Hydrogen Production from Hydrous Hydrazine Decomposition Using Ir Catalysts: Effect of the Preparation Method and the Support
Abstract
1. Introduction
2. Results
2.1. Effect of the Preparation Method
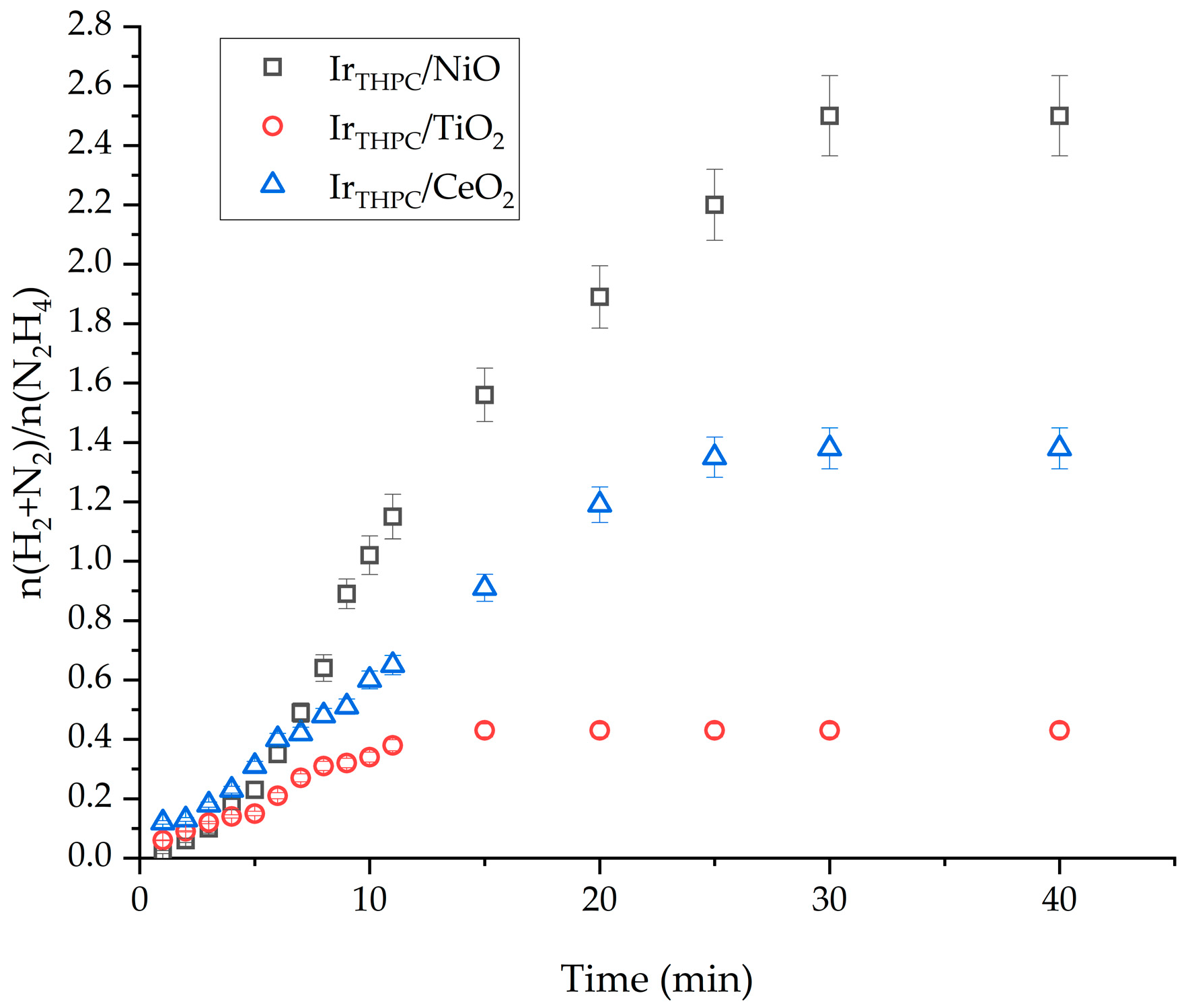
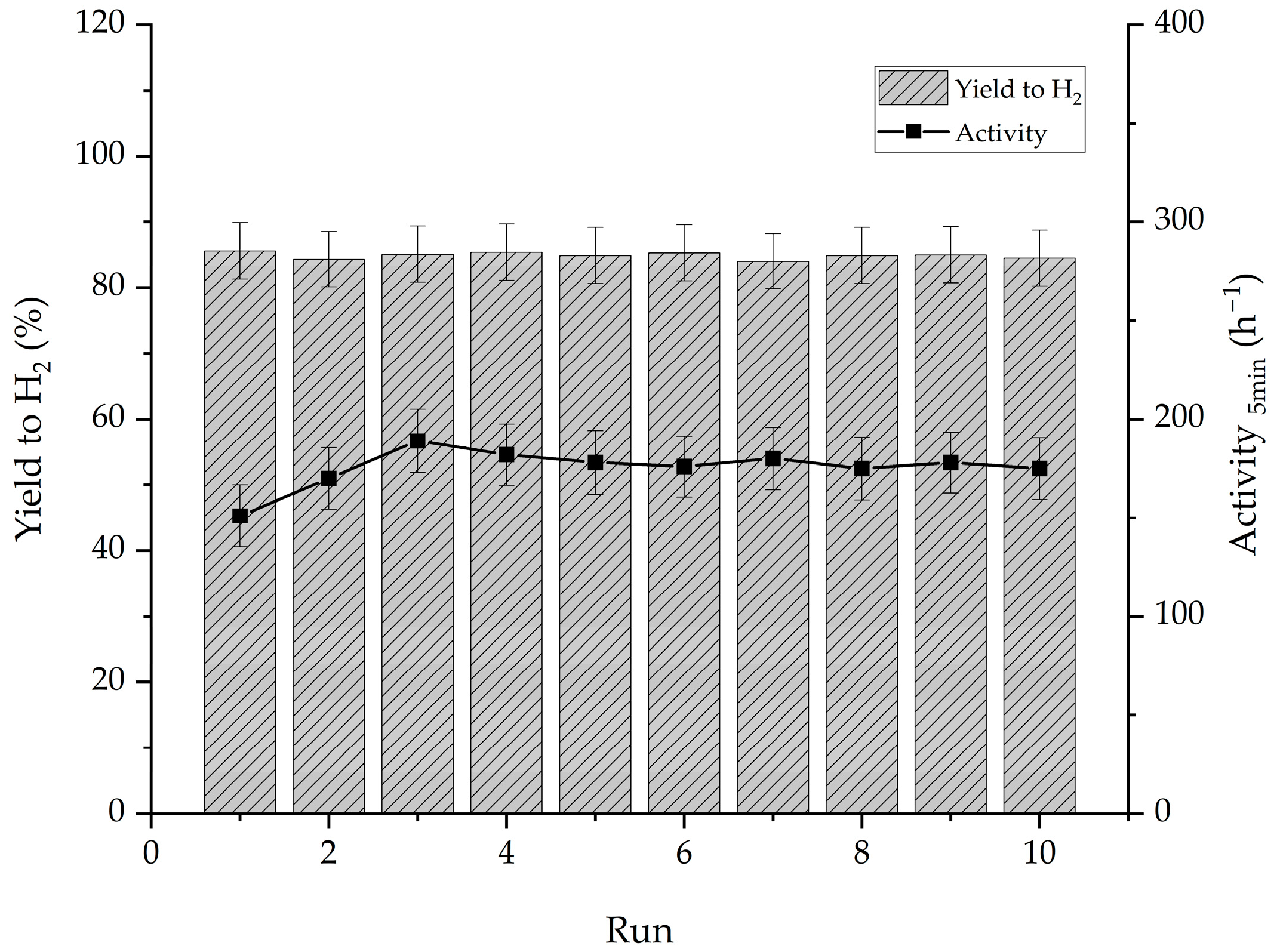
2.2. Influence of the Support Material
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Supported Ir Catalyst Preparation using Deposition–Precipitation/NaOH
3.1.2. Supported Ir Catalyst Preparation Using Deposition–Precipitation/Urea
3.1.3. Supported Ir Catalyst Preparation Using THPC/NaOH Colloidal Method
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Ramachandran, R.; Menon, R.K. An overview of industrial uses of hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Xu, H. Catalytic decomposition of hydrous hydrazine for hydrogen production. Sustain. Energy Fuels 2018, 3, 343–365. [Google Scholar] [CrossRef]
- Matyshak, V.A.; Silchenkova, O.N. Catalytic Decomposition of Hydrazine and Hydrazine Derivatives to Produce Hydrogen-Containing Gas Mixtures: A Review. Kinet. Catal. 2022, 63, 339–350. [Google Scholar] [CrossRef]
- Zhang, P.-X.; Wang, Y.-G.; Huang, Y.-Q.; Zhang, T.; Wu, G.-S.; Li, J. Density functional theory investigations on the catalytic mechanisms of hydrazine decompositions on Ir(111). Catal. Today 2011, 165, 80–88. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, X.; Cheng, R.; Li, N.; Sun, J.; Wang, X.; Zhang, T. Catalytic decomposition of hydrazine on iron nitride catalysts. Catal. Commun. 2006, 7, 187–191. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Xia, L.; Li, T.; Zheng, M.; Wu, Z.; Wang, X.; Wei, Z.; Xin, Q.; Li, C. Catalytic Decomposition of Hydrazine over Supported Molybdenum Nitride Catalysts in a Monopropellant Thruster. Catal. Lett. 2002, 79, 21–25. [Google Scholar] [CrossRef]
- McKay, H.L.; Jenkins, S.J.; Wales, D.J. Dissociative Chemisorption of Hydrazine on an Fe{211} Surface. J. Phys. Chem. C 2011, 115, 17812–17828. [Google Scholar] [CrossRef]
- Song-Il, O.; Yan, J.-M.; Wang, H.-L.; Wang, Z.-L.; Jiang, Q. High catalytic kinetic performance of amorphous CoPt NPs induced on CeO for H2 generation from hydrous hydrazine. Int. J. Hydrogen Energy 2014, 39, 3755–3761. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Wang, A.; Wang, X.; Chen, X.; Delgado, J.J.; Zhang, T. A Noble-Metal-Free Catalyst Derived from Ni-Al Hydrotalcite for Hydrogen Generation from N2H4·H2O Decomposition. Angew. Chem. Int. Ed. 2012, 51, 6191–6194. [Google Scholar] [CrossRef]
- Kang, W.; Varma, A. Hydrogen generation from hydrous hydrazine over Ni/CeO2 catalysts prepared by solution combustion synthesis. Appl. Catal. B Environ. 2018, 220, 409–416. [Google Scholar] [CrossRef]
- Sanabria-Chinchilla, J.; Asazawa, K.; Sakamoto, T.; Yamada, K.; Tanaka, H.; Strasser, P. Noble Metal-Free Hydrazine Fuel Cell Catalysts: EPOC Effect in Competing Chemical and Electrochemical Reaction Pathways. J. Am. Chem. Soc. 2011, 133, 5425–5431. [Google Scholar] [CrossRef]
- Huang, W.; Liu, X. The “on–off” switch for on-demand H2 evolution from hydrous hydrazine over Ni8Pt1/C nano-catalyst. Fuel 2022, 315, 123210. [Google Scholar] [CrossRef]
- Yao, Q.; Long, J.; Yang, K.; Li, X.; Huang, B.; Chen, X.; Lu, Z.-H. Alkali-assisted synthesis of ultrafine NiPt nanoparticles immobilized on La2O2CO3 for highly efficient dehydrogenation of hydrous hydrazine and hydrazine borane. Catal. Today 2022, 400–401, 49–58. [Google Scholar] [CrossRef]
- Singh, S.K.; Zhang, X.-B.; Xu, Q. Room-Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage. J. Am. Chem. Soc. 2009, 131, 9894–9895. [Google Scholar] [CrossRef]
- Singh, S.K.; Iizuka, Y.; Xu, Q. Nickel-palladium nanoparticle catalyzed hydrogen generation from hydrous hydrazine for chemical hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 11794–11801. [Google Scholar] [CrossRef]
- Singh, S.K.; Xu, Q. Complete Conversion of Hydrous Hydrazine to Hydrogen at Room Temperature for Chemical Hydrogen Storage. J. Am. Chem. Soc. 2009, 131, 18032–18033. [Google Scholar] [CrossRef]
- Wood, S.E.; Bryant, J.T. Decomposition of Hydrazine on Shell 405 Catalyst at High Presure. Prod. R&D 1973, 12, 117–122. [Google Scholar] [CrossRef]
- Jang, Y.B.; Kim, T.H.; Sun, M.H.; Lee, J.; Cho, S.J. Preparation of iridium catalyst and its catalytic activity over hydrazine hydrate decomposition for hydrogen production and storage. Catal. Today 2009, 146, 196–201. [Google Scholar] [CrossRef]
- Bellomi, S.; Barlocco, I.; Chen, X.; Delgado, J.J.; Arrigo, R.; Dimitratos, N.; Roldan, A.; Villa, A. Enhanced stability of sub-nanometric iridium decorated graphitic carbon nitride for H2 production upon hydrous hydrazine decomposition. Phys. Chem. Chem. Phys. 2022, 25, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Vasanthkumar, M.S. Iridium-decorated multiwall carbon nanotubes and its catalytic activity with Shell 405 in hydrazine decomposition. J. Nanoparticle Res. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Motta, D.; Barlocco, I.; Bellomi, S.; Villa, A.; Dimitratos, N. Hydrous Hydrazine Decomposition for Hydrogen Production Using of Ir/CeO2: Effect of Reaction Parameters on the Activity. Nanomaterials 2021, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Guczi, L.; Horváth, D.; Pászti, Z.; Tóth, L.; Horváth, Z.E.; Karacs, A.; Petõ, G. Modeling Gold Nanoparticles: Morphology, Electron Structure, and Catalytic Activity in CO Oxidation. J. Phys. Chem. B 2000, 104, 3183–3193. [Google Scholar] [CrossRef]
- Henry, C.R. Morphology of supported nanoparticles. Prog. Surf. Sci. 2005, 80, 92–116. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Cerium-Oxide-Modified Nickel as a Non-Noble Metal Catalyst for Selective Decomposition of Hydrous Hydrazine to Hydrogen. ACS Catal. 2015, 5, 1623–1628. [Google Scholar] [CrossRef]
- He, L.; Liang, B.; Huang, Y.; Zhang, T. Design strategies of highly selective nickel catalysts for H2 production via hydrous hydrazine decomposition: A review. Natl. Sci. Rev. 2017, 5, 356–364. [Google Scholar] [CrossRef]
- Campisi, S.; Motta, D.; Barlocco, I.; Stones, R.; Chamberlain, T.W.; Chutia, A.; Dimitratos, N.; Villa, A. Furfural Adsorption and Hydrogenation at the Oxide-Metal Interface: Evidence of the Support Influence on the Selectivity of Iridium-Based Catalysts. ChemCatChem 2022, 14, e202101700. [Google Scholar] [CrossRef]
- Wang, W.; Villa, A.; Kuebel, C.; Hahn, H.; Wang, D. Tailoring the 3D Structure of Pd Nanocatalysts Supported on Mesoporous Carbon for Furfural Hydrogenation. Chemnanomat 2018, 4, 1125–1132. [Google Scholar] [CrossRef]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-ray photoelectron spectra of Ir, IrO2 and IrCl3 revisited. Surf. Interface Anal. 2017, 49, 794–799. [Google Scholar] [CrossRef]
- Lykhach, Y.; Kubát, J.; Neitzel, A.; Tsud, N.; Vorokhta, M.; Skála, T.; Dvořák, F.; Kosto, Y.; Prince, K.C.; Matolín, V.; et al. Charge transfer and spillover phenomena in ceria-supported iridium catalysts: A model study. J. Chem. Phys. 2019, 151, 204703. [Google Scholar] [CrossRef]
- Lee, S.; Fan, C.; Wu, T.; Anderson, S.L. Hydrazine Decomposition over Irn/Al2O3 Model Catalysts Prepared by Size-Selected Cluster Deposition. J. Phys. Chem. B 2004, 109, 381–388. [Google Scholar] [CrossRef]
- Fan, C.; Wu, T.; Kaden, W.E.; Anderson, S.L. Cluster size effects on hydrazine decomposition on Irn/Al2O3/NiAl(110). Surf. Sci. 2006, 600, 461–467. [Google Scholar] [CrossRef]
- Parapat, R.Y.; Saputra, O.H.I.; Ang, A.P.; Schwarze, M.; Schomäcker, R. Support effect in the preparation of supported metal catalysts via microemulsion. RSC Adv. 2014, 4, 50955–50963. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, J.; Zhang, Y.; Pan, C.; Dong, Y.; Zhu, Y. Metal-support interaction for heterogeneous catalysis: From nanoparticles to single atoms. Mater. Today Nano 2020, 12, 100093. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chem. Rev. 2019, 120, 1250–1349. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.E.; Chinchilla, L.E.; Chutia, A.; Botton, G.A.; Mohammed, K.M.H.; Dimitratos, N.; Wells, P.P.; Villa, A. Dual-Site-Mediated Hydrogenation Catalysis on Pd/NiO: Selective Biomass Transformation and Maintenance of Catalytic Activity at Low Pd Loading. ACS Catal. 2020, 10, 5483–5492. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Chen, Y.; Li, W.; Lin, L.; Li, M.; Deng, Y.; Wang, X.; Ge, B.; Yang, C.; et al. Tuning the Selectivity of Catalytic Carbon Dioxide Hydrogenation over Iridium/Cerium Oxide Catalysts with a Strong Metal–Support Interaction. Angew. Chem. Int. Ed. 2017, 56, 10761–10765. [Google Scholar] [CrossRef]
- Lu, X.; Francis, S.; Motta, D.; Dimitratos, N.; Roldan, A. Mechanistic study of hydrazine decomposition on Ir(111). Phys. Chem. Chem. Phys. 2020, 22, 3883–3896. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, X.; Du, Y.; Chen, R.; Xing, W. Catalytic activity of palladium nanoparticles immobilized on an amino-functionalized ceramic membrane support. Chin. J. Catal. 2014, 35, 1990–1996. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]


| Catalyst | XPS | TEM | Ir Loading (wt%) | Activity (h−1) | H2 Yield (%) | |||
|---|---|---|---|---|---|---|---|---|
| Ir 4f | Ir at (%) | Ir NP Size (nm) | ||||||
| Ir0 | IrIV | |||||||
| IrDPNaOH/CeO2 | BE | 61.6 | 62.6 | 0.39 | 0.9 ± 0.2 | 0.70 ± 0.10 | 1541 | 38.9 |
| % | 87.6 | 12.4 | ||||||
| IrDPurea/CeO2 | BE | 61.6 | 62.8 | 0.41 | 3.6 ± 0.5 | 0.98 ± 0.06 | 741 | 0.7 |
| % | 70.6 | 29.4 | ||||||
| IrTHPC/CeO2 | BE | 61.7 | 62.6 | 0.86 | 1.1 ± 0.3 | 1.04 ± 0.13 | 1740 | 36.6 |
| % | 87.1 | 12.9 | ||||||
| IrTHPC/TiO2 | BE | 60.9 | - | 0.40 | 1.3 ± 0.2 | 0.98 ± 0.08 | 984 | 4.5 |
| % | 100 | - | ||||||
| IrTHPC/NiO | BE | 60.9 | 62.2 | 1.73 | 1.2 ± 0.3 | 1.00 ± 0.10 | 151 | 83.9 |
| % | 84.9 | 15.1 | ||||||
| IrTHPC/NiO after stability tests | BE | 61.2 | 62.4 | 1.73 | 1.2 ± 0.3 | 1.00 ± 0.10 | - | - |
| % | 87.2 | 13.8 | 1.64 | n.d. | 0.98 ± 0.13 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellomi, S.; Motta, D.; Stucchi, M.; Prati, L.; Dimitratos, N.; Villa, A. Hydrogen Production from Hydrous Hydrazine Decomposition Using Ir Catalysts: Effect of the Preparation Method and the Support. Catalysts 2024, 14, 119. https://doi.org/10.3390/catal14020119
Bellomi S, Motta D, Stucchi M, Prati L, Dimitratos N, Villa A. Hydrogen Production from Hydrous Hydrazine Decomposition Using Ir Catalysts: Effect of the Preparation Method and the Support. Catalysts. 2024; 14(2):119. https://doi.org/10.3390/catal14020119
Chicago/Turabian StyleBellomi, Silvio, Davide Motta, Marta Stucchi, Laura Prati, Nikolaos Dimitratos, and Alberto Villa. 2024. "Hydrogen Production from Hydrous Hydrazine Decomposition Using Ir Catalysts: Effect of the Preparation Method and the Support" Catalysts 14, no. 2: 119. https://doi.org/10.3390/catal14020119
APA StyleBellomi, S., Motta, D., Stucchi, M., Prati, L., Dimitratos, N., & Villa, A. (2024). Hydrogen Production from Hydrous Hydrazine Decomposition Using Ir Catalysts: Effect of the Preparation Method and the Support. Catalysts, 14(2), 119. https://doi.org/10.3390/catal14020119










