Abstract
Developing sustainable TiO2-based photocatalysts for environmental remediation is an increasingly significant area of research. However, a limited understanding of the long-term ecological impact of these photocatalysts poses a barrier to their practical and industrial-scale applications. To address this challenge, this work employed a green synthesis approach to prepare an Ag/TiO2 photocatalyst designed to improve environmental compatibility and enhance efficiency in pollutant degradation. Ag/TiO2 was synthesized using mushroom biomass as a natural capping to evaluate its effectiveness in the degradation of ciprofloxacin (CIP) and azo Carmine G dye (ACGD). The mushroom biomass served as a renewable cost-effective support for Ag incorporation, while the Ag modification of TiO2 could enhance the photocatalyst’s performance. Structural, chemical, and morphological characterization techniques were applied and showed that the Ag/TiO2 particles consisted of irregularly shaped nanoparticles. The CIP removal reached 82.46% after 300 min and ACGD removal efficiency went up to 83.64%. The enhanced performance is attributed to the unique electronic and structural properties of Ag-modified TiO2. This study highlights the potential of Ag/TiO2 synthesized via green methods as a high-performance photocatalyst for the effective remediation of pharmaceutical and dye pollutants in wastewater treatment applications.
1. Introduction
Titanium dioxide (TiO2) is a widely studied semiconductor material known for its exceptional properties such as defect chemistry [1], optical properties and photocatalytic characteristics [2], and ecological applications [3], making it a popular choice for environmental applications including pollutant degradation and water purification [4,5]. TiO2 crystals exist mainly in three different crystalline forms, namely anatase, rutile, and brookite [6]. Rutile TiO2 offers distinct advantages in photocatalysis applications [7]; so, it could be considered a valuable component in environmental remediation applications. With a band gap of approximately 3.0 eV, rutile TiO2 can absorb a larger portion of the solar spectrum compared to anatase and allow it to operate more effectively under visible light irradiation. This feature is particularly beneficial for large-scale applications where reliance on natural sunlight can enhance energy efficiency and reduce operational costs. Rutile’s crystal structure also promotes greater thermal stability [8], which is essential in industrial processes that could involve fluctuating temperatures. In addition, rutile exhibits strong photo-stability [9], which contributes to the catalyst’s durability and longevity during repeated cycles of pollutant degradation. The presence of rutile in a TiO2-based photocatalyst could further improve charge transport properties [10]. Therefore, it could facilitate the migration of photogenerated electrons and holes to the photocatalyst surface. This enhancement in charge mobility reduces the expected recombination rates, thereby boosting the photocatalytic efficiency of the applied material. The incorporation of co-catalysts such as silver into TiO2 crystals could provide a synergistic effect [11]. This resembles what could occur in the incorporation of small content from metals to higher content from another metal like what was reported before in the incorporation of Cr in Co/C material [12]. This synergistic effect could lead to increased material performance for sustainable or environmental applications [13].
Incorporating silver (Ag) into semiconductor materials (such as TiO2) has gained significant attention due to its ability to improve photocatalytic efficiency [14]. Silver serves as an electron sink [15], capturing photogenerated electrons from TiO2 and thereby reducing electron–hole recombination. Additionally, Ag nanoparticles on the TiO2 surface can form a Schottky barrier [16] that further promotes charge separation, enhancing the efficiency of redox reactions. Ag also introduces localized surface plasmon resonance (LSPR) [11], allowing TiO2 to absorb light in the visible range, which expands the photocatalyst’s applicability under sunlight. By enhancing light absorption, improving charge separation, and reducing recombination rates, silver-modified TiO2 substrates have shown enhanced photocatalytic performance in various environmental applications [17].
Ciprofloxacin (CIP) is a commonly used antibiotic due to its widespread medical and veterinary applications, and it frequently contaminates aquatic environments through wastewater discharge [18]. CIP is persistent, biologically active, and can contribute to the development of antibiotic-resistant bacteria [19]; thus, its impact can pose serious risks to human health and ecosystems. Conventional wastewater treatment processes often fail to remove CIP efficiently, which underscores the need for advanced treatment methods like photocatalysis. TiO2 has been shown to effectively degrade CIP through photocatalytic oxidation, breaking down the antibiotic into less harmful by-products [20]. The reactive oxygen species generated by TiO2 photocatalysis can attack the chemical structure of CIP, leading to effective mineralization and removal [19,20]. This capability can combine with low toxicity and stability, which makes it a suitable photoactive substrate for CIP removal from wastewater.
Carmine G is an azo dye widely used in textiles, food coloring, and cosmetics, and it frequently exists in industrial effluents. Azo dyes are characterized by their nitrogen–nitrogen double bond (–N=N–) and are often resistant to conventional biodegradation processes, which poses challenges for wastewater treatment [21]. The persistence of Carmine G and other azo dyes in the environment can lead to toxicity, carcinogenicity, and ecological imbalance [22]. Therefore, the degradation of such dyes in wastewater is crucial. TiO2 photocatalysis is highly effective in breaking down azo dyes like Carmine G due to its strong oxidative capabilities, which target the azo bond and other chromophore groups [23]. Under UV or visible light, TiO2 produces ROS that can mineralize the dye into environmentally benign compounds. Therefore, it could be considered an attractive solution for dye-contaminated wastewater treatment, and the modification of TiO2 to provide enhanced azo dye degradation is preferred.
The combination of Ag and TiO2 has emerged as a promising approach to enhance the photocatalytic performance of TiO2 for pollutant degradation. Ag/TiO2 composites offer a synergistic effect, where Ag nanoparticles can improve light absorption, particularly in the visible range. Additionally, it can act as an electron trap that improves charge separation [24] and reduces recombination rates [25]. This dual effect allows Ag/TiO2 photocatalysts to achieve higher degradation efficiencies for environmental pollutants such as antibiotics or azo dyes. Moreover, the green synthesis of Ag/TiO2 using natural templates like mushroom biomass further underscores the sustainability and environmental compatibility of this approach. By providing a more efficient and sustainable method for degrading contaminants like CIP and Carmine G, Ag/TiO2 represents a significant advancement in photocatalytic materials for environmental remediation. In this study, we developed a sustainable Ag/TiO2 photocatalyst using mushroom biomass as a natural template combined with silver nitrate and TiO2 to enhance photocatalytic performance in the degradation of ciprofloxacin (CIP) and Carmine G azo dye (ACGD). The choice of mushroom biomass as a green template offers several advantages including environmental sustainability, cost-effectiveness, and the potential to introduce beneficial structural and electronic modifications to the TiO2 framework. This approach aligns with green chemistry principles by reducing reliance on synthetic and toxic reagents, thus making the synthesis process more environmentally friendly. Mushroom biomass could serve as a renewable and naturally abundant resource, readily available as agricultural waste or through low-cost cultivation. This makes it a sustainable alternative to synthetic capping agents typically used in conventional nanoparticle synthesis. Additionally, the mushroom biomass acts as a natural reducing, capping, and stabilizing agent, eliminating the need for additional synthetic chemicals (e.g., toxic reducing agents like sodium borohydride or hydrazine). This contributes to a safer and more environmentally friendly synthesis process. While Ag nanoparticles and TiO2 could be synthesized by conventional methods, the incorporation of mushroom biomass in the synthesis process eliminates the need for synthetic stabilizers and toxic chemicals commonly used in Ag nanoparticle synthesis. In addition, the use of water as a solvent and mushroom biomass as a biogenic capping agent aligns with green chemistry principles by reducing hazardous waste generation and minimizing energy-intensive steps. Unlike many conventional methods, this approach leverages biological materials to reduce the environmental footprint of the synthesis process. For example, the use of mushroom biomass avoids the release of harmful by-products associated with synthetic reagents. After that, the characterization of the Ag/TiO2 catalyst was extensively studied and silver incorporation was approved. The degradation of CIP and ACGD was evaluated based on the synthesized Ag/TiO2 nanoparticles and showed significant pollutant removal efficiency. These results highlight the potential of Ag/TiO2 synthesized through a sustainable methodology and as an effective photocatalyst for addressing environmental pollutants in wastewater treatment applications.
2. Results and Discussion
2.1. Surface Morphology
The Ag/TiO2 photocatalyst was synthesized through a green strategy using silver nitrate, P-25 TiO2 nanoparticles, and mushroom extract, which acted as a natural reducing agent and stabilizer [26]. In this process, the mushroom extract played a crucial role in reducing silver ions (Ag+) from silver nitrate to metallic silver Ag(0) [27], while simultaneously capping and stabilizing the silver particles on the surface of the TiO2 nanoparticles. This eco-friendly method was chosen to avoid the use of harsh chemicals or toxic reducing agents, thus providing a non-precious, efficient, and environmentally sustainable photocatalytic material. The resulting material (Ag/TiO2) was characterized using several techniques, including TEM, SEM, FT-IR, XPS, and XRD. These techniques were employed to analyze the morphology, surface chemical content, and crystal structure of the synthesized TiO2 material. SEM analysis (as shown in Figure 1) was performed at different magnifications (1.5 KX, 3.0 KX, 10 KX, and 14 KX) to reveal the material’s surface morphology and particle distribution. The results demonstrated that the Ag/TiO2 particles consisted of irregularly shaped nanoparticles, with some degree of aggregation. The particles had varying sizes from 15 to 90 nm, and their aggregation resulted in the formation of larger monolithic microparticles. These microparticles are likely formed due to interactions between the reduced silver nanoparticles and the TiO2 surface. Surface interactions between the two materials contributed to a clustering of the nanoparticles into larger particles, which could be driven by a reduction in surface free energy. This tendency for aggregation has been observed in previous studies, where nanoparticles with reduced surface free energy tend to adhere to one another, resulting in larger particle formation [28]. TEM analysis further confirmed the findings from SEM, as displayed in Figure 2. TEM images revealed that the Ag/TiO2 particles were made up of small aggregated nanoparticles with sizes in the nanometer range. These nanoparticles exhibited irregular shapes (like the SEM results) and were seen to cluster together to form larger particles. The irregular morphology of the particles suggests that the synthesis method which involved the mushroom extract effectively produced Ag/TiO2 with nanoscale features and varying particle sizes.
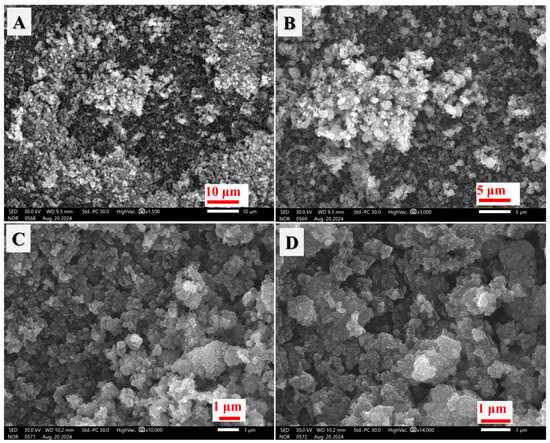
Figure 1.
SEM images of the synthesized Ag/TiO2 at different magnifications: (A) 1.5 KX; (B) 3.0 KX; (C) 10 KX; and (D) 14 KX.
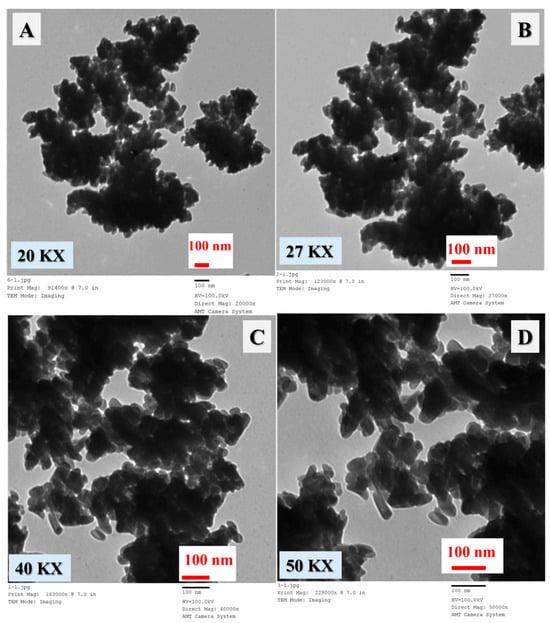
Figure 2.
TEM images of the synthesized Ag/TiO2 at different magnifications: (A) 20 KX; (B) 27 KX; (C) 40 KX; and (D) 50 KX.
2.2. Material Chemistry
The chemical composition of the Ag/TiO2 material was investigated through EDX analysis, as presented in Figure 3A. The analysis confirmed the chemical presence of titanium (Ti), oxygen (O), carbon (C), and silver (Ag), demonstrating the successful design of the Ag/TiO2 nanomaterial. The elemental composition was quantitatively assessed in terms of atomic percentages, revealing that the synthesized material consists of 30.29% Ti, 69.19% O, and 0.52% Ag. These elements were detected at characteristic energy levels of 4.51 keV for Ti, 0.53 keV for O, and 2.99 keV for Ag. The relatively high oxygen content suggests that the nanomaterial contains a significant number of oxygen atoms within the bulk structure. The low percentage of Ag was intentionally controlled during synthesis to ensure that the primary properties of TiO2 as a semiconductor remained largely unaltered, with only minimal modifications at its surface to enhance its performance. This controlled incorporation of silver was crucial to maintaining the photocatalytic efficiency and other functional characteristics of TiO2. Elemental mapping is shown in Figure 3C and illustrates the spatial distribution of Ti, O, and Ag. The Ti and O elements exhibit uniform mapping across the material, indicating their even dispersion throughout the nanomaterial. In contrast, Ag is distributed in distinct regions, suggesting that the silver is primarily localized on the surface of the TiO2 nanomaterial. This could be attributed to the reduction of Ag ions on the solid TiO2 surface, which leads to localized silver deposition. Overall, the EDX results confirm the successful preparation of the Ag/TiO2 nanomaterial using a mushroom-assisted synthesis approach with a well-defined elemental composition and distribution.
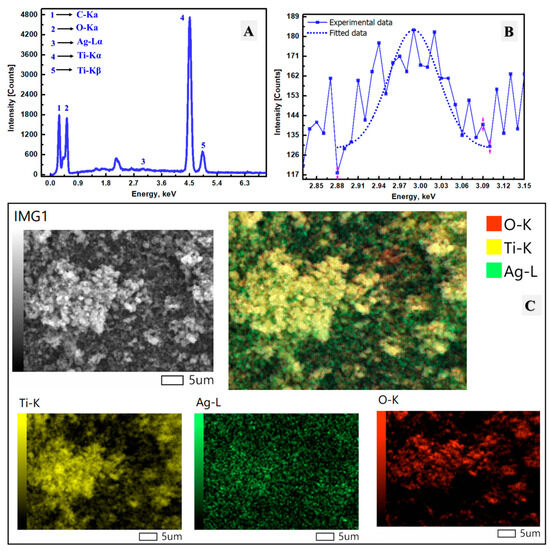
Figure 3.
(A) EDX analysis of the prepared Ag/TiO2, (B) EDX analysis with focus on the Ag area of Ag/TiO2, and (C) elemental mapping of Ag/TiO2, including the map of Ti, O, and Ag.
The elemental composition and chemical states of the Ag/TiO2 nanomaterial synthesized via mushroom-mediated reduction in the presence of silver nitrate and TiO2 nanoparticles were examined through XPS, which provided insight into the chemical surface properties and bonding environment. The XPS survey spectrum is shown in Figure 4A and reveals distinct peaks at binding energies of 285.94 eV, 368.03 eV, 459.09 eV, and 530.35 eV, corresponding to C 1s, Ag 3d, Ti 2p, and O 1s, respectively, confirming the incorporation of carbon, silver, titanium, and oxygen [29,30]. The carbon presence could be derived from the remaining/ adsorbed organic compounds within the mushroom extract [31]. This result suggests the mushroom’s role as a reducing and capping agent, which could stabilize Ag on the TiO2 surface. Ag content was observed at a concentration of 0.46%, which aligns well with the previously studied EDX data (0.52%), and this further validates that Ag is integrated into both the surface and bulk of the composite. To explore the chemical states and electronic environments of the Ti, O, and Ag species, high-resolution XPS spectra were analyzed and deconvoluted. In the Ti 2p spectrum (Figure 4B), major peaks at 458.9 eV and 464.8 eV are attributable to the Ti 2p3/2 and Ti 2p1/2 spin–orbit states, with a separation of 5.9 eV. This energy separation is indicative of Ti in the +4 oxidation state (characteristic of TiO2) [32]. Minor fitted peaks at 458.7 eV, 459.1 eV, 460.5 eV, 463.7 eV, 464.8 eV, and 465.9 eV could correspond to slight variations in the Ti-O bonding environment, possibly due to slight surface hydroxylation or interactions with Ag [33]. For O 1s (Figure 4C), peak deconvolution shows contributions around 530.2 eV, 531.7 eV, and 532.8 eV. The main peak at 530.2 eV corresponds to lattice oxygen in TiO2 (Ti-O-Ti bonds), while the higher binding energy peaks at 531.7 eV and 532.8 eV are attributable to surface-adsorbed hydroxyl groups (Ti-OH) and oxygen species associated with Ag-O bonds, suggesting that Ag incorporation influences the surface chemistry [34]. The Ag 3d spectrum (Figure 4D) displays two primary peaks at 367.7 eV and 373.7 eV, which correspond to the Ag 3d5/2 and Ag 3d3/2 levels, respectively [35]. The energy separation was found at 6.0 eV spin–orbit splitting, which is typical of metallic Ag [36]. However, slight shifts in peak positions could indicate the partial oxidation of Ag or strong interactions with TiO2. This could suggest Ag exists in both the bulk and surface of the prepared TiO2 nanomaterial. This bonding structure implies that Ag could interact with TiO2 and form weakly bonded Ag-O linkages on the TiO2 material. Overall, the XPS analysis affirms the effective integration of Ag into the TiO2 matrix and highlights the distribution of titanium, oxygen, and silver within the material chemistry, which could emphasize that surface modification can be achieved through the mushroom-assisted synthesis.
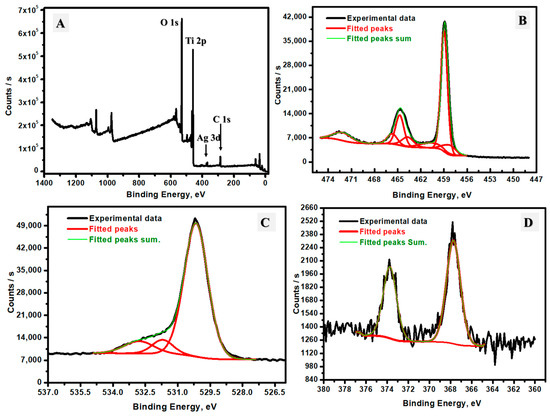
Figure 4.
(A) Total survey of XPS analysis, (B) Ti-2p XPS fine-fitting analysis, (C) O-1s XPS fine-fitting analysis, and (D) Ag-3d XPS fine-fitting analysis.
2.3. Function Groups and Crystallinity Analysis
The FT-IR spectra of TiO2 nanoparticles before and after Ag modification were analyzed in the range of 400–4000 cm−1 (Figure 5) to identify functional groups and chemical bonds based on their vibrational energies. In the unmodified TiO2 spectrum, the characteristic broad peak observed at 3427.1 cm−1 can be attributed to the O–H stretching vibrations of surface humidity [37], while the peak at 1639 cm−1 corresponds to the H–O–H bending vibrations from adsorbed water molecules [38]. The clear peak at 685.83 cm−1 is associated with the Ti–O–Ti stretching vibrations, which are integral to the TiO2 framework structure [39]. After Ag modification, notable changes are observed in the Ag/TiO2 FT-IR spectrum with new peaks appearing at 3678 cm−1, 2971.9 cm−1, 2910.7 cm−1, 1385.9 cm−1, 1228.7 cm−1, 1047.1 cm−1, 871.57 cm−1, and 485.8 cm−1. The peak at 3678 cm−1 suggests the presence of O–H vibrations, possibly due to additional hydroxyl groups from the mushroom extract coating, while peaks at 2971.9 cm−1 and 2910.7 cm−1 are likely due to C–H stretching vibrations from residual organic compounds introduced by the mushroom. The peaks at 1385.9 cm−1 and 1228.7 cm−1 could be attributed to C–O and C–N stretching vibrations, respectively. In addition, the significant shift and decrease in intensity of the Ti–O peaks (originally at 685.83 cm−1) to 485.8 cm−1 in Ag/TiO2 suggests modifications in the Ti–O–Ti lattice vibrations, likely due to lattice distortions from Ag insertion and the formation of Ag–O beside Ti–O bonds [40]. The overall decline in the intensity and broad characteristics of the O–H and Ti–O peaks support a reduction in hydroxyl groups, which could result from Ag interaction on the TiO2 surface, altering the functional chemistry of the TiO2 to form Ag-modified TiO2.
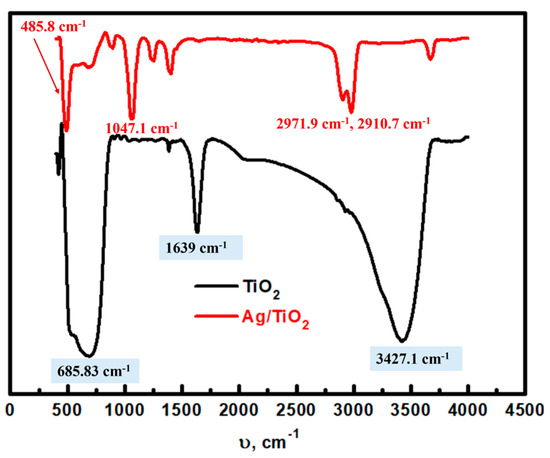
Figure 5.
FT-IR analysis of TiO2 and the prepared Ag/TiO2 material.
The XRD analysis was performed to examine the crystal structure of both TiO2 and Ag/TiO2 with the diffraction patterns displayed in Figure 6 and Table 1. For the unmodified TiO2, the characteristic peaks identified at 2θ positions of 27.47°, 36.12°, 39.22°, 41.27°, 44.015°, 54.251°, 56.683°, 62.84°, 64.06°, 68.91°, and 69.81° indicate the presence of the rutile phase, which aligns with the Joint Committee on Powder Diffraction Standards (JCPDS) reference number 00-004-0551 [32,41]. These diffraction angles correspond to the (110), (101), (200), (111), (210), (211), (220), (002), (310), (301), and (112) planes, respectively, confirming the rutile phase as the dominant structure in the TiO2 sample. Upon modification with silver, the Ag/TiO2 sample exhibited a similar set of peaks, observed at 27.398°, 35.99°, 39.27°, 41.29°, 44.16°, 54.35°, 56.55°, 62.73°, 64.12°, 68.97°, and 69.74°, corresponding to the same planes of (110), (101), (200), (111), (210), (211), (220), (002), (310), (301), and (112), indicating the preservation of the rutile phase upon Ag incorporation. However, a new peak at 32.2246° was detected, which aligns with JCPDS 00-052-1202, suggesting the formation of AgTiO3 [42]. This peak could imply the partial formation of a composite phase due to the interaction between Ag and TiO2, potentially attributed to the mushroom-based reduction process that facilitated silver deposition. The Scherrer equation, based on the full width at half maximum (FWHM) of the (110) rutile plane, was applied to determine the crystallite size. For pure TiO2, the crystallite size was approximately 104.0 nm. After silver modification, this size reduced to 69.29 nm, while the 2θ position for the (110) plane shifted slightly from 27.47° to 27.398°. This reduction in crystallite size and shift in peak position could be attributed to lattice strain induced by the incorporation of silver atoms into the TiO2 matrix. The smaller Ag atoms can enter substitutional or interstitial sites, causing lattice distortions that increase strain and, in turn, contribute to grain size reduction [43]. The lattice parameters of the tetragonal TiO2 structure were calculated for both samples. For unmodified TiO2, the lattice constants were determined to be a = b = 2.958 Å and c = 4.592 Å. In Ag/TiO2, slight increases in the a and b parameters to 2.969 Å and in the c parameter to 4.604 Å were observed. This expansion of lattice parameters in Ag/TiO2 is likely due to the partial substitution or surface attachment of Ag, which could lead to a slight lattice distortion and an increase in unit cell dimensions. This effect could result from either substitutional doping, where Ag occupies Ti sites, or interstitial positioning, where Ag is inserted between TiO2 lattice planes. Each of these introduces minor lattice strain and results in the observed lattice expansion. The XRD analysis reveals that the Ag modification of TiO2 results in the formation of a mixed-phase structure with reduced crystallite size, lattice strain, slight lattice expansion, and the introduction of a secondary phase (AgTiO3).
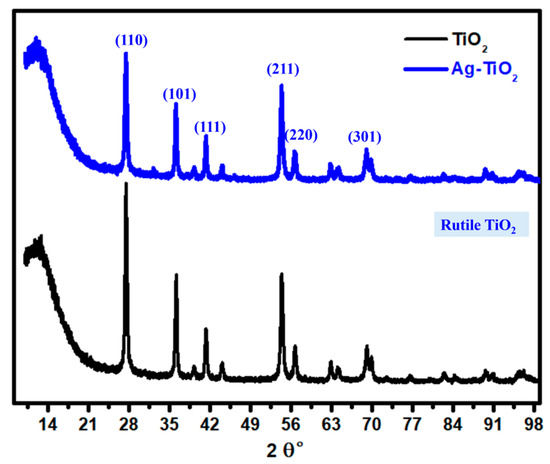
Figure 6.
XRD analysis of TiO2 and the prepared Ag/TiO2 material.

Table 1.
The XRD analysis of Ag/TiO2 peaks, including the parameter details.
2.4. Mechanism of Ag/TiO2 Material Formation
The formation of Ag/TiO2 follows a green synthetic route wherein the mushroom extract serves as both a reducing and capping agent for the silver nanoparticles [44], as shown in Scheme 1. The bioactive compounds present in the mushroom extract such as polysaccharides, proteins, and phenolic compounds could reduce silver ions (Ag+) from silver nitrate into metallic silver (Ag°) through a redox reaction. These bioactive compounds also prevent excessive aggregation of the silver particles by stabilizing them on the TiO2 surface [45]. Therefore, the mushroom extract plays a dual role in the synthesis, facilitating both the reduction of silver ions and the stabilization of the formed nanoparticles. The reduction process occurs when the functional groups in the mushroom extract such as hydroxyl and carboxyl groups donate electrons to the silver ions, which leads to converting them into metallic silver. The mushroom-derived compounds adsorb onto the surface of the silver nanoparticles, acting as a protective barrier to prevent uncontrolled growth and agglomeration. This results in the formation of well-dispersed silver nanoparticles on the TiO2 surface. The interaction between the mushroom-derived stabilizing agents and the TiO2 surface enhances the stability of the Ag/TiO2 material and prevents further particle aggregation. In short, the green synthesis of Ag/TiO2 using mushroom extract as a reducing and stabilizing agent offers an environmental approach to enhance TiO2 characteristics.
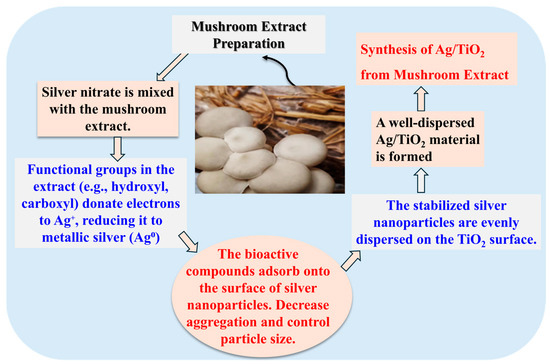
Scheme 1.
Preparation of the studied Ag/TiO2 material, including mechanistic aspects.
2.5. Photocatalytic Degradation of Ciprofloxacin (CIP)
The degradation of CIP is crucial due to its environmental and health impacts, particularly its contribution to antimicrobial resistance and aquatic toxicity [46]. TiO2 materials have high photocatalytic efficiency and have been extensively studied for pollutant degradation [47,48]. However, unmodified TiO2 is limited by its wide bandgap (3.0–3.2 eV), which restricts absorption primarily to the UV region. Therefore, the silver modification was suggested to address this limitation. In this study, the Ag/TiO2 photocatalyst material was synthesized using eco-friendly mushroom extract, silver nitrate, and TiO2, and their effectiveness in degrading CIP under a metal halide lamp was compared to that of pristine TiO2 and CIP photolysis under the same irradiation conditions (Figure 7). The absorption spectrum of CIP without the photocatalyst exhibited peaks at 273 nm and 325 nm, with absorbance values of 1.421 and 0.57, respectively, indicating a high CIP concentration. With the introduction of pure rutile TiO2 as a photocatalyst, these peaks showed a notable decrease in absorbance values to 0.65 and 0.26 at 273 nm and 325 nm, respectively. However, the Ag/TiO2 photocatalyst demonstrated more reduction in the absorbance values to 0.48 at 273 nm and 0.19 at 325 nm, which illustrates the enhanced degradation of CIP. Scheme 2 displays the photodegradation steps. The improved performance of Ag/TiO2 over pure TiO2 can be attributed to several key factors stemming from Ag incorporation. Firstly, Ag acts as an electron sink, which facilitates electron transfer from TiO2 and inhibits electron–hole recombination [49]. By prolonging the lifetime of photogenerated charge carriers, Ag enables more effective oxidation and reduction reactions on the TiO2 surface, which can enhance degradation efficiency. Secondly, Ag nanoparticles on the TiO2 surface can form a Schottky barrier and further promote charge separation, which enhances photocatalytic activity [50]. Additionally, Ag doping broadens TiO2’s light absorption into the visible range due to localized surface plasmon resonance effects, where Ag nanoparticles absorb visible light and transfer energy to TiO2. This phenomenon extends the spectral response of TiO2 and allows it to utilize a larger fraction of solar irradiation. The spectrum for Ag/TiO2-treated CIP also revealed high absorbance in the 200–236 nm range, which can be interpreted by CIP degradation by-products and confirming that Ag modification enhances the breakdown of CIP molecules. Thus, Ag/TiO2 presents a promising approach for antibiotic degradation in wastewater by combining the stability of TiO2 with enhanced light absorption and improved charge separation introduced by Ag. This synergy effectively enhances the oxidative degradation pathways available for CIP under visible light.
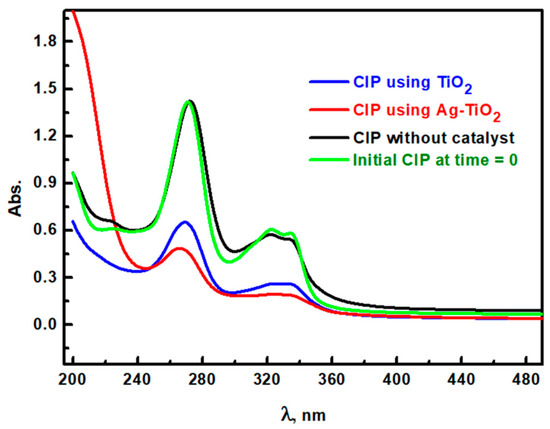
Figure 7.
UV-visible spectroscopy of CIP at time = 0 (initial) and after 30 min from irradiation of simulated light without catalyst and with TiO2 and Ag/TiO2.
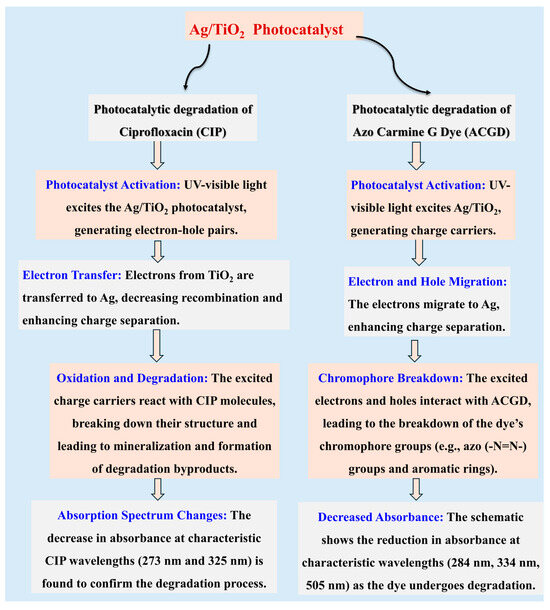
Scheme 2.
Photocatalytic degradation of pollutants based on the studied Ag/TiO2 material, including mechanistic aspects.
The degradation of CIP in aqueous media at different times up to 300 min was studied via UV-visible spectroscopy (Figure 8). CIP’s characteristic UV-Vis peaks (particularly at 273 nm and 325 nm) have a progressive decline in absorbance as the reaction time extends up to 270 min. Initially, the minor peak at 325 nm disappeared after 90 min, which indicates the prompt breakdown of specific molecular structures associated with this band. The primary peak at 273 nm decreases more gradually, requiring 270 min to disappear completely. This result suggests that certain intermediate compounds could be generated during CIP degradation and are relatively stable and resistant to further breakdown. This delayed response in the peak of 273 nm could be attributed to the existence of the structure having π-π* electron transitions within the initially degraded CIP molecule. The degradation pathways could stabilize certain reaction intermediates absorbing around 273 nm before they undergo complete oxidative degradation. The emergence of higher spectral absorbance in the 200–240 nm range as degradation progresses confirms the formation of intermediates, highlighting the Ag/TiO2 performance in enhancing the CIP breakdown dynamics. Therefore, these findings indicate that Ag/TiO2 could accelerate CIP degradation for advanced environmental remediation applications.
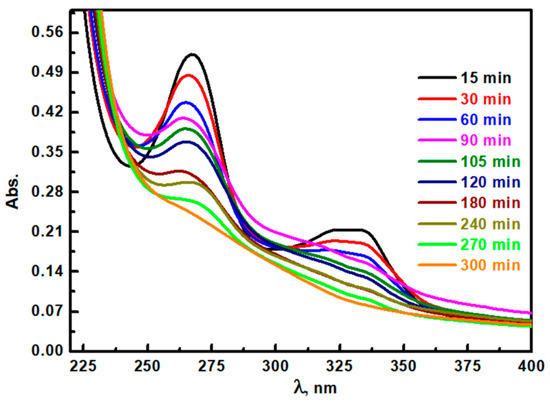
Figure 8.
Photocatalytic degradation of CIP pollutant at different times from irradiation of simulated light using Ag/TiO2 photocatalyst.
The photocatalytic degradation efficiency of Ag/TiO2 for ciprofloxacin (CIP) removal in aqueous media was assessed over a range of reaction times up to 330 min, providing a detailed understanding of the material’s performance in degrading this antibiotic pollutant (Figure 9). The efficiency of CIP removal was calculated using the following equation [51,52]: Degradation Efficiency (%) = (Ao − At/Ao) × 100, where Ao is the initial absorbance of CIP at its characteristic peak (273 nm) and At is the absorbance at time t. After 15 min, the removal efficiency reached 62.88%, and this value progressively increased as the reaction continued, achieving 82.46% efficiency at 300 min. However, a slight decrease in efficiency to 80.04% was observed after 330 min, which could be attributed to limitations in the calculation method because it relies solely on the absorbance of CIP’s main peak at 273 nm. This peak is characteristic of π-π* electron transitions in CIP’s aromatic ring structure and does not fully account for potential interactions with intermediate degradation products that may also absorb in this region without a clear peak, resulting in a subtle overestimation or fluctuation in apparent efficiency. Nevertheless, the overall removal efficiency of approximately 80% demonstrates the efficacy of the Ag/TiO2 photocatalyst synthesized from mushroom extract and supports its potential as a green and sustainable strategy for the treatment of biomedical wastewater containing CIP. This approach not only emphasizes the role of biogenic synthesis routes but also showcases the promising application of Ag/TiO2 in addressing antibiotic contamination in water, which is critical for reducing environmental impact and antimicrobial resistance associated with residual pharmaceuticals.
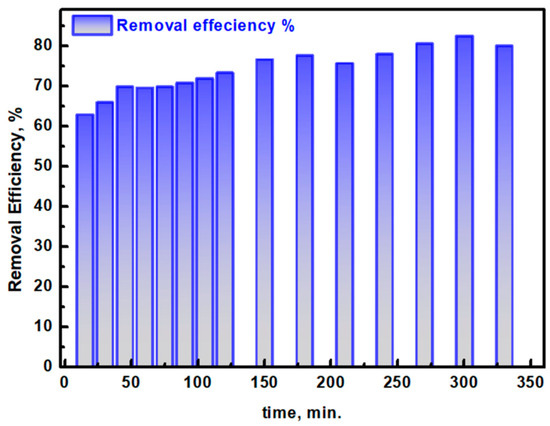
Figure 9.
The estimated removal degradation (%) of CIP pollutant at different times from the irradiation of simulated light using the Ag/TiO2 photocatalyst.
The reaction kinetics for CIP degradation by the Ag/TiO2 photocatalyst were analyzed under the assumption of first-order kinetics, as depicted in Figure 10. Pearson’s correlation coefficient (R) was found at 0.98411. This high R value confirms that the reaction closely follows first-order kinetic behavior, where the reaction rate is proportional to the concentration of CIP. The slight deviation observed in Pearson’s R value could be attributed to minor experimental variations such as the heterogeneous nature of the photocatalytic process, which could introduce small inconsistencies in the degradation rate over time. The adherence to first-order kinetics suggests that the primary rate-limiting step is likely the surface reaction between CIP molecules and the reactive sites on the Ag/TiO2 photocatalyst. This behavior is typical in photocatalytic degradation processes, where the concentration of the substrate directly influences the degradation rate. The rate constant (k1) was calculated as 0.00243 min−¹, underscoring the efficiency of the Ag/TiO2 material in promoting CIP degradation. This kinetic model provides a strong foundation for predicting CIP removal rates under similar experimental conditions and highlights the suitability of Ag/TiO2 as a high-performance photocatalyst for the effective degradation of antibiotic contaminants.
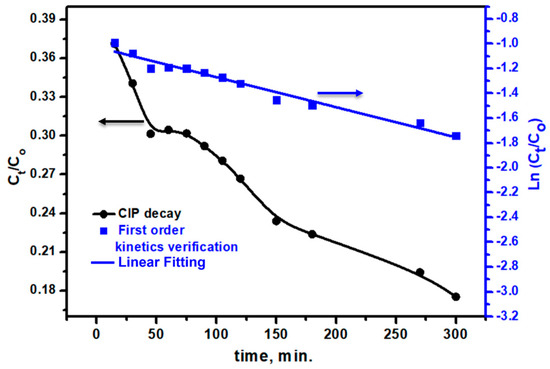
Figure 10.
The Co/Ct and first order-kinetics verification of the CIP pollutant degradation at different times from the irradiation of simulated light using the Ag/TiO2 photocatalyst.
2.6. Photocatalytic Degradation of Azo Carmine G Dye (ACGD)
The degradation of azo Carmine G Dye (ACGD) is of critical importance due to its environmental persistence and potential toxicity in aquatic systems. Therefore, Ag-modified TiO2 (Ag/TiO2) was selected as a photocatalyst to enhance the photodegradation of ACGD, given that silver doping is known to improve TiO2’s photocatalytic properties. The photocatalytic activity was assessed using a metal halide lamp, with performance compared against pristine TiO2 under identical irradiation conditions (see Figure 11). The initial absorption spectrum of ACGD, without any photocatalyst, displayed distinct peaks at 284 nm, 334 nm, and 505 nm, with absorbance values of 0.896, 0.613, and 1.0482, respectively, indicating a high concentration of ACGD. These spectral features are likely attributed to specific chromophore groups in the dye structure. Chromophores are parts of a molecule that absorbs UV-visible light due to their conjugated double-bond systems or aromatic rings, causing electronic transitions. For ACGD, the key chromophores are likely to include the azo (−N=N−) groups, aromatic rings, and possibly other conjugated functional groups. Each of these groups has characteristic absorption behaviors in the UV-visible spectrum, which provide many UV-visible peaks for the ACGD aqueous solution. The decline in the absorbance peaks indicates a possible breakdown or structural alteration of these chromophores, thus confirming ACGD degradation. Scheme 2 displays the photodegradation steps. Upon the addition of pure rutile TiO2 as a photocatalyst, the absorbance values at these wavelengths decreased to 0.78, 0.535, and 0.9039, respectively, indicating partial degradation. However, with Ag/TiO2 as the photocatalyst, a more significant reduction in ACGD concentration was seen. The absorbance values further declined to 0.70, 0.45, and 0.72 at 284 nm, 334 nm, and 505 nm, respectively, after 15 min of irradiation, which could suggest enhanced dye degradation (Figure 11). The improved performance of Ag/TiO2 could be attributed to Ag modification, which acts as an electron sink and creates a Schottky barrier at the TiO2 surface, as well as extends TiO2’s light absorption range into the visible spectrum [53,54]. These combined effects underscore the effectiveness of Ag/TiO2 in achieving high ACGD degradation rates.
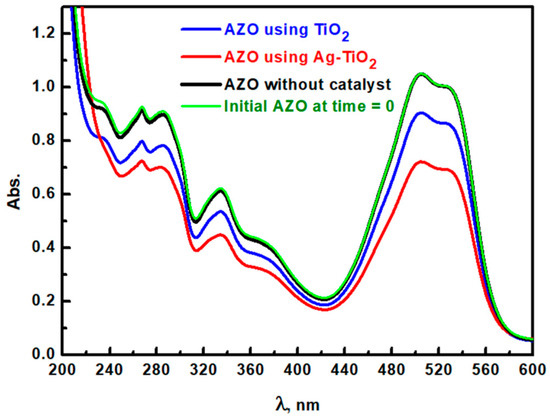
Figure 11.
UV-visible spectroscopy of ACGD at time = 0 (initial) and after 30 min from irradiation of simulated light without catalyst and with TiO2 and Ag/TiO2.
The removal of ACGD in aqueous solutions at different times up to 270 min was studied via UV-visible spectroscopy (Figure 12). ACGD’s characteristic UV-Vis peaks (particularly at 505 nm, 334 nm, and 284 nm) have a progressive decline in absorbance as the reaction time extends up to 270 min. Initially, the minor peak at 334 nm disappeared after 60 min, which indicates the prompt breakdown of specific molecular structures associated with this band. The primary peak at 285 nm has a clear shift to the UV region, which indicated the formed intermediates after ACGD degradation. The main peak at 505 nm has a clear decrease gradually with time progress and is removed after 270 min. Therefore, these findings indicate that Ag/TiO2 is an acceptable photocatalyst for ACGD degradation towards environmental remediation uses.
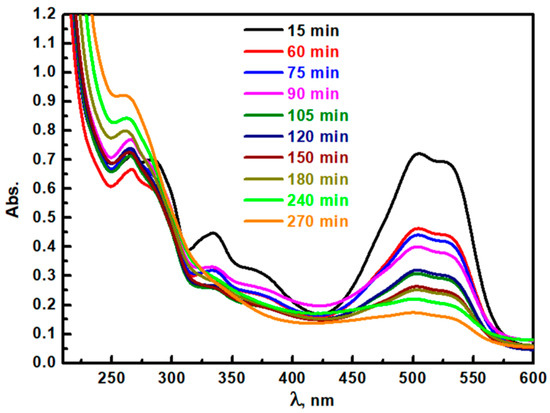
Figure 12.
Photocatalytic degradation of ACGD pollutant at different times from irradiation of simulated light using Ag/TiO2 photocatalyst.
The photocatalytic degradation efficiency of Ag/TiO2 against ACGD removal was assessed over a range of reaction times up to 300 min, providing a detailed understanding of the material’s performance in degrading this ACGD pollutant (Figure 13). The efficiency of ACGD removal was calculated using the following equation [51,52]: Degradation Efficiency (%) = (Ao − At/Ao) × 100, where Ao is the initial absorbance of ACGD at its characteristic peak (505 nm) and At is the absorbance at time t. After 15 min, the removal efficiency reached 28.08%, and this value progressively increased as the reaction continued, achieving 83.64% efficiency at 300 min. Therefore, Ag/TiO2 is an outstanding photocatalyst against ACGD pollutants, as well as previously discussed biomedical pollutants (CIP). Additionally, the reaction kinetics for ACGD degradation by the Ag/TiO2 photocatalyst were analyzed under the assumption of first-order kinetics, as depicted in Figure 14. Pearson’s correlation coefficient (R) was found at 0.94976. This R value confirms that the reaction follows first-order kinetic behavior, where the reaction rate is proportional to the concentration of ACGD. The adherence to first-order kinetics suggests that the primary rate-limiting step is likely the surface reaction between ACGD molecules and the reactive sites on the Ag/TiO2 photocatalyst. This behavior is typical in photocatalytic degradation processes, where the concentration of the substrate directly influences the degradation rate. The rate constant (k1) was calculated as 0.00533 min−1, underscoring the efficiency of the Ag/TiO2 material in promoting ACGD degradation. This kinetic model provides a strong foundation for predicting ACGD removal rates under similar experimental conditions and highlights the suitability of Ag/TiO2 as a high-performance photocatalyst for the effective degradation of dye contaminants. Table 2 presents a comparative analysis of the degradation efficiencies of CIP and ACGD using some reported photocatalysts, as well as the performance of the mushroom-assisted Ag/TiO2 introduced in this work. Among the listed photocatalysts, the CuS/BiVO4 binary heterojunction shows the highest degradation efficiency (86.70%) [55], closely followed by the introduced Ag/TiO2 photocatalyst (82.46%). The presented Ag/TiO2 photocatalyst outperforms other materials, such as Bi2WO6/Ta3N5 (81.10%) [56] and Y-NaBi (MoO4)2 (80.60%) [57], by achieving a comparable degradation efficiency while utilizing a sustainable synthesis approach. This highlights the potential of mushroom-assisted synthesis in producing highly efficient photocatalysts. Photocatalysts like Ag2O (25%) and hydrothermal TiO2 (45.61%) show significantly lower degradation efficiencies, indicating the benefits of incorporating Ag nanoparticles and the synergistic effects introduced by the mushroom-mediated synthesis. Additionally, the introduced Ag/TiO2 photocatalyst achieves a degradation efficiency of 83.64%, which surpasses that of other reported photocatalysts, including G/N-doped ZnO nanocomposite (66.76%) [58] and G-SrO nanoparticles (52.50%) [59]. This superior performance can be attributed to the enhanced visible light absorption and the unique features of mushroom-mediated Ag/TiO2. Therefore, the mushroom-assisted synthesis method introduces a novel and green approach to synthesizing Ag/TiO2 photocatalysts. The natural reducing, capping, and stabilizing agents derived from mushrooms likely contribute to improved photocatalytic activity by creating more active sites and better dispersing Ag nanoparticles on the TiO2 surface. The Ag/TiO2 photocatalyst demonstrates competitive performance not only for CIP but also for ACGD, highlighting its versatility in degrading different pollutants. At last, the degradation of CIP and removal of ACGD in aqueous media based on Ag/TiO2 was compared with reported material for that purpose, including Bi2WO6/Ta3N5, CuS/BiVO4 binary heterojunction, TiO2/montmorillonite, hydrothermal TiO2, γ-Fe2O3/Bi2WO6 nanocomposite, NaBi (MoO4)2, Y-NaBi (MoO4)2, pure Ag2O, G/N-doped ZnO nanocomposite, and G-SrO nanoparticles, and the performance of Ag/TiO2 provides a considerable site for it compared to the previously reported photocatalysts.
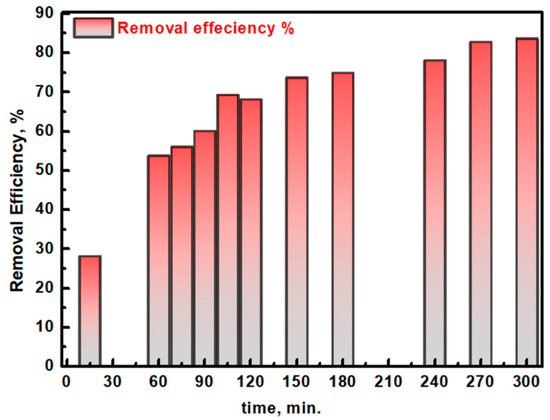
Figure 13.
The estimated removal degradation (%) of the ACGD pollutant at different times from the irradiation of simulated light using the Ag/TiO2 photocatalyst.
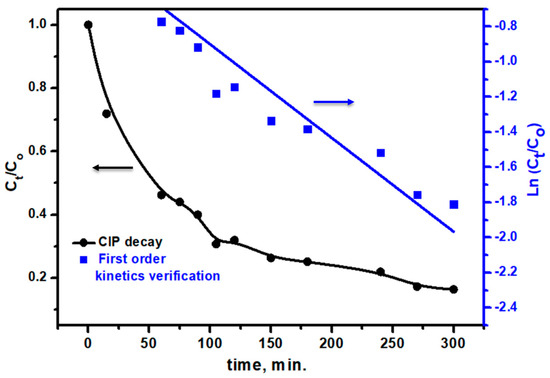
Figure 14.
The Co/Ct and first order-kinetics verification of the ACGD pollutant degradation at different times from the irradiation of simulated light using the Ag/TiO2 photocatalyst.

Table 2.
Degradation of CIP and in the presence of the reported photocatalysts.
3. Materials and Methods
3.1. Chemical Materials
Ciprofloxacin (CIP), azo Carmine G dye (ACGD), TiO2, commercial P25 (≥99.5%), and silver precursor (AgNO3) were purchased from Shanghai Macklin Biochemical Co., Ltd. and used without further purification. Silver salt was dissolved in distilled water and was sourced from a commercial supplier. The utilized mushroom extract was found by dissolving the solid mushroom, as reported in a previous study [27], in hot water.
3.2. Sustainable Synthesis of Ag/TiO2 by Mushroom-Assisted Methodology
The mushroom extract was used after soaking 15 g of mushroom, as reported previously [27], in 500 mL of de-ionized water after thoroughly washing and chopping it into small pieces (to facilitate the dissolution of active mushroom ingredients). The mushroom pieces were left to soak overnight and then filtered using traditional filter paper. The silver precursor solution (1 mM) was added to the filtrate to induce the formation of silver nanoparticles (Ag NPs) under ambient conditions. The silver–mushroom extract mixture was kept at 30 °C for one day. The color change from colorless to brown indicated the formation of Ag NPs. After that, commercial TiO2 was added to form a suspension having both TiO2 and Ag NPs, followed by continuous shaking overnight. The mixture was then centrifuged at 15,000 rpm for 15 min. The synthesized Ag/TiO2 material was purified by washing with sterilized water to remove any impurities.
3.3. Characterization
The morphology of the prepared material was examined with a scanning electron microscope (SEM; Model: JEOL-JSM-5410, Tokyo, Japan), followed by EDX analysis and elemental mapping via the same instrument. Additionally, the morphology of the synthesized Ag/TiO2 sample was also assessed using a transmission electron microscope (TEM) at 200 KeV, Model-JEOL-1230. The crystallinity of TiO2 and Ag/TiO2 samples were identified via X-ray diffraction using an X’Pert PRO with Cu-Kα radiation (wavelength 0.1540 nm) on a PANalytical diffractometer. FT-IR analysis was checked by the use of a Bruker FT-IR spectrometer within the range of 400 to 4000 cm−1. To gain a comprehensive understanding of surface chemistry, X-ray photoelectron spectroscopy (XPS) was utilized. The XPS data were obtained using a K-ALPHA instrument (Thermo Fisher Scientific, Lenexa, USA), featuring monochromatic Al K-alpha X-ray radiation with an energy range of 0 to 1350 eV and a spot size of 400 microns.
3.4. Photocatalytic Measurements
All photocatalytic experiments were conducted using a metal halide lamp as a solar simulator, coupled with a magnetic stirrer in a closed system. A specific quantity of TiO2 or Ag/TiO2 was combined with 200 mL of 50 ppm aqueous ciprofloxacin (CIP) solution in a dark setting for 60 min to achieve adsorption–desorption equilibrium. Following this, the mixture was exposed to direct simulated sunlight at 25 °C. After a designated reaction period, a 4 mL sample of the solution was extracted using a filtered syringe and analyzed by UV–Vis spectroscopy. The degradation or removal efficiency for various CIP or ACGD solutions was calculated with the following formula: Efficiency (%) = (Co − Cf)/Co × 100, where Co represents the CIP or ACGD concentration, with subscripts “o” and “f” denoting initial and final states, respectively [63].
4. Conclusions
In this study, the Ag/TiO2 photocatalyst synthesized using mushroom biomass as a natural template in combination with silver nitrate and TiO2 was evaluated to be an effective and environmentally sustainable material for the degradation of both ciprofloxacin (CIP) and azo Carmine G dye (ACGD). The mushroom biomass served as a renewable and cost-effective resource to support Ag incorporation, while the use of Ag with TiO2 enhanced the catalyst’s photocatalytic properties by promoting greater electron–hole separation, reducing recombination rates, and broadening the light absorption spectrum into the visible range. The results demonstrated that the Ag/TiO2 particles consisted of irregularly shaped nanoparticles, with some degree of aggregation. The particles had varying sizes, and their aggregation resulted in the formation of larger monolithic microparticles. The XRD analysis reveals that the Ag modification of TiO2 results in the formation of a mixed-phase structure with reduced crystallite size, lattice strain, slight lattice expansion, and the introduction of a secondary phase (AgTiO3). The photocatalytic efficiency of Ag/TiO2 was evident through its high degradation rates for CIP and ACGD, demonstrating rapid and significant removal of these contaminants. After 15 min, the removal efficiency of ciprofloxacin reached 62.88%, and this value progressively increased as the reaction continued, achieving 82.46% efficiency at 300 min. For azo dye degradation, the removal efficiency reached 28.08% after 15 min, and this value progressively increased as the reaction continued, achieving 83.64% efficiency at 300 min. The photocatalytic performance could be due to the unique structure and electronic properties that could exist in Ag/TiO2 material. Therefore, this work underscores the potential of Ag/TiO2 synthesized through green methods as a sustainable and high-performance photocatalyst for tackling a range of environmental pollutants, including pharmaceutical and dye contaminants commonly found in wastewater.
Author Contributions
Conceptualization, H.M.A.E.-L., C.-Q.Z., M.M.K. and I.M.A.M.; methodology, H.M.A.E.-L. and I.M.A.M.; software, C.-Q.Z., M.M.K. and I.M.A.M.; validation, M.M.K. and I.M.A.M.; formal analysis, H.M.A.E.-L., C.-Q.Z., M.M.K. and I.M.A.M.; investigation, H.M.A.E.-L. and I.M.A.M.; resources, C.-Q.Z. and M.M.K.; data curation, C.-Q.Z., M.M.K. and I.M.A.M.; writing—original draft preparation, H.M.A.E.-L., C.-Q.Z., M.M.K. and I.M.A.M.; writing—review and editing, H.M.A.E.-L., M.M.K. and I.M.A.M.; visualization, C.-Q.Z. and M.M.K.; supervision, H.M.A.E.-L.; project administration, H.M.A.E.-L. and C.-Q.Z.; funding acquisition, H.M.A.E.-L. and C.-Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the Annual Funding track by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, grant number KFU242608. Additionally, this work was supported by Shenzhen polytechnic university funding 6024310030K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the authors.
Acknowledgments
This work was supported through the Annual Funding track by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, grant number KFU242608. Additionally, this work was supported by Shenzhen polytechnic university funding 6024310030K. The authors also extend profound gratitude to Sohag, King Faisal, and Shenzhen polytechnic universities for essential support of the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bak, T.; Sherif, S.A.; Black, D.S.; Nowotny, J. Defect Chemistry of Titanium Dioxide (Rutile). Progress Toward Sustainable Energy. Chem. Rev. 2024, 124, 11848–11914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Dong, X.; Xi, X.; Dong, P. Recent advances in TiO2-based photocatalytic concrete: Synthesis strategies, structure characteristics, multifunctional applications, and CFD simulation. Chem. Eng. J. 2024, 496, 154186. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, I.M.A.; Park, C.H.; Kim, C.S. Design of novel electrode for capacitive deionization using electrospun composite titania/zirconia nanofibers doped-activated carbon. Mater. Lett. 2018, 213, 62–66. [Google Scholar] [CrossRef]
- Puri, N.; Gupta, A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environ. Res. 2023, 227, 115786. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L.; Gao, Y.; Ji, L.; Yang, Z.; Wang, H.; Shang, M.; Du, J.; Yang, X. TiO2/p-BC Composite Photocatalyst for Efficient Removal of Tetracycline from Aqueous Solutions under Simulated Sunlight. Catalysts 2024, 14, 357. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, J.; Cheng, G. Recent advances in brookite phase TiO2-based photocatalysts toward CO2 reduction. Fuel 2024, 357, 129806. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Li, M.; Cheng, Q.; Deng, J.; Wang, P.; Cai, M.; Sun, S. Facet-Dependent Photocatalytic Behavior of Rutile TiO2 for the Degradation of Volatile Organic Compounds: In Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy and Density Functional Theory Investigations. Langmuir 2024, 40, 2120–2129. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, L.; Lin, G.; Xi, S. Enhanced photoelectrocatalysis in porous single crystalline rutile titanium dioxide electrodes. J. Mater. Chem. A 2024, 12, 3879–3885. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Najafabadi, A.H.; Zehtabi, F.; Kouchehbaghi, N.H.; Sharma, S.; Markowska-Szczupak, A.; Kowalska, E. Apatite-coated Ag/AgBr/TiO2 nanocomposites: Insights into the antimicrobial mechanism in the dark and under visible-light irradiation. Appl. Surf. Sci. 2023, 617, 156574. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, W.-W.; Song, Y.-C.; Wang, Z.; Yuan, C.; Han, L.-L.; Liu, J.-Q.; Yang, Y.; Liu, P. Improvement of the dielectric properties of rutile TiO2 ceramics at megahertz. J. Eur. Ceram. Soc. 2023, 43, 1500–1508. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, J.; Wang, S.; Zhang, Z.; Li, J.; Xu, X.; Wang, Y.; Ye, Z.; Zhang, H. LSPR effect enabled Ag-TiO2 nanotube arrays for high sensitivity and selectivity detection of acetone under visible light. J. Alloys Compd. 2024, 1003, 175533. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Motlak, M.; Fouad, H.; Barakat, N.A.M. Cobalt/Chromium Nanoparticles-Incorporated Carbon Nanofibers as Effective Nonprecious Catalyst for Methanol Electrooxidation in Alkaline Medium. Nano 2016, 11, 1650049. [Google Scholar] [CrossRef]
- Guo, C.; Han, C.-Y.; Zhou, K.-H.; Huo, G.-N.; Zhang, S.-S.; Wang, G.-C.; Zhang, S.-M.; Huang, W.-P.; Zhu, B.-L. Fast monitoring of organic dye degradation coupled with a colorimetric approach in a Ag-modified TiO2 nanotube photocatalytic system. Ceram. Int. 2023, 49, 12653–12661. [Google Scholar] [CrossRef]
- Janbandhu, S.Y.; Patra, U.; Sukhadeve, G.K.; Kumar, R.; Gedam, R.S. Photocatalytic performance of glasses embedded with Ag-TiO2 quantum dots on photodegradation of indigo carmine and eosin Y dyes in sunlight. Inorg. Chem. Commun. 2023, 148, 110317. [Google Scholar] [CrossRef]
- Muneeb, A.; Rafique, M.S.; Murtaza, M.G.; Arshad, T.; Shahadat, I.; Rafique, M.; Nazir, A. Fabrication of Ag–TiO2 nanocomposite employing dielectric barrier discharge plasma for photodegradation of methylene blue. Phys. B Condens. Matter 2023, 665, 414995. [Google Scholar] [CrossRef]
- Chen, W.; Xiong, J.; Wen, Z.; Chen, R.; Cheng, G. Synchronistic embedding of oxygen vacancy and Ag nanoparticles into potholed TiO2 nanoparticles-assembly for collaboratively promoting photocatalytic CO2 reduction. Mol. Catal. 2023, 542, 113138. [Google Scholar] [CrossRef]
- Lopes, F.C.S.; Rocha, M.d.G.C.d.; Bargiela, P.; Ferreira, H.S.; Pires, C.A.d.M. Ag/TiO2 photocatalyst immobilized onto modified natural fibers for photodegradation of anthracene. Chem. Eng. Sci. 2020, 227, 115939. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, S.; Li, G.; Dong, Y.; Wang, Q.; Zhang, L.; Ren, Z.; Wang, P. Superoxide radical induced redox processes for simultaneous reduction of Cr (VI) and oxidation of ciprofloxacin in wastewater. Appl. Catal. B 2024, 343, 123565. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Hou, Y.; Wu, J.; Ting, Y.; Nie, C.; Tong, M. Elimination of representative antibiotic-resistant bacteria, antibiotic resistance genes and ciprofloxacin from water via photoactivation of periodate using FeS2. J. Hazard. Mater. 2024, 476, 134982. [Google Scholar] [CrossRef]
- Raikar, L.G.; Patel, A.; Gandhi, J.; Gupta, K.; Prakash, H. Nano-TiO2 immobilized polyvinylidene fluoride based spongy-spheres for ciprofloxacin photocatalytic degradation: Antibacterial activity removal, mechanisms, UVA LED irradiation and easy recovery. Environ. Sci. Nano 2024, 11, 3729–3743. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Li, L.; Su, C.; Song, B.; Zhang, H.; Guo, S.; Pan, F. Progress and prospects of Mg-based amorphous alloys in azo dye wastewater treatment. J. Magnes. Alloys 2024, 12, 873–889. [Google Scholar] [CrossRef]
- Damotharan, K.; Sudhakaran, G.; Ramu, M.; Krishnan, M.; S, K.R.N.; Arockiaraj, J. Biochemical processes mediating neurotoxicity induced by synthetic food dyes: A review of current evidence. Chemosphere 2024, 364, 143295. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Mukaba, J.-L.; Ndayambaje, G.; Tijani, J.O.; Eze, C.P.; Ntsa, E.; Myint, M.T.Z.; Kyaw, H.H.; Al-Abri, M.; Dobretsov, S.; et al. Innovative carbon-nitrogen-TiO2 catalyst immobilised on stainless steel mesh for effective removal of orange II organic dye in the DCDBD plasma actuator. Catal. Commun. 2023, 183, 106778. [Google Scholar] [CrossRef]
- Chen, F.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Atomic-Level Charge Separation Strategies in Semiconductor-Based Photocatalysts. Adv. Mater. 2021, 33, 2005256. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Hao, Z.; Feng, T.; Li, Y.; Dong, B.; Cao, L. Plasma Ag-loaded bandgap variational Zn1-xMgxO-enhanced charge separation for photoelectrochemical water oxidation of CuWO4 photoanodes. Chem. Eng. J. 2023, 455, 140861. [Google Scholar] [CrossRef]
- Manimaran, K.; Yanto, D.H.Y.; Anita, S.H.; Nurhayat, O.D.; Selvaraj, K.; Basavarajappa, S.; Hashem, M.I.; Palanisamy, G.; Lin, M.-C.; Kumarasamy, K. Synthesis and characterization of Hypsizygus ulmarius extract mediated silver nanoparticles (AgNPs) and test their potentiality on antimicrobial and anticancer effects. Environ. Res. 2023, 235, 116671. [Google Scholar] [CrossRef]
- Youssef, M.S.; Ahmed, S.I.; Mohamed, I.M.; Abdel-Kareem, M.M. Biosynthesis, Spectrophotometric Follow-Up, Characterization, and Variable Antimicrobial Activities of Ag Nanoparticles Prepared by Edible Macrofungi. Biomolecules 2023, 13, 1102. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Wu, X.-Y.; Zhu, J.-H.; El-Lateef, H.M.A.; Mousa, H.M.; Xing, F. Microstructure and interface analyses of novel external anode mortar incorporated calcined hydrotalcite nanoparticles towards an enhanced impressed current cathodic protection. J. Taiwan Inst. Chem. Eng. 2023, 145, 104803. [Google Scholar] [CrossRef]
- Ali, T.; Ahmad, N.; Nabeel, M.I.; Xiao, H.-M.; Hussain, D. Facile and scalable growth of bimetallic 3D Ag/MIL125-NH2 on cotton fabric for multifaceted anti-wetting and self-healing characteristics. Carbon 2024, 228, 119395. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; Abd El-Lateef, H.M.; Khalaf, M.M.; Mohamed, I.M.A. Steel protection in acidified 3.5% NaCl by novel hybrid composite of CoCrO3/polyaniline: Chemical fabrication, physicochemical properties, and corrosion inhibition performance. Constr. Build. Mater. 2022, 317, 125918. [Google Scholar] [CrossRef]
- Aziz, N.A.; Salleh, N.H.M.; Gopinath, S.C.B.; Kamaruddin, A.H.; Ridzuan, M.I.; Ahmad, N.A. Valorization of Momordica charantia seeds into phytogenically synthesized silver nanoparticles for the protection of oyster mushrooms against Trichoderma pleuroticola. J. Environ. Chem. Eng. 2023, 11, 111064. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Dao, V.-D.; Yasin, A.S.; Choi, H.-S.; Khalil, K.; Barakat, N.A.M. Facile synthesis of GO@SnO2/TiO2 nanofibers and their behavior in photovoltaics. J. Colloid Interface Sci. 2017, 490, 303–313. [Google Scholar] [CrossRef]
- Montakhab, E.; Rashchi, F.; Sheibani, S. Enhanced photocatalytic activity of TiO2 nanotubes decorated with Ag nanoparticles by simultaneous electrochemical deposition and reduction processes. Appl. Surf. Sci. 2023, 615, 156332. [Google Scholar] [CrossRef]
- Ban, C.; Wang, Y.; Ma, J.; Feng, Y.; Wang, X.; Qin, S.; Jing, S.; Duan, Y.; Zhang, M.; Tao, X.; et al. Metal–oxygen hybridization in Agcluster/TiO2 for selective CO2 photoreduction to CH4. Chem. Eng. J. 2024, 488, 150845. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, H.; Gao, F.; Yu, L.; Shang, K.; Fang, M.; Ma, B.; Tan, X.; Wang, S.; Wang, X. Magnetic Field Induced Dielectric Polarization to Enhance Raman Detection of Nitrite by NiFe2O4@Ag. Adv. Funct. Mater. 2024, 34, 2414319. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, M.; Meng, L.; Su, S.; Ding, W.; Yuan, S.; Li, H.; Li, X. Plasma Ag nanoparticles loaded on Bi2MoO6 to enhance surface oxygen vacancies for efficient nitrogen conversion to ammonia. J. Mater. Chem. C 2024, 12, 10070–10082. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Touny, A.H.; Saleh, M.M.; Mohamed, I.M.A. Electrocatalytic performance of inorganic nanoflakes nickel phosphates under adjusted synthetic parameters towards urea and methanol oxidation in alkaline media. Microchem. J. 2021, 163, 105901. [Google Scholar] [CrossRef]
- Belkassa, K.; Khelifa, M.; Batonneau-Gener, I.; Marouf-Khelifa, K.; Khelifa, A. Understanding of the mechanism of crystal violet adsorption on modified halloysite: Hydrophobicity, performance, and interaction. J. Hazard. Mater. 2021, 415, 125656. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.M.A.; Shehata, A.G.; Al-Anazi, A.; Khairy, M.; Newair, E.F. A template-assisted method for synthesizing TiO2 nanoparticles and Ni/TiO2 nanocomposites for urea electrooxidation. Mater. Chem. Phys. 2024, 316, 129112. [Google Scholar] [CrossRef]
- Uthiravel, V.; Narayanamurthi, K.; Raja, V.; Anandhabasker, S.; Kuppusamy, K. Green synthesis and characterization of TiO2 and Ag-doped TiO2 nanoparticles for photocatalytic and antimicrobial applications. Inorg. Chem. Commun. 2024, 170, 113327. [Google Scholar] [CrossRef]
- Singh, G.; Nimanpure, S.; Acharyya, N.; Rane, S.; Roychowdhury, D.; Singh, B.P.; Tawale, J.S.; Sharma, R.; Jewariya, M. High-performing TiO2 flower-like nanostructures based on flexible MWCNTs for dual-band terahertz absorption. J. Mater. Chem. C 2023, 11, 14199–14206. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Abinaya, M.; Chen, S.-M.; Sethupathi, V.; Muthuraj, V. Ultrasound assisted synthesis of silver titanate for the differential pulse voltammetric determination of antibiotic drug metronidazole. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 134, 114865. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, Y.; Zhang, S.; Shi, R.; Zhang, T. Strain engineering: A boosting strategy for photocatalysis. Adv. Mater. 2022, 34, 2200868. [Google Scholar] [CrossRef]
- Amr, M.; Abu-Hussien, S.H.; Ismail, R.; Aboubakr, A.; Wael, R.; Yasser, M.; Hemdan, B.; El-Sayed, S.M.; Bakry, A.; Ebeed, N.M.; et al. Utilization of biosynthesized silver nanoparticles from Agaricus bisporus extract for food safety application: Synthesis, characterization, antimicrobial efficacy, and toxicological assessment. Sci. Rep. 2023, 13, 15048. [Google Scholar] [CrossRef]
- Rathi, V.H.; Jeice, A.R.; Jayakumar, K. Green synthesis of Ag/CuO and Ag/TiO2 nanoparticles for enhanced photocatalytic dye degradation, antibacterial, and antifungal properties. Appl. Surf. Sci. Adv. 2023, 18, 100476. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, D.K. Insight into the fluoroquinolone resistance, sources, ecotoxicity, and degradation with special emphasis on ciprofloxacin. J. Water Process Eng. 2021, 43, 102218. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic degradation by TiO2-conjugated/coordination polymer heterojunction: Preparation, mechanisms, and prospects. Appl. Catal. B 2024, 344, 123605. [Google Scholar] [CrossRef]
- Bopape, D.A.; Mathobela, S.; Matinise, N.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of CuO-TiO2 Nanoparticles for the Degradation of Organic Pollutants: Physical, Optical and Electrochemical Properties. Catalysts 2023, 13, 163. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhou, Y.; Wang, J.; Li, A.; Corvini, P.F.-X. Boosting light harvesting and charge separation over hollow double-shelled Ag@SrTiO3-TiO2 with Z-scheme heterostructure for highly efficient photocatalytic reduction of nitrate to N2. Chem. Eng. J. 2023, 457, 140992. [Google Scholar] [CrossRef]
- da Silva, M.P.C.; Gomes, A.M.C.; Oliveira, A.F.; Huanca, D.R. Enhanced Schottky barrier engineering in silver-doped TiO2/p-Si heterojunctions: Insights into electrical behavior and charge carrier dynamics. Mater. Chem. Phys. 2024, 328, 129995. [Google Scholar] [CrossRef]
- Mutukwa, D.; Taziwa, R.T.; Tichapondwa, S.M.; Khotseng, L. Optimisation, Synthesis, and Characterisation of ZnO Nanoparticles Using Leonotis ocymifolia (L. ocymifolia) Leaf Extracts for Antibacterial and Photodegradation Applications. Int. J. Mol. Sci. 2024, 25, 11621. [Google Scholar] [CrossRef]
- Khadka, D.; Gautam, P.; Dahal, R.; Ashie, M.D.; Paudyal, H.; Ghimire, K.N.; Pant, B.; Poudel, B.R.; Bastakoti, B.P.; Pokhrel, M.R. Evaluating the Photocatalytic Activity of Green Synthesized Iron Oxide Nanoparticles. Catalysts 2024, 14, 751. [Google Scholar] [CrossRef]
- Sabzehparvar, M.; Kiani, F.; Tabrizi, N.S. Mesoporous-assembled TiO2-NiO-Ag nanocomposites with p-n/Schottky heterojunctions for enhanced photocatalytic performance. J. Alloys Compd. 2021, 876, 160133. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. rGO-wrapped Ag-doped TiO2 nanofibers for photocatalytic CO2 reduction under visible light. J. Clean. Prod. 2022, 374, 134022. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Hu, S.; Wang, H.; Jiang, W.; Chen, X. Facile construction of novel Bi2WO6/Ta3N5 Z-scheme heterojunction nanofibers for efficient degradation of harmful pharmaceutical pollutants. Chem. Eng. J. 2020, 402, 126165. [Google Scholar] [CrossRef]
- Yang, W.; He, X.; He, T.; Li, Y.; Yang, J.; Huang, H.; Cui, S. Broad spectral response of Y-NaBi(MoO4)2@NaYF4:Yb, Tm photocatalysts for efficient degradation of ciprofloxacin: Upconversion mechanism and theoretical calculations. Ceram. Int. 2024, 50, 46904–46915. [Google Scholar] [CrossRef]
- El-Sayed, F.; Hussien, M.S.A.; AlAbdulaal, T.H.; Ismail, A.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S.; Khairy, Y.; Ali, T.E.; Ibrahim, M.A. Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures. Crystals 2022, 12, 535. [Google Scholar] [CrossRef]
- El-Sayed, F.; Hussien, M.S.A.; AlAbdulaal, T.H.; Abdel-Aty, A.-H.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S.; Ibrahim, E.H.; Ibrahim, M.A.; Elhaes, H. Study of catalytic activity of G-SrO nanoparticles for degradation of cationic and anionic dye and comparative study photocatalytic and electro & photo-electrocatalytic of anionic dye degradation. J. Mater. Res. Technol. 2022, 20, 959–975. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef]
- Song, S.Y.; Chen, H.D.; Li, C.X.; Shi, D.S.; Ying, Y.; Han, Y.B.; Xu, J.C.; Hong, B.; Jin, H.X.; Jin, D.F.; et al. Magnetic Bi2WO6 nanocomposites: Synthesis, magnetic response and their visible-light-driven photocatalytic performance for ciprofloxacin. Chem. Phys. 2020, 530, 110614. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Bahnemann, D.W. Controlled synthesis of Ag2O/g-C3N4 heterostructures using soft and hard templates for efficient and enhanced visible-light degradation of ciprofloxacin. Ceram. Int. 2021, 47, 31073–31083. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Hadadi, A.; Manseri, A.; Bouzaza, A.; Assadi, A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).