Cyclopentadienyl Amidinate Ligand Directing Effects in the Enantioselective Living Coordinative Chain Transfer Polymerization of 1,5-Hexadiene
Abstract
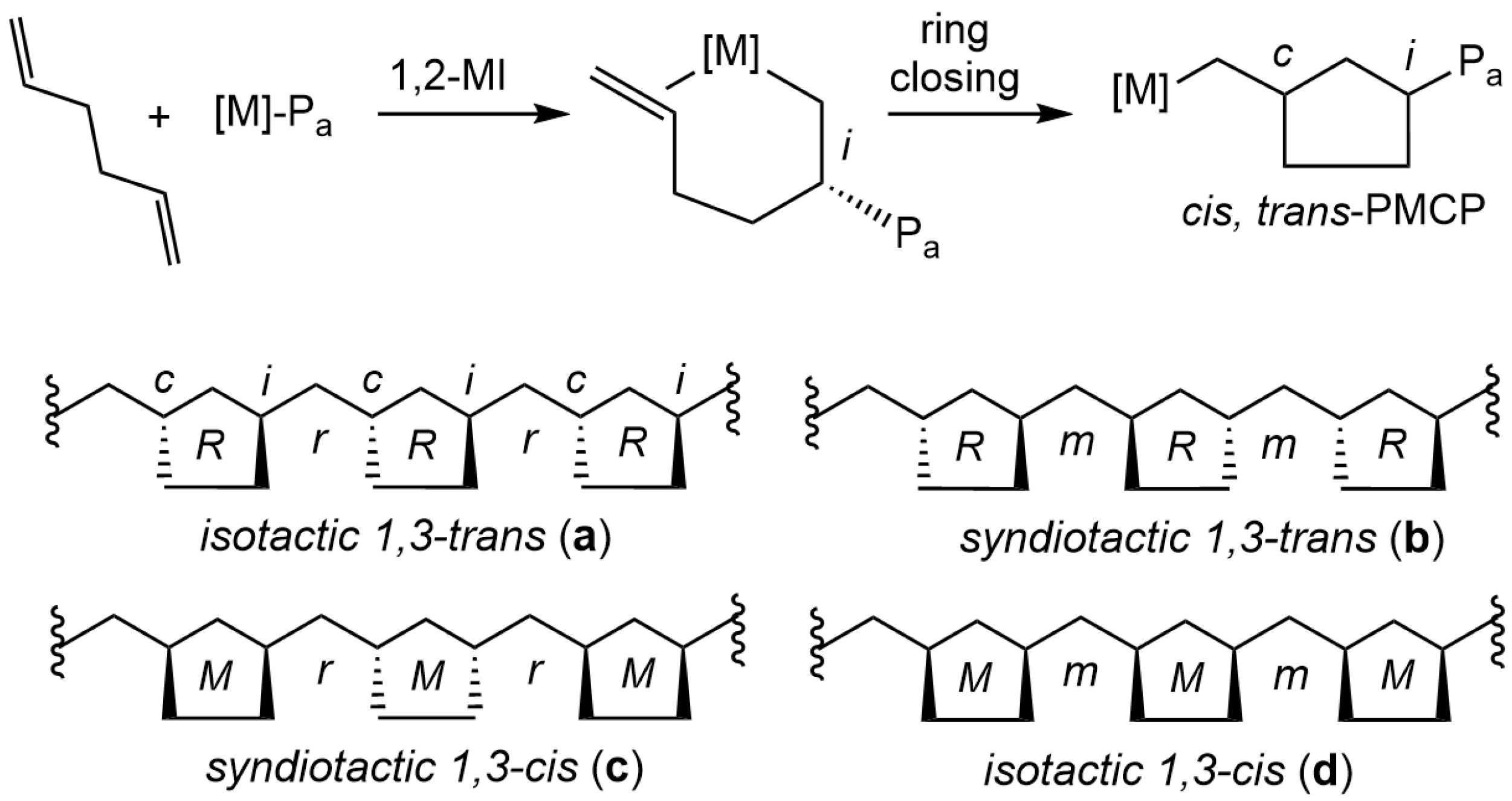
1. Introduction
2. Results and Discussion
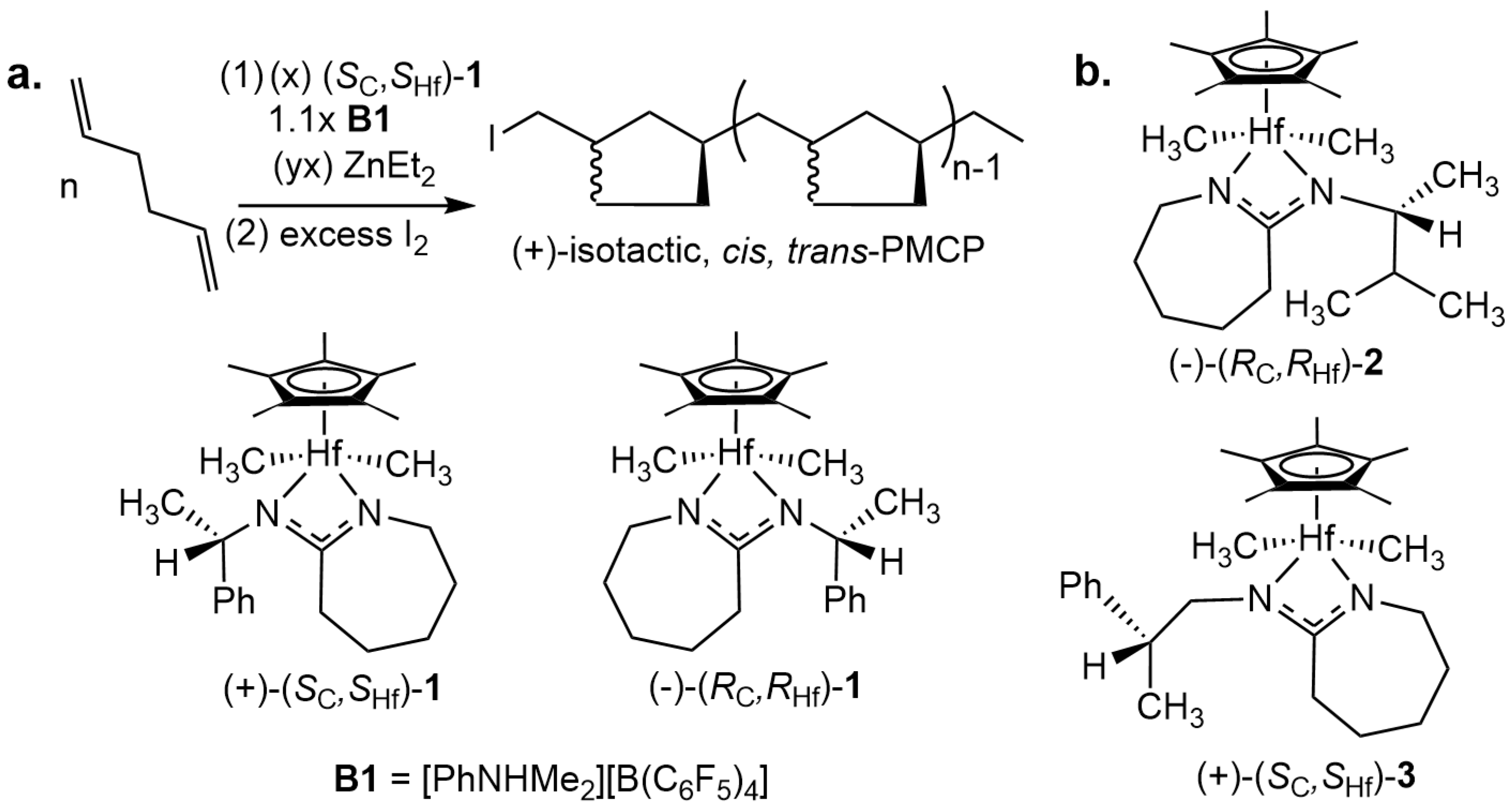
2.1. Synthesis of New CPAM Hafnuium Pre-Initiators 2 and 3
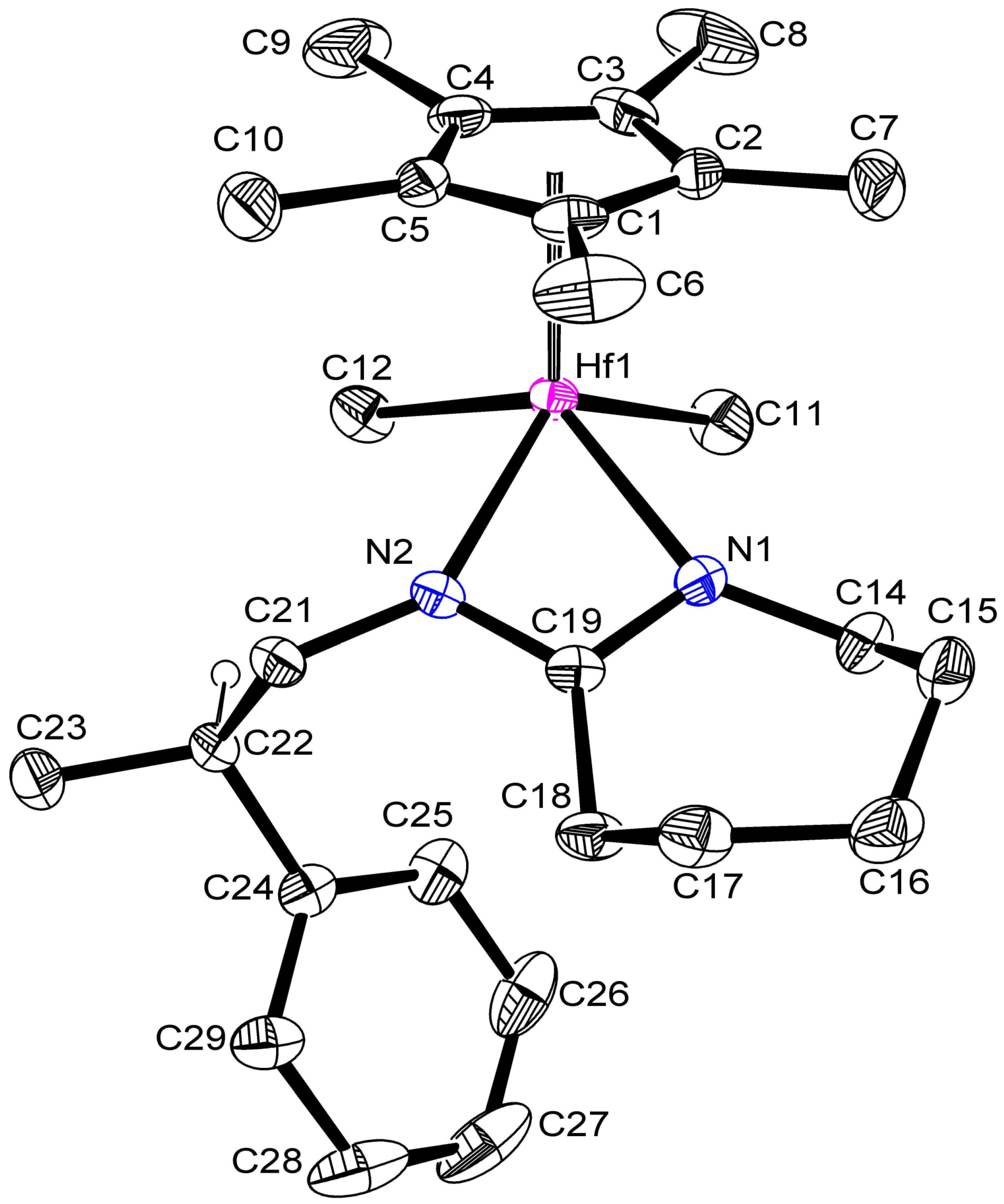
2.2. Structural Characterization of 2 and 3
2.3. Living Coordinative (Chain Transfer) Polymerization of 1,5-Hexadiene by (RC, RHf)-2 and (SC, SHf)-3 as Pre-Initiators
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vasile, C. (Ed.) Handbook of Polyolefins; Marcel Dekker, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Kaminsky, W. (Ed.) Polyolefins: 50 Years after Ziegler and Natta I: Polyethylene and Polypropylene. In Advances in Polymer Science ; Springer: Berlin/Heidelberg, Germany, 2013; Volume 257. [Google Scholar]
- Kaminsky, W. (Ed.) Polyolefins: 50 Years after Ziegler and Natta II: Polyolefins by Metallocenes and Other Single-Site Catalysts. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 258. [Google Scholar]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Al-AliAlMa’adeed, M.; Krupa, I. (Eds.) Polyolefin Compounds and Materials; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Resconi, L.; Waymouth, R.M. Diastereoselectivity in the Homogeneous Cyclopolymerization of 1,5-Hexadiene. J. Am. Chem. Soc. 1990, 112, 4953–4954. [Google Scholar] [CrossRef]
- Resconi, L.; Coates, G.W.; Mogstad, A.; Waymouth, R.M. Stereospecific Cyclopolymerization with Group 4 Metallocenes. J. Macromol. Sci. Chem. 1991, 28, 1225–1234. [Google Scholar] [CrossRef]
- Cavallo, L.; Gurerra, G.; Corradini, P.; Resconi, L.; Waymouth, R.M. Model Catalytic Sites for Olefin Polymerization and Diastereoselectivity in the Cyclopolymerization of 1,5-Hexadiene. Macromolecules 1993, 26, 260–267. [Google Scholar] [CrossRef]
- Jeremic, D.; Wang, Q.Y.; Quyoum, R.; Baird, M.C. Polymerization of Norbornene and 1,5-Hexadiene by [CpTiMe2][[MeB(C6F5)3]. J. Organomet. Chem. 1995, 497, 143–147. [Google Scholar] [CrossRef]
- Mitani, M.; Oouchi, K.; Hayakawa, M.; Yamada, T.; Mukaiyama, T. Stereoselective Cyclopolymerization of 1,5-Hexadiene Using Novel Bis(ferrocenyl)zirconocene Catalyst. Chem. Lett. 1995, 24, 905–906. [Google Scholar] [CrossRef]
- Kim, I.L.; Shin, Y.S.; Lee, J.K.; Won, M.S. Cyclopolymerization of 1,5-Hexadiene Catalyzed by Various Stereospecific Metallocene Compounds. J. Polym. Sci. A Polym. Chem. 2000, 38, 1520–1527. [Google Scholar] [CrossRef]
- Hustad, P.D.; Coates, G.W. Insertion/Isomerization Polymerization of 1,5-Hexadiene: Synthesis of Functional Propylene Copolymers and Block Copolymers. J. Am. Chem. Soc. 2002, 124, 11578–11579. [Google Scholar] [CrossRef]
- Volkis, V.; Averbuj, C.; Eisen, M.S. Reactivity of Group 4 Benzamidinate Complexes towards Mono- and Bis-substituted Silanes and 1,5-Hexadiene. J. Organomet. Chem. 2007, 692, 1940–1950. [Google Scholar] [CrossRef]
- Edson, J.B.; Coates, G.W. Cyclopolymerization of Nonconjugated Dienes with a Tridentate Phenoxyamine Hafnium Complex Supported by a sp3-C Donor: Isotactic and Diastereoselective cis-Ring Closure. Macromol. Rapid Commun. 2009, 30, 1900–1906. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Liu, J.; Cui, D.; Men, Y.; Li, Y. Stereospecific Cyclopolymerization of α,ω-Diolefins by Pyridylamidohafnium Catalyst with the Highest Activity. Macromolecules 2011, 44, 1062–1065. [Google Scholar] [CrossRef]
- Coates, G.W.; Waymouth, R.M. Enantioselective Cyclopolymerization: Optically Active Poly(methylene-1,3-cyclopentane). J. Am. Chem. Soc. 1991, 113, 6270–6271. [Google Scholar] [CrossRef]
- Coates, G.W.; Waymouth, R.M. Enantioselective Cyclopolymerization of 1,5-Hexadiene Catalyzed by Chiral Zirconocenes: A Novel Strategy for the Synthesis of Optically Active Polymers with Chirality in the Main Chain. J. Am. Chem. Soc. 1993, 115, 91–98. [Google Scholar] [CrossRef]
- Yeori, A.; Goldberg, I.; Kol, M. Cyclopolymerization of 1,5-Hexadiene by Enantiomerically-Pure Zirconium Salan Complexes. Polymer Optical Activity Reveals α-Olefin Face Preference. Macromolecules 2007, 40, 8521–8523. [Google Scholar] [CrossRef]
- Nakata, N.; Watanabe, T.; Toda, T.; Ishii, A. Enantio- and Stereoselective Cyclopolymerization of Hexa-1,5-Diene Catalyzed by Zirconium Complexes Possessing Optically Active Bis(phenolato) Ligands. Macromol. Rapid Commun. 2016, 37, 1820–1824. [Google Scholar] [CrossRef]
- Naga, N.; Shimura, H.; Sone, M. Liquid Crystalline Features of Optically Active Poly(methylene-1,3-cyclopentane). Macromolecules 2008, 41, 7448–7452. [Google Scholar] [CrossRef]
- Naga, N.; Shimura, H.; Sone, M. Liquid Crystalline Features of Optically Active Poly(methylene-1,3-cyclopentane). Macromolecules 2009, 42, 7631–7633. [Google Scholar] [CrossRef]
- Tonelli, A.E.; Schilling, F.C. 13C NMR Chemical Shifts and the Microstructure of Polymers. Acc. Chem. Res. 1981, 14, 233–238. [Google Scholar] [CrossRef]
- Mogstad, A.L.; Waymouth, R.M. Chain transfer to aluminum in the homogenous cyclopolymerization of 1,5-hexadiene. Macromolecules 1992, 25, 2282–2284. [Google Scholar] [CrossRef]
- Mogstad, A.L.; Waymouth, R.M. Synthesis of diblock polyolefins and polyester copolymers using zirconium and aluminum catalysts. Macromolecules 1994, 27, 2313–2315. [Google Scholar] [CrossRef]
- Wallace, M.A.; Zavalij, P.Y.; Sita, L.R. Enantioselective living coordinative chain transfer polymerization: Production of optically active end-group-functionalized (+)- or (–)-poly(methylene-1,3-cyclopentane) via a homochiral C1-symmetric caproamidinate hafnium initiator. ACS Catal. 2020, 10, 8496–8502. [Google Scholar] [CrossRef]
- Wallace, M.A.; Wentz, C.M.; Sita, L.R. Optical purity as a programmable variable for controlling polyolefin tacticity in living coordinative chain transfer polymerization: Application to the stereomodulated LCCTP of α,ω-nonconjugated dienes. ACS Catal. 2021, 11, 4583–4592. [Google Scholar] [CrossRef]
- Jayaratne, K.C.; Keaton, R.J.; Henningsen, D.A.; Sita, L.R. Living Ziegler-Natta Cyclopolymerization of Nonconjugated Dienes: New Classes of Microphase-Separated Polyolefin Block Copolymers via a Tandem Polymerization/Cyclopolymerization Strategy. J. Am. Chem. Soc. 2000, 122, 10490–10491. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, J.; Sita, L.R. Living Coordinative Chain-Transfer Polymerization and Copolymerization of Ethene, α-Olefins, and α,ω-Nonconjugated Dienes using Dialkylzinc as “Surrogate” Chain-Growth Sites. Macromolecules 2008, 41, 7829–7833. [Google Scholar] [CrossRef]
- Crawford, K.E.; Sita, L.R. Stereoengineering of Poly(1,3-methylenecyclohexane) via Two-State Living Coordination Polymerization of 1,6-Heptadiene. J. Am. Chem. Soc. 2013, 135, 8778–8781. [Google Scholar] [CrossRef]
- Crawford, K.E.; Sita, L.R. Regio- and Stereospecific Cyclopolymerization of Bis(2-propenyl)diorganosilanes and the Two-State Stereoengineering of 3,5-cis-isotactic Poly(3,5-methylene-1-silacyclohexane)s. ACS Macro Lett. 2014, 3, 506–509. [Google Scholar] [CrossRef]
- Crawford, K.E.; Sita, L.R. De Novo Design of a New Class of “Hard-Soft” Amorphous, Microphase-Separated, Polyolefin Block Copolymer Thermoplastic Elastomers. ACS Macro Lett. 2015, 4, 921–925. [Google Scholar] [CrossRef]
- Burgenson, W.R.; Wentz, C.M.; Sita, L.R. Tailoring the glass transition temperature in a series of poly(methylene-1,3-cyclopentane-stat-cyclohexane) statistical copolymers. ACS Catal. 2023, 12, 101–106. [Google Scholar] [CrossRef]
- Wei, J.; Duman, L.M.; Redman, D.W.; Yonke, B.L.; Zavalij, P.Y.; Sita, L.R. N-Substituted Iminocaprolactams as Versatile and Low Cost Ligands in Group 4 Metal Initiators for the Living Coordinative Chain Transfer Polymerization of α-Olefins. Organometallics 2017, 36, 4202–4207. [Google Scholar] [CrossRef]
- Benson, R.E.; Cairns, T.L. Chemical Reactions of Caprolactam. J. Am. Chem. Soc. 1948, 70, 2115–2118. [Google Scholar] [CrossRef]
- Benson, R.E.; Carins, T.L. O-Methylcaprolactam. Org. Synth. 1951, 31, 72–74. [Google Scholar]
- Steinlandt, P.S.; Zhang, L.; Meggers, E. Metal Stereogenicity in Asymmetric Transition Metal Catalysts. Chem. Rev. 2023, 123, 4764–4794. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H. Optically Active Organometallic Compounds of Transition Elements with Chiral Metal Atoms. Angew. Chem. Int. Ed. 1999, 38, 1194–1208. [Google Scholar] [CrossRef]
- Amouri, H.; Gruselle, M. Chirality in Transition Metal Chemistry; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Brunner, H. Stability of the Metal Configuration in Chiral Metal Half-Sandwich Compounds. Eur. J. Inorg. Chem. 2001, 2001, 905–912. [Google Scholar] [CrossRef]
- Bauer, E.B. Chiral-at-metal complexes and their catalytic applications in organic synthesis. Chem. Soc. Rev. 2012, 41, 3153–3167. [Google Scholar] [CrossRef]


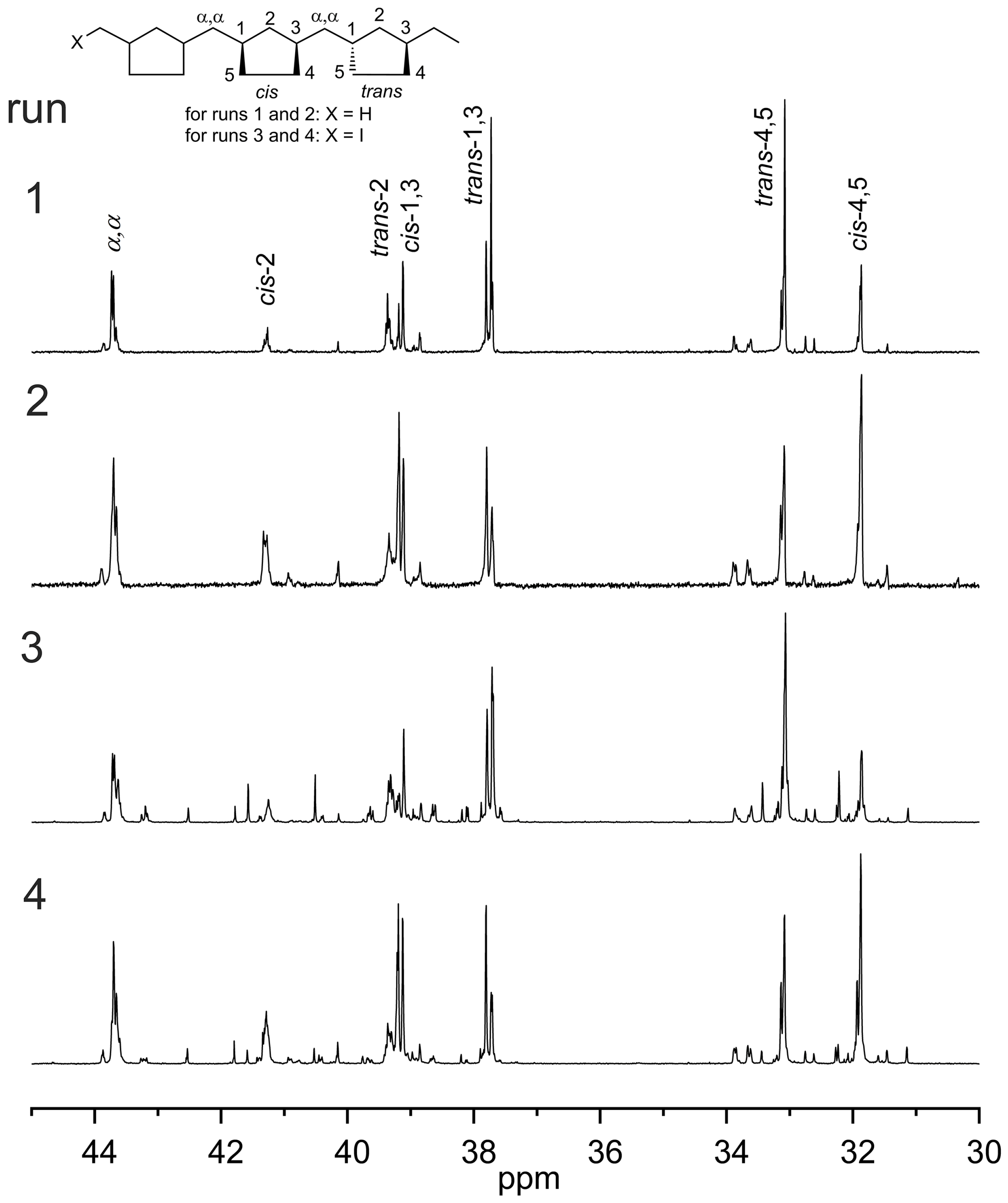
| Run | Pre-Init. | ZnEt2 (eq) | Yield (g) | Mn (kDa) b | Ð b | %trans c | Tg d | Tm d | [α]D e (°) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (RC, RHf)-2 | 0 | 0.49 | 9.23 | 1.44 | 70.4 | −4.77 | 78.0 | −5.8/8.5 |
| 2 | (SC, SHf)-3 | 0 | 0.51 | 24.2 | 1.36 | 41.1 | 2.95 | 94.8 | 3.2/−11.7 |
| 3 | (RC, RHf)-2 | 5 | 0.85 | 1.44 | 1.18 | 72.7 | n.o. f | n.o. | 7.2/−3.4 |
| 4 | (SC, SHf)-3 | 5 | 0.59 | 2.73 | 1.18 | 41.7 | n.o. | n.o. | 2.6/−9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burrows, C.M.; Zavalij, P.Y.; Sita, L.R. Cyclopentadienyl Amidinate Ligand Directing Effects in the Enantioselective Living Coordinative Chain Transfer Polymerization of 1,5-Hexadiene. Catalysts 2024, 14, 944. https://doi.org/10.3390/catal14120944
Burrows CM, Zavalij PY, Sita LR. Cyclopentadienyl Amidinate Ligand Directing Effects in the Enantioselective Living Coordinative Chain Transfer Polymerization of 1,5-Hexadiene. Catalysts. 2024; 14(12):944. https://doi.org/10.3390/catal14120944
Chicago/Turabian StyleBurrows, Cole M., Peter Y. Zavalij, and Lawrence R. Sita. 2024. "Cyclopentadienyl Amidinate Ligand Directing Effects in the Enantioselective Living Coordinative Chain Transfer Polymerization of 1,5-Hexadiene" Catalysts 14, no. 12: 944. https://doi.org/10.3390/catal14120944
APA StyleBurrows, C. M., Zavalij, P. Y., & Sita, L. R. (2024). Cyclopentadienyl Amidinate Ligand Directing Effects in the Enantioselective Living Coordinative Chain Transfer Polymerization of 1,5-Hexadiene. Catalysts, 14(12), 944. https://doi.org/10.3390/catal14120944











