Enhanced Visible-Light Photocatalysis Activity of TiO2/Ag Nanocomposites Prepared by the Ultrasound-Assisted Sol–Gel Method: Characterization and Degradation–Mineralization of Cationic and Anionic Dyes
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of TiO2/Ag Composite Photocatalysts
2.3. Nanoparticles Characterization
2.4. Photocatalysis Tests of BM and AO7 Under Visible Light
3. Results and Discussion
3.1. Characterization of TiO2/Ag Composites
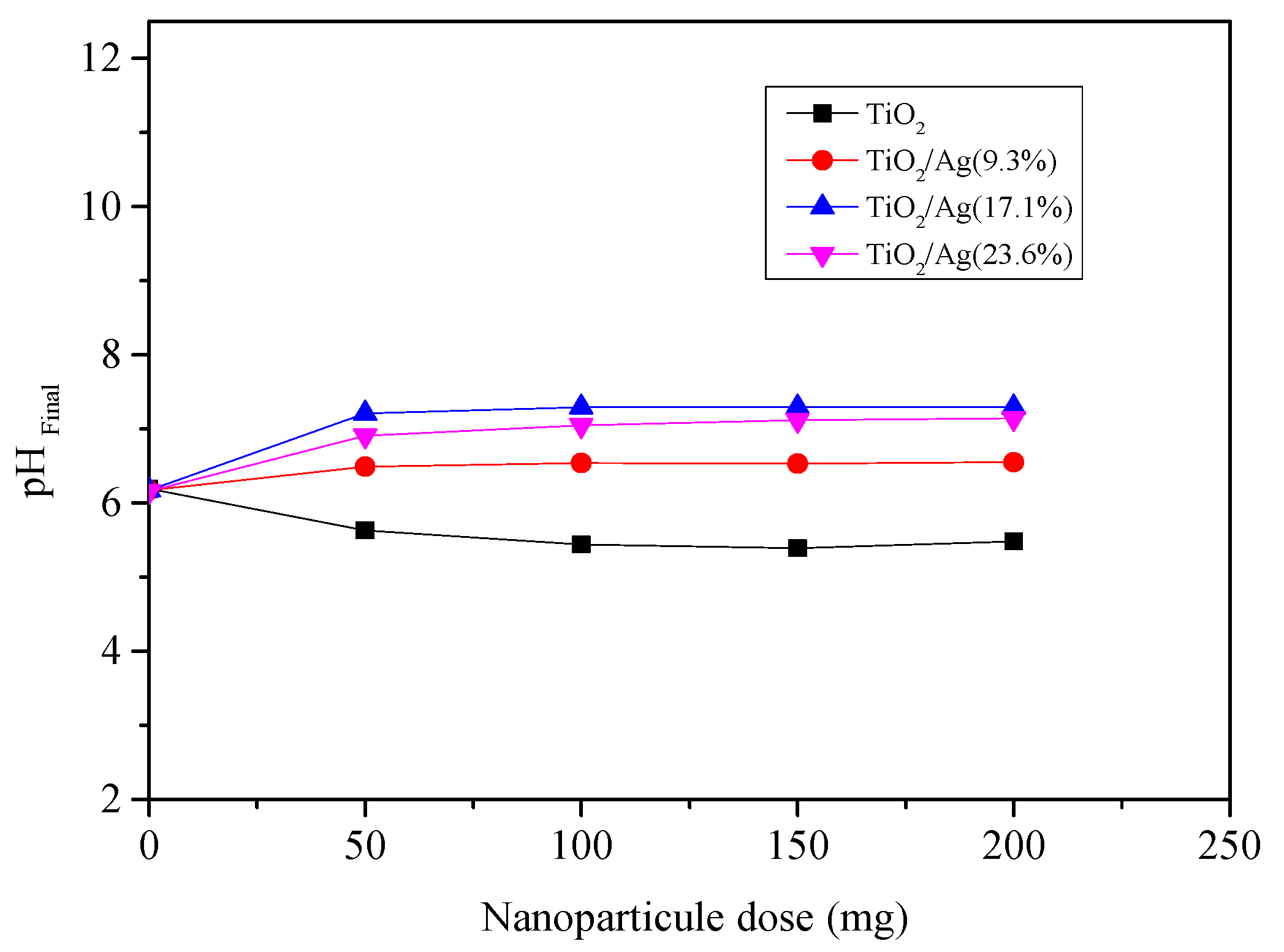
3.1.1. pHPZC
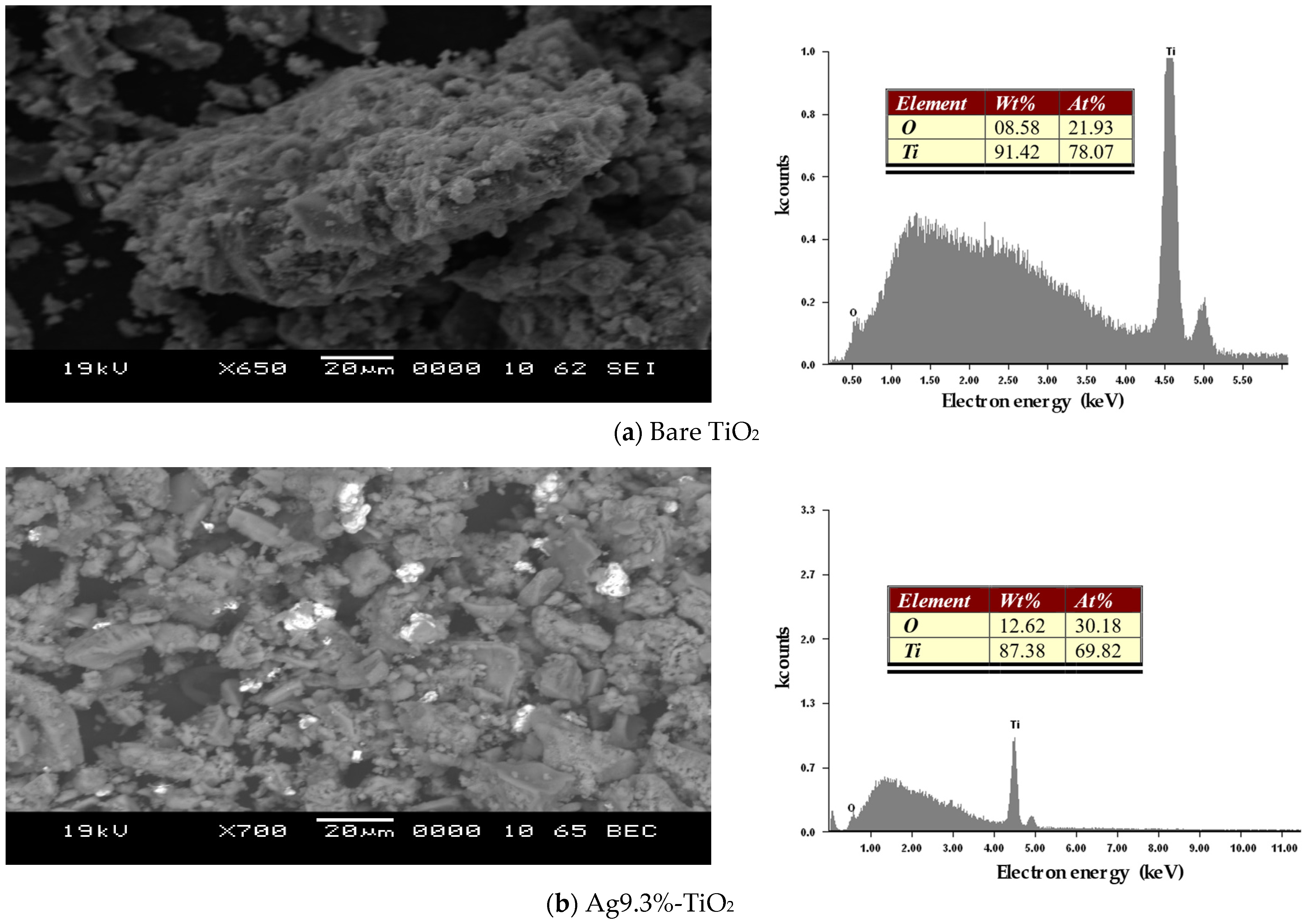
3.1.2. SEM/EDX
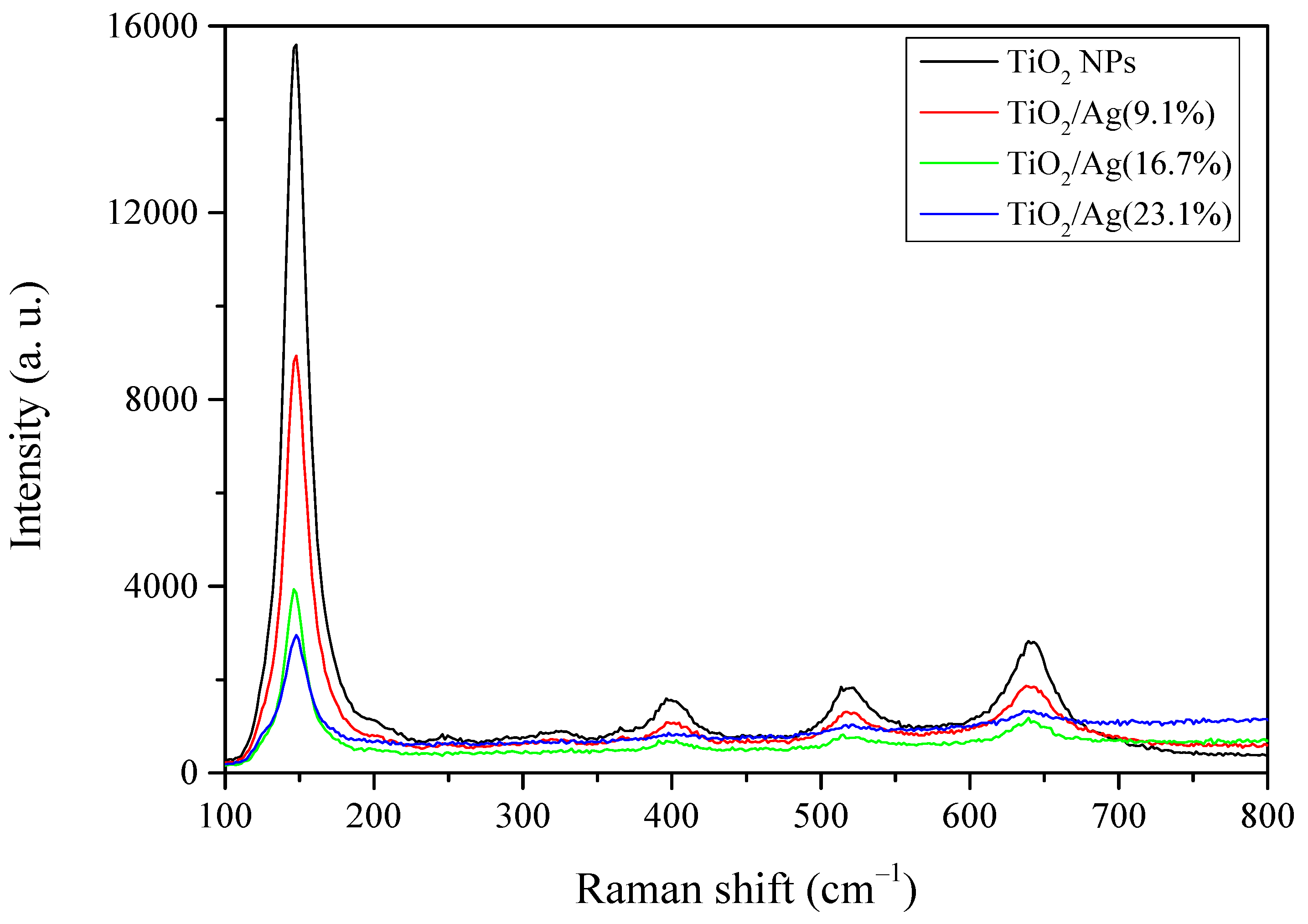
3.1.3. Raman Spectroscopy
3.2. Photocatalytic Activity
3.2.1. Effect of Ag-Doping TiO2 Nanoparticle on Photocatalytic Degradation of MB and AO7 Dyes Under Visible-Light Irradiation
3.2.2. Kinetic Studies
3.2.3. Stability Tests
3.2.4. Effect of Water Matrix Nature on Photocatalytic Activity
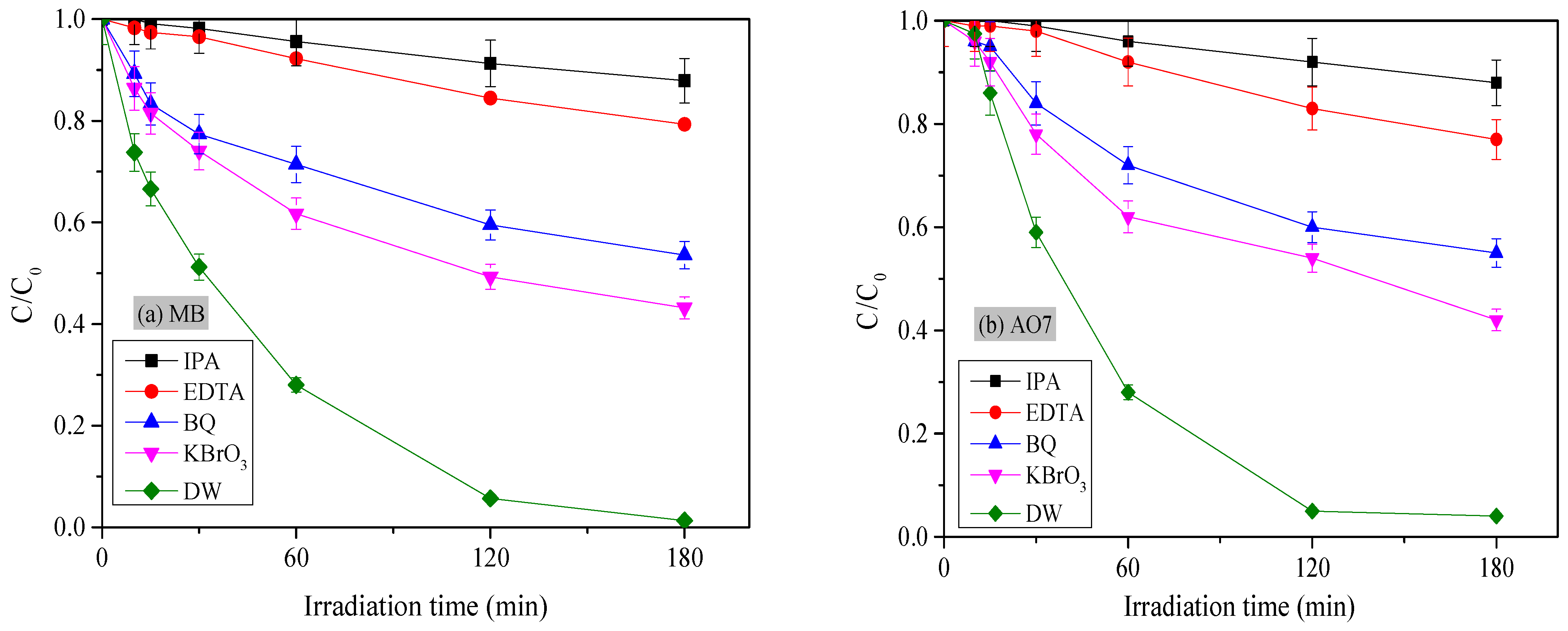
3.2.5. Proposed Photocatalytic Reaction Mechanism on TiO2/Ag9.3%: Role of Reactive Oxygen Species in AO7 and MB Degradation
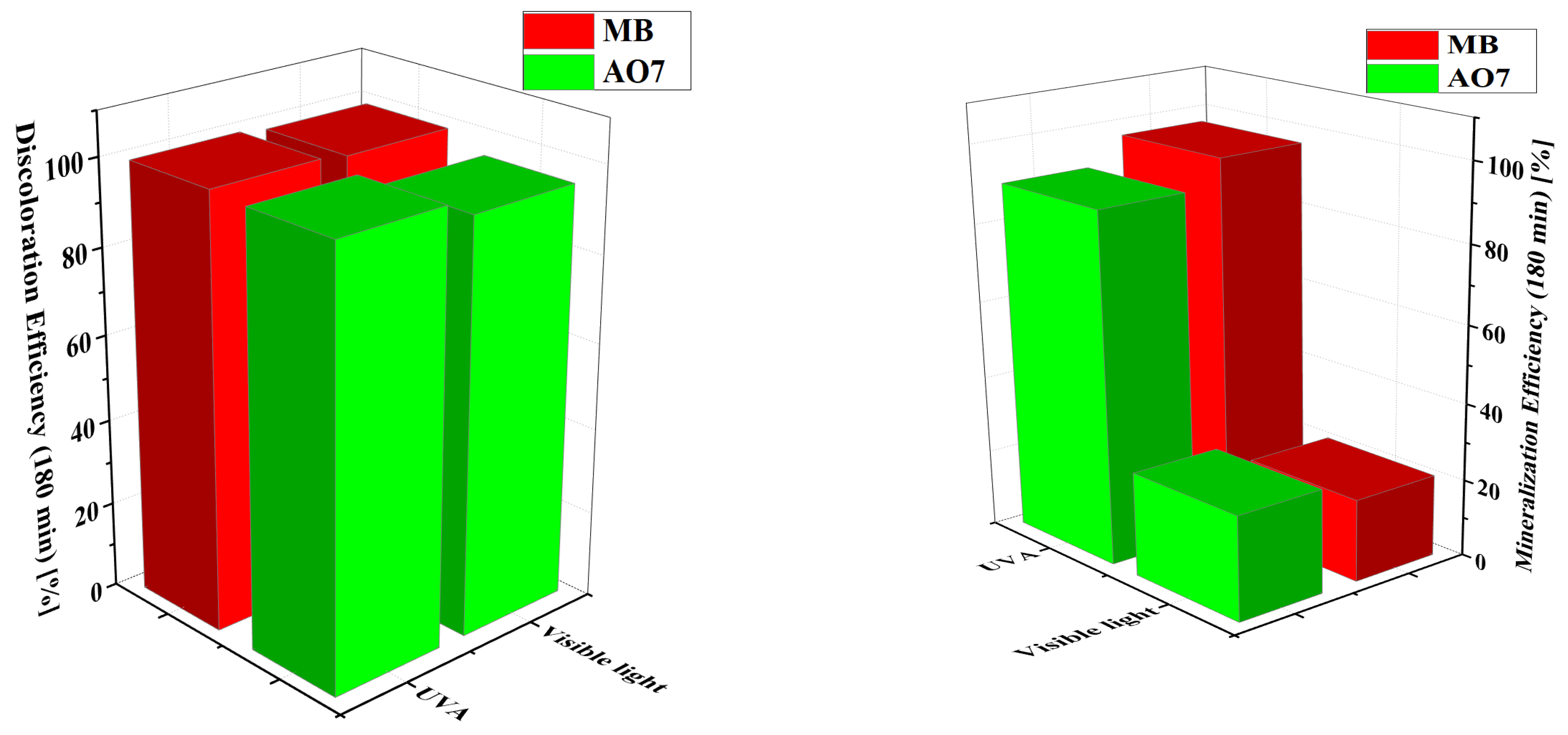
3.2.6. Mineralization Studies by TOC Analysis
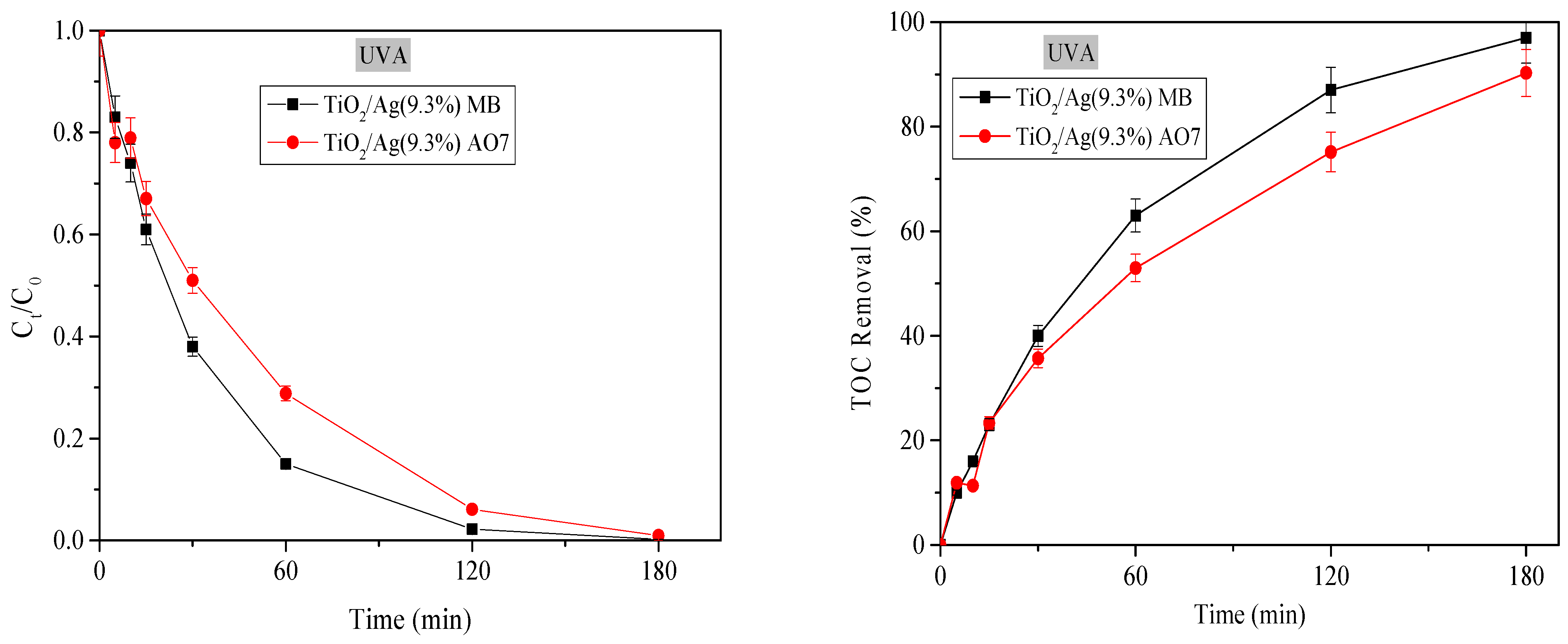
3.2.7. Photocatalytic Mineralization of MB and AO7 Dyes Under UV-A Irradiation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, S.; Mishra, A.K.; Bhat, M.A.; Bhat, M.A.; Jan, A.T. Pollutants in Aquatic System: A Frontier Perspective of Emerging Threat and Strategies to Solve the Crisis for Safe Drinking Water. Environ. Sci. Pollut. Res. Int. 2023, 30, 113242–113279. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.; Musteret, C.P.; Afrasinei, M.A. The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials. Appl. Sci. 2024, 14, 2184. [Google Scholar] [CrossRef]
- Jerin, I.; Rahman, M.A.; Khan, A.H.; Hossain, M.M. Photocatalytic Degradation of Methylene Blue under Visible Light Using Carbon-Doped Titanium Dioxide as Photocatalyst. Desalination Water Treat. 2024, 320, 100711. [Google Scholar] [CrossRef]
- Cardito, A.; Carotenuto, M.; Sacco, O.; Albarano, L.; Vaiano, V.; Iannece, P.; Libralato, G.; Spica, V.R.; Lofrano, G. UV Light Assisted Degradation of Acid Orange Azo Dye by ZVI-ZnS and Effluent Toxicity Effects. Environ. Pollut. 2024, 343, 123226. [Google Scholar] [CrossRef]
- Sannino, D.; Morante, N.; Sacco, O.; Mancuso, A.; De Guglielmo, L.; Di Capua, G.; Femia, N.; Vaiano, V. Visible Light-Driven Degradation of Acid Orange 7 by Light Modulation Techniques. Photochem. Photobiol. Sci. 2023, 22, 185–193. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Liyanaarachchi, C.; Samarakoon, U. Efficient Photocatalysis of Cu Doped TiO2/g-C3N4 for the Photodegradation of Methylene Blue. Arab. J. Chem. 2023, 16, 104749. [Google Scholar] [CrossRef]
- Nabeel, M.I.; Hussain, D.; Ahmad, N.; Xiao, H.-M.; Musharra, S.G. Improved Visible Light-Driven Photocatalytic Degradation of an Industrial Dye Acid Orange 7 Using Metal-Free Sulfur-Doped Graphitic Carbon Nitride. Environ. Sci. Nano 2023, 10, 2810–2830. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, X.; Zhang, Y.; Li, Y.; Shao, W.; Fu, J.; Lin, Q.; Tan, J.; Gao, S.; Ye, W.; et al. Highly Efficient Harvesting of Vibration Energy for Complex Wastewater Purification Using Bi5Ti3FeO15 with Controlled Oxygen Vacancies. Chem. Eng. J. 2023, 453, 139919. [Google Scholar] [CrossRef]
- Fitch, A.; Balderas-Hernandez, P.; Ibanez, J.G. Electrochemical Technologies Combined with Physical, Biological, and Chemical Processes for the Treatment of Pollutants and Wastes: A Review. J. Environ. Chem. Eng. 2022, 10, 107810. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions; Springer: Berlin/Heidelberg, Germany, 2023; Volume 30, ISBN 0123456789. [Google Scholar]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Khader, E.H.; Muslim, S.A.; Saady, N.M.C.; Ali, N.S.; Salih, I.K.; Mohammed, T.J.; Albayati, T.M.; Zendehboudi, S. Recent Advances in Photocatalytic Advanced Oxidation Processes for Organic Compound Degradation: A Review. Desalination Water Treat. 2024, 318, 100384. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced Oxidation Process: A Sustainable Technology for Treating Refractory Organic Compounds Present in Industrial Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 25477–25505. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Lago, A.; Silva, B.; Barros, Ó.; Neves, I.C.; Tavares, T. Immobilization of Biogenic Metal Nanoparticles on Sustainable Materials—Green Approach Applied to Wastewater Treatment: A Systematic Review. Environ. Sci. Nano 2024, 11, 36–60. [Google Scholar] [CrossRef]
- Ahtasham Iqbal, M.; Akram, S.; Khalid, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Mushtaq, M.; Chinibayeva, N.; Hosseini-Bandegharaei, A. Advanced Photocatalysis as a Viable and Sustainable Wastewater Treatment Process: A Comprehensive Review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and Application of Titanium Dioxide Photocatalysis for Energy, Decontamination and Viral Disinfection: A Review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Van Thuan, D.; Ngo, H.L.; Thi, H.P.; Chu, T.T.H. Photodegradation of Hazardous Organic Pollutants Using Titanium Oxides -Based Photocatalytic: A Review. Environ. Res. 2023, 229, 116000. [Google Scholar] [CrossRef]
- Kubiak, A. Impact of LED Radiation Intensity on Gold Nanoparticles Photodeposition on TiO2 with Physicochemical and Photocatalytic Characterization. Sci. Rep. 2024, 14, 20563. [Google Scholar] [CrossRef]
- Rossi, L.; Villabrille, P.I.; Pastrana-Martínez, L.M.; Caregnato, P.; Rosso, J.A. Photocatalytic Performance of Palladium and Carbon Modified TiO2 Using Solar Radiation. J. Photochem. Photobiol. A Chem. 2023, 437, 114461. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Gilani, M.R.H.S.; Maaz, M.; Akhter, P.; Hussain, M.; Jeong, K.E.; Kwon, E.E.; Bae, S.; Park, Y.K. Advancements in TiO2-Based Photocatalysis for Environmental Remediation: Strategies for Enhancing Visible-Light-Driven Activity. Chemosphere 2024, 349, 140703. [Google Scholar] [CrossRef]
- Mohsin, M.; Bhatti, I.A.; Zeshan, M.; Yousaf, M.; Iqbal, M. Prospects, Challenges, and Opportunities of the Metals-Modified TiO2 Based Photocatalysts for Hydrogen Generation under Solar Light Irradiation: A Review. FlatChem 2023, 42, 100547. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification Strategies of TiO2 Based Photocatalysts for Enhanced Visible Light Activity and Energy Storage Ability: A Review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Li, M.; Li, J. Advances in the Preparation and Biological Applications of Core@shell Nanocrystals Based on Quantum Dots and Noble Metal. RSC Adv. 2024, 14, 26308–26324. [Google Scholar] [CrossRef] [PubMed]
- Harun-Ur-Rashid, M.; Pal, K.; Imran, A.B. Hybrid Nanocomposite Fabrication of Nanocatalyst with Enhanced and Stable Photocatalytic Activity. Top. Catal. 2024, 67, 17–45. [Google Scholar] [CrossRef]
- Piwoński, J.; Kisielewska, A.; Piwoński, I. Preparation and Photocatalytic Activity of TiO2 Photonic Crystals Modified by Bimetallic Ag–Pt Nanostructures. Catal. Sci. Technol. 2024, 14, 4274–4292. [Google Scholar]
- Zhou, G.; Meng, H.; Cao, Y.; Kou, X.; Duan, S.; Fan, L.; Xiao, M.; Zhou, F.; Li, Z.; Xing, Z. Surface Plasmon Resonance-Enhanced Solar-Driven Photocatalytic Performance from Ag Nanoparticles-Decorated Ti3+ Self-Doped Porous Black TiO2 Pillars. J. Ind. Eng. Chem. 2018, 64, 188–193. [Google Scholar] [CrossRef]
- Nie, J.; Schneider, J.; Sieland, F.; Zhou, L.; Xia, S.; Bahnemann, D.W. New Insights into the Surface Plasmon Resonance (SPR) Driven Photocatalytic H2 Production of Au-TiO2. RSC Adv. 2018, 8, 25881–25887. [Google Scholar] [CrossRef]
- Wang, J.; Fazil, P.; Ali Shah, M.I.; Zada, A.; Anwar, N.; Zain, G.G.; Khan, W.; Jan, F.; Lei, T.; Ateeq, M. Surface Plasmon Assisted Photocatalytic Hydrogen Generation with Ag Decorated G-C3N4 Coupled SnO2 Nanophotocatalyst under Visible-Light Driven Photocatalysis. Int. J. Hydrogen Energy 2023, 48, 21674–21685. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cheng, Z.E.; Tahir, A.A.; Luo, W.; Wang, C. Au Surface Plasmon Resonance Promoted Charge Transfer in Z-Scheme System Enables Exceptional Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2022, 310, 121322. [Google Scholar] [CrossRef]
- Li, J.; Duan, Y.; Wang, L.; Ma, J. Preparation of Core-Shell Structure Ag@TiO2 Plasma Photocatalysts and Reduction of Cr(VI): Size Dependent and LSPR Effect. Environ. Res. 2024, 248, 118265. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Shailajha, S. Plasmonic Core–Shell Nanoparticles of Ag@TiO2 for Photocatalytic Degradation of Rhodamine B. Appl. Nanosci. 2023, 13, 3677–3692. [Google Scholar] [CrossRef]
- Xu, H.; Li, G.; Liu, N.; Zhu, K.; Zhu, G.; Jin, S. Ag @ Hierarchical TiO2 Core-Shell Nanostructures for Enhanced Photocatalysis. Mater. Lett. 2015, 142, 324–327. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Wong, K.; Jiang, X.C.; Yu, A.B. Hybrid Ag@TiO2 Core-Shell Nanostructures with Highly Enhanced Photocatalytic Performance. Nanotechnology 2013, 24, 415601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Lee, D.-S. Photocatalytic Destruction of Methylene Blue on Ag@TiO2 with Core/Shell Structure. OALib 2014, 1, 1–14. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.N.K.; Cheng, C.K. Schottky Barrier and Surface Plasmonic Resonance Phenomena towards the Photocatalytic Reaction: Study of Their Mechanisms to Enhance Photocatalytic Activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef]
- Wu, L.; Ma, S.; Chen, P.; Li, X. The Mechanism of Enhanced Charge Separation and Photocatalytic Activity for Au@TiO2 Core-Shell Nanocomposite. Int. J. Environ. Anal. Chem. 2023, 103, 201–211. [Google Scholar] [CrossRef]
- Dimitratos, N.; Alshammari, K.; Niu, Y.; Palmer, R.E.; Dimitratos, N. Optimization of Sol-Immobilized Bimetallic Au–Pd/TiO2 Catalysts: Reduction of 4-Nitrophenol to 4-Aminophenol for Wastewater Remediation. Philos. Trans. R. Soc. A 2020, 24, 20200057. [Google Scholar]
- Yilleng, M.T.; Artioli, N.; Rooney, D.; Manyar, H. Continuous Flow Photocatalytic Degradation of Phenol Using Palladium@Mesoporous TiO2 Core@Shell Nanoparticles. Water 2023, 15, 2975. [Google Scholar] [CrossRef]
- He, Z.; Zhang, C.; Meng, R.; Luo, X.; Chen, M.; Lu, H.; Yang, Y. Influence of Ag@SiO2 with Different Shell Thickness on Photoelectric Properties of Hole-Conductor-Free Perovskite Solar Cells. Nanomaterials 2020, 10, 2364. [Google Scholar] [CrossRef]
- Morante, N.; Folliero, V.; Dell’Annunziata, F.; Capuano, N.; Mancuso, A.; Monzillo, K.; Galdiero, M.; Sannino, D.; Franci, G. Characterization and Photocatalytic and Antibacterial Properties of Ag- and TiOx-Based (x = 2, 3) Composite Nanomaterials under UV Irradiation. Materials 2024, 17, 2178. [Google Scholar] [CrossRef]
- Yang, X.; Liang, J.; Fu, H.; Ran, X.; An, X. Fabrication of Au-Ag@TiO2 Ternary Core-Shell Nanostructures with Enhanced Sunlight Photocatalytic Activity. Powder Technol. 2022, 404, 117463. [Google Scholar] [CrossRef]
- Misra, M.; Singh, N.; Gupta, R.K. Enhanced Visible-Light-Driven Photocatalytic Activity of Au@Ag Core-Shell Bimetallic Nanoparticles Immobilized on Electrospun TiO2 Nanofibers for Degradation of Organic Compounds. Catal. Sci. Technol. 2017, 7, 570–580. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. C—J. Carbon Res. 2018, 4, 62. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D.; Ciambelli, P. Visible Light Driven Mineralization of Spiramycin over Photostructured N-Doped TiO2 on up Conversion Phosphors. J. Environ. Sci. 2017, 54, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Photocatalytic Removal of Spiramycin from Wastewater under Visible Light with N-Doped TiO2 Photocatalysts. Chem. Eng. J. 2015, 261, 3–8. [Google Scholar] [CrossRef]
- Liza, T.Z.; Tusher, M.M.H.; Anwar, F.; Monika, M.F.; Amin, K.F.; Asrafuzzaman, F.N.U. Effect of Ag-Doping on Morphology, Structure, Band Gap and Photocatalytic Activity of Bio-Mediated TiO2 Nanoparticles. Results Mater. 2024, 22, 100559. [Google Scholar] [CrossRef]
- Jaihindh, D.P.; Chen, C.C.; Fu, Y.P. Reduced Graphene Oxide-Supported Ag-Loaded Fe-Doped TiO2 for the Degradation Mechanism of Methylene Blue and Its Electrochemical Properties. RSC Adv. 2018, 8, 6488–6501. [Google Scholar] [CrossRef]
- Suriya, P.; Prabhu, M.; Jagannathan, K. Synthesis and Structural, Optical and Photovoltaic Characteristics of Pure and Ag Doped TiO2 Nanoparticles for Dye Sensitized Solar Cell Application. Mater. Today Proc. 2022, 65, 100–105. [Google Scholar] [CrossRef]
- Tzevelekidis, P.; Theodosiou, M.; Papadopoulou, A.; Sakellis, E.; Boukos, N.; Alexandros, K.; Bikogiannakis, G.K.; Efthimiadou, E.K.; Mitsopoulou, C.A. Visible-Light-Activated Antibacterial and Antipollutant Properties of Biocompatible Cu-Doped and Ag-Decorated TiO2 Nanoparticles. Heliyon 2019, 10, 115147. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Alvarado-Gil, J.J.; Oskam, G.; Rodríguez-Gattorno, G. Influence of Brookite Impurities on the Raman Spectrum of TiO2 Anatase Nanocrystals. J. Phys. Chem. C 2018, 122, 19921–19930. [Google Scholar] [CrossRef]
- Tao, X.; Ruan, P.; Zhang, X.; Sun, H.; Zhou, X. Microsphere Assembly of TiO2 Mesoporous Nanosheets with Highly Exposed (101) Facets and Application in a Light-Trapping Quasi-Solid-State Dye-Sensitized Solar Cell. Nanoscale 2015, 7, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Machado, W.A.; França, M.D.; Borges, K.A.; Paniago, R.M.; Patrocinio, A.O.T.; Machado, A.E.H. Structural Characterization of Ag-Doped TiO2 with Enhanced Photocatalytic Activity. RSC Adv. 2015, 5, 103752–103759. [Google Scholar] [CrossRef]
- Mills, A.; O’Rourke, C.; Kalousek, V.; Rathousky, J. Adsorption and Photocatalytic and Photosensitised Bleaching of Acid Orange 7 on Multilayer Mesoporous Films of TiO2. J. Hazard. Mater. 2012, 211–212, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Belayachi, H.; Bestani, B.; Benderdouche, N.; Belhakem, M. The Use of TiO2 Immobilized into Grape Marc-Based Activated Carbon for RB-5 Azo Dye Photocatalytic Degradation. Arab. J. Chem. 2019, 12, 3018–3027. [Google Scholar] [CrossRef]
- Bibi, S.; Shah, S.S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.A.; Sheikh Saleh Mushab, M.; Sarwar, S. Cu-Doped Mesoporous TiO2 Photocatalyst for Efficient Degradation of Organic Dye via Visible Light Photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Ouhssain, A.; Kadmiri, I.M.; Benzeid, H.; Zari, N.; Qaiss, A.; Bouhfid, R. Photocatalytic and Bactericidal Behaviors of Ag/TiO2 Doped Biochar through Ball–Milling Approach. J. Photochem. Photobiol. A Chem. 2023, 444, 114971. [Google Scholar] [CrossRef]
- Asrafuzzaman, F.N.U.; Amin, K.F.; Gafur, M.A.; Gulshan, F. Mangifera Indica Mediated Biogenic Synthesis of Undoped and Doped TiO2 Nanoparticles and Evaluation of Their Structural, Morphological, and Photocatalytic Properties. Results Mater. 2023, 17, 100384. [Google Scholar] [CrossRef]
- Radić, N.; Ilić, M.; Stojadinović, S.; Milić, J.; Avdalović, J.; Šaponjić, Z. Photocatalytically Active Ag-Doped TiO2 Coatings Developed by Plasma Electrolytic Oxidation in the Presence of Colloidal Ag Nanoparticles. J. Phys. Chem. Solids 2024, 188, 111918. [Google Scholar] [CrossRef]
- Bellè, U.; Pelizzari, F.; Lucotti, A.; Castiglioni, C.; Ormellese, M.; Pedeferri, M.; Diamanti, M.V. Immobilized Nano-TiO2 Photocatalysts for the Degradation of Three Organic Dyes in Single and Multi-Dye Solutions. Coatings 2020, 10, 919. [Google Scholar] [CrossRef]
- Rathi, V.H.; Jeice, A.R.; Jayakumar, K. Green Synthesis of Ag/CuO and Ag/TiO2 Nanoparticles for Enhanced Photocatalytic Dye Degradation, Antibacterial, and Antifungal Properties. Appl. Surf. Sci. Adv. 2023, 18, 100476. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Bonelli, B.; Esposito, S.; Freyria, F.S.; Blangetti, N.; Sannino, D. Visible Light-Driven Photocatalytic Activity and Kinetics of Fe-Doped TiO2 Prepared by a Three-Block Copolymer Templating Approach. Materials 2021, 14, 3105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; Chen, W.; Li, L.; Zen, M.; Qin, S.; Sun, S. Photodecolorization of Rhodamine B on Tungsten-Doped TiO2/Activated Carbon under Visible-Light Irradiation. J. Hazard. Mater. 2012, 227–228, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Elias, M.; Sarker, D.R.; Diba, Z.R.; Mithun, J.M.; Azad, M.A.K.; Siddiquey, I.A.; Rahman, M.M.; Uddin, J.; Uddin, M.N. Synthesis of Fe- or Ag-Doped TiO2–MWCNT Nanocomposite Thin Films and Their Visible-Light-Induced Catalysis of Dye Degradation and Antibacterial Activity. Res. Chem. Intermed. 2018, 44, 2667–2683. [Google Scholar] [CrossRef]
- Ratshiedana, R.; Fakayode, O.J.; Mishra, A.K.; Kuvarega, A.T. Visible-Light Photocatalytic Degradation of Tartrazine Using Hydrothermal Synthesized Ag-Doped TiO2 Nanoparticles. J. Water Process Eng. 2021, 44, 102372. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, Y.; Chen, L.; Chen, S.; Ma, L.; Wu, Y.; Wang, Q. Construction of Ternary Ag/Ti3C2/TiO2 Photocatalysts for Rhodamine B Degradation under Visible Light Radiation. Opt. Mater. 2022, 134, 113201. [Google Scholar] [CrossRef]
- Garg, S.; Kataria, J.; Sharma, S.; Choudhary, M.K. Kinetic Investigation of Invigorated Photocatalytic Performance of Phytogenically Prepared Ag@TiO2 Composites. Mater. Chem. Phys. 2023, 294, 127005. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Torres-Pinto, A.; Pedrosa, M.; Munoz, M.; de Pedro, Z.M.; Silva, C.G.; Faria, J.L.; Casas, J.A.; Silva, A.M.T. Application of G-C3N4-PVDF Membrane for the Photocatalytic Degradation of Micropollutants in Continuous Flow Mode: Impact of Water Matrix. J. Environ. Chem. Eng. 2023, 11, 110586. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Cortés-Lagunes, S.; Mahjoub, O.; Serrano-Lázaro, A.; Garduño-Jiménez, A.; Zanella, R. Tapping the Tunisian Sunlight’s Potential to Remove Pharmaceuticals in Tap Water and Secondary Effluents: A Comparison of Ag2O/TiO2 and BiOI Photocatalysts and Toxicological Insights. Sep. Purif. Technol. 2024, 335, 126221. [Google Scholar] [CrossRef]
- Bhuyan, A.; Ahmaruzzaman, M. Ternary 3D/2D/3D Direct Dual Z-Scheme MOF-on-MOF-Derived α-Fe2O3/g-C3N4/Fe-MOF Photocatalyst for Boosted Sunlight-Driven Removal of Metronidazole: Effect of Coexisting Ions, Mechanistic Insights, and Water Matrices. Environ. Sci. Nano 2024. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Inorganic–Organic Hybrid Quantum Dots for AOP-Mediated Photodegradation of Ofloxacin and Para-Nitrophenol in Diverse Water Matrices. NPJ Clean Water 2023, 6, 78. [Google Scholar] [CrossRef]
- Hernández-Laverde, M.; Morante, N.; Gutiérrez, B.L.; Murcia, J.J.; Monzillo, K.; Sannino, D.; Vaiano, V. Solar Light Elimination of Bacteria, Yeast and Organic Pollutants by Effective Photocatalysts Based on Ag/Cr-TiO2 and Pd/Cr-TiO2. Nanomaterials 2024, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ying, Z.; Li, H.; Liu, X.; Ma, D.; Yu, H. Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation. Toxins 2024, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Almulhem, N.K.; Awada, C.; Shaalan, N.M. Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment. Separations 2023, 10, 162. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, K.; Wen, X.; Yang, J.; Li, J. AgTNP@TiO2@Ag Core-Satellite Composites for Sensitive Sensing and in Situ Monitoring Photodegradation of Organic Dyes by Portable Raman Spectrometer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 306, 123562. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; An, W.; Zhao, H.; He, H.; Fu, Y.; Shen, X. Enhancing Visible-Light Degradation Performance of g-C3N4 on Organic Pollutants by Constructing Heterojunctions via Combining Tubular g-C3N4 with Bi2O3 Nanosheets. J. Alloys Compd. 2023, 934, 167928. [Google Scholar] [CrossRef]
- Sebti, A.; Boutra, B.; Trari, M.; Igoud, S. Solar Photodegradation of Solophenyl Red 3BL and Neuro-Fuzzy Modeling: Kinetic, Mechanism and Mineralization Studies. React. Kinet. Mech. Catal. 2022, 135, 2207–2229. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, Y.; Hu, Z.; Zhang, W.; Zhang, X.; Ji, X.; Wang, X. Modification of ZIF-8 Nanocomposite by a Gd Atom Doped TiO2 for High Efficiency Photocatalytic Degradation of Neutral Red Dye: An Experimental and Theoretical Study. J. Mol. Liq. 2023, 380, 121729. [Google Scholar] [CrossRef]
- Mottola, S.; Mancuso, A.; Sacco, O.; De Marco, I.; Vaiano, V. Photocatalytic Performance Assessment of Fe-N Co-Doped TiO2/β-Cyclodextrin Hybrid Systems Prepared by Supercritical Antisolvent Micronization for Organic Dyes Removal. J. Supercrit. Fluids 2023, 201, 106005. [Google Scholar] [CrossRef]







| Sample Name | Sample Description |
|---|---|
| TiO2 | Pure sol–gel TiO2 |
| TiO2/Ag(9.3%) | TiO2 (3.271 g), Ag (337.33 mg) |
| TiO2/Ag(17.1%) | TiO2 (3.271 g), Ag (674.66 mg) |
| TiO2/Ag(23.6%) | TiO2 (3. 271 g), Ag (1.012 g) |
| Sample Name | pHpzc | Gap (eV) | SBET (m2 g−1) |
|---|---|---|---|
| TiO2 | 5.48 | 3.13 | 96 |
| TiO2/Ag(9.3%) | 6.55 | 2.84 | 89 |
| TiO2/Ag(17.1%) | 7.29 | 2.49 | 82 |
| TiO2/Ag(23.6%) | 7.14 | 2.62 | 71 |
| MB | AO7 | |||||
|---|---|---|---|---|---|---|
| Nanoparticles | kapp (min−1) | t1/2 (min) | R2 | kapp (min−1) | t1/2 (min) | R2 |
| TiO2 | - | - | 9.253 × 10−4 | 749.07 | 0.9531 | |
| TiO2/Ag(9.3%) | 2.451 × 10−2 | 28.28 | 0.9986 | 1.895 × 10−2 | 36.58 | 0.9682 |
| TiO2/Ag(17.1%) | 1.340 × 10−2 | 51.73 | 0.989 | 1.028 × 10−2 | 67.43 | 0.934 |
| TiO2/Ag(23.6%) | 1.192 × 10−2 | 58.15 | 0.9717 | 1.057 × 10−2 | 65.58 | 0.9659 |
| Doped Nano-Particulate TiO2 | Dye | Source Illumination | Discoloration (%) | kapp (min−1) | Reference |
|---|---|---|---|---|---|
| Ag–TiO2 (50/50) | Methyl orange | Simulating sunlight lamp (300 W) | 88 | 2.43 × 10−3 | [59] |
| Fe(2.5wt)-TiO2 | AO7 | Visible LED (10 W) | ~90 | 4.2 × 10−3 | [62] |
| W0.6%–TiO25%–AC | Rhodamine B | Visible light | - | 1.86 × 10−2 | [63] |
| Ag5%-TiO2 | E102 tartrazine | Hg lamp (400 W) | 76 | 4.15 × 10−3 | [53] |
| Fe–TiO2–MWCNT | MB | Tungsten Lamp (200 W) | 58 | 3 × 10−3 | [64] |
| Ag3%-TiO2 | Tartrazine | Solar Simulator with Xenon light source | 87 | 8.38 × 10−3 | [65] |
| Ag/Ti3C2/TiO2 | Rhodamine-B | Visible light (300 W Xe lamp) | 97 | 4.73 × 10−3 | [66] |
| Ag3 wt%@TiO2 | Rhodamine-B | Sunlight | 99 | 4.77 × 10−2 | [67] |
| Ag3%-TiO2 | MB | Visible light (Xe lamp) | 94 | 5.57 × 10−2 | [61] |
| TiO2/Ag(9.3%) | MB | Visible light (8 W × 2) | 99 | 2.451 × 10−2 | Current work |
| TiO2/Ag(9.3%) | AO7 | Visible light (8 W × 2) | 95 | 1.895 × 10−2 | Current work |
| Cycle | kapp, min−1 (MB) | kapp, min−1 (AO7) |
|---|---|---|
| I cycle | 0.0238 | 0.0219 |
| II cycle | 0.0238 | 0.0168 |
| III cycle | 0.0187 | 0.0157 |
| IV cycle | 0.0185 | 0.0181 |
| V cycle | 0.0192 | 0.0193 |
| Parameter | Value |
|---|---|
| Conductivity (µS cm−1) | 471 |
| Sodium (ppm) | 3.25 |
| Potassium (ppm) | 1.12 |
| Calcium (ppm) | 85.9 |
| Magnesium (ppm) | 16.1 |
| Chlorides, Cl− (ppm) | 4.8 |
| Sulfates, SO42− (ppm) | 17.1 |
| Bicarbonates, HCO3− (ppm) | 319 |
| Nitrates, NO3− (ppm) | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudechiche, N.; Morante, N.; Sannino, D.; Monzillo, K.; Trari, M.; Sadaoui, Z. Enhanced Visible-Light Photocatalysis Activity of TiO2/Ag Nanocomposites Prepared by the Ultrasound-Assisted Sol–Gel Method: Characterization and Degradation–Mineralization of Cationic and Anionic Dyes. Catalysts 2024, 14, 883. https://doi.org/10.3390/catal14120883
Boudechiche N, Morante N, Sannino D, Monzillo K, Trari M, Sadaoui Z. Enhanced Visible-Light Photocatalysis Activity of TiO2/Ag Nanocomposites Prepared by the Ultrasound-Assisted Sol–Gel Method: Characterization and Degradation–Mineralization of Cationic and Anionic Dyes. Catalysts. 2024; 14(12):883. https://doi.org/10.3390/catal14120883
Chicago/Turabian StyleBoudechiche, Noreddine, Nicola Morante, Diana Sannino, Katia Monzillo, Mohamed Trari, and Zahra Sadaoui. 2024. "Enhanced Visible-Light Photocatalysis Activity of TiO2/Ag Nanocomposites Prepared by the Ultrasound-Assisted Sol–Gel Method: Characterization and Degradation–Mineralization of Cationic and Anionic Dyes" Catalysts 14, no. 12: 883. https://doi.org/10.3390/catal14120883
APA StyleBoudechiche, N., Morante, N., Sannino, D., Monzillo, K., Trari, M., & Sadaoui, Z. (2024). Enhanced Visible-Light Photocatalysis Activity of TiO2/Ag Nanocomposites Prepared by the Ultrasound-Assisted Sol–Gel Method: Characterization and Degradation–Mineralization of Cationic and Anionic Dyes. Catalysts, 14(12), 883. https://doi.org/10.3390/catal14120883








