassy carbon electrode. J. Chin. Chem. Soc. 2019, 66, 515–521. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallon, E. Carbon material and cobalt-substitution effects in the electrochemical behavior of LaMnO3 for ORR and OER. Nanomaterials 2020, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Kasatkin, P.E.; Härk, E.; Jäger, R.; Lust, E. Oxygen reduction reaction in alkaline solution: Influence of catalyst loading and carbon support characteristics. ECS Trans. 2015, 64, 115–123. [Google Scholar] [CrossRef]
- Vaik, K.; Schiffrin, D.J.; Tammeveski, K. Electrochemical reduction of oxygen on anodically pre-treated and chemically grafted glassy carbon electrodes in alkaline solutions. Electrochem. Commun. 2004, 6, 1–5. [Google Scholar] [CrossRef]
- Kaskiala, T. Determination of oxygen solubility in aqueous sulphuric acid media. Miner. Eng. 2002, 15, 853–857. [Google Scholar] [CrossRef]
- Tammeveski, K.; Kontturi, K.; Nichols, R.J.; Potter, R.J.; Schiffrin, D.J. Surface redox catalysis for O2 reduction on quinone-modified glassy carbon electrodes. J. Electroanal. Chem. 2001, 515, 101–112. [Google Scholar] [CrossRef]
- Baur, J.E.; Spaine, T.W. Electrochemical deposition of iridium(IV) oxide from alkaline solutions of iridium(III) oxide. J. Electroanal. Chem. 1998, 443, 208–216. [Google Scholar] [CrossRef]


| Electrodes | Medium | Peak Potential/V | Onset Potential/V | Reference |
|---|---|---|---|---|
| Co3O4/GC | 0.5 M KOH | −0.46 | −0.36 | [14] |
| FeS2-CNT/GC | 0.1 M NaOH | 0.49 | 0.60 | [15] |
| GNR/GC | 0.1 M KOH | −0.3 | 0.8 | [38] |
| DWCNT/GC | 0.1 M KOH | −0.5 | −0.4 | [39] |
| Pr6O11/GC | 0.1 M NaOH | - | 0.84 | [40] |
| Fe-S-graphene/GC | 0.1 M KOH | 0.8 V | 1.0 | [41] |
| LaMn1−xCoxO3/GC | 0.1 M KOH | 0.5 V | 0.7 | [42] |
| Pt-C/GC | 0.1 M KOH | −0.3 | 0.10 | [43] |
| Oxidized GC | 0.1 M KOH | −0.5 | - | [44] |
| Activated GC | 0.1 M NaOH | −0.34 | −0.22 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddika, M.; Hosen, N.; Althomali, R.H.; Al-Humaidi, J.Y.; Rahman, M.M.; Hasnat, M.A. Kinetics of Electrocatalytic Oxygen Reduction Reaction over an Activated Glassy Carbon Electrode in an Alkaline Medium. Catalysts 2024, 14, 164. https://doi.org/10.3390/catal14030164
Siddika M, Hosen N, Althomali RH, Al-Humaidi JY, Rahman MM, Hasnat MA. Kinetics of Electrocatalytic Oxygen Reduction Reaction over an Activated Glassy Carbon Electrode in an Alkaline Medium. Catalysts. 2024; 14(3):164. https://doi.org/10.3390/catal14030164
Chicago/Turabian StyleSiddika, Munira, Nazmul Hosen, Raed H. Althomali, Jehan Y. Al-Humaidi, Mohammed M. Rahman, and Mohammad A. Hasnat. 2024. "Kinetics of Electrocatalytic Oxygen Reduction Reaction over an Activated Glassy Carbon Electrode in an Alkaline Medium" Catalysts 14, no. 3: 164. https://doi.org/10.3390/catal14030164
APA StyleSiddika, M., Hosen, N., Althomali, R. H., Al-Humaidi, J. Y., Rahman, M. M., & Hasnat, M. A. (2024). Kinetics of Electrocatalytic Oxygen Reduction Reaction over an Activated Glassy Carbon Electrode in an Alkaline Medium. Catalysts, 14(3), 164. https://doi.org/10.3390/catal14030164









